Neuroprotective Bioactive Compounds from Marine Algae and Their By-Products Against Cerebral Ischemia–Reperfusion Injury: A Comprehensive Review
Abstract
1. Introduction
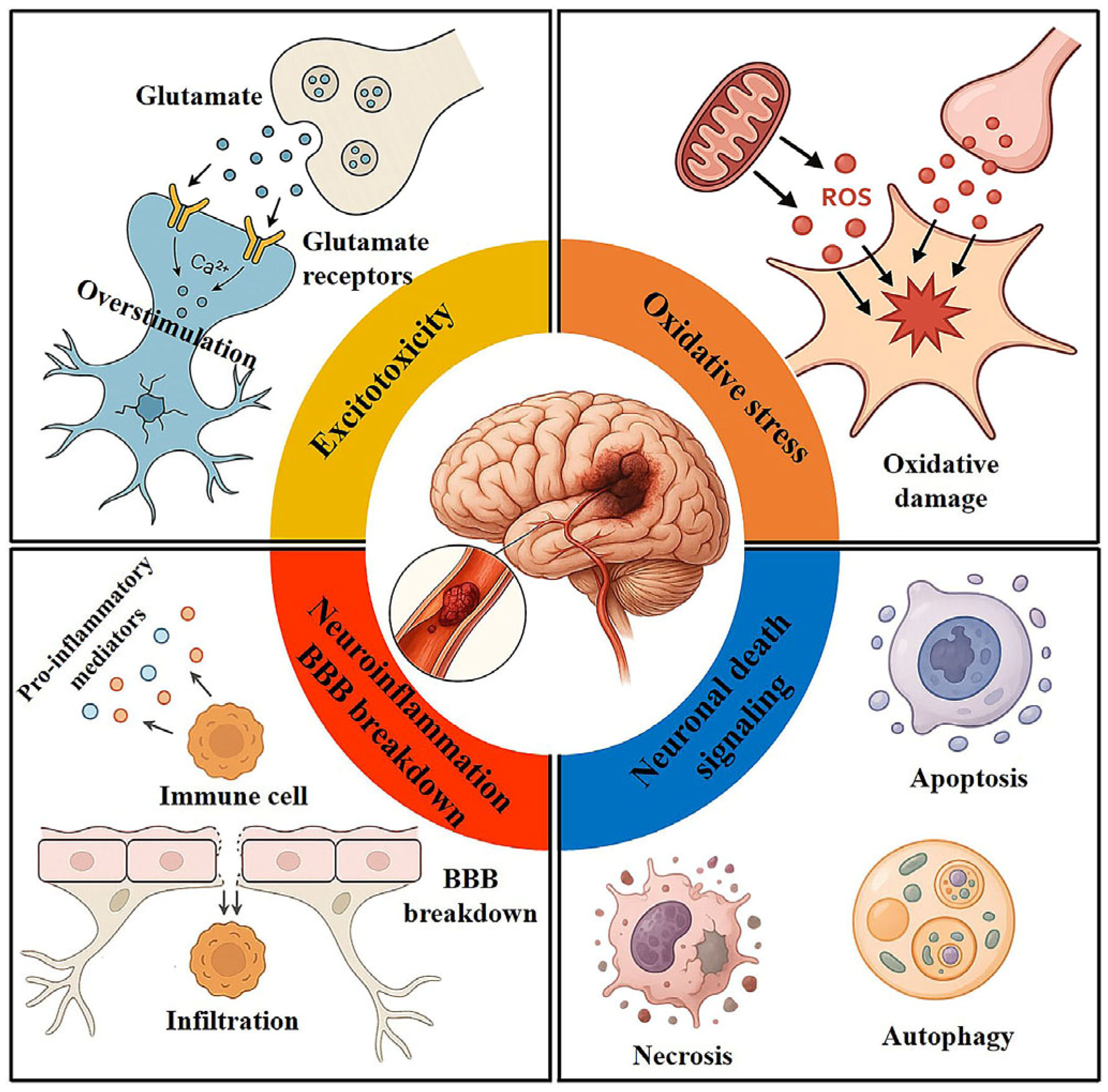
2. Pathophysiology of Cerebral I/R Injury
2.1. Excitotoxicity
2.2. Oxidative Stress
2.3. Inflammatory Response
2.4. Apoptosis and Necrosis
2.5. Autophagy
2.6. BBB Breakdown
2.7. Other Pathophysiological Mechanisms
3. Neuroprotective Effects and Underlying Mechanisms of Bioactive Compounds Found in Marine Algae
3.1. Polysaccharides
3.1.1. Fucoidan
3.1.2. Laminarin
3.1.3. Porphyran
| Compound | Algal Origin (If Any) | Effective Dose | Experimental Model | Significant Findings | Signaling Pathway | Pharmacological Markers | Reference |
|---|---|---|---|---|---|---|---|
| Fucoidan | Brown algae | 50 mg/kg | Rat model of transient focal cerebral I/R with LPS | ↓Infarct volume; ↓neutrophil infiltration; ↓inflammation | N/A | ↓MPO; ↓TNF-α and IL-8 | [76] |
| Fucoidan | Brown algae | 80 and 160 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓inflammation; ↓oxidative; ↓apoptosis | MAPK pathway | ↓IL-1β, IL-6, TNF-α, and MPO; ↓MDA; ↑SOD; ↓phospho-p53 and Bax; ↑Bcl-2; ↑phospho-p38, phospho-ERK, and phospho-JNK | [74] |
| Fucoidan | Fucus vesiculosus (brown algae) | 50 mg/kg | Gerbil model of transient global cerebral I/R | ↓Hyperactivity; ↓neuronal death; ↓glial cell activation; ↓oxidative stress | N/A | ↑GFAP and Iba-1; ↓DHE and 4-HNE; ↑SOD1 and SOD2 | [19] |
| Fucoidan | Fucus vesiculosus (brown algae) | 50 mg/kg | Obese gerbil model of transient global cerebral I/R | ↓Neuronal death; ↓oxidative stress | N/A | ↓DHE, 8-OHG, and 4-HNE; ↑SOD1 and SOD2 | [73] |
| Fucoidan + cerebrolysin | Fucus vesiculosus (brown algae) | 50 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓BBB integrity; ↓inflammation | N/A | ↓Evans blue dye extravasation; ↓TNF-α, NF-κB, IL-1α, IL-1β, IL-6, Iba-1, CD68, and COX-2; ↑IL-10 and CD31 | [75] |
| Fucoidan + enriched environment | Brown algae | 50 mg/kg | Rat model of transient global cerebral I/R | ↑Cognitive deficits; ↓neuronal death; ↓inflammation; ↓oxidative stress; ↑synaptic markers | N/A | ↓GFAP, IL-1β, IL-6, NF-κB, and TNF-α; ↓LPO; ↑SOD, CAT, GSH, GST, and GPX; ↑BDNF, SYP, and PSD-95 | [77] |
| Laminarin | Laminaria digitate (brown algae) | 50 and 100 mg/kg | Gerbil model of transient global cerebral I/R | ↓Neuronal death; ↓glial cell activation | N/A | ↓GFAP and Iba-1 | [79] |
| Laminarin | Laminaria digitate (brown algae) | 50 mg/kg | Aged gerbil model of transient global cerebral I/R | ↓Neuronal death; ↓oxidative stress; ↓inflammation | N/A | ↓DHE and 4-HNE; ↑SOD1 and SOD2; ↓TNF-α and IL-1β; ↑IL-4 and IL-13 | [80] |
| Laminarin | Brown algae | 0.5, 2.5, and 5 µg/mL | OGD/R model in PC12 cells | ↑Cell viability; ↓oxidative stress; ↓inflammation ↓apoptosis | PI3K/Akt pathway | ↑PCNA and Ki67; ↓ROS, LDH, and MPO; ↓TNF-α, IL-1β, and IL-6; ↓Bax and caspase-3; ↑Bcl-2; ↑PI3K, phospho-AKT, and PTEN | [78] |
| Laminarin | - | 10 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; transcriptomic changes | N/A | N/A | [81] |
| Porphyran | Porphyra yezoensis (red algae) | 100 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓oxidative stress; ↓inflammation | N/A | ↑SOD, CAT, and GSH; ↓IL-1β, IL-6, TNF-α, and nuclear NF-κB | [82] |
| Porphyran | - | 50 mg/kg | Gerbil model of transient global cerebral I/R | ↓Hyperactivity; ↓neuronal death; ↓microglial activation and proliferation; ↓inflammation | N/A | ↓Iba-1; ↑NLRP3, ASC, cleaved caspase-1, IL-1β, and IL-18 | [83] |
| Porphyran | - | 50 mg/kg | Gerbil model of transient global cerebral I/R | ↑Cognitive function; ↓cholinergic dysfunction; ↓microglial activation; ↓inflammation; ↓BBB leakage | N/A | ↓Ach; ↓Iba-1, IL-1β, IL-6, and TNF-α; ↓IgG | [84] |
3.2. Carotenoids
3.2.1. Astaxanthin
3.2.2. Fucoxanthin
3.2.3. Lutein
3.2.4. Zeaxanthin
| Compound | Algal Origin (If Any) | Effective Dose | Experimental Model | Significant Findings | Signaling Pathway | Pharmacological Markers | Reference |
|---|---|---|---|---|---|---|---|
| Astaxanthin | - | 0.1 mM in 20 μL | Rat model of transient focal cerebral I/R | ↑Locomotor activity; ↓infarct volume; ↓oxidative stress; ↓excitotoxicity; ↓apoptosis | N/A | ↓Aconitase and MDA; ↓glutamate; ↓TUNEL and Cyt c | [87] |
