Effect of Oxygen Concentration on the Corrosion Behaviour of Coated and Uncoated 316L Stainless Steel in Liquid Lead
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Thin-Film Coatings for Corrosion Tests
2.2. Microstructure and Surface Observation
2.3. Mechanical Properties
2.4. Corrosion Testing of Materials in Static Liquid Lead at Different Oxygen Concentrations
3. Results and Discussion
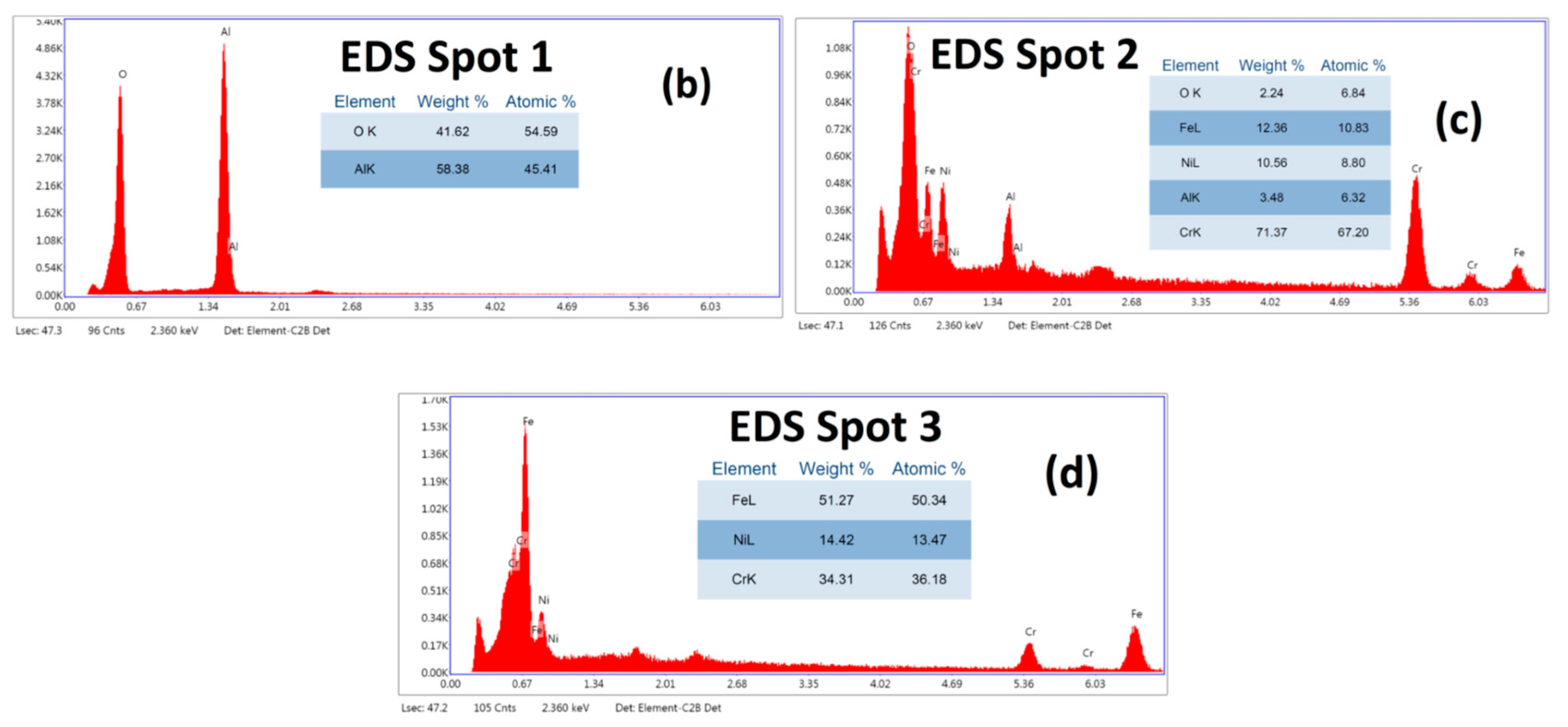
3.1. Coating Analysis
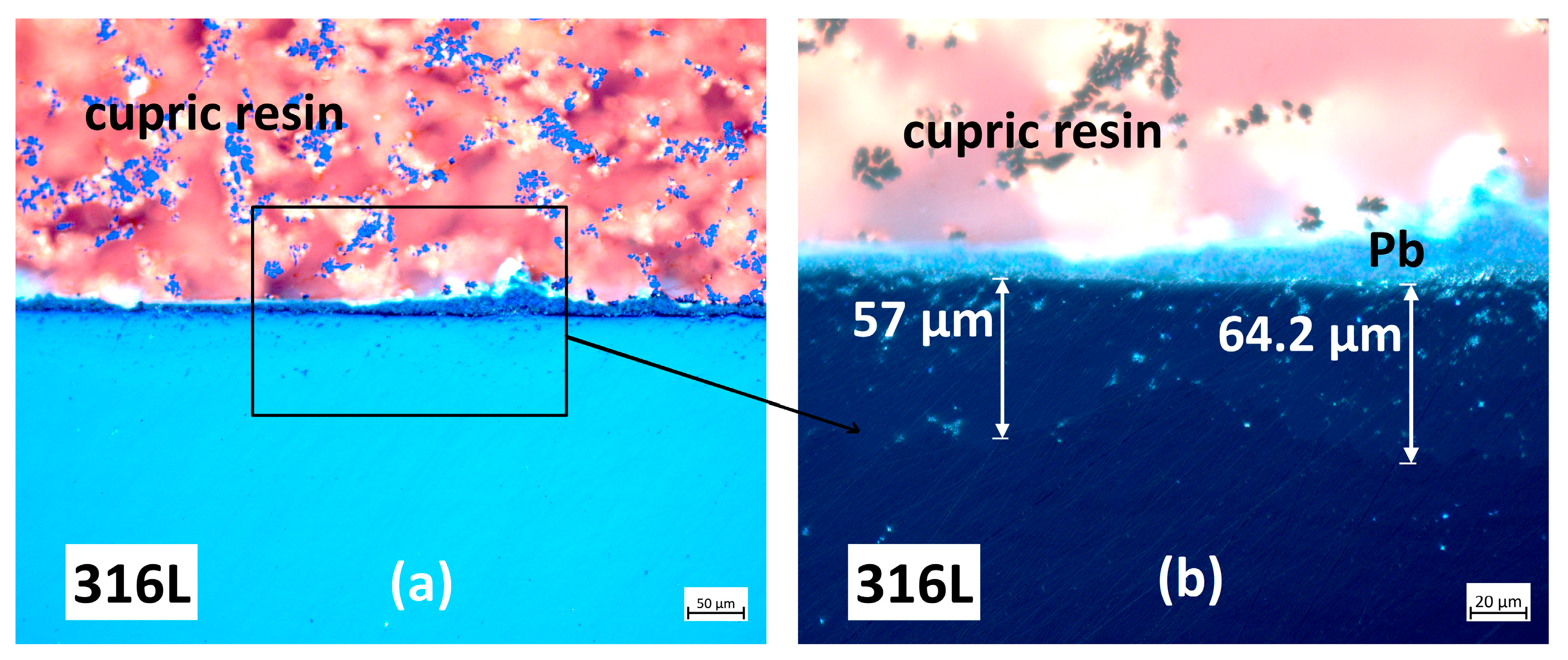
