Morphological and Structural Analysis of Pyrolytic Carbon from Simple Thermal Methane Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Pyrolysis Process
2.2. SEM
2.3. Raman Spectroscopy
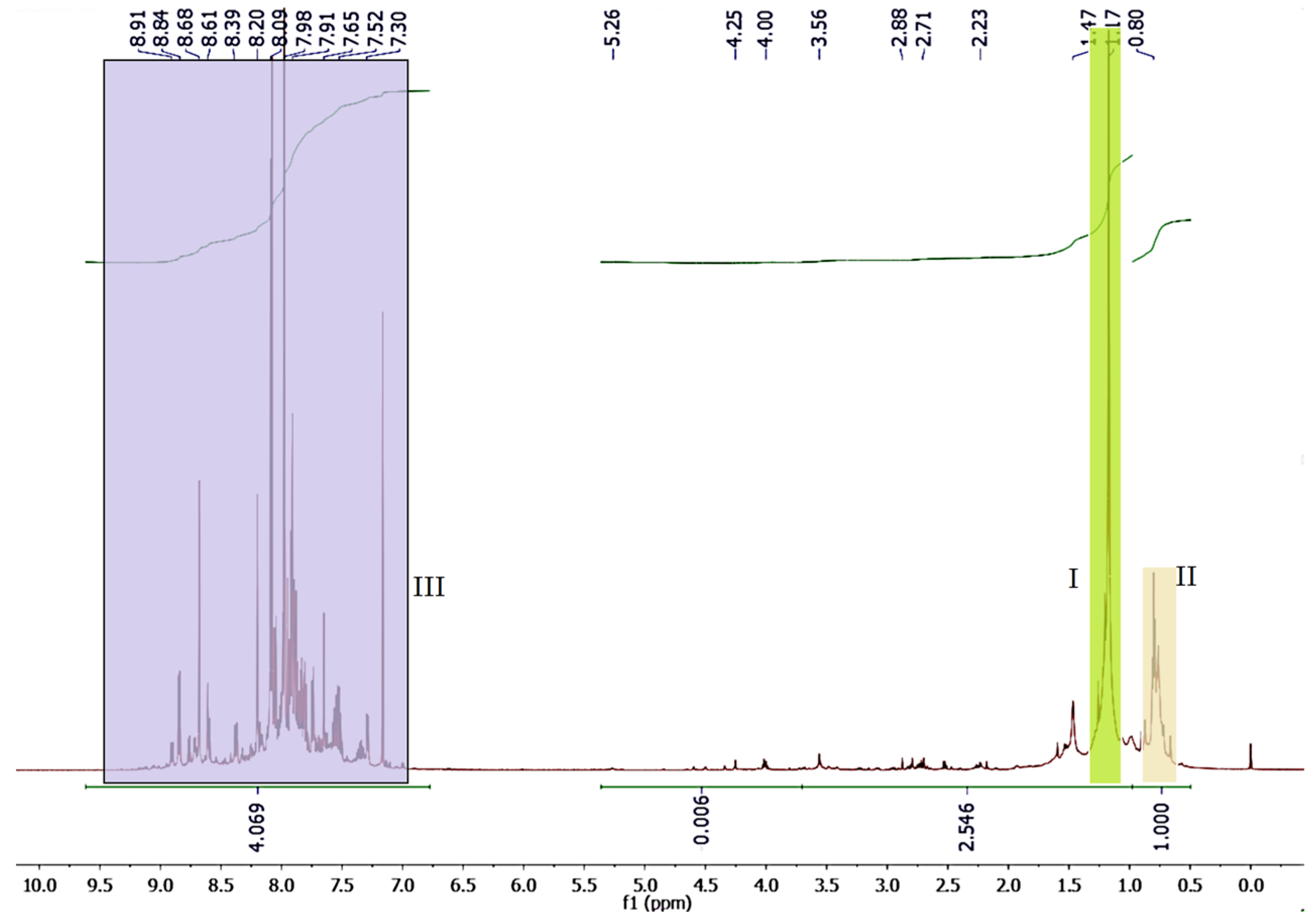
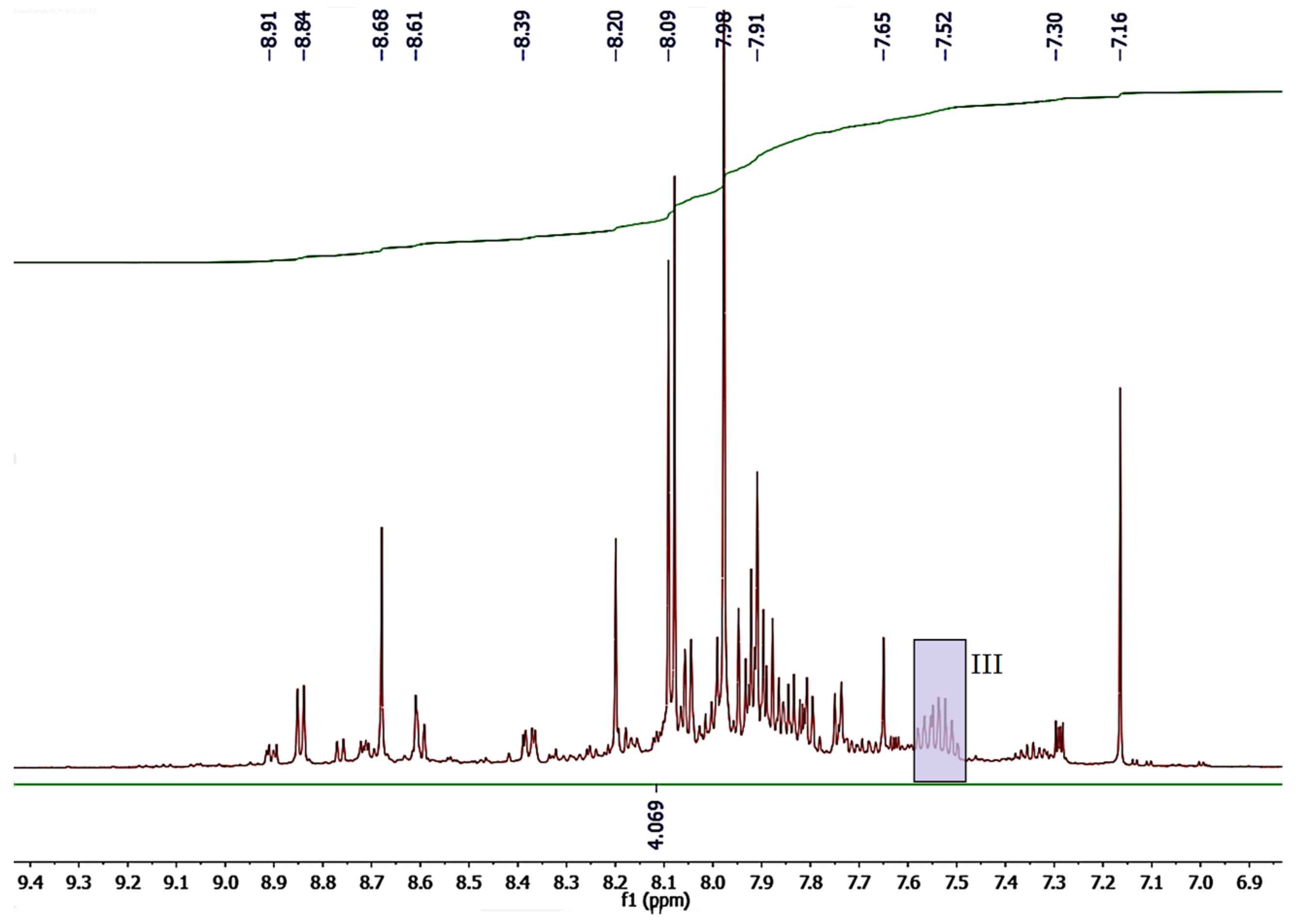
2.4. NMR
2.5. IR
2.6. Textural Studies
- Specific surface area according to the Brunauer–Emmet–Teller (BET) methodology.
- Total pore volume Vt for a relative pressure of 0.99.
- Volume of Vmez mesopores (pores with a width greater than 2 nm and less than 50 nm) by DFT.
2.7. TGA
3. Results
3.1. Methane Pyrolysis
3.2. SEM
3.3. Raman Spectroscopy
3.4. IR Study
3.5. NMR Study
3.6. Textural Studies
3.7. TGA Study
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Grubert, E. Water consumption from electrolytic hydrogen in a carbon-neutral US energy system. Clean. Prod. Lett. 2023, 4, 100037. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; di Berardino, S.; Amorim, F.; Gírio, F.; Rangel, C.; de Leão, T.P. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Postels, S.; Abánades, A.; von der Assen, N.; Rathnam, R.K.; Stückrad, S.; Bardow, A. Life cycle assessment of hydrogen production by thermal cracking of methane based on liquid-metal technology. Int. J. Hydrogen Energy 2016, 41, 23204–23212. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroǧlu, T.N. “Green” path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies. Int. J. Hydrogen Energy 2008, 33, 6804–6839. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Steinberg, M. Fossil fuel decarbonization technology for mitigating global warming. Int. J. Hydrogen Energy 1999, 24, 771–777. [Google Scholar] [CrossRef]
- Parkinson, B.; Matthews, J.W.; McConnaughy, T.B.; Upham, D.C.; McFarland, E.W. Techno-Economic Analysis of Methane Pyrolysis in Molten Metals: Decarbonizing Natural Gas. Chem. Eng. Technol. 2017, 40, 1022–1030. [Google Scholar] [CrossRef]
