New Data on Phase Composition and Geochemistry of the Muschelkalk Carbonate Rocks of the Upper Silesian Province in Poland
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Macroscopic Description
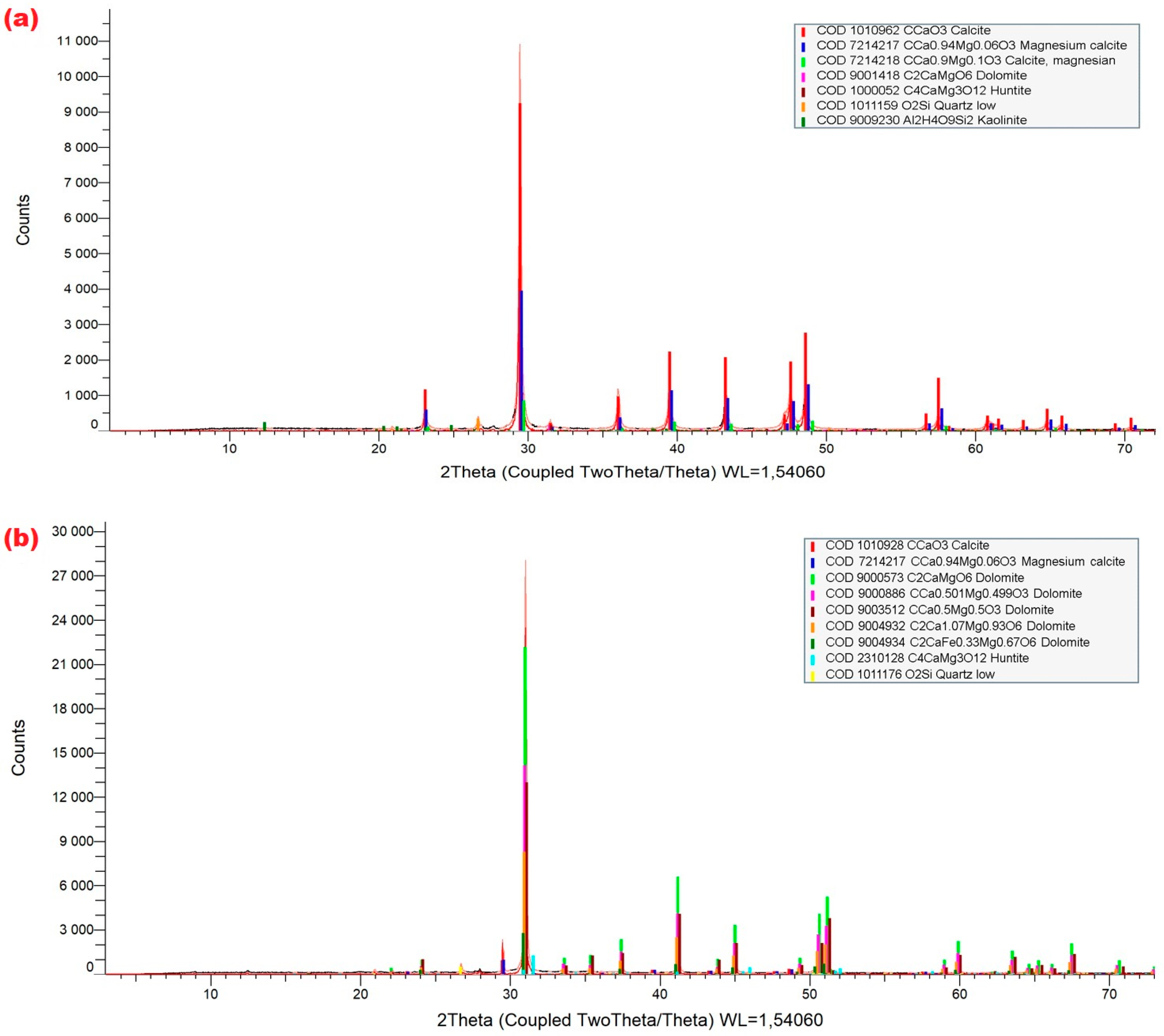
3.2. Results of X-Ray Diffraction (XRD)
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Results
3.4. Results of X-Ray Fluorescence Analysis (XRF)
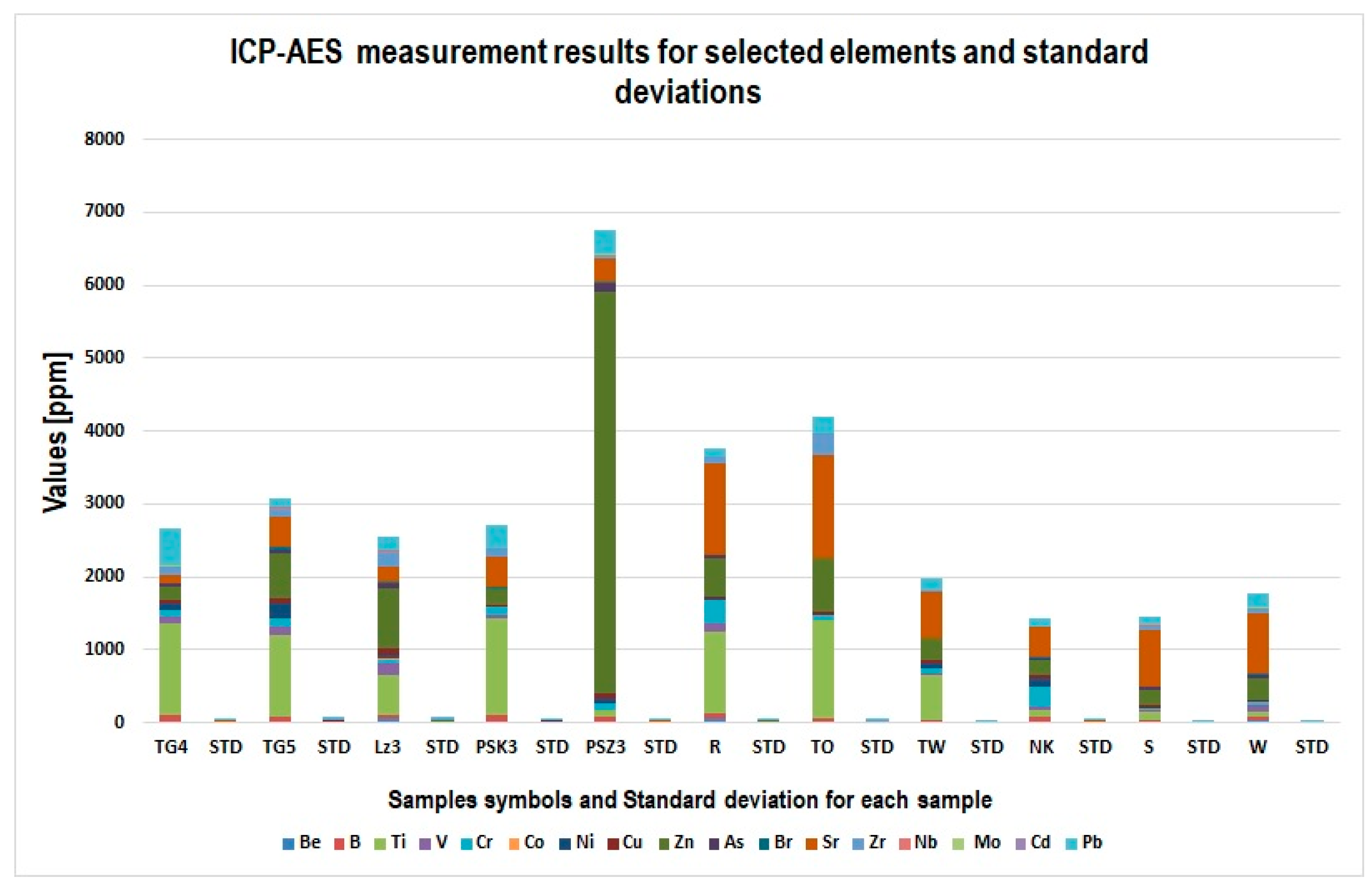
3.5. Results of ICP-AES Spectrometry
4. Discussion
4.1. Analysis of Research Results
4.2. Carbonate Phases with Magnesium
4.3. Trace Elements
4.4. Genesis of Studied Rocks Formation
5. Conclusions
- It is confirmed that the rocks of the Gogolin Unit (Lower Muschelkalk), the Diplopore Dolomite Unit (Middle Muschelkalk), the Tarnowice Unit (Upper Muschelkalk—lower part of the profile) and the Boruszowice Unit (Upper Muschelkalk—upper part of the profile) have diverse mineral compositions and carbonate phases. These results corroborate data obtained from previous studies involving selected rock samples from the Tarnowice and Boruszowice Units, as well as the Gogolin unit in Opole Silesia.