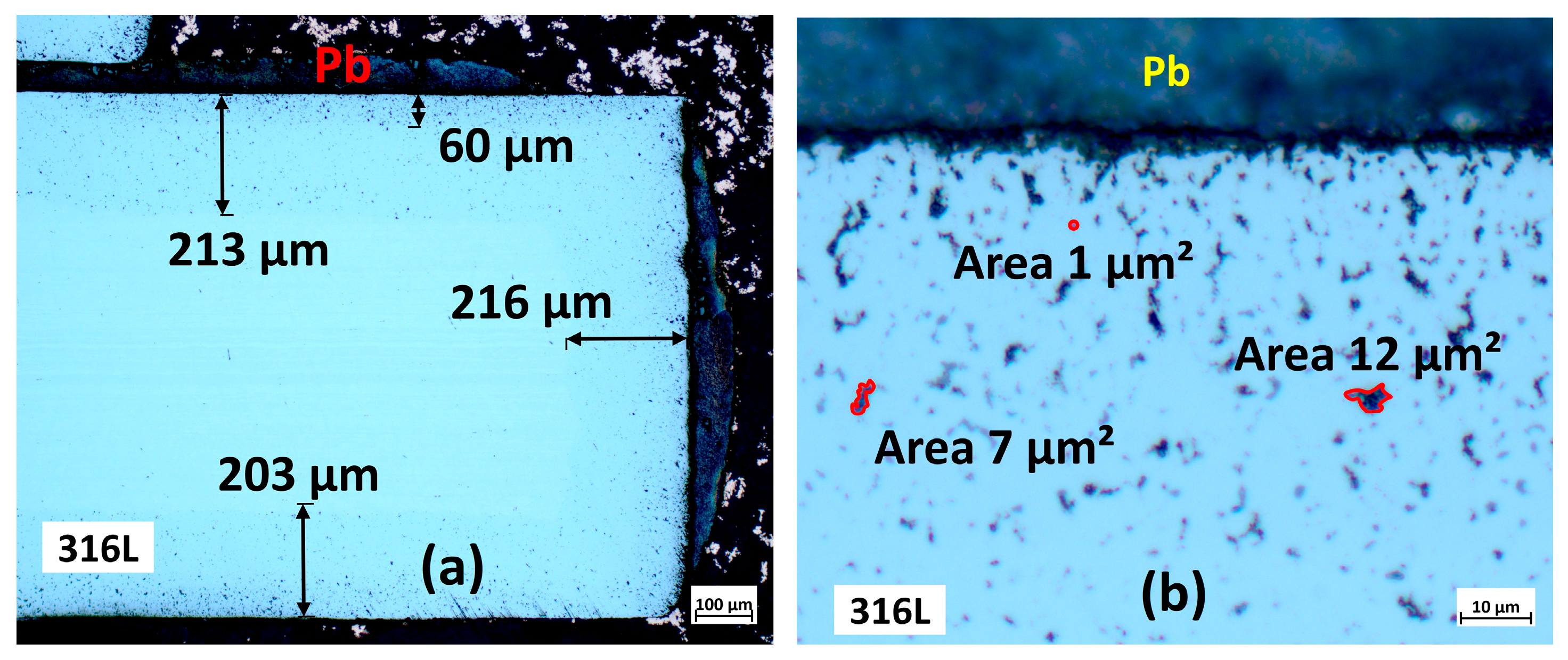
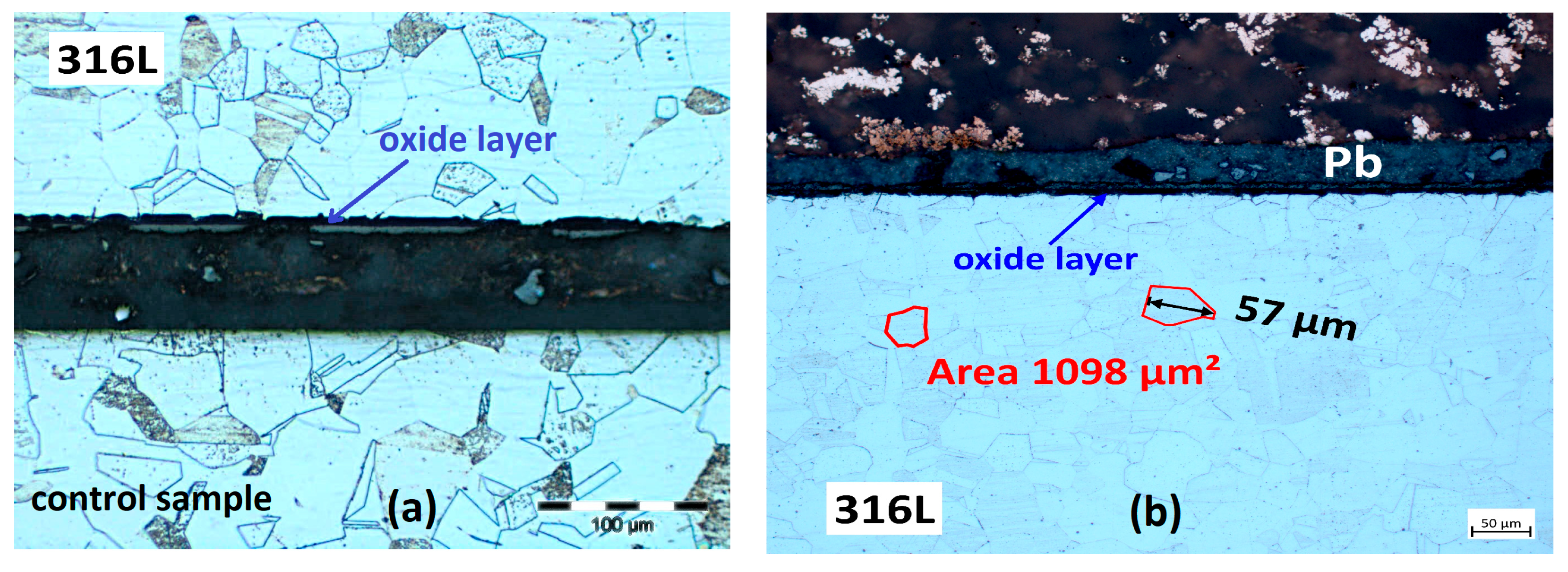
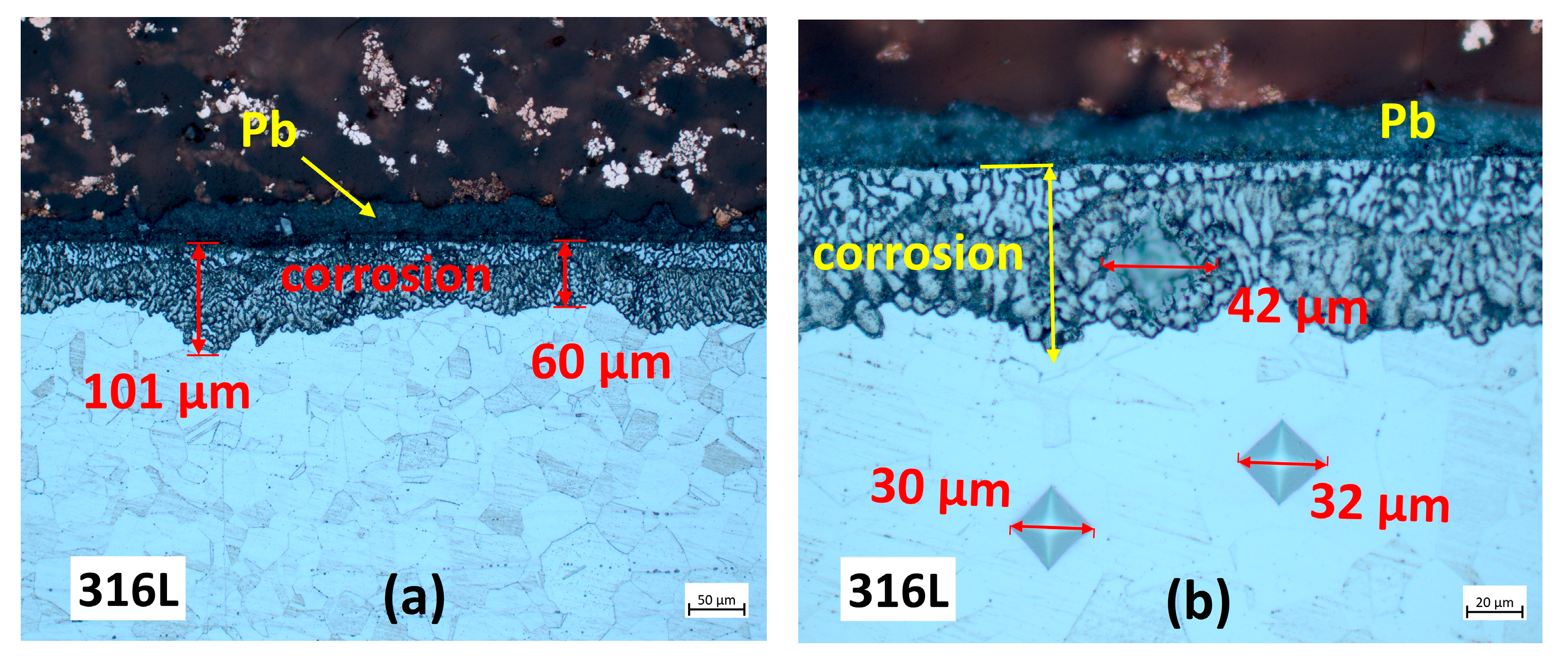