- Holmen, A.; Olsvik, O.; Rokstad, O.A. Pyrolysis of natural gas: Chemistry and process concepts. Fuel Process Technol. 1995, 42, 249–267. [Google Scholar] [CrossRef]
- Abánades, A.; Ruiz, E.; Ferruelo, E.M.; Hernández, F.; Cabanillas, A.; Martínez-Val, J.M.; Rubio, J.A.; López, C.; Gavela, R.; Barrera, G.; et al. Experimental analysis of direct thermal methane cracking. Int. J. Hydrogen Energy 2011, 36, 12877–12886. [Google Scholar] [CrossRef]
- Poirier, M.G.; Sapundzhiev, C. Catalytic decomposition of natural gas to hydrogen for fuel cell applications. Int. J. Hydrogen Energy 1997, 22, 429–433. [Google Scholar] [CrossRef]
- Steinberg, M. Production of hydrogen and methanol from natural gas with reduced CO2 emission. Int. J. Hydrogen Energy 1998, 23, 419–425. [Google Scholar] [CrossRef]
- Billaud, F.; Gueret, C.; Weill, J. Thermal decomposition of pure methane at 1263 K. Experiments and mechanistic modelling. Thermochim. Acta 1992, 211, 303–322. [Google Scholar] [CrossRef]
- Guéret, C.; Daroux, M.; Billaud, F. Methane pyrolysis: Thermodynamics. Chem. Eng. Sci. 1997, 52, 815–827. [Google Scholar] [CrossRef]
- Wojtasik, M.; Burnus, Z.; Markowski, J.; Żak, G.; Lubowicz, J. Methane pyrolysis—Influence of selected parameters on the course of the process. Naft-Gaz 2023, 79, 484–489. [Google Scholar] [CrossRef]
- Wojtasik, M. Katalityczna dekompozycja metanu jako źródło cennego wodoru. Przem. Chem. 2022, 1, 33–37. [Google Scholar] [CrossRef]
- Vedele, P.; Sartoretti, E.; Torretti, G.; Novara, C.; Salomone, F.; Giorgis, F.; Antonini, M.; Bensaid, S. Thermocatalytic methane pyrolysis over iron-based catalysts for turquoise hydrogen production: Activity and kinetic studies. Chem. Eng. J. 2025, 514, 163392. [Google Scholar] [CrossRef]