- The following mineral phases: low-magnesium calcite (CaCO3); two varieties of high-magnesium calcite: (Ca0.94Mg0.06CO3 and Ca0.9Mg0.1CO3, two varieties of ordered dolomite with the formulas: CaMg(CO3)2 and (Ca0.5Mg0.5)2(CO3)2, two types of protodolomite: Ca0.501Mg0.449)2(CO3)2 and Ca1.07Mg0.93(CO3)2, a dolomite phase with iron substitution (CaMg0.67Fe0.33)(CO3)2 and a huntite with the formula CaMg3(CO3)4 were identified.
- The following rocks were found to be limestones: the rocks of the Gogolin Unit (Twardowice, Toporowice, Świerklaniec and Niezdara-Krzyżowa), the rock from the Diplopore Dolomite Unit (Wojkowice), and one rock from the Tarnowice Unit (R). The remaining rocks of the Tarnowice Unit (Piekary Śląskie, the vicinity of the Lazarówka Quarry in Bytom and Tarnowskie Góry) and the Boruszowice Unit (TGO4, from Tarnowskie Góry) are dolomites.
- The following trace elements were identified: Al, Si, Fe, Mn, K, Na, S, Cl, Ti, Cr, Ni, Zn, Rb, Zr, Pb, As, V, Be, B, Co, Cu, Br, Mo i Cd, which indicate minerals such as silicates, aluminosilicates, oxides and sulfides. Apart from strontium (Sr) and barium (Ba), these elements are not primary components of carbonate rocks.
- Based on the data, it was concluded that calcite phases (low- and high-magnesium) were formed in carbonate mud during its compaction (the early stage of diagenesis, or the eogenetic stage), in an epicontinental, shallow, warm, stagnant marine reservoir with increased seawater salinity and the participation of meteoric waters. Some of the magnesium in the seawater came from the release of magnesium from the skeletons of dead organisms. The dolomite phases were formed during the compaction of the sediment. The dolomite mineralisation is probably the result of metasomatic processes. Huntite formed with the participation of waters from the vadose zone (aeration).
- According to the theory of the rocks origin the formation and diagenesis of the Gogolin limestones (Lower Muschelkalk) probably occurred in the deep-sea zone, the marine phreatic zone, and the shallow-sea bottom and shallow-subsurface areas (the eogenetic stage of diagenesis). In some areas, this process occurred with the participation of meteoric waters under conditions of initial marine transgression. The limestones of the Diplopore Dolomite formations (Middle Muschelkalk) probably formed and underwent diagenesis under conditions of marine regression. The dolomites of the Tarnowice Unit (Middle Muschelkalk), meanwhile, may have formed as a result of metasomatic processes, similarly to ore-bearing dolomites. The Boruszowice layers are a typical example of a formation resulting from a drying sea.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matysik, M. Reefal environments and sedimentary processes of the Anisian Karchowice Beds in Upper Silesia, Southern Poland. Ann. Soc. Geol. Pol. 2010, 80, 123–145. [Google Scholar]
- Senkowiczowa, H.; Szyperko-Śliwczyńska, A. Stratigraphy and palaeography of the Triassic. Geol. Inst. Bull. 1972, 252, 133–151. [Google Scholar]
- Senkowiczowa, H. Possibilities for formalising the lithostratigraphic division of the Middle and Upper Triassic of the Silesian-Krakow Upland. Geol. Q. 1980, 24, 787–804. [Google Scholar]
- Stanienda-Pilecki, K. Crystals structures of carbonate phases with Mg in Triassic rocks, mineral formation and transitions. Sci. Rep. 2023, 13, 18759. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K.; Jendruś, R. Geochemical and Mineralogical Characteristics of Triassic Dolomites from Upper Silesia, Poland. Minerals 2024, 14, 371. [Google Scholar] [CrossRef]
- Stanienda- Pilecki, K.; Nowińska, K.; Nowrot, A.; Szewczenko, J. Middle Triassic Limestones as a Source of Trace Elements and REY. Materials 2024, 17, 3668. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K.; Łukowiec, D. New data on the identification of magnesium-rich carbonate phases using Fourier Transform Infrared Spectroscopy. Mar. Pet. Geol. 2025, 182, 107585. [Google Scholar] [CrossRef]
- Niedźwiedzki, R. Litostratigraphy of the Górażdże Formation and the Dziewkowice Formation in Opole Silesia; Geological and Mineralogical Works of the University of Wrocław; University of Wrocław: Wrocław, Poland, 2000; Volume 71. [Google Scholar]
- Niedźwiedzki, R.; Szulc, J.; Zarankiewicz, M. Stone Treasures of the Saint Anne Region. Geological Guide. 2013. Available online: www.annaland.pl (accessed on 20 July 2025).