| Astaxanthin | - | 50 and 80 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; | N/A | N/A | [88] |
| Astaxanthin | - | 10, 25, 50, and 100 µM | OGD/R model in SH-SY5Y cells | ↑Cell viability; ↓oxidative stress | N/A | ↓Nitrite and iNOS; ↑HO-1and Hsp70↑ | [89] |
| 30 mg/kg | Rat model of transient global cerebral I/R | ↓Neuronal death; ↓apoptosis | N/A | ↓PARP-1 | |||
| Astaxanthin | - | 5 and 10 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓oxidative stress; ↓apoptosis; ↑neural regeneration | Nrf2-ARE pathway | ↓MDA; ↑SOD; ↑Nrf2, HO-1, and NQO1; ↓Bax; ↑Bcl-2; ↑GFAP, MAP-2, BDNF, and GAP43 | [92] |
| Astaxanthin | - | 10 mg/kg | Mouse model of repeated cerebral I/R | ↑Learning and memory; ↓neuronal death; ↓oxidative stress; ↓apoptosis | N/A | ↓MDA; ↑GSH and SOD; ↓Cyt c, cleaved caspase-3, and Bax; ↑Bcl-2 | [91] |
| Astaxanthin | - | 20, 40, and 80 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓cerebral edema; ↓oxidative stress; ↑neurotrophic factors | N/A | ↓MDA; ↑SOD, CAT, and GPX; ↑BDNF and NGF | [93] |
| Astaxanthin | - | 30 mg/kg | Mouse model of permanent focal cerebral ischemia | ↑Motor function; ↑axonal regeneration and reconnection | cAMP/PKA/CREB pathway | ↑GAP43; ↑cAMP, PKA, and phospho-CREB | [94] |
| Astaxanthin | - | 25 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓neuronal loss; ↓oxidative stress; ↓inflammation; ↓apoptosis | N/A | ↑TAS, Nrf2, and Hsp70; ↓TOS, OSI, LPO, 8-OHdG, and AOPP; ↓MPO, TNF-α, and IL-6; ↓caspase-3, -8, and -9 | [95] |
| Astaxanthin | - | 5, 10, 20, and 40 µM | OGD/R model in SH-SY5Y cells | ↑Cell viability; ↓oxidative stress; ↓apoptosis | PI3K/Akt/GSK3β/Nrf2 pathway | ↓ROS and MDA; ↑SOD; ↓Bax and cleaved caspase-3; ↑Bcl-2; ↑phospho-GSK3β, phospho-AKT, nuclear Nrf2, and HO-1 | [90] |
| Astaxanthin | - | 100 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓brain edema; ↓infarct volume; ↓oxidative stress; ↓inflammation; ↓apoptosis | Nrf2/HO-1 pathway | ↓MDA; ↑CAT, SOD, and GPX; ↓TNF-α, IL-1β, and IL-6; ↓Bax; ↑Bcl-2, ↑nuclear Nrf2 and HO-1; ↓cytosolic Nrf2 | [96] |
| Astaxanthin | - | 25, 45, and 65 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓oxidative stress; ↓inflammation; ↓apoptosis; ↓excitotoxicity | N/A | ↓MDA and TOS; ↑GSH, CAT, GPX, and SOD; ↓TNF-α, and NF-κB; ↓p53, PUMA, Bax, and caspase-3; ↑Bcl-2; ↑GLT-1 | [97] |
| Astaxanthin | - | 100 mg/kg | Gerbil model of transient global cerebral I/R | ↓Neuronal death; ↓oxidative stress | N/A | ↓8-OHdG and 4-HNE; ↑SOD1 and SOD2 | [20] |
| Fucoxanthin | - | 5, 10, and 20 µM | OGD/R model in rat cortical neurons | ↓Apoptosis; ↓oxidative stress | Nrf2/HO-1 pathway | ↓Bax, and cleaved caspase-3; ↑Bcl-2; ↓ROS and MDA; ↑SOD; ↑nuclear Nrf2 and HO-1 | [98] |
| 30, 60, and 90 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓brain edema ↓apoptosis; ↓oxidative stress; | Nrf2/HO-1 pathway | ↓Bax, and cleaved caspase-3; ↑Bcl-2; ↑SOD; ↑nuclear Nrf2 and HO-1 | |||
| Apo-9′-fucoxanthinone | Sargassum fusiforme (brown algae) | 2.5, 5, and 10 µM | OGD/R model in SH-SY5Y cells | ↑Cell viability; ↓apoptosis | PI3K/Akt/GSK3β pathway | ↓Bax, and cleaved caspase-3; ↑Bcl-2; ↑phospho-PI3K, phospho-Akt, and phospho-GSK3β | [99] |
| 15 and 30 mg/kg | Mouse model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓inflammation; ↓apoptosis | PI3K/Akt/GSK3β pathway | ↓IL-1β, IL-6, IKK, and nuclear NF-κB; ↑IκB; ↓Bax, cleaved caspase-3, and TUNEL; ↑Bcl-2; ↑phospho-PI3K, phospho-Akt, and phospho-GSK3β | |||
| Lutein | - | 0.2 mg/kg | Mouse model of transient focal cerebral I/R | ↑Survival rate; ↓infarct volume; ↓apoptosis; ↓oxidative/nitrosative stress; ↓inflammation | PI3K/Akt, MAPK/ERK, and NF-κB pathways | ↓TUNEL; ↑Bcl-2; ↓NT and PAR; ↓COX-2, phospho-IκB, and nuclear NF-κB; ↑Hsp70 and phospho-Akt; ↓phospho-ERK | [103] |
| Lutein | - | 7.5, 15, and 30 mg/kg | Mouse model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓apoptosis; ↓oxidative stress | N/A | ↓TUNEL; ↑GSH, SOD, GPX, and CAT; ↓MDA, protein carbonyl content, and 8-OHdG | [104] |
| Zeaxanthin | - | 2 mg/kg | Mouse model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓brain edema; ↓oxidative stress | N/A | ↑BAP; ↓d-ROMs and hydroperoxide | [107] |
3.3. Polyphenols
3.3.1. Dieckol
3.3.2. Phlorotannins from Ecklonia cava
3.4. Sterols: β-sitosterol