- Zhou, N.; Zhao, D.; Su, Q.; Li, Q.; Zha, W.; Feng, S. Catalytic performance of modified carbon black on methane decomposition for hydrogen production. RSC Adv. 2024, 14, 15656–15663. [Google Scholar] [CrossRef]
- Shivtsov, D.M.; Veselov, G.B.; Afonnikova, S.D.; Ayupov, A.B.; Shubin, Y.V.; Bauman, Y.I.; Mishakov, I.V.; Shelepova, E.V.; Vedyagin, A.A. Production of hydrogen from methane over nickel-copper oxide catalysts prepared via mechanochemical activation under varied conditions. Int. J. Hydrogen Energy 2025, 149, 150082. [Google Scholar] [CrossRef]
- Chen, L.; Song, Z.; Zhang, S.; Chang, C.-K.; Chuang, Y.-C.; Peng, X.; Dun, C.; Urban, J.J.; Guo, J.; Chen, J.-L.; et al. Ternary NiMo-Bi liquid alloy catalyst for efficient hydrogen production from methane pyrolysis. Science 2023, 381, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Hofberger, C.; Dietrich, B.; Krumholz, R.; Noglik, A.P.; Olbricht, M.; Schatzmann, S.; Stoppel, L.; Richter, M.; Uhlenbruck, N.; Wetzel, T. Technical Aspects of Natural Gas Pyrolysis in Liquid Metal Bubble Column Reactors. Energy Technol. 2024, 12, 2400183. [Google Scholar] [CrossRef]
- Fulcheri, L.; Rohani, V.J.; Wyse, E.; Hardman, N.; Dames, E. An energy-efficient plasma methane pyrolysis process for high yields of carbon black and hydrogen. Int. J. Hydrogen Energy 2023, 48, 2920–2928. [Google Scholar] [CrossRef]
- Moghaddam, A.L.; Hejazi, S.; Fattahi, M.; Kibria, G.; Thomson, M.J.; AlEisa, R.; Khan, M.A. Methane pyrolysis for hydrogen production: Navigating the path to a net zero future. Energy Environ. Sci. 2025, 18, 2747–2790. [Google Scholar] [CrossRef]
- Kim, S.C.; Lim, M.S.; Chun, Y.N. Hydrogen-rich gas production from a biomass pyrolysis gas by using a plasmatron. Int. J. Hydrogen Energy 2013, 38, 14458–14466. [Google Scholar] [CrossRef]