- Pettijohn, F.J. Sedimentary Rocks, 3rd ed.; Harper and Row: New York, NY, USA, 1975. [Google Scholar]
- Stanienda, K. Possibilities of Dolomitisation in Triassic Limestones of the ‘Tarnów Opolski’ Deposit; Silesian University of Technology Press: Gliwice, Poland, 2011; ISBN 978-83-7335-872-0. [Google Scholar]
- Stanienda, K. Diagenesis of the Triassic Limestone from the Opole Silesia in the Aspect of Magnesian Calcite Presence; Silesian University of Technology Press: Gliwice, Poland, 2013; ISBN 978-83-7880-071-2. [Google Scholar]
- Stanienda, K. Huntite in the Triassic limestones of Opolski Silesia. Miner. Resour. Manag. 2013, 9, 79–98. [Google Scholar] [CrossRef]
- Stanienda, K. Mineral phases in carbonate rocks of the Gogolin Beds from the area of Opole Silesia. Miner. Resour. Manag. 2014, 30, 17–42. [Google Scholar] [CrossRef]
- Stanienda, K. Carbonate phases rich in magnesium in the Triassic limestones of the East part of Germanic Basin. Carbonates Evaporites 2016, 31, 387–405. [Google Scholar] [CrossRef]
- Stanienda, K. Mineral phases in carbonate rocks of the Górażdże Beds from the area of Opole Silesia. Miner. Resour. Manag. 2016, 32, 67–92. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. Carbonate minerals with magnesium in Triassic Terebratula limestone in the term of limestone with magnesium application as a sorbent in desulfurization of flue gases. Arch. Min. Sci. 2017, 62, 459–482. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. Magnesium calcite in Muschelkalk limestones of the Polish part of the Germanic Basin. Carbonates Evaporites 2018, 33, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Stanienda-Pilecki, K. The Use of Limestones Built of Carbonate Phases with Increased Mg Content in Processes of Flue Gas Desulfurization. Minerals 2021, 11, 1044. [Google Scholar] [CrossRef]
- Szulc, J. International Workshop—Field Seminar The Muschelkalk-Sedimentary Environments, Facies and Diagenesis—Excursion GuideBook and Abstracts; An excursion guidebook and abstract collection from an international workshop focused on the Muschelkalk sedimentary environments. The publication was based in Kraków–Opole; Polish Geological Society: Kraków, Poland, 1990; pp. 1–32. [Google Scholar]
- Szulc, J. Early alpinie tectonics and lithofacies succesion in the Silesian part of the Muschelkalk Basin. A synopsis. In Muschelkalk; Hagdorn, H., Seilacher, A., Eds.; Goldschneck: Stuttgart, Germany, 1993; pp. 19–28. [Google Scholar]
- Szulc, J. Middle triassic evolution of the Northern Peri-Tethys area is influenced by early opening of the Tethys Ocean. Ann. Soc. Geol. Pol. 2000, 70, 1–48. [Google Scholar]
- Szulc, J. Stratigraphy and correlation with Tethys and other Germanic subbasins. In Proceedings of the International Workshop on the Triassic of Southern Poland: Pan-European Correlation of Epicontinental Triassic 4th Meeting, Fieldtrip Guide, Sosnowiec, Poland, 3–8 September 2007; Polish Geological Society: Cracow, Poland, 2007; pp. 26–28. Available online: http://www.ptgeol.pl/wp-content/uploads/Szulc-Becker_2007-Workshop.pdf (accessed on 1 September 2025).
- Szulc, J. The Triassic of the Silesia-Krakow area. In Proceedings of the 42nd Speleological Symposium, Krakow, Poland, 10 September 2008. [Google Scholar]