| Compound | Algal Origin (If Any) | Effective Dose | Experimental Model | Significant Findings | Signaling Pathway | Pharmacological Markers | Reference |
|---|---|---|---|---|---|---|---|
| Dieckol | Ecklonia cava (brown algae) | 10, 20, 30, 40, and 50 µM | Glutamate excitotoxicity model in primary cortical neurons and HT22 neurons | ↑Cell viability; ↓morphological deterioration; ↓oxidative stress; ↓mitochondrial dysfunction | Nrf2/HO-1 pathway | ↓Intracellular ROS; ↑ATP and ΔΨm; ↓mitochondrial Ca2+ overload and ROS generation; ↑nuclear Nrf2 and HO-1 | [18] |
| Phlorotannin-rich extract | Ecklonia cava (brown algae) | 10 and 50 mg/kg | Rat model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓brain edema; ↓apoptosis | N/A | ↓TUNEL | [110] |
| β-sitosterol | - | 0.1, 1, and 10 µM | OGD/R model in primary cortical neurons | ↑Neuronal activity; ↓LDH release; ↓apoptosis | N/A | ↓LDH; ↓Annexin V | [112] |
| 2, 10, and 50 mg/kg | Mouse model of transient focal cerebral I/R | ↓Neurological deficits; ↓infarct volume; ↓brain edema; ↓endoplasmic reticulum stress; ↓cholesterol-induced apoptosis | cholesterol overload/endoplasmic reticulum stress/apoptosis pathways | ↓TUNEL; ↓GRP78/Bip, caspase-12, and caspase-3; ↓Bax; ↑Bcl-2; ↓phospho-JNK2, phospho-STAT3, NPC1L1, and cholesterol |
4. Future Perspectives
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| Akt | Protein kinase B |
| AOPP | Advanced oxidation protein products |
| ARE | Antioxidant response element |
| ASC | Apoptosis-associated speck-like protein containing a caspase recruitment domain |
| Bax | Bcl-2 associated X protein |
| BBB | Blood–brain barrier |
| Bcl-2 | B-cell lymphoma-2 |
| BDNF | brain-derived neurotrophic factor |
| cAMP | Cyclic adenosine monophosphate |
| CD68 | Cluster of differentiation 68 |
| COX | Cyclooxygenase |
| CREB | cAMP-response element-binding protein |
| Cyt c | Cytochrome c |
| DHE | Dihydroethidium |
| ΔΨm | Mitochondrial membrane potential |
| ERK | Extracellular signal-regulated kinase |
| FasL | Fas ligand |
| GAP43 | Growth-associated protein 43 |
| GFAP | Glial fibrillary acidic protein |
| GLT-1 | Glutamate transporter-1 |
| GPX | Glutathione peroxidase |
| GRP78 | Glucose-regulated protein 78 |
| Bip | Binding immunoglobulin protein |
| GSH | Glutathione |
| GST | Glutathione-s-transferase |
| GSK3β | Glycogen synthase kinase 3β |
| HO-1 | Heme oxygenase-1 |
| Hsp70 | Heat shock protein 70 |
| I/R | Ischemia–reperfusion |
| Iba-1 | Ionized calcium binding adapter molecule 1 |
| IKK | Inhibitory kappa B kinase |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IκB | Inhibitory kappa B |
| JNK | c-Jun N-terminal kinase |
| LC3 | Microtubule-associated protein light chain 3 |
| LDH | Lactate dehydrogenase |
| LPO | Lipid peroxidation |
| LPS | Lipopolysaccharide |
| MAP-2 | Microtubule-associated protein 2 |
| MAPKs | Mitogen-activated protein kinases |
| MDA | Malondialdehyde |
| MOMP | Mitochondrial outer membrane permeabilization |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor-kappa B |
| NGF | Nerve growth factor |
| NLRP3 | Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing protein-3 |
| NPC1L1 | Niemann-Pick C1 like 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OGD/R | Oxygen glucose deprivation-reoxygenation |
| OSI | Oxidative stress index |
| PARP-1 | Poly(ADP-ribose) polymerase 1 |
| PCNA | Proliferating cell nuclear antigen |
| PI3K | Phosphoinositide 3-kinase |
| PKA | Protein kinase A |
| PSD-95 | Postsynaptic density protein 95 |
| PTEN | Phosphatase and tensin homolog |
| PUMA | p53 upregulated modulator of apoptosis |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| SYP | Synaptophysin |
| TAS | Total antioxidant status |
| TNF | Tumor necrosis factor |
| TOS | Total oxidant status |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| ULK1 | Unc-51 like autophagy activating kinase 1 |
| 4-HNE | 4-hydroxy-2-noneal |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| 8-OHG | 8-hydroxyguanine |
References
- Pu, L.; Wang, L.; Zhang, R.; Zhao, T.; Jiang, Y.; Han, L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke 2023, 54, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Lee, D.Y.; Basith, S.; Manavalan, B.; Paik, M.J.; Rybinnik, I.; Mouradian, M.M.; Ahn, J.H.; Lee, G. Metabolome Changes in Cerebral Ischemia. Cells 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Grabb, M.C.; Zipfel, G.J.; Choi, D.W. Brain tissue responses to ischemia. J. Clin. Investig. 2000, 106, 723–731. [Google Scholar] [CrossRef]