- Fulcheri, L.; Schwob, Y. From methane to hydrogen, carbon black and water. Int. J. Hydrogen Energy 1995, 20, 197–202. [Google Scholar] [CrossRef]
- Fabry, F.; Flamant, G.; Fulcheri, L. Carbon black processing by thermal plasma. Analysis of the particle formation mechanism. Chem. Eng. Sci. 2001, 56, 2123–2132. [Google Scholar] [CrossRef]
- Bromberg, L.; Cohn, D.R.; Rabinovich, A.; Alexeev, N. Plasma catalytic reforming of methane. Int. J. Hydrogen Energy 1999, 24, 1131–1137. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Yan, K.; Qin, X.; Feng, T.; Wang, J.; Wang, F.; Song, Z. Methane dry reforming with microwave heating over carbon-based catalyst obtained by agriculture residues pyrolysis. J. CO2 Util. 2018, 28, 41–49. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Hrabovský, M.; Van Oost, G.; Pohořelý, M.; Jeremiáš, M. Progress in waste utilization via thermal plasma. Prog. Energy Combust. Sci. 2020, 81, 100873. [Google Scholar] [CrossRef]
- Sekiguchi, H.; Mori, Y. Steam plasma reforming using microwave discharge. Thin Solid Films 2003, 435, 44–48. [Google Scholar] [CrossRef]
- Deminsky, M.; Jivotov, V.; Potapkin, B.; Rusanov, V. Plasma-assisted production of hydrogen from hydrocarbons. Pure Appl. Chem. 2002, 74, 413–418. [Google Scholar] [CrossRef]
- Jasiński, M.; Dors, M.; Mizeraczyk, J. Production of hydrogen via methane reforming using atmospheric pressure microwave plasma. J. Power Sources 2008, 181, 41–45. [Google Scholar] [CrossRef]
- Moisan, M.; Zakrzewski, Z.; Rostaing, J.C. Waveguide-based single and multiple nozzle plasma torches: The TIAGO concept. Plasma Sources Sci. Technol. 2001, 10, 387–394. [Google Scholar] [CrossRef]
- Otsuka, K.; Takenaka, S.; Ohtsuki, H. Production of pure hydrogen by cyclic decomposition of methane and oxidative elimination of carbon nanofibers on supported-Ni-based catalysts. Appl. Catal. A Gen. 2004, 273, 113–124. [Google Scholar] [CrossRef]