- Biernat, S.; Wilanowski, S.; Lewandowski, J. Detailed Geological Map of Poland. Sheet 910—Bytom (M-34-50-D); National Geological Institute: Warsaw, Poland, 1954. [Google Scholar]
- EN ISO 22475-1; Geotechnical investigation and testing—Sampling methods and groundwater measurements—Part 1: Technical principles for the sampling of soil, rock and groundwater. International Standard: Geneva, Switzerland, 2021.
- Böttcher, M.E.; Gehlken, P.L.; Steele, F.D. Characterization of inorganic and biogenic magnesian calcites by Fourier Transform infrared spectroscopy. Solid State Ion. 1997, 101, 1379–1385. [Google Scholar] [CrossRef]
- Ji, J.; Ge, Y.; Balsam, W.; Damuth, J.E.; Chen, J. Rapid identification of dolomite using a Fourier Transform Infrared Spectrophotometer (FTIR): A fast method for identifying Heinrich events in IODP Site U1308. Mar. Geol. 2009, 258, 60–68. [Google Scholar] [CrossRef]
- Ahn, D.J.; Berman, A.; Charych, D. Probing the dynamics of template-directed calcite crystallization with in situ FTIR. J. Phys. Chem. 1996, 100, 12455–12461. [Google Scholar] [CrossRef]
- Newman, R. Some application of infrared spectroscopy in the examination of painting materials. J. Am. Inst. Conserv. 1979, 19, 42–46. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Mielczarski, J.A.; Barrea, O.; Schott, J. Surface spaciation models of calcite and dolomite aqueous solution interfaces and their spectroscopic evaluation. Langmuir 2000, 16, 2677–2688. [Google Scholar] [CrossRef]
- Ramseyer, K.; Miano, T.M.; D’Orazio, V.; Wildberger, A.; Wagner, T.; Geister, J. Nature and origin of organic matter in carbonates from speleothems, marine cements and coral skeletons. Org. Geochem. 1997, 26, 361–378. [Google Scholar] [CrossRef]
- Deelman, J.C. Low-Temperature Formation of Dolomite and Magnesite; Compact Disc Publications: Eindhoven, The Netherlands, 2011; Available online: http://www.jcdeelman.demon.nl/dolomite/files/13_Chapter6.pdf (accessed on 18 March 2025).
- Boggs, S., Jr. Petrology of Sedimentary Rocks, 2nd ed.; Cambridge University Press: London, UK, 2010. [Google Scholar]
- Mackenzie, F.T.; Andersson, A.J. The Marine carbon system and ocean acidification during Phanerozoic Time. Geochem. Perspect. 2013, 2, 1–3. [Google Scholar] [CrossRef]
- Morse, J.W.; Mackenzie, F.T. Geochemistry of Sedimentary Carbonates; Elsevier: Amsterdam, The Netherlands, 1990; p. 707. [Google Scholar]
- Morse, J.W.; Andersson, A.J.; Mackenzie, F.T. Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and “ocean acidification”: Role of high Mg-calcites. Geochim. Cosmochim. Acta 2006, 70, 5814–5830. [Google Scholar] [CrossRef]
- Polański, A. Fundamentals of Geochemistry; Geological Publications: Warsaw, Poland, 1988. [Google Scholar]
- Deelman, J.C. Magnesite, Dolomite and Carbonate Groups; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2021. [Google Scholar]
- Tucker, M.E.; Wright, V.P. Carbonate Sedimentology; Blackwell Scientific Publications: Oxford, UK, 1990; pp. 366–372. [Google Scholar]
- Chilingar, G.V. Classification of limestones and dolomites on basis of Ca/Mg ratio. J. Sed. Petrol. 1957, 27, 187–189. [Google Scholar]
- Książkiewicz, M. Dynamic Geology; Geological Publications: Warsaw, Poland, 1968. [Google Scholar]