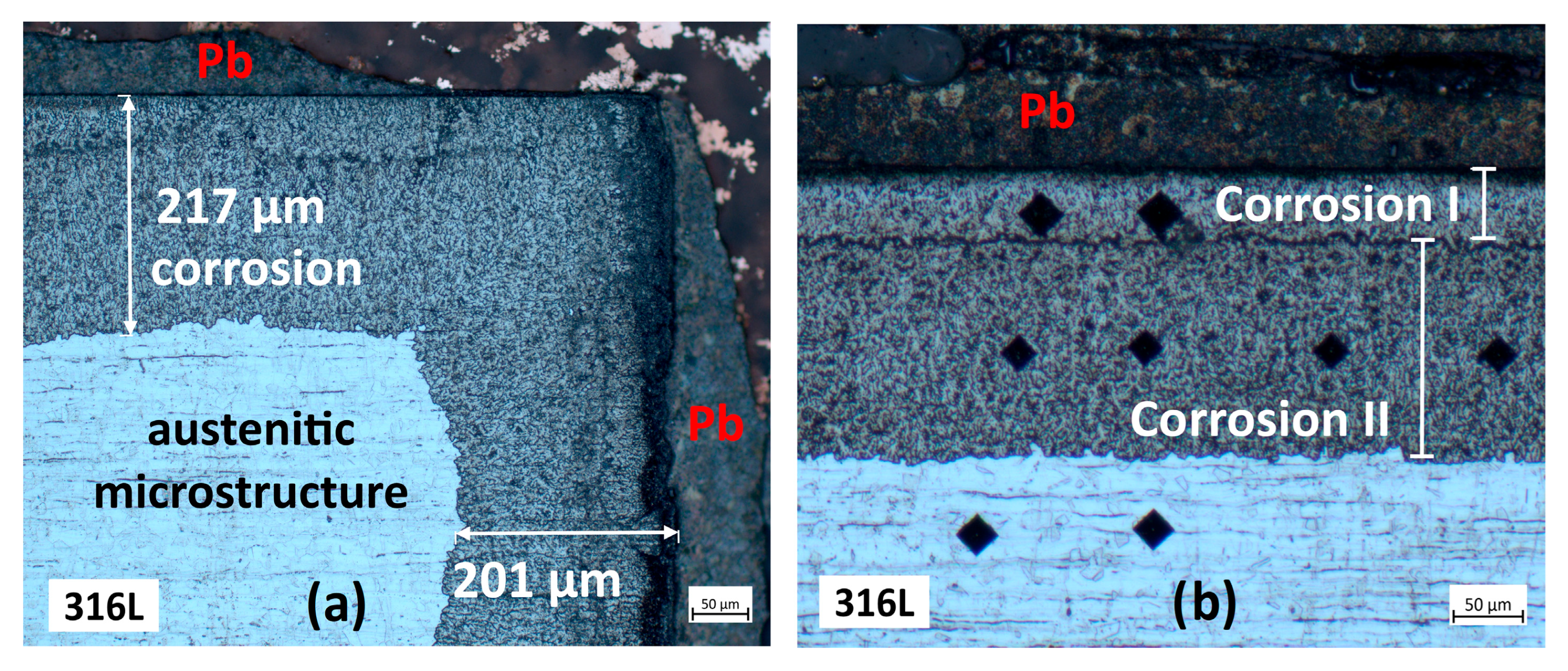
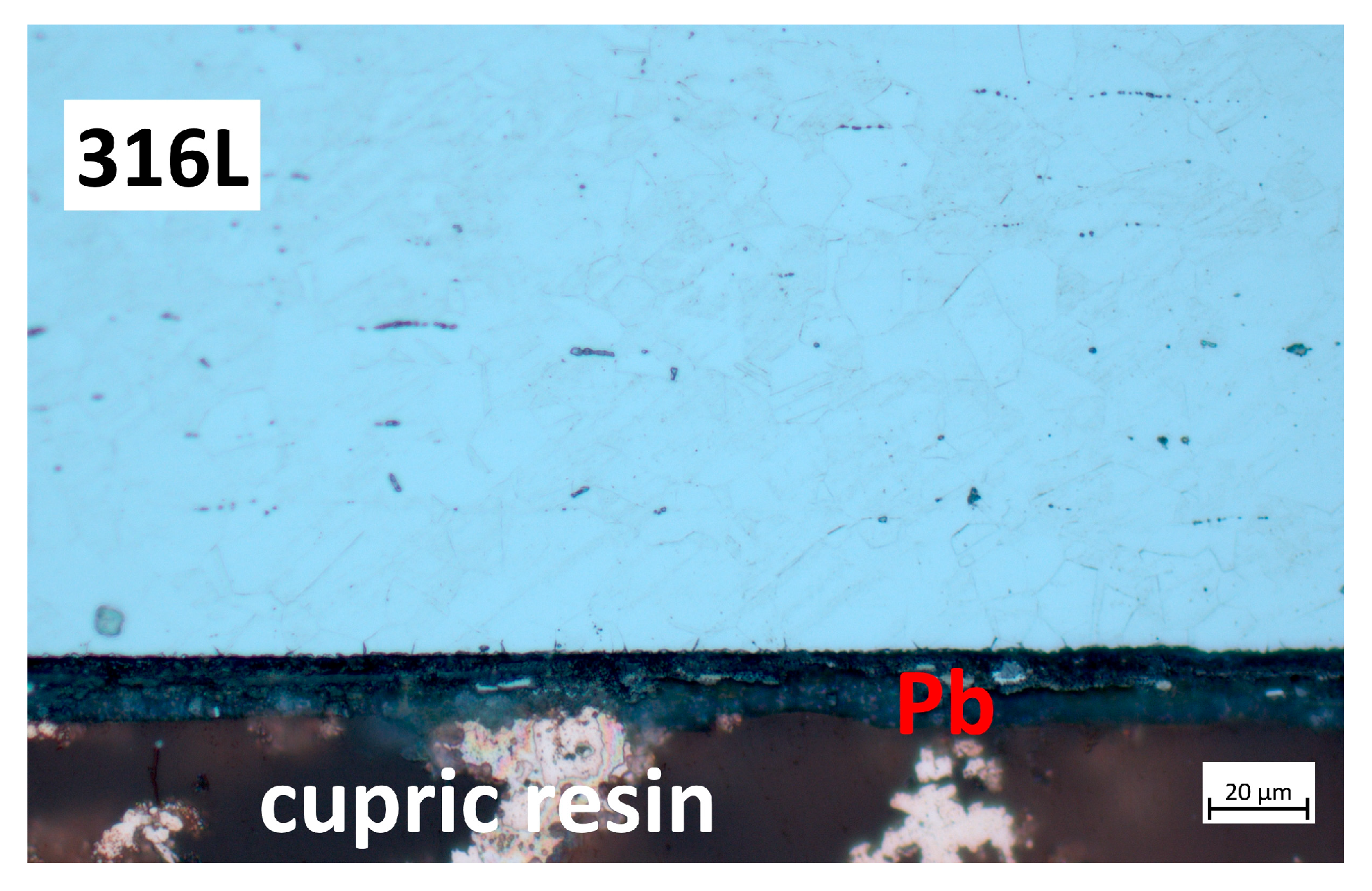
3.2. Characterisation Using