- Wojtasik, K.; Wojtasik, M.; Suchanek, K.; Zięba, M.; Karasiński, P.; Pakieła, W.; Żak, G.; Krasodomski, W. Effect of pyrolytic carbon addition on the structural and optical properties of TiO2 composite thin films. Sci. Rep. 2025, 15, 8078. [Google Scholar] [CrossRef]
- Potylitsyna, A.R.; Bauman, Y.I.; Ayupov, A.B.; Plyusnin, P.E.; Shubin, Y.V.; Stoyanovskii, V.O.; Vedyagin, A.A.; Mel’GUnov, M.S.; Korenev, S.V.; Mishakov, I.V. Turbostratic carbon nanofibers produced from C2HCl3 over self-dispersing Ni-catalyst doped with W and Mo. Diam. Relat. Mater. 2024, 148, 111416. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, J.; Li, Q.; Wang, Y.; Cui, S.; Yang, J.; Li, J. Nano-turbostratic carbon-decorated SiOC ceramic aerogels with ultra-broadband electromagnetic wave absorption properties. J. Non Cryst. Solids 2024, 624, 122725. [Google Scholar] [CrossRef]
- Granieri, S.F.; Rovera, E.; Cecchetti, M.; Pagano, G.M.; Prato, M.; Pasquale, L.; Brescia, R.; Ivanov, Y.P.; Divitini, G.; Casalegno, A.; et al. High-power density turbostratic carbon nano-onion functionalized carbon paper electrodes for vanadium flow batteries. J. Energy Storage 2025, 121, 116567. [Google Scholar] [CrossRef]
- Yeh, I.C.; Tran, N.T.; Knorr, D.B. Effects of high-temperature annealing on structural and mechanical properties of amorphous carbon materials investigated by molecular dynamics simulations. Carbon 2025, 234, 120006. [Google Scholar] [CrossRef]
- Wang, S.; Lee, W.J.; Li, C.; Kuan, B.; Burke, N.; Patel, J. The pyrolysis of natural gas: A study of carbon deposition and the suitability of reactor materials. AIChE J. 2019, 65, 1035–1046. [Google Scholar] [CrossRef]