- Śliwiński, S. Dolomitisation of carbonate formations in the Silesian-Krakow region. Geol. Rev. 1981, 10, 532–535. [Google Scholar]
- Migaszewski, Z. The origin of dolomites. Geol. Q. 1990, 34, 202. [Google Scholar]
- Migaszewski, Z. Current views on the origin of dolomites. Geol. Rev. 1990, 3, 111–117. [Google Scholar]
- Gad, P.; Rosenbaum, S. Tarnowskie Góry: Underground; Department of Culture and City Promotion: Tarnowskie Góry, Poland, 2010; ISBN 978-83-61458-73-9. [Google Scholar]
- Tschermak, G.; Becke, F. Mineralogy Handbook; Mianowski Institute for the Promotion of Science: Warsaw, Poland, 1931. [Google Scholar]
- Feist-Burkhardt, S.; Gotz, A.E.; Szulc, J.; Borkhataria, R.; Geluk, M.; Haas, J.; Hornung, J.; Jordan, P.; Kempf, O.; Michalik, J.; et al. Triassic. In the Geology of Central Europe. Volume 2: Mesozoic and Cenozoic; McCann, T., Ed.; The Geological Society: London, UK, 2008. [Google Scholar]
- Assmann, P. Die Stratigraphie der Oberschlesischen Trias. Teil 2. Der Muschelkalk; Abhandlungen des Reichsamtes fur Bodenforschung, Neue Folge, Heft 208; Reichsamt für Bodenforschung: Berlin, Germany, 1944; pp. 1–124. (In German) [Google Scholar]
- Makowski, H. Historical Geology; Geological Publishing House: Warsaw, Poland, 1977. [Google Scholar]
- Czermiński, J. Geological Structure of Poland Volume II Catalogue of Fossils, Part 2, Mesozoic; Geological Publishing House: Warsaw, Poland, 1970. [Google Scholar]
- Bujoczek, M.; Gładysz, K.; Stępień, A. The Tarnowskie Góry Underground—A Living Organism in a Selected Section of the Fryderyk Mine (Blachówka—Western—Urban Glückhilfe); Project Report for Tarnowskie Góry Cave Mountaineering Club: Tarnowskie Góry, Poland, 2013. [Google Scholar]
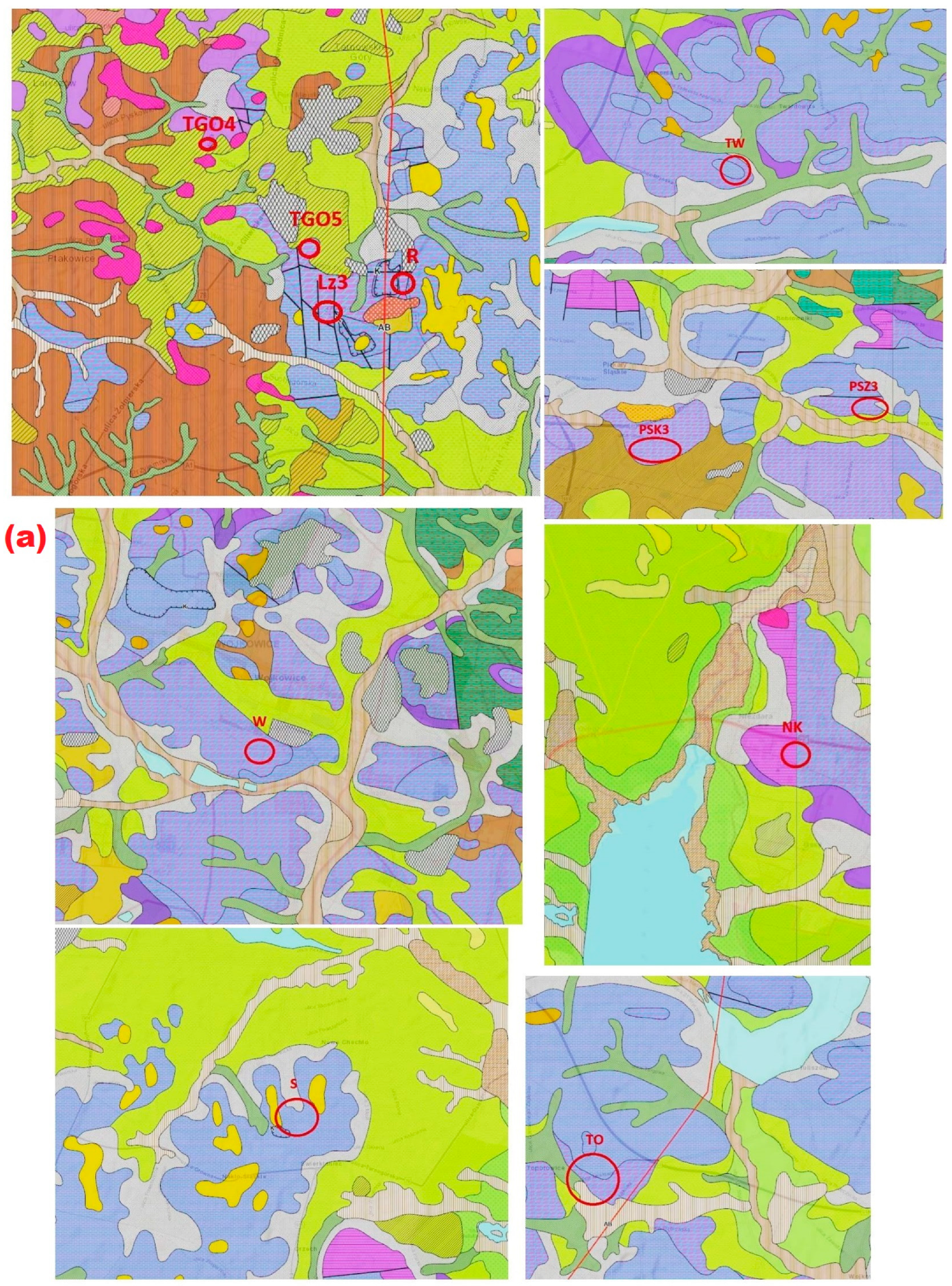
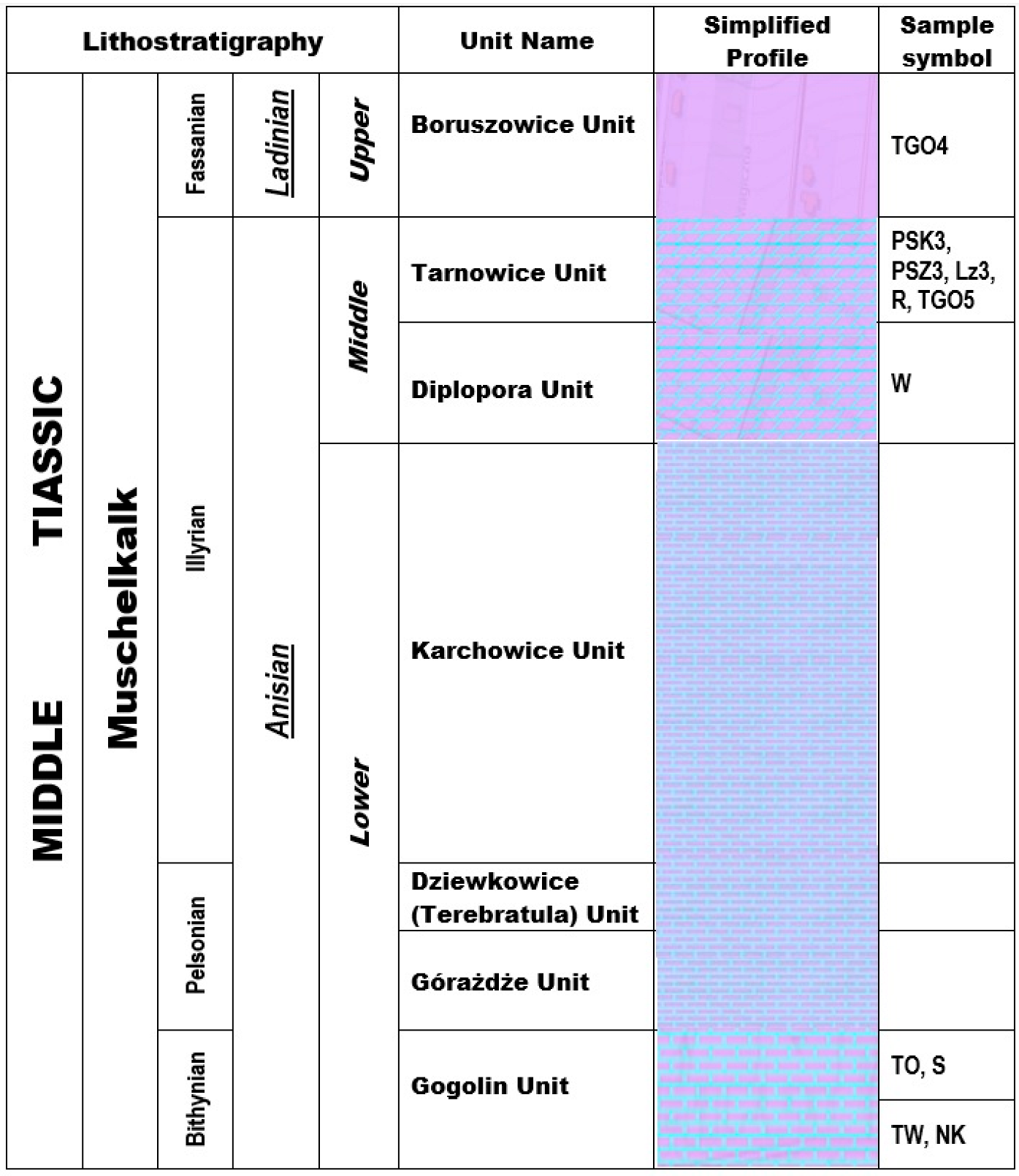

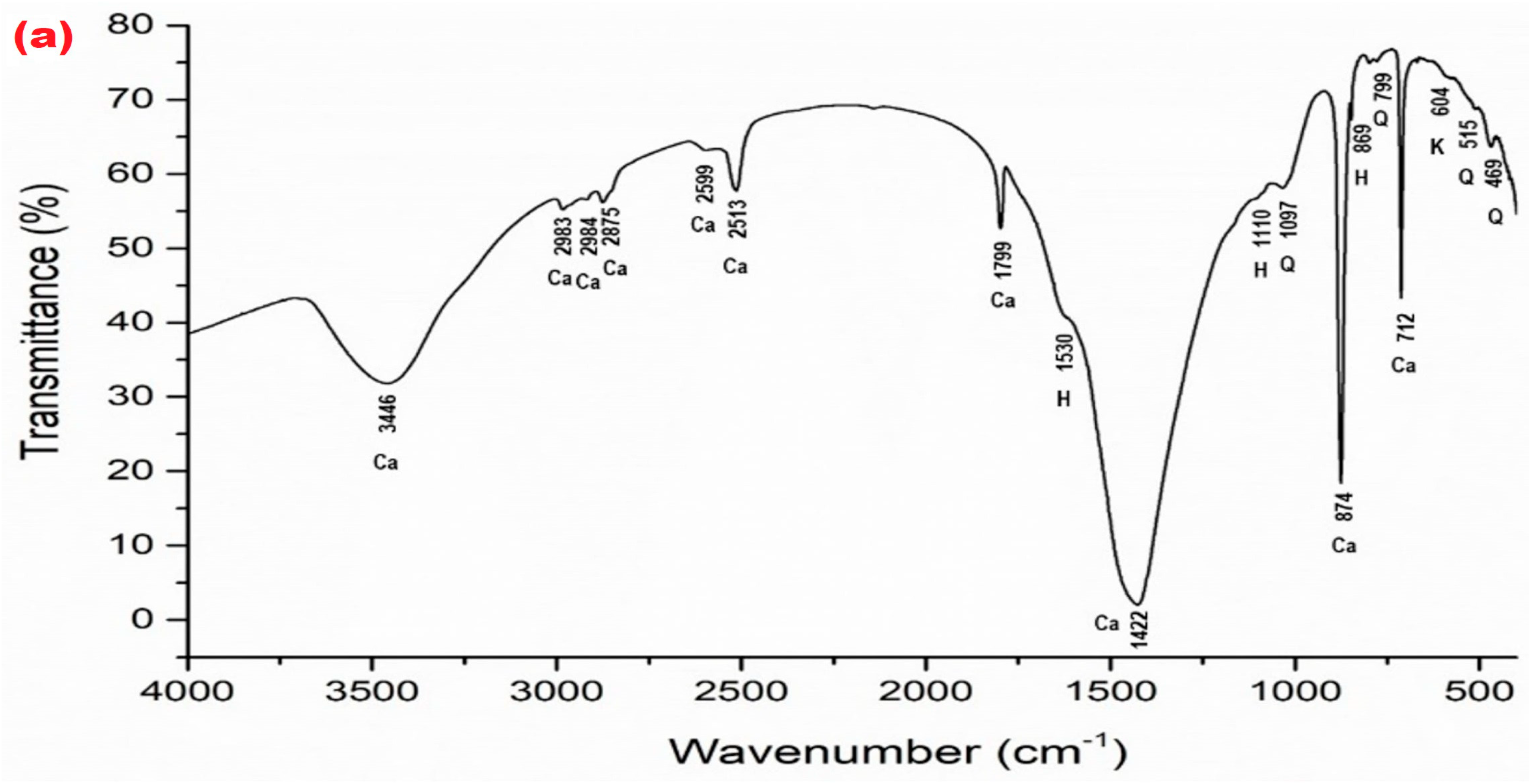
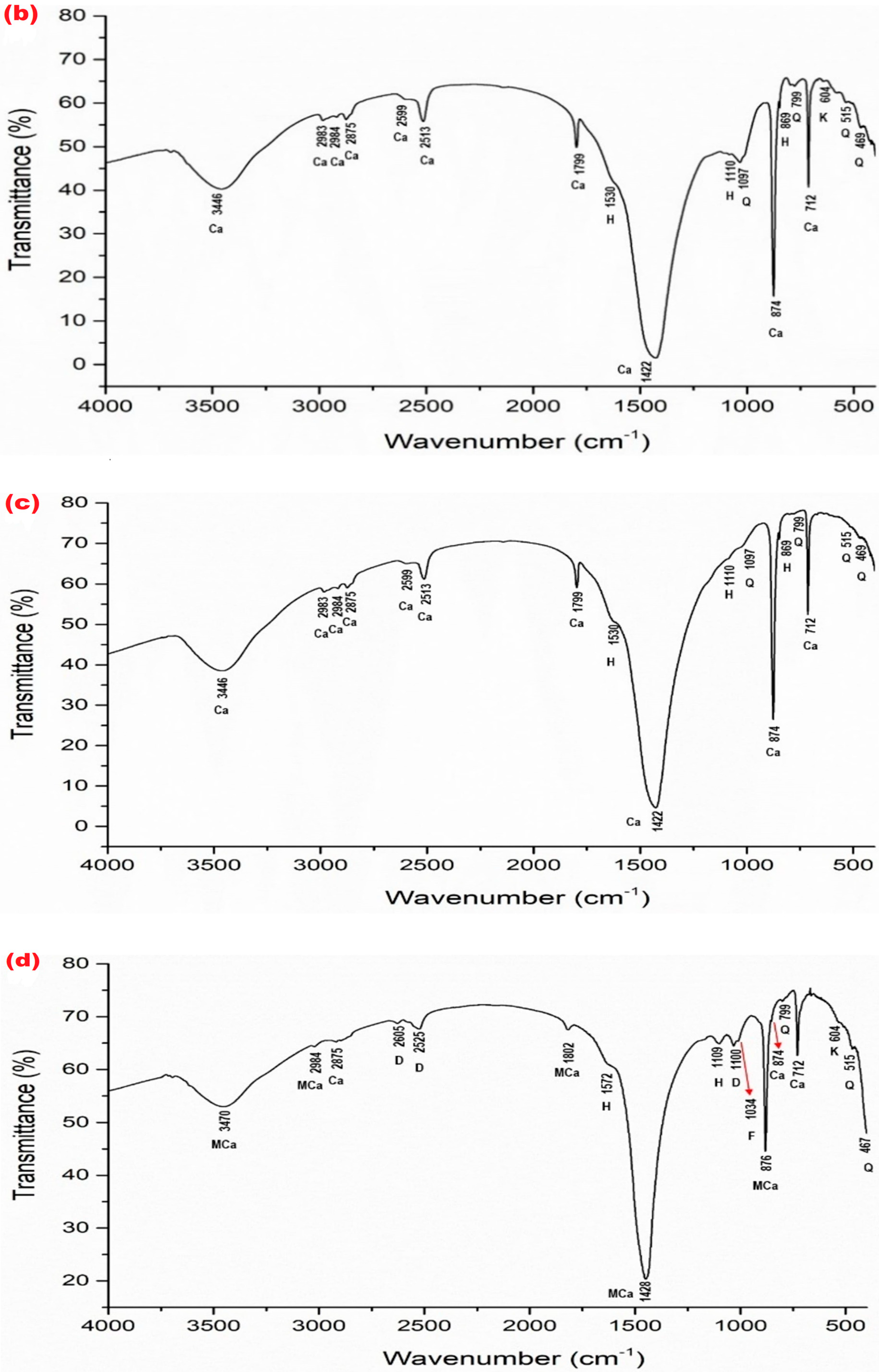
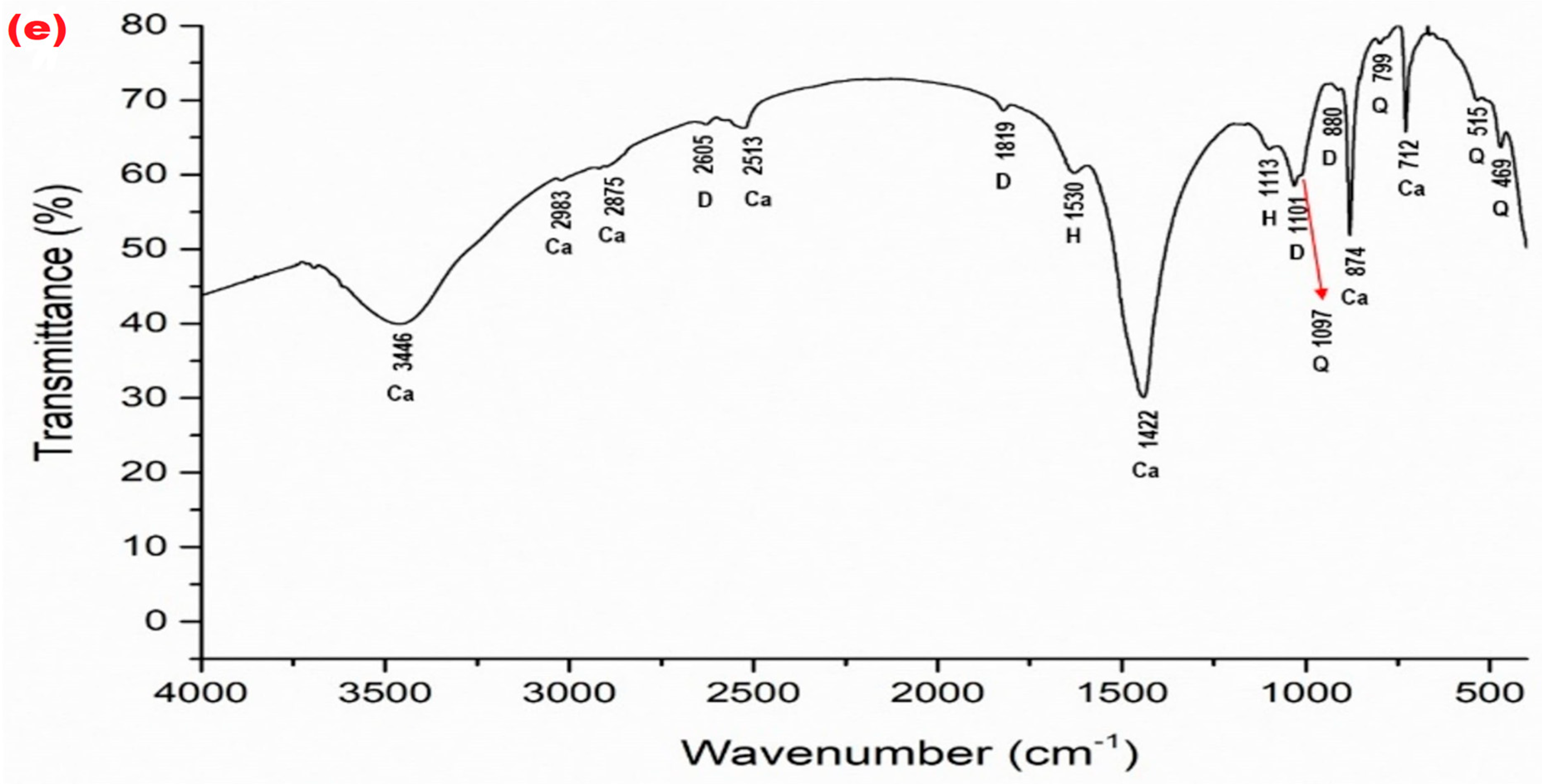
| Mineral Phase | Sample Symbol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TW | NK | TO | S | W | PSK3 | PSZ3 | R | Lz3 | TGO5 | TGO4 | |
| Content in [%] | |||||||||||
| Low-Mg calcite CaCO3 | 80 | 87 | 81 | 85 | 73 | 0 | 1 | 69 | 3 | 3 | 1 |
| High-Mg Calcite 1 Ca0.94Mg0.06CO3 | 13 | 9 | 1 | 1 | 20 | 0 | 0 | 24 | 1 | 1 | 1 |
| High-Mg Calcite 2 Ca0.9Mg0.1CO3 3 | 0 | 2 | 0 | 7 | 4 | 0 | 0 | 3 | 0 | 0 | 0 |
| Ordered Dolomite 1 Ca,Mg(CO3)2 | 1 | 1 | 1 | 1 | 1 | 32 | 32 | 1 | 33 | 57 | 31 |
| Ordered Dolomite 2 Ca0.50,Mg0.50(CO3)2 | 0 | 0 | 0 | 0 | 0 | 41 | 0 | 0 | 32 | 0 | 20 |
| Protodolomite 1 Ca0.501,Mg0.499(CO3)2 | 0 | 0 | 0 | 0 | 0 | 22 | 44 | 0 | 0 | 0 | 9 |
| Protodolomite 2 Ca1.07,Mg0.93(CO3)2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 |
| Fe Dolomite CaMg0.67Fe0.33(CO3)2 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 0 | 28 | 32 | 8 |
| Huntite CaMg3(CO3)4 | 1 | 0 | 1 | 1 | 0 | 2 | 2 | 1 | 2 | 4 | 0 |
| Quartz SiO2 | 3 | 1 | 14 | 3 | 0 | 3 | 0 | 0 | 1 | 3 | 0 |
| Kaolinite Al2(H2O)2Si2O7 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 0 |
| total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Sample Symbol | Low-Mg Calcite CaCO3 | High-Mg Calcite (Ca,Mg)CO3 | Dolomite Ca,Mg(CO3)2 | Huntite CaMg3(CO3)4 | Quartz SiO2 | Feldspars | Clay Minerals |
|---|---|---|---|---|---|---|---|