Optical Microscopy
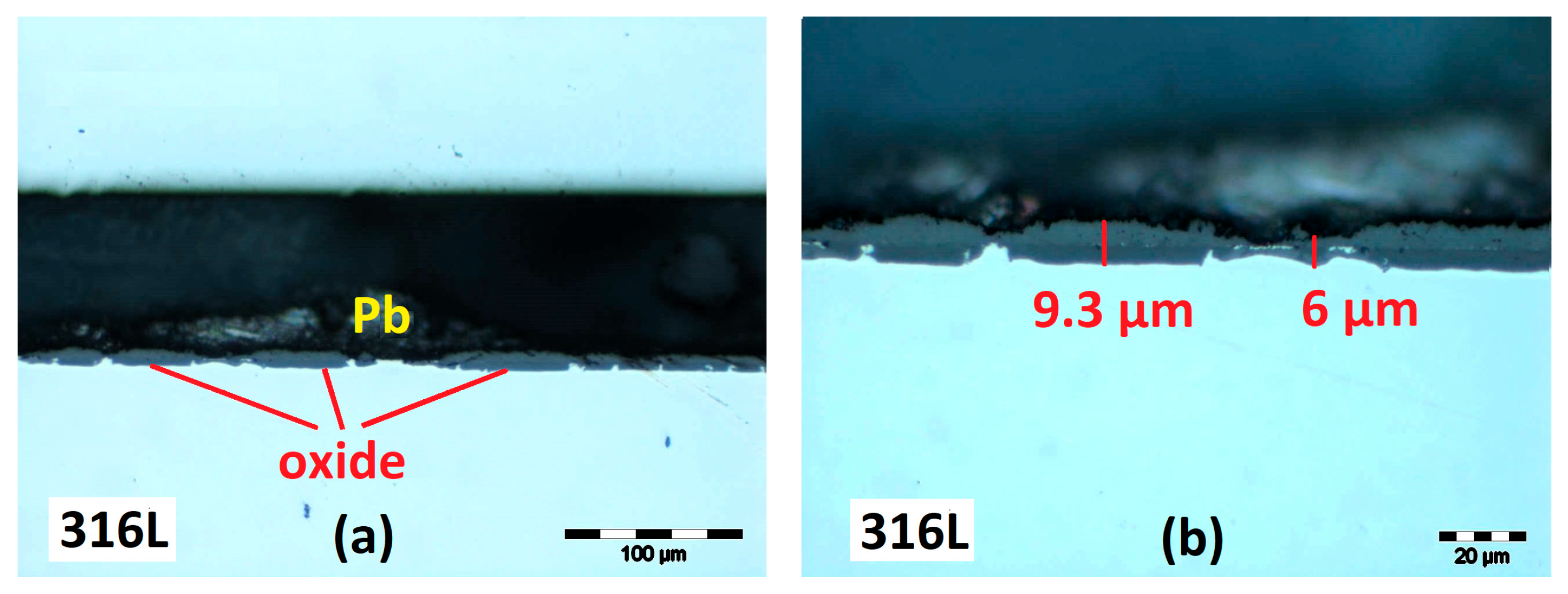
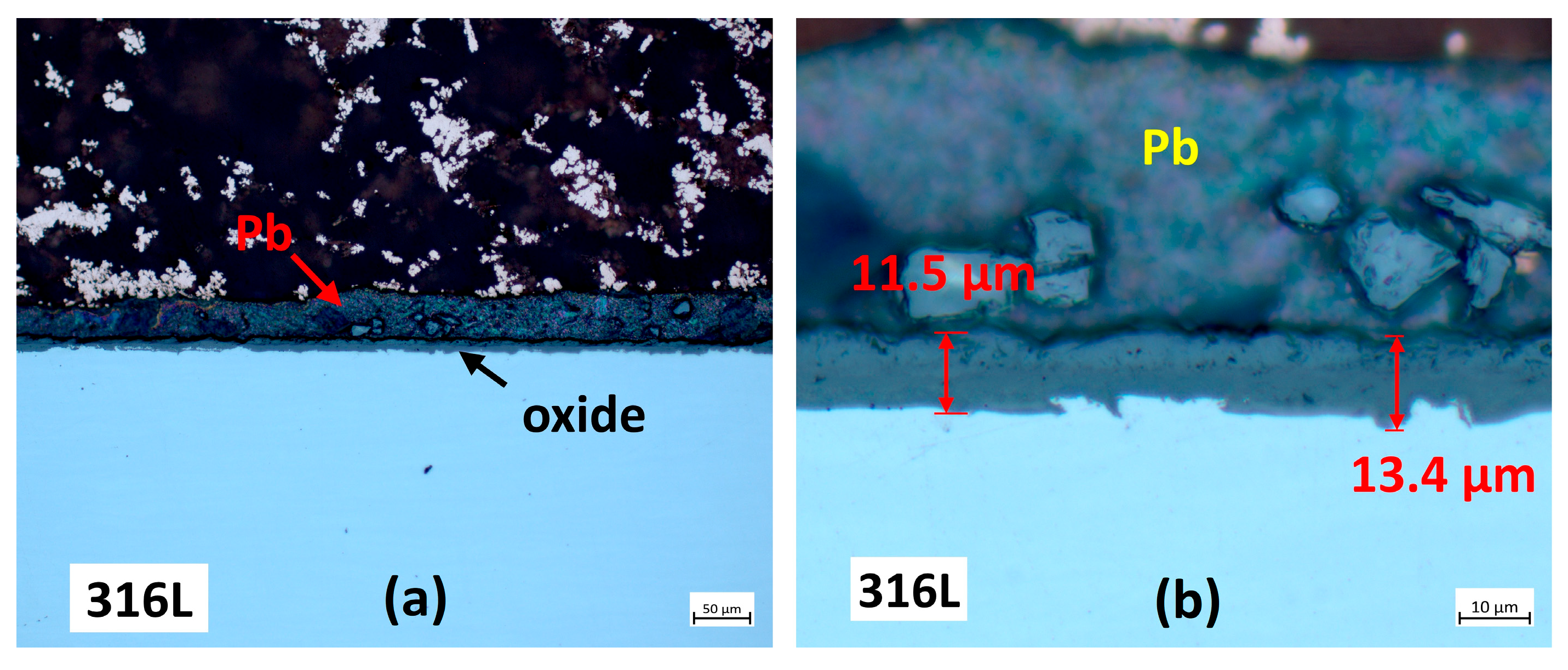
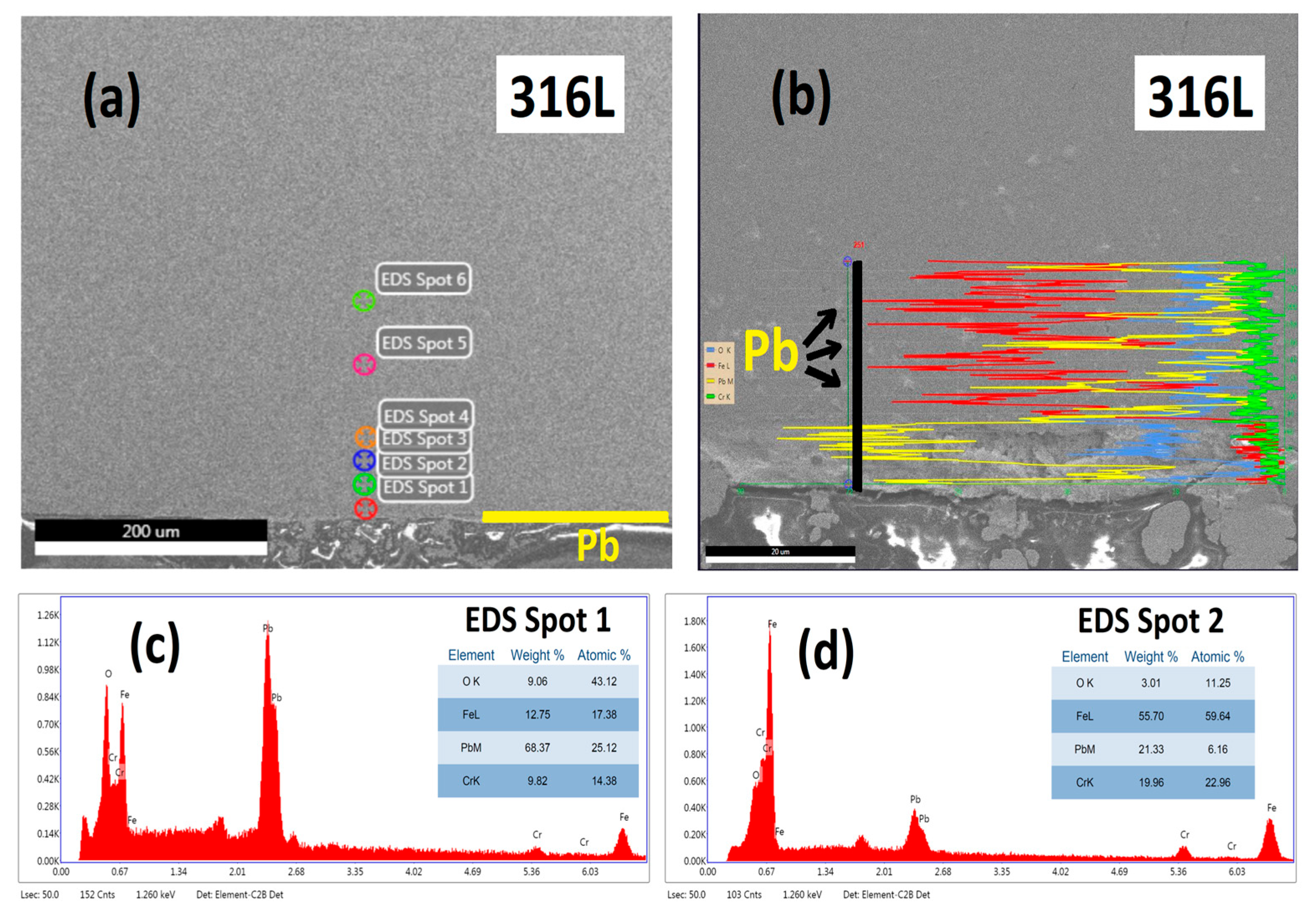