- Awasthi, V.A.; Dhankar, V.; Singh, S. Novel therapeutic targets for reperfusion injury in ischemic stroke: Understanding the role of mitochondria, excitotoxicity and ferroptosis. Vascul Pharmacol. 2024, 156, 107413. [Google Scholar] [CrossRef]
- Park, C.W.; Ahn, J.H.; Lee, T.K.; Park, Y.E.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Park, Y.; Cho, J.H.; et al. Post-treatment with oxcarbazepine confers potent neuroprotection against transient global cerebral ischemic injury by activating Nrf2 defense pathway. Biomed. Pharmacother. 2020, 124, 109850. [Google Scholar] [CrossRef]
- Ugale, R.; Vatte, S.; Girdhar, P.; Anandani, D. Deferoxamine prevents BBB disruption, neuroinflammation and apoptotic changes in early hours of ischemic reperfusion injury. Neurochem. Int. 2025, 188, 106009. [Google Scholar] [CrossRef]
- Sweeney, M.I.; Yager, J.Y.; Walz, W.; Juurlink, B.H. Cellular mechanisms involved in brain ischemia. Can. J. Physiol. Pharmacol. 1995, 73, 1525–1535. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, M.; Wang, Y.; Wang, Q.; Wu, J. Cell Death Mechanisms in Cerebral Ischemia-Reperfusion Injury. Neurochem. Res. 2022, 47, 3525–3542. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.; Li, H.; Zhao, R.; Huang, Q.; Liu, J. Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur. J. Med. Chem. 2019, 162, 132–146. [Google Scholar] [CrossRef]
- Shehzad, U. Medical Management of Acute Cerebral Ischemia. Dela J. Public. Health 2023, 9, 20–26. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Yu, Z. Ischemia-reperfusion Injury in the Brain: Mechanisms and Potential Therapeutic Strategies. Biochem. Pharmacol. 2016, 5, 213. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020, 216, 107695. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tao, J.; Zhao, Q.; Xu, C.; Zhang, Q. Confirmation of potential neuroprotective effects of natural bioactive compounds from traditional medicinal herbs in cerebral ischemia treatment. J. Integr. Neurosci. 2020, 19, 373–384. [Google Scholar] [CrossRef]
- Cadar, E.; Popescu, A.; Dragan, A.M.; Pesterau, A.M.; Pascale, C.; Anuta, V.; Prasacu, I.; Velescu, B.S.; Tomescu, C.L.; Bogdan-Andreescu, C.F.; et al. Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Mar. Drugs 2025, 23, 152. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef]
- Cui, Y.; Amarsanaa, K.; Lee, J.H.; Rhim, J.K.; Kwon, J.M.; Kim, S.H.; Park, J.M.; Jung, S.C.; Eun, S.Y. Neuroprotective mechanisms of dieckol against glutamate toxicity through reactive oxygen species scavenging and nuclear factor-like 2/heme oxygenase-1 pathway. Korean J. Physiol. Pharmacol. 2019, 23, 121–130. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, J.H.; Song, M.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Kim, J.D.; Cho, J.H.; Hwang, I.K.; et al. Pretreated fucoidan confers neuroprotection against transient global cerebral ischemic injury in the gerbil hippocampal CA1 area via reducing of glial cell activation and oxidative stress. Biomed. Pharmacother. 2019, 109, 1718–1727. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, T.K.; Kim, D.W.; Ahn, J.H.; Lee, C.H.; Kim, J.D.; Shin, M.C.; Cho, J.H.; Lee, J.C.; Won, M.H.; et al. Astaxanthin Confers a Significant Attenuation of Hippocampal Neuronal Loss Induced by Severe Ischemia-Reperfusion Injury in Gerbils by Reducing Oxidative Stress. Mar. Drugs 2022, 20, 267. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Paschen, W. Glutamate excitotoxicity in transient global cerebral ischemia. Acta Neurobiol Exp (Wars) 1996, 56, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Bano, D.; Nicotera, P. Ca2+ signals and neuronal death in brain ischemia. Stroke 2007, 38, 674–676. [Google Scholar] [CrossRef]