| TW (Figure 5a) | v3 = 1422 v4 = 712 v2 = 874 v1 + v3 = 2513 v1 + v4 = 1799 other bonds—2599, 2875, 2983, 2984, 3446 | – | – | v3 = 1530 v1 = 1110 v2 = 869 | 1097 799 515 469 | – | kaolinite Al2Si2O5(OH)4 604 |
| TO (Figure 5b) | v3 = 1422 v4 = 712 v2 = 874 v1 + v3 = 2513 v1 + v4 = 1789 other bonds—2599, 2875, 2983, 2984, 3446 | – | – | v3 = 1530 v1 = 1110 v2 = 869 | 1097 799 515 469 | – | kaolinite Al2Si2O5(OH)4 605 |
| W (Figure 5c) | v3 = 1422 v4 = 712 v2 = 874 v1 + v3 = 2513 v1 + v4 = 1799 other bonds—2599, 2875, 2983, 2984, 3446 | – | – | v3 = 1530 v1 = 1110 v2 = 869 | 1097 799 515 469 | – | – |
| Lz3 (Figure 5d) | v4 = 712 v2 = 874 other bonds–2984 | v3 = 1428 v2 = 876 v1 + v4 = 1802 other bonds—2984, 3470 | v1 = 1100 v1 + v4 = 1819 other bonds—2525, 2605 | v3 = 1572 v1 = 1109 | 799 515 467 | 1034 | kaolinite Al2Si2O5(OH)4 604 |
| TGO4 (Figure 5e) | v3 = 1422 v4 = 712 v2 = 874 v1 + v3 = 2513 v1 + v4 = 1797 other bonds–2875, 2983, 3446 | – | v4 = 728 v2 = 880 v1 = 1101 v1 + v4 = 1819 other bonds–2605 | v3 = 1530 v1 = 1113 | 1097 799 515 469 | – | – |
| Element | Sample Symbol [%Mass] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG4 | TG5 | Lz3 | PSK3 | PSZ3 | R | TO | TW | NK | S | W | |
| Na | 0.04 | 0.04 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg | 21.00 | 20.00 | 22.00 | 27.00 | 28.00 | 0.59 | 0.57 | 0.59 | 0.87 | 0.51 | 0.33 |
| Al | 3.90 | 2.90 | 1.70 | 2.80 | 0.42 | 1.90 | 1.80 | 0.84 | 0.66 | 0.58 | 0.33 |
| Si | 8.50 | 6.60 | 3.80 | 6.70 | 0.58 | 4.20 | 6.10 | 2.30 | 1.50 | 1.60 | 0.88 |
| K | 1.30 | 0.73 | 0.12 | 0.80 | 0.00 | 0.85 | 1.70 | 0.54 | 0.40 | 0.41 | 0.19 |
| Ca | 59.00 | 66.00 | 57.00 | 60.00 | 66.00 | 66.00 | 88.00 | 95.00 | 96.00 | 96.00 | 97.00 |
| Mn | 0.32 | 0.15 | 1.20 | 0.18 | 0.28 | 0.04 | 0.06 | 0.04 | 0.04 | 0.03 | 0.02 |
| Fe | 5.10 | 3.40 | 13.00 | 2.00 | 3.20 | 1.40 | 1.20 | 0.60 | 0.81 | 0.58 | 0.50 |
| Element | Sample Symbol [%Mass] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG4 | TG5 | Lz3 | PSK3 | PSZ3 | R | TO | TW | NK | S | W | |
| S | 0.00 | 0.10 | 0.00 | 0.14 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.03 | 0.14 |
| Cl | 0.08 | 0.06 | 0.08 | 0.13 | 0.13 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ti | 0.16 | 0.13 | 0.06 | 0.15 | 0.00 | 0.13 | 0.14 | 0.06 | 0.00 | 0.00 | 0.00 |
| Cr | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.03 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 |
| Ni | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Zn | 0.18 | 0.06 | 0.74 | 0.22 | 0.52 | 0.05 | 0.07 | 0.03 | 0.02 | 0.02 | 0.02 |
| Rb | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sr | 0.01 | 0.04 | 0.02 | 0.04 | 0.03 | 0.11 | 0.14 | 0.06 | 0.04 | 0.07 | 0.08 |
| Zr | 0.01 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ba | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.86 |
| Pb | 0.05 | 0.01 | 0.17 | 0.03 | 0.03 | 0.00 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 |
| As | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| V | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Oxide | Sample Symbol [%Mass] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG4 | TG5 | Lz3 | PSK3 | PSZ3 | R | TO | TW | NK | S | W | |