3.2.1. Cross-Section Analysis
3.2.2. Microstructural Analysis
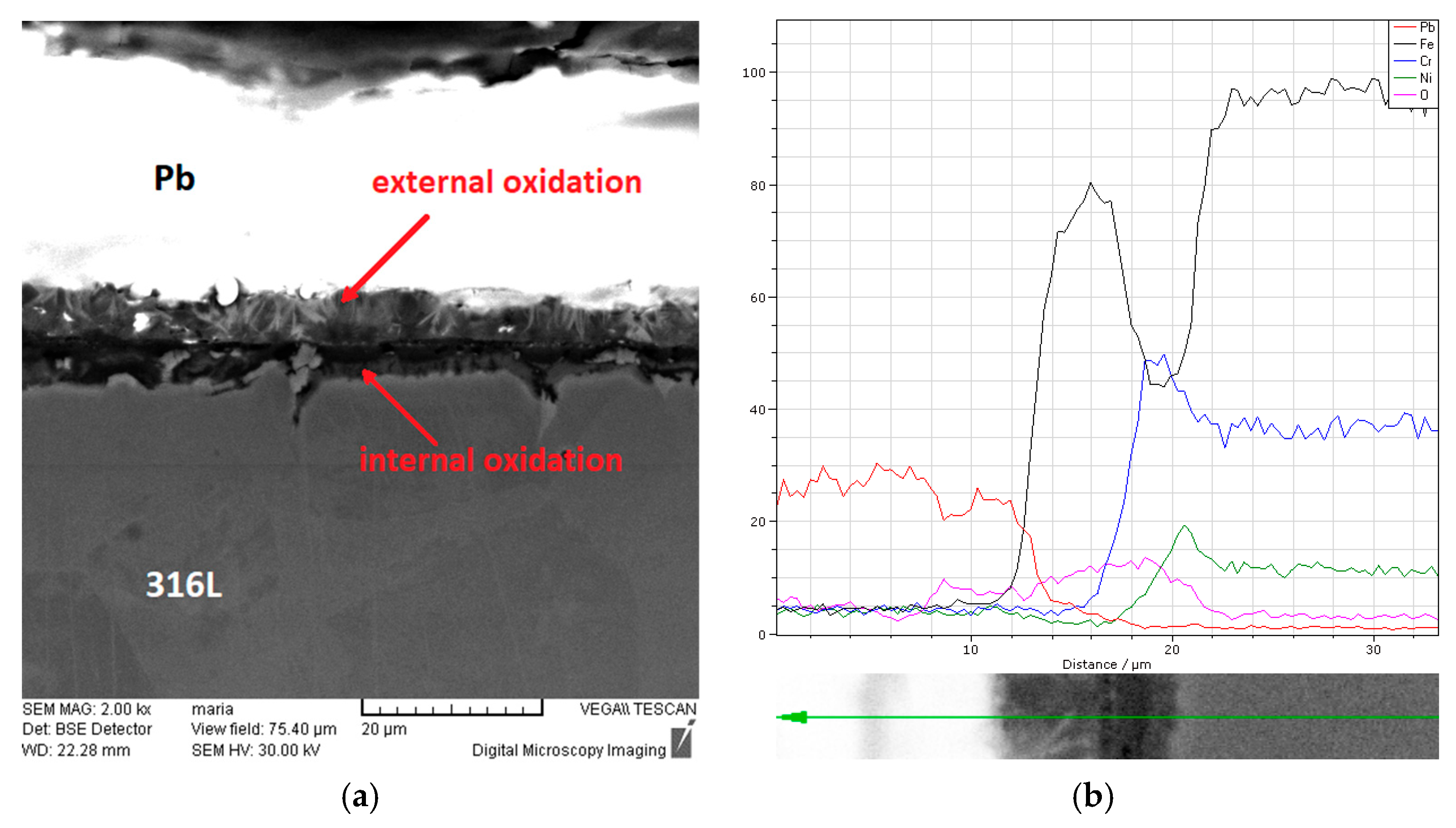