- Neves, D.; Salazar, I.L.; Almeida, R.D.; Silva, R.M. Molecular mechanisms of ischemia and glutamate excitotoxicity. Life Sci. 2023, 328, 121814. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Sugawara, T.; Chan, P.H. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid. Redox Signal 2003, 5, 597–607. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Chan, P.H. Role of oxidants in ischemic brain damage. Stroke 1996, 27, 1124–1129. [Google Scholar] [CrossRef]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, J.; Hosseini, L.; Mobed, A.; Zangbar, H.S.; Jafarzadeh, J.; Pasban, J.; Shahabi, P. The Impact of Cerebral Ischemia on Antioxidant Enzymes Activity and Neuronal Damage in the Hippocampus. Cell Mol. Neurobiol. 2023, 43, 3915–3928. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef]
- Kawabori, M.; Yenari, M.A. Inflammatory responses in brain ischemia. Curr. Med. Chem. 2015, 22, 1258–1277. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: Critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol. Dis. 2010, 38, 376–385. [Google Scholar] [CrossRef]
- Shichita, T.; Sakaguchi, R.; Suzuki, M.; Yoshimura, A. Post-ischemic inflammation in the brain. Front. Immunol. 2012, 3, 132. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, T.K.; Kim, D.W.; Ahn, J.H.; Shin, M.C.; Cho, J.H.; Won, M.H.; Kang, I.J. Neuroprotective Effects of Aucubin against Cerebral Ischemia and Ischemia Injury through the Inhibition of the TLR4/NF-kappaB Inflammatory Signaling Pathway in Gerbils. Int. J. Mol. Sci. 2024, 25, 3461. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Sciacca, R.; Di Raimondo, D.; Renda, C.; Pinto, A.; Licata, G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr. Top. Med. Chem. 2009, 9, 1240–1260. [Google Scholar] [CrossRef]
- Unal-Cevik, I.; Kilinc, M.; Can, A.; Gursoy-Ozdemir, Y.; Dalkara, T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke 2004, 35, 2189–2194. [Google Scholar] [CrossRef]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef]
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef]
- Rami, A.; Kogel, D. Apoptosis meets autophagy-like cell death in the ischemic penumbra: Two sides of the same coin? Autophagy 2008, 4, 422–426. [Google Scholar] [CrossRef]
- Aboutaleb, N.; Shamsaei, N.; Khaksari, M.; Erfani, S.; Rajabi, H.; Nikbakht, F. Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation. J. Physiol. Sci. 2015, 65, 435–443. [Google Scholar] [CrossRef]
- Yaidikar, L.; Thakur, S. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol. Cell Biochem. 2015, 402, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Luo, Y.; Nagayama, T.; Pei, W.; Stetler, R.A.; Graham, S.H.; Chen, J. Cloning and characterization of rat caspase-9: Implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia. J. Cereb. Blood Flow. Metab. 2002, 22, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Noshita, N.; Sugawara, T.; Fujimura, M.; Morita-Fujimura, Y.; Chan, P.H. Manganese Superoxide Dismutase Affects Cytochrome c Release and Caspase-9 Activation After Transient Focal Cerebral Ischemia in Mice. J. Cereb. Blood Flow. Metab. 2001, 21, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Klopfenstein, J.D.; Gujrati, M.; Rao, J.S.; Veeravalli, K.K. Temporal regulation of apoptotic and anti-apoptotic molecules after middle cerebral artery occlusion followed by reperfusion. Mol. Neurobiol. 2014, 49, 50–65. [Google Scholar] [CrossRef]
- Plesnila, N.; Zhu, C.; Culmsee, C.; Groger, M.; Moskowitz, M.A.; Blomgren, K. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 2004, 24, 458–466. [Google Scholar] [CrossRef]
- Kulkarni, A.; Chen, J.; Maday, S. Neuronal autophagy and intercellular regulation of homeostasis in the brain. Curr. Opin. Neurobiol. 2018, 51, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, R.; Pietrangelo, D.; Cotugno, M.; Forte, M.; Rubattu, S. Role of autophagy in ischemic stroke: Insights from animal models and preliminary evidence in the human disease. Front. Cell Dev. Biol. 2024, 12, 1360014. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, Y.; Liu, K.; Sun, X. Autophagy: A double-edged sword for neuronal survival after cerebral ischemia. Neural Regen. Res. 2014, 9, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gan, Q.; Yang, Y.; Reis, C.; Zhang, Z.; Xu, S.; Zhang, T.; Sun, C. Mitophagy in Cerebral Ischemia and Ischemia/Reperfusion Injury. Front. Aging Neurosci. 2021, 13, 687246. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, T.; Guo, J.; Liu, Y.; Cui, G.; Gu, L.; Su, L.; Zhang, Y. Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS ONE 2012, 7, e46092. [Google Scholar] [CrossRef]