| Na2O | 0.02 | 0.02 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MgO | 13.25 | 12.72 | 14.20 | 16.77 | 17.26 | 0.54 | 0.41 | 0.40 | 0.59 | 0.35 | 0.22 |
| Al2O3 | 2.80 | 2.10 | 1.25 | 1.98 | 0.29 | 1.98 | 1.49 | 0.65 | 0.51 | 0.45 | 0.25 |
| SiO2 | 6.92 | 5.41 | 3.16 | 5.37 | 0.46 | 4.96 | 5.71 | 2.03 | 1.31 | 1.41 | 0.77 |
| K2O | 0.60 | 0.34 | 0.06 | 0.36 | 0.00 | 0.56 | 0.90 | 0.27 | 0.20 | 0.20 | 0.09 |
| CaO | 31.41 | 35.42 | 31.05 | 31.44 | 34.33 | 50.95 | 53.87 | 54.77 | 54.89 | 55.22 | 55.43 |
| MnO | 0.16 | 0.07 | 0.60 | 0.09 | 0.13 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | 0.01 |
| Fe2O3 | 2.50 | 1.68 | 6.51 | 0.96 | 1.53 | 0.99 | 0.68 | 0.32 | 0.43 | 0.31 | 0.26 |
| LOI | 42.34 | 42.24 | 43.17 | 43.03 | 45.92 | 39.98 | 36.91 | 41.54 | 42.06 | 42.05 | 42.96 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Sample Symbol | Ca [%] | CaO [%] | Mg [%] | MgO [%] | MgCO3 [%] | Ca/Mg |
|---|---|---|---|---|---|---|
| TG4 | 59.00 | 38.28 | 21.00 | 13.25 | 27.83 | 2.81 |
| TG5 | 66.00 | 35.42 | 20.00 | 12.72 | 26.71 | 3.30 |
| Lz3 | 57.00 | 31.05 | 22.00 | 14.20 | 29.82 | 2.59 |
| PSK3 | 60.00 | 31.44 | 27.00 | 16.77 | 35.22 | 2.22 |
| PSZ3 | 66.00 | 34.33 | 28.00 | 17.26 | 36.25 | 2.36 |
| R | 91.00 | 50.95 | 0.59 | 0.54 | 1.13 | 154.24 |
| TO | 88.00 | 53.87 | 0.57 | 0.41 | 0,86 | 154.38 |
| TW | 95.00 | 54.77 | 0.59 | 0.40 | 0.84 | 161.02 |
| NK | 96.00 | 54.98 | 0.87 | 0.59 | 1.24 | 110.34 |
| S | 96.00 | 55.22 | 0.51 | 0.35 | 0.74 | 188.23 |
| W | 97.00 | 55.43 | 0.33 | 0.22 | 0.46 | 293.93 |
| Rock Name | Ca/Mg Ratio |
|---|---|
| magnesium dolomite | 1.0–1.5 |
| dolomite | 1.5–1.7 |
| calcareous dolomite | 1.7–2.0 |
| limestone dolomite | 2.0–3.5 |
| highly dolomitic limestone | 3.5–16.0 |
| dolomitic limestone | 16.0–60.0 |
| dolomitic limestone | 60.0–105.0 |
| calcite limestone | more than 105.0 |
| Rock Name | Dolomite Content [%] | MgCO3 Content [%] | MgO Content [%} |
|---|---|---|---|
| limestone | 0.0–10 | 0.0–4.4 | 0.0–2.1 |
| dolomitic limestone | 10–50 | 4.4–22.7 | 2.1–10.8 |
| calcareous dolomite | 50–90 | 22.7–41.0 | 10.8–19.5 |
| dolomite | 90–100 | 44.0–45.4 | 19.5–21.6 |
| Formation Name | Name of Variety | Sample Symbol | Ca/Mg | MgO [%] | MgCO3 [%] | Name of the Rock in the Classification of Chilingar G.V. (1957) [41] | Name of the Rock in the Classification of Pettijohn F.J. (1975) [10] |
|---|---|---|---|---|---|---|---|
| Lower Gogolin Unit | Rock from Twardowice | TW | 161.02 | 0.40 | 0.84 | Limestone | Calcite limestone |
| Rock from the vicinity of Niezdara-Krzyżowa | NK | 110.34 | 0.59 | 1.24 | Limestone | Calcite limestone | |
| Upper Gogolin Unit | Rock from Toporowice | TO | 154.38 | 0.41 | 0,86 | Limestone | Calcite limestone |
| Rock from the vicinity of Świerklaniec | S | 188.23 | 0.35 | 0.74 | Limestone | Calcite limestone | |
| Diplopore Dolomites Unit | Rock from Wojkowice | W | 293.93 | 0.22 | 0.46 | Limestone | Calcite limestone |
| Tarnowice Unit | Rocks from Piekary Śląskie | PSK3 | 2.22 | 16.77 | 35.22 | Calcareous dolomite | Calcareous dolomite |
| PSZ3 | 2.36 | 17.26 | 36.25 | Calcareous dolomite | Calcareous dolomite | ||
| Rock from Radzionków | Rd | 154.24 | 0.54 | 1.13 | Limestone | Calcite limestone | |
| Rock from the Lazarówka Quarry | Lz3 | 2.59 | 14.20 | 29.82 | Calcareous dolomite | Calcareous dolomite | |