- Prabowo, J.; Lai, L.; Chivers, B.; Burke, D.; Dinh, A.H.; Ye, L.; Wang, Y.; Wang, Y.; Wei, L.; Chen, Y. Solid carbon co-products from hydrogen production by methane pyrolysis: Current understandings and recent progress. Carbon 2024, 216, 118507. [Google Scholar] [CrossRef]
- Xu, H.; Abuseada, M.; Ju, Y.S.; Spearrin, R.M.; Fisher, T.S. Local Thermochemical Mechanisms in Direct Solar Graphite Synthesis from Methane. Energy Fuels 2024, 38, 18087–18089. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Hu, J.; Lei, Y.; Zhao, R.; Gao, C.; Wang, H.; Li, B.; Kang, F.; Zhai, D. Key Factor Determining the Cyclic Stability of the Graphite Anode in Potassium-Ion Batteries. ACS Nano 2022, 16, 12511–12519. [Google Scholar] [CrossRef] [PubMed]
- Moisan, M.; Grenier, R.; Zakrzewski, Z. The electromagnetic performance of a surfatron-based coaxial microwave plasma torch. Spectrochim. Acta Part B Spectrosc. 1995, 50, 781–789. [Google Scholar] [CrossRef]
- Srisuma, P.; Suwattanapongtada, N.; Opasanon, N.; Charoensuppanimit, P.; Kerdnawee, K.; Termvidchakorn, C.; Tanthapanichakoon, W.; Charinpanitku, T. A fundamental exploration on carbon nanotube formation via pyrolysis of ferrocene and glycerol: Experimental and theoretical viewpoints. Eng. Sci. Technol. Int. J. 2021, 24, 1373–1382. [Google Scholar] [CrossRef]
- Ziemiaski, P.P.; van Bokhoven, J.; Pan, Z.; Krumeich, F.; Ziemiański, P.P.; van Bokhoven, J.A. Tunable Synthesis of Carbon Nanotubes via Methane Catalytic Pyrolysis by Adjusting Mo Incorporation in Fe/MgO. Phys. Chem. Chem. Phys. 2025, 27, 18302–18308. [Google Scholar] [CrossRef]
- Wang, I.W.; Kutteri, D.A.; Gao, B.; Tian, H.; Hu, J. Methane Pyrolysis for Carbon Nanotubes and COx-Free H2 over Transition-Metal Catalysts. Energy Fuels 2019, 33, 197–205. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Sharafian, A.; Katsu, K.; Mérida, W. Temperature effects on the properties of solid carbon from natural gas pyrolysis in molten tin. Carbon Lett. 2024, 34, 1899–1913. [Google Scholar] [CrossRef]