- Wang, J.F.; Mei, Z.G.; Fu, Y.; Yang, S.B.; Zhang, S.Z.; Huang, W.F.; Xiong, L.; Zhou, H.J.; Tao, W.; Feng, Z.T. Puerarin protects rat brain against ischemia/reperfusion injury by suppressing autophagy via the AMPK-mTOR-ULK1 signaling pathway. Neural Regen. Res. 2018, 13, 989–998. [Google Scholar] [CrossRef]
- Hara, T.; Takamura, A.; Kishi, C.; Iemura, S.; Natsume, T.; Guan, J.L.; Mizushima, N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell Biol. 2008, 181, 497–510. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Wang, J.; Zhang, Z.; Zhai, Y.; Yang, G.Y.; Sun, X. Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem. Biophys. Res. Commun. 2014, 444, 182–188. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, H.; Bai, X.; Lu, Y.; Dong, H.; Xiong, L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 2011, 1402, 109–121. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Ronaldson, P.T.; Davis, T.P. The role of oxidative stress in blood-brain barrier disruption during ischemic stroke: Antioxidants in clinical trials. Biochem. Pharmacol. 2024, 228, 116186. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Tepper, D.; Leonard, A. Corrigendum: Matrix Metalloproteinases and Blood-Brain Barrier Disruption in Acute Ischemic Stroke. Front. Neurol. 2018, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hawkins, K.E.; Dore, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Brzica, H.; Abdullahi, W.; Ibbotson, K.; Ronaldson, P.T. Role of Transporters in Central Nervous System Drug Delivery and Blood-Brain Barrier Protection: Relevance to Treatment of Stroke. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517693802. [Google Scholar] [CrossRef]
- Kanemitsu, H.; Suematsu, M.; Ishii, T.; Aoki, M.; Nakagomi, T.; Kishino, N.; Iwasawa, T.; Iwahashi, M.; Tamura, A. [Changes in acetylcholine level and its related enzyme activities in rat brain following focal ischemia]. No Shinkei 1998, 50, 39–44. [Google Scholar]
- Qu, J.F.; Chen, Y.K.; Luo, G.P.; Zhao, J.H.; Zhong, H.H.; Yin, H.P. Severe Lesions Involving Cortical Cholinergic Pathways Predict Poorer Functional Outcome in Acute Ischemic Stroke. Stroke 2018, 49, 2983–2989. [Google Scholar] [CrossRef]
- Rosenzweig, S.; Carmichael, S.T. Age-dependent exacerbation of white matter stroke outcomes: A role for oxidative damage and inflammatory mediators. Stroke 2013, 44, 2579–2586. [Google Scholar] [CrossRef]
- Stanzione, R.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Forte, M.; Volpe, M.; Rubattu, S. Pathogenesis of Ischemic Stroke: Role of Epigenetic Mechanisms. Genes. 2020, 11, 89. [Google Scholar] [CrossRef]
- Jung, J.E.; Kim, G.S.; Chen, H.; Maier, C.M.; Narasimhan, P.; Song, Y.S.; Niizuma, K.; Katsu, M.; Okami, N.; Yoshioka, H.; et al. Reperfusion and neurovascular dysfunction in stroke: From basic mechanisms to potential strategies for neuroprotection. Mol. Neurobiol. 2010, 41, 172–179. [Google Scholar] [CrossRef]
- Zhu, J.; Mo, J.; Liu, K.; Chen, Q.; Li, Z.; He, Y.; Chang, Y.; Lin, C.; Yu, M.; Xu, Y.; et al. Glymphatic System Impairment Contributes to the Formation of Brain Edema After Ischemic Stroke. Stroke 2024, 55, 1393–1404. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Shin, M.C.; Kim, D.W.; Kim, H.; Song, M.; Lee, T.K.; Lee, J.C.; Kim, H.; Cho, J.H.; Kim, Y.M.; et al. Antioxidant Properties of Fucoidan Alleviate Acceleration and Exacerbation of Hippocampal Neuronal Death Following Transient Global Cerebral Ischemia in High-Fat Diet-Induced Obese Gerbils. Int. J. Mol. Sci. 2019, 20, 554. [Google Scholar] [CrossRef] [PubMed]
- Che, N.; Ma, Y.; Xin, Y. Protective Role of Fucoidan in Cerebral Ischemia-Reperfusion Injury through Inhibition of MAPK Signaling Pathway. Biomol. Ther. 2017, 25, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Nambi, P.; Sathyamoorthy, Y.; Kaliyappan, K.; Radhakrishnan, R. Fucoidan (A Sulfated Polysaccharide) and Cerebroprotein in Combination Alleviate the Neuroinflammation-mediated Neural Damage and Functional Deficits in the Focal Cerebral Ischemia Model of Rat. Neuroscience 2023, 524, 52–64. [Google Scholar] [CrossRef]
- Kang, G.H.; Yan, B.C.; Cho, G.S.; Kim, W.K.; Lee, C.H.; Cho, J.H.; Kim, M.; Kang, I.J.; Won, M.H.; Lee, J.C. Neuroprotective effect of fucoidin on lipopolysaccharide accelerated cerebral ischemic injury through inhibition of cytokine expression and neutrophil infiltration. J. Neurol. Sci. 2012, 318, 25–30. [Google Scholar] [CrossRef]