| Rock from the Tarnowskie Góry area | TG5 | 3.30 | 12.72 | 26.71 | Calcareous dolomite | Calcareous dolomite | |
| Boruszowice Unit | Rock from the Tarnowskie Góry area | TG4 | 2.81 | 13.25 | 27.83 | Calcareous dolomite | Calcareous dolomite |
| Element | Detection Limit | Sample Symbol (Measured Value for Element)/Standard Deviation—STD [Values in ppm] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TW/STD | NK/STD | TO/ STD | S/ STD | W/ STD | PSK3/STD | PSZ3/STD | R/ STD | Lz3/ STD | TGO5/STD | TGO4/STD | ||
| Be | 0.002 | 0/- | 0/- | 0/- | 25/1 | 30/1 | 15/1 | 20/1 | 60/1 | 55/1 | 0/- | 10/0.5 |
| B | 0.005 | 35/1 | 80/2 | 55/1 | 25/1 | 45/1 | 95/3 | 65/1 | 75/1 | 55/1 | 85/1 | 95/2 |
| Ti | 0.005 | 610/5 | 90/2 | 1355/10 | 95/2 | 80/2 | 1321/12 | 85/2 | 1125/10 | 545/7 | 1120/10 | 1250/10 |
| V | 0.007 | 45/1 | 55/1 | 0/- | 20/1 | 95/2 | 55/2 | 0/- | 95/3 | 160/7 | 110/6 | 95/2 |
| Cr | 0.001 | 55/1 | 280/5 | 65/1 | 25/1 | 45/1 | 105/5 | 100/1 | 321/4 | 55/1 | 110/6 | 98/1 |
| Co | 0.01 | 0/- | 0/- | 6/0.2 | 0/- | 0/- | 8/0.1 | 0/- | 0/- | 10/0.5 | 5/0.2 | 0/- |
| Ni | 0.001 | 65/1 | 98/3 | 0/- | 0/- | 0/- | 0/- | 70/2 | 25/1 | 55/1 | 210/4 | 85/2 |
| Cu | 0.002 | 55/1 | 60/2 | 45/1 | 55/1 | 10/q | 25/1 | 65/1 | 20/1 | 95/4 | 65/2 | 55/1 |
| Zn | 0.01 | 295/5 | 210/7 | 720/5 | 215/6 | 310/4 | 210/3 | 5500/15 | 530/7 | 820/8 | 620/8 | 190/5 |
| As | 0.57 | 0/- | 15/1 | 0/- | 25/1 | 35/1 | 21/1 | 115/3 | 60/2 | 55/1 | 35/1 | 25/1 |
| Br | 0.36 | 10/1 | 11/1 | 5/0.1 | 10/1 | 20/1 | 10/0.8 | 20/1 | 0/- | 25/1 | 55/4 | 10/0.5 |
| Sr | 0.001 | 625 4 | 410/5 | 1422/9 | 780/5 | 830/5 | 410/3 | 320/5 | 1250/10 | 205/9 | 410/8 | 120/5 |
| Zr | 0.001 | 25/1 | 0/- | 295/4 | 55/1 | 75/2 | 120/2 | 55/1 | 95/3 | 220/9 | 120/5 | 110/5 |
| Nb | 0.005 | 0/- | 0/- | 5/0.1 | 9/1 | 5/0.1 | 0/- | 10/0.5 | 3/0.1 | 4/0.3 | 11/0.7 | 5/0.2 |
| Mo | 0.03 | 4/0.09 | 10/1 | 5/0.1 | 7/0.2 | 5/0.1 | 6/0.1 | 10/0.5 | 0/- | 2/0.1 | 10/0.7 | 5/0.1 |
| Cd | 0.01 | 0.5/0.01 | 0.5/0.02 | 2/0.08 | 1/0.01 | 0.2/0.01 | 0.2/0.01 | 2/0.01 | 0.3/0.01 | 1.1/0.1 | 0.7/0.01 | 0.5/0.01 |
| Pb | 0.014 | 155/2 | 110/2 | 210/2 | 110/3 | 200/5 | 320/5 | 310/4 | 95/2 | 190/4 | 120/5 | 510/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanienda-Pilecki, K.J.; Jendruś, R. New Data on Phase Composition and Geochemistry of the Muschelkalk Carbonate Rocks of the Upper Silesian Province in Poland. Appl. Sci. 2025, 15, 10751. https://doi.org/10.3390/app151910751
Stanienda-Pilecki KJ, Jendruś R. New Data on Phase Composition and Geochemistry of the Muschelkalk Carbonate Rocks of the Upper Silesian Province in Poland. Applied Sciences. 2025; 15(19):10751. https://doi.org/10.3390/app151910751
Chicago/Turabian StyleStanienda-Pilecki, Katarzyna J., and Rafał Jendruś. 2025. "New Data on Phase Composition and Geochemistry of the Muschelkalk Carbonate Rocks of the Upper Silesian Province in Poland" Applied Sciences 15, no. 19: 10751. https://doi.org/10.3390/app151910751
APA StyleStanienda-Pilecki, K. J., & Jendruś, R. (2025). New Data on Phase Composition and Geochemistry of the Muschelkalk Carbonate Rocks of the Upper Silesian Province in Poland. Applied Sciences, 15(19), 10751. https://doi.org/10.3390/app151910751