- Krasodomski, W.; Wojtasik, M.; Markowski, J.; Żak, G. Dekarbonizacja metanu—Kierunki zagospodarowania węgla popirolitycznego. Naft-Gaz 2022, 78, 56–63. [Google Scholar] [CrossRef]
- Dadsetan, M.; Latham, K.G.; Khan, M.F.; Zaher, M.H.; Manzoor, S.; Bobicki, E.R.; Titirici, M.; Thomson, M.J. Characterization of carbon products from microwave-driven methane pyrolysis. Carbon Trends 2023, 12, 100277. [Google Scholar] [CrossRef]
- He, Y.; Jing, X.; Qin, L.; Wang, D.; Wu, C.; Liu, M.; Yang, M.; Huang, Z. Insights into carbon formation over molten salt-promoted NiO/Al2O3 during methane pyrolysis. Carbon Lett. 2024, 34, 1471–1480. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Z.; Zhu, Y.; Liu, J.; Yang, B.; Yu, Q.; Xie, X. Mechanism for One-Pot Synthesis of 0D-2D Carbon Materials in the Bubbles Inside Molten Salts. Adv. Funct. Mater. 2022, 32, 2202381. [Google Scholar] [CrossRef]
- Lott, P.; Mokashi, M.B.; Müller, H.; Heitlinger, D.J.; Lichtenberg, S.; Shirsath, A.B.; Janzer, C.; Tischer, S.; Maier, L.; Deutschmann, O. Hydrogen Production and Carbon Capture by Gas-Phase Methane Pyrolysis: A Feasibility Study. ChemSusChem 2023, 16, e202201720. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, K.A.; Dollimore, D.; Dollimore, J. The surface area of graphite calculated from adsorption isotherms and heats of wetting experiments. Carbon 1966, 4, 281–287. [Google Scholar] [CrossRef]

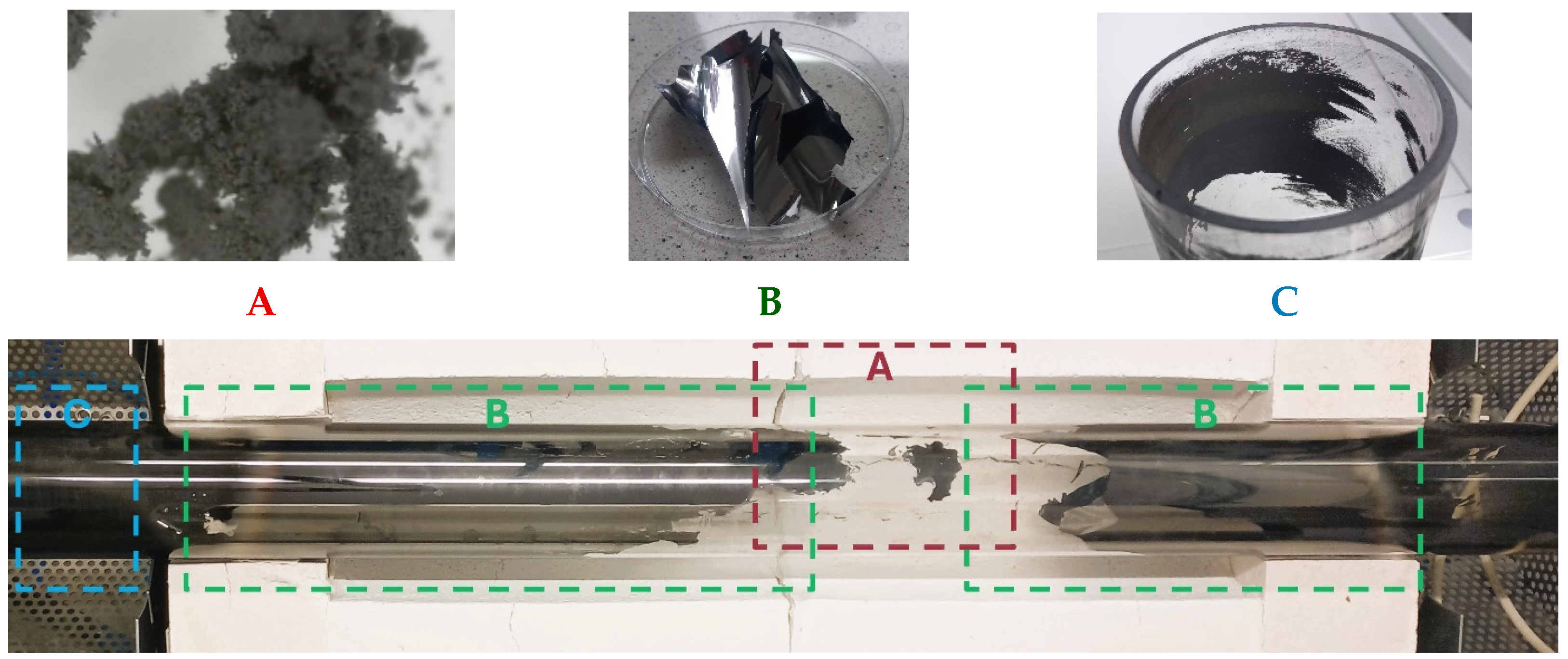
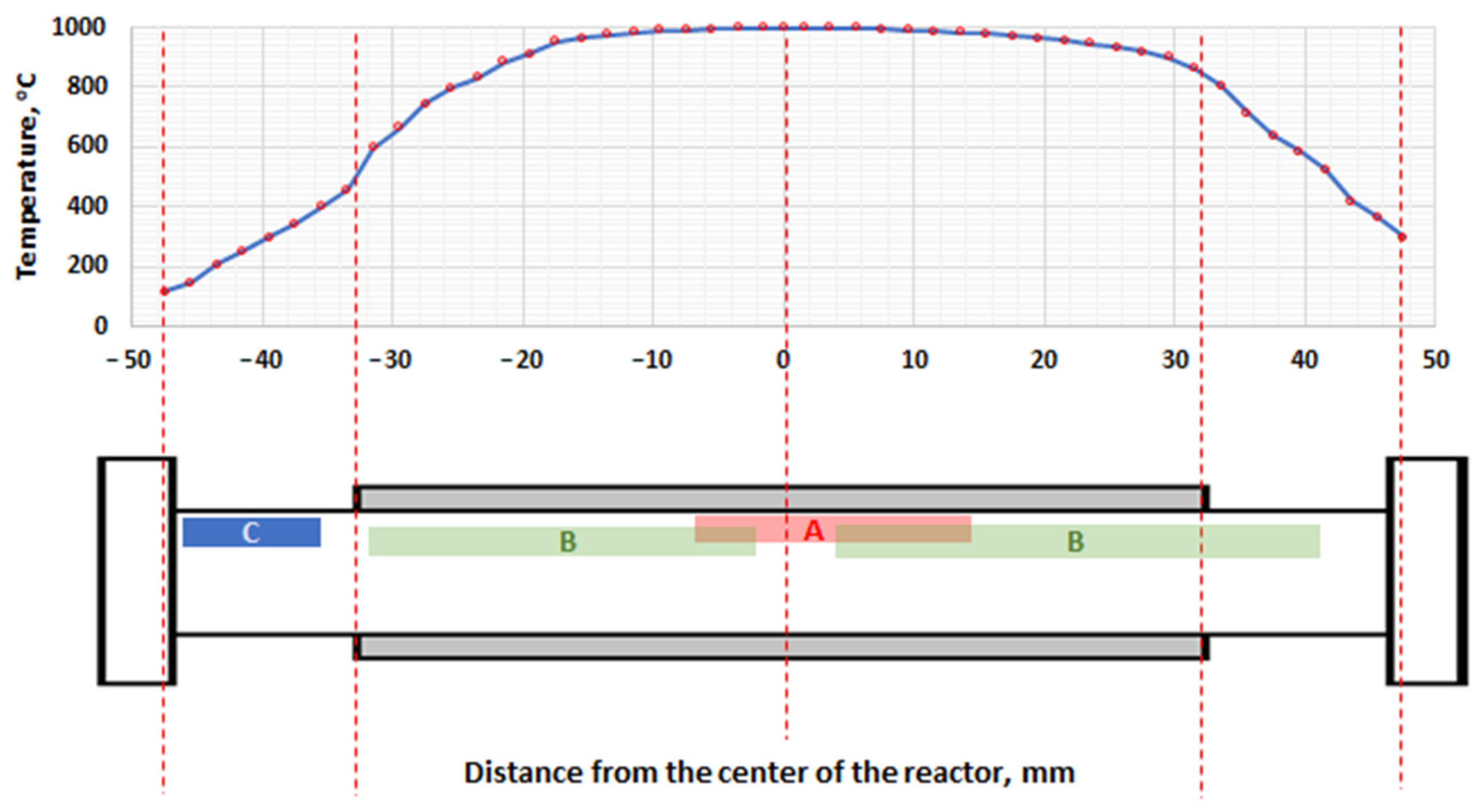

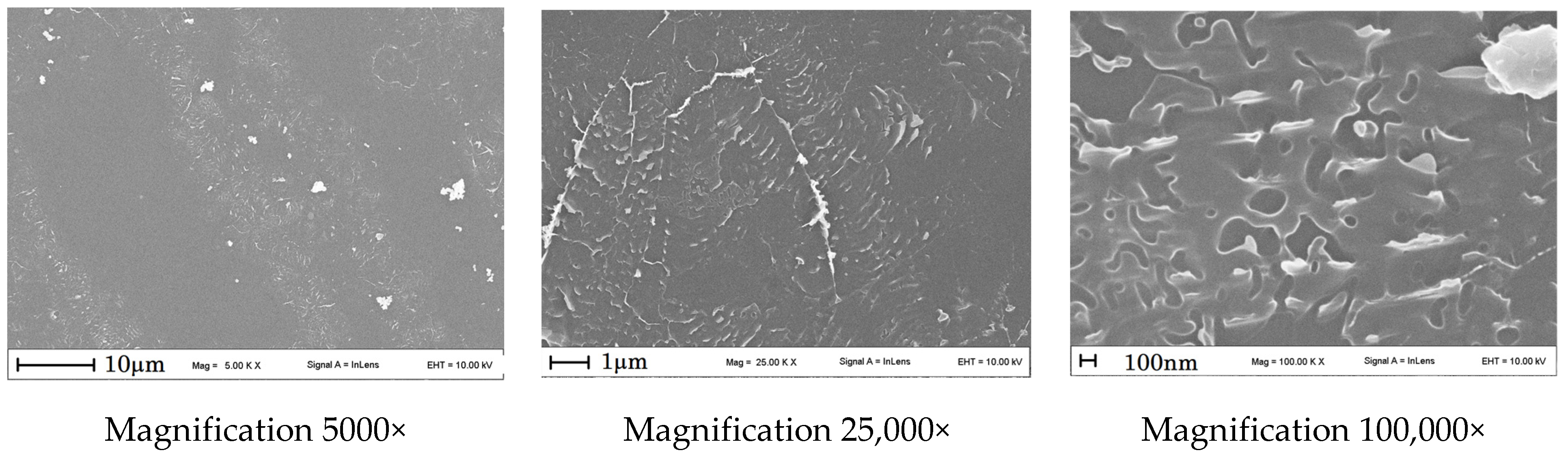
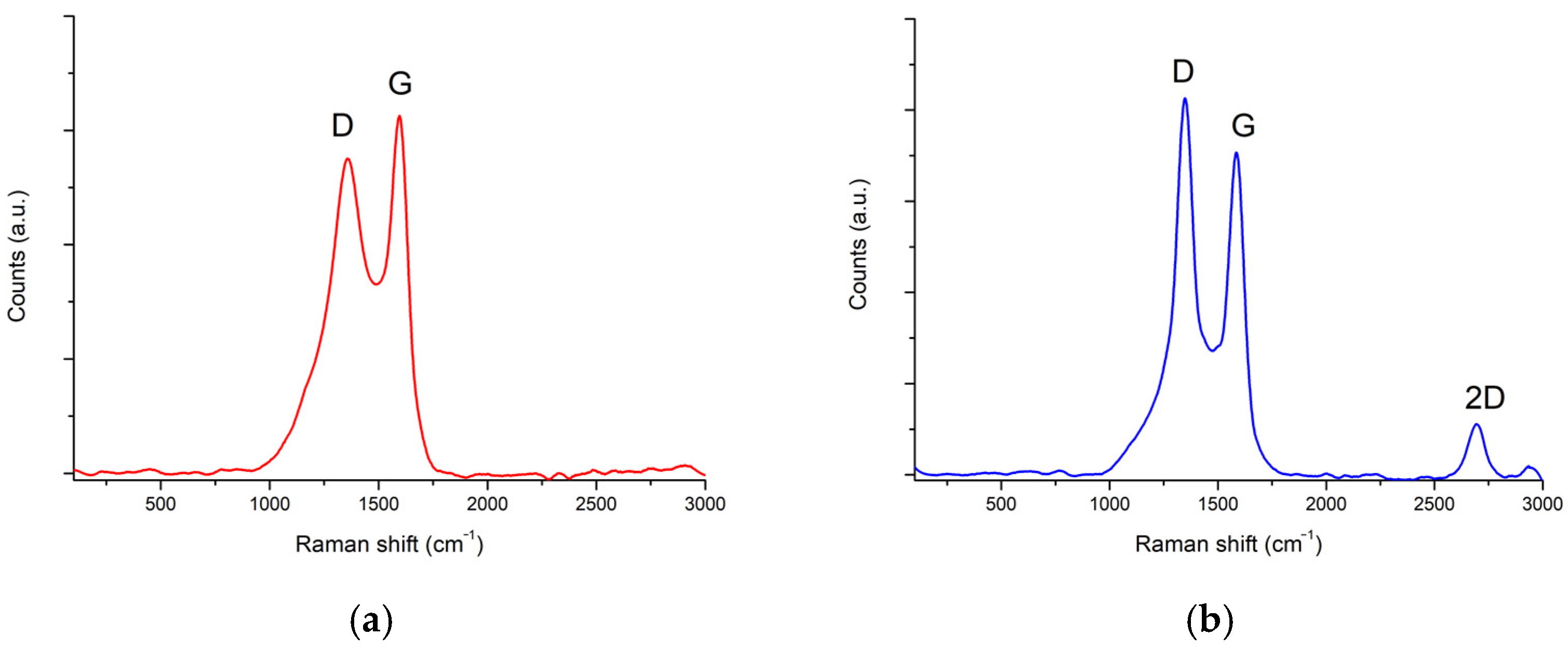



| Temperatures, °C | Catalyst Type | Carbon Morphology |
|---|---|---|
| 500–700 | Ni-based | Fiber structure |
| 650–950 | Fe-based | Fiber structure |