- Kharkongor, R.; Stephen, J.; Khan, U.; Radhakrishnan, R. Exposure to an enriched environment and fucoidan supplementation ameliorate learning and memory function in rats subjected to global cerebral ischemia. Neurosci. Lett. 2025, 847, 138094. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Zhao, W.; Wang, D. Laminarin alleviates the ischemia/reperfusion injury in PC12 cells via regulation of PTEN/PI3K/AKT pathway. Adv. Polym. Technol. 2022, 2022, 9999339. [Google Scholar] [CrossRef]
- Lee, T.K.; Ahn, J.H.; Park, C.W.; Kim, B.; Park, Y.E.; Lee, J.C.; Park, J.H.; Yang, G.E.; Shin, M.C.; Cho, J.H.; et al. Pre-Treatment with Laminarin Protects Hippocampal CA1 Pyramidal Neurons and Attenuates Reactive Gliosis Following Transient Forebrain Ischemia in Gerbils. Mar. Drugs 2020, 18, 52. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, J.H.; Lee, T.K.; Park, C.W.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Cho, J.H.; Lee, C.H.; et al. Laminarin Pretreatment Provides Neuroprotection against Forebrain Ischemia/Reperfusion Injury by Reducing Oxidative Stress and Neuroinflammation in Aged Gerbils. Mar. Drugs 2020, 18, 213. [Google Scholar] [CrossRef]
- Luo, J.; Chen, D.; Qin, B.; Kong, D. Molecular mechanisms for the prevention and promoting the recovery from ischemic stroke by nutraceutical laminarin: A comparative transcriptomic approach. Front. Nutr. 2022, 9, 999426. [Google Scholar] [CrossRef]
- Sun, C.; Wu, F.; Chen, D.; Ge, J. Therapeutic effects of polysaccharides extracted from Porphyra yezoensis in rats with cerebral ischemia/reperfusion injury. Arch. Biol. Sci. 2018, 70, 233–239. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, T.K.; Ahn, J.H.; Yang, S.R.; Shin, M.C.; Cho, J.H.; Won, M.H.; Kang, I.J.; Park, J.H. Porphyran Attenuates Neuronal Loss in the Hippocampal CA1 Subregion Induced by Ischemia and Reperfusion in Gerbils by Inhibiting NLRP3 Inflammasome-Mediated Neuroinflammation. Mar. Drugs 2024, 22, 170. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, T.K.; Park, J.H.; Kim, D.W.; Lee, C.H.; Won, M.H.; Kang, I.J. Therapeutic potential of porphyran in mitigating ischemia-reperfusion injury in gerbil hippocampus. Histol. Histopathol. 2025, in press. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kuo, C.C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef]
- Lu, Y.P.; Liu, S.Y.; Sun, H.; Wu, X.M.; Li, J.J.; Zhu, L. Neuroprotective effect of astaxanthin on H(2)O(2)-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, Y.J.; Kwon, K.H. Neuroprotective Effects of Astaxanthin in Oxygen-Glucose Deprivation in SH-SY5Y Cells and Global Cerebral Ischemia in Rat. J. Clin. Biochem. Nutr. 2010, 47, 121–129. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, C.; Zhang, S.; Xu, Y. Neuroprotective effects of astaxanthin against oxygen and glucose deprivation damage via the PI3K/Akt/GSK3beta/Nrf2 signalling pathway in vitro. J. Cell Mol. Med. 2020, 24, 8977–8985. [Google Scholar] [CrossRef]
- Xue, Y.; Qu, Z.; Fu, J.; Zhen, J.; Wang, W.; Cai, Y.; Wang, W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bull. 2017, 131, 221–228. [Google Scholar] [CrossRef]
- Pan, L.; Zhou, Y.; Li, X.F.; Wan, Q.J.; Yu, L.H. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res. Bull. 2017, 130, 211–220. [Google Scholar] [CrossRef]
- Nai, Y.; Liu, H.; Bi, X.; Gao, H.; Ren, C. Protective effect of astaxanthin on acute cerebral infarction in rats. Hum. Exp. Toxicol. 2018, 37, 929–936. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhu, X.L.; Sun, M.H.; Dang, Y.K. Effects of astaxanthin onaxonal regeneration via cAMP/PKA signaling pathway in mice with focal cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cakir, E.; Cakir, U.; Tayman, C.; Turkmenoglu, T.T.; Gonel, A.; Turan, I.O. Favorable Effects of Astaxanthin on Brain Damage due to Ischemia- Reperfusion Injury. Comb. Chem. High. Throughput Screen. 2020, 23, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.B.; Zou, M.; Zhao, L.; Zhang, Y.K. Astaxanthin attenuates acute cerebral infarction via Nrf-2/HO-1 pathway in rats. Curr. Res. Transl. Med. 2021, 69, 103271. [Google Scholar] [CrossRef] [PubMed]
- Taheri, F.; Sattari, E.; Hormozi, M.; Ahmadvand, H.; Bigdeli, M.R.; Kordestani-Moghadam, P.; Anbari, K.; Milanizadeh, S.; Moghaddasi, M. Dose-Dependent Effects of Astaxanthin on Ischemia/Reperfusion Induced Brain Injury in MCAO Model Rat. Neurochem. Res. 2022, 47, 1736–1750. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Tian, F.; Yuan, C.; Wang, H.; Yue, H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 1484–1489. [Google Scholar] [CrossRef]
- Qi, Y.; Tang, S.; Jin, S.; Wang, J.; Zhang, Y.; Xu, X.; Zhu, H.; Zhang, J.; Xu, X.; Zhao, M.; et al. Neuroprotective effect of apo-9′-fucoxanthinone against cerebral ischemia injury by targeting the PI3K/AKT/GSK-3beta pathway. Eur. J. Pharmacol. 2025, 991, 177348. [Google Scholar] [CrossRef]
- Iyer, S.; Bhat, I.; Bangera Sheshappa, M. Lutein and the Underlying Neuroprotective Promise against Neurodegenerative Diseases. Mol. Nutr. Food Res. 2024, 68, e2300409. [Google Scholar] [CrossRef]
- Montuori, E.; Lima, S.; Marchese, A.; Scargiali, F.; Lauritano, C. Lutein Production and Extraction from Microalgae: Recent Insights and Bioactive Potential. Int. J. Mol. Sci. 2024, 25, 2892. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, T.; Zhou, Z.G.; Jiang, Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. Adv. Biochem. Eng. Biotechnol. 2016, 153, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yang, D.; Fu, Z.J.; Woo, T.; Wong, D.; Lo, A.C. Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiol. Dis. 2012, 45, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Liu, T.; Dai, X.L.; Zheng, Q.S.; Hui, B.D.; Jiang, Z.F. Treatment with lutein provides neuroprotection in mice subjected to transient cerebral ischemia. J. Asian Nat. Prod. Res. 2014, 16, 1084–1093. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Roberts, J.E.; Dennison, J. The Photobiology of Lutein and Zeaxanthin in the Eye. J. Ophthalmol. 2015, 2015, 687173. [Google Scholar] [CrossRef]
- La Russa, D.; Manni, G.; Di Santo, C.; Pieroni, B.; Pellegrino, D.; Barba, F.J.; Bagetta, G.; Fallarino, F.; Montesano, D.; Amantea, D. Zeaxanthin exerts anti-inflammatory effects in vitro and provides significant neuroprotection in mice subjected to transient middle cerebral artery occlusion. PharmaNutrition 2024, 27, 100368. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins-bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, N.S.; Jeong, Y.G.; Lee, J.H.; Kim, E.J.; Han, S.Y. Protective efficacy of an Ecklonia cava extract used to treat transient focal ischemia of the rat brain. Anat. Cell Biol. 2012, 45, 103–113. [Google Scholar] [CrossRef]
- Sohn, S.I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in Seaweeds: An Overview on Biosynthesis to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Elsayed, K.N.; Radwan, M.M.; Hassan, S.H.; Abdelhameed, M.S.; Ibraheem, I.B.; Ross, S.A. Phytochemical and biological studies on some Egyptian seaweeds. Nat. Prod. Commun. 2012, 7, 1209–1210. [Google Scholar] [CrossRef]
- Tang, X.; Yan, T.; Wang, S.; Liu, Q.; Yang, Q.; Zhang, Y.; Li, Y.; Wu, Y.; Liu, S.; Ma, Y.; et al. Treatment with beta-sitosterol ameliorates the effects of cerebral ischemia/reperfusion injury by suppressing cholesterol overload, endoplasmic reticulum stress, and apoptosis. Neural Regen. Res. 2024, 19, 642–649. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H. Neuroprotective Bioactive Compounds from Marine Algae and Their By-Products Against Cerebral Ischemia–Reperfusion Injury: A Comprehensive Review. Appl. Sci. 2025, 15, 10791. https://doi.org/10.3390/app151910791
Park JH. Neuroprotective Bioactive Compounds from Marine Algae and Their By-Products Against Cerebral Ischemia–Reperfusion Injury: A Comprehensive Review. Applied Sciences. 2025; 15(19):10791. https://doi.org/10.3390/app151910791
Chicago/Turabian StylePark, Joon Ha. 2025. "Neuroprotective Bioactive Compounds from Marine Algae and Their By-Products Against Cerebral Ischemia–Reperfusion Injury: A Comprehensive Review" Applied Sciences 15, no. 19: 10791. https://doi.org/10.3390/app151910791
APA StylePark, J. H. (2025). Neuroprotective Bioactive Compounds from Marine Algae and Their By-Products Against Cerebral Ischemia–Reperfusion Injury: A Comprehensive Review. Applied Sciences, 15(19), 10791. https://doi.org/10.3390/app151910791