| 850–950 | carbonaceous | Fiber structure or turbostratic carbon |
| 650–1050 | other metal catalyst | graphitic carbon or turbostratic carbon |
| Above 1100 | without catalyst | amorphous carbon |
| CH4 | H2 | C2 + C3 * | N2 | O2 | |
|---|---|---|---|---|---|
| Content, % (m/m) | 34.0 | 19.3 | 0.1 | 46.6 | 0.1 |
| Normalized content (without nitrogen), % (m/m) | 63.67 | 36.14 | 0.19 | - | 0.19 |
| Parameters | Value |
|---|---|
| Methane conversion, % | 36.5 |
| Process efficiency, % | 36.1 |
| Process selectivity, % | 98.9 |
| Carbon | Area A | Area B |
|---|---|---|
| Specific surface area SBET, m2/g | 125.270 | 1.990 |
| Pore width (KJS), nm | 10.49 | 6.56 |
| Total pore volume, cm3/g | 0.3420 | 0.0060 |
| Mesopore volume, cm3/g | 0.3390 | 0.0048 |
| Mesoporosity, % | 99.12 | 60.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtasik, M.; Krasodomski, W.; Żak, G.; Wojtasik, K.; Pakieła, W. Morphological and Structural Analysis of Pyrolytic Carbon from Simple Thermal Methane Pyrolysis. Appl. Sci. 2025, 15, 10742. https://doi.org/10.3390/app151910742
Wojtasik M, Krasodomski W, Żak G, Wojtasik K, Pakieła W. Morphological and Structural Analysis of Pyrolytic Carbon from Simple Thermal Methane Pyrolysis. Applied Sciences. 2025; 15(19):10742. https://doi.org/10.3390/app151910742
Chicago/Turabian StyleWojtasik, Michał, Wojciech Krasodomski, Grażyna Żak, Katarzyna Wojtasik, and Wojciech Pakieła. 2025. "Morphological and Structural Analysis of Pyrolytic Carbon from Simple Thermal Methane Pyrolysis" Applied Sciences 15, no. 19: 10742. https://doi.org/10.3390/app151910742
APA StyleWojtasik, M., Krasodomski, W., Żak, G., Wojtasik, K., & Pakieła, W. (2025). Morphological and Structural Analysis of Pyrolytic Carbon from Simple Thermal Methane Pyrolysis. Applied Sciences, 15(19), 10742. https://doi.org/10.3390/app151910742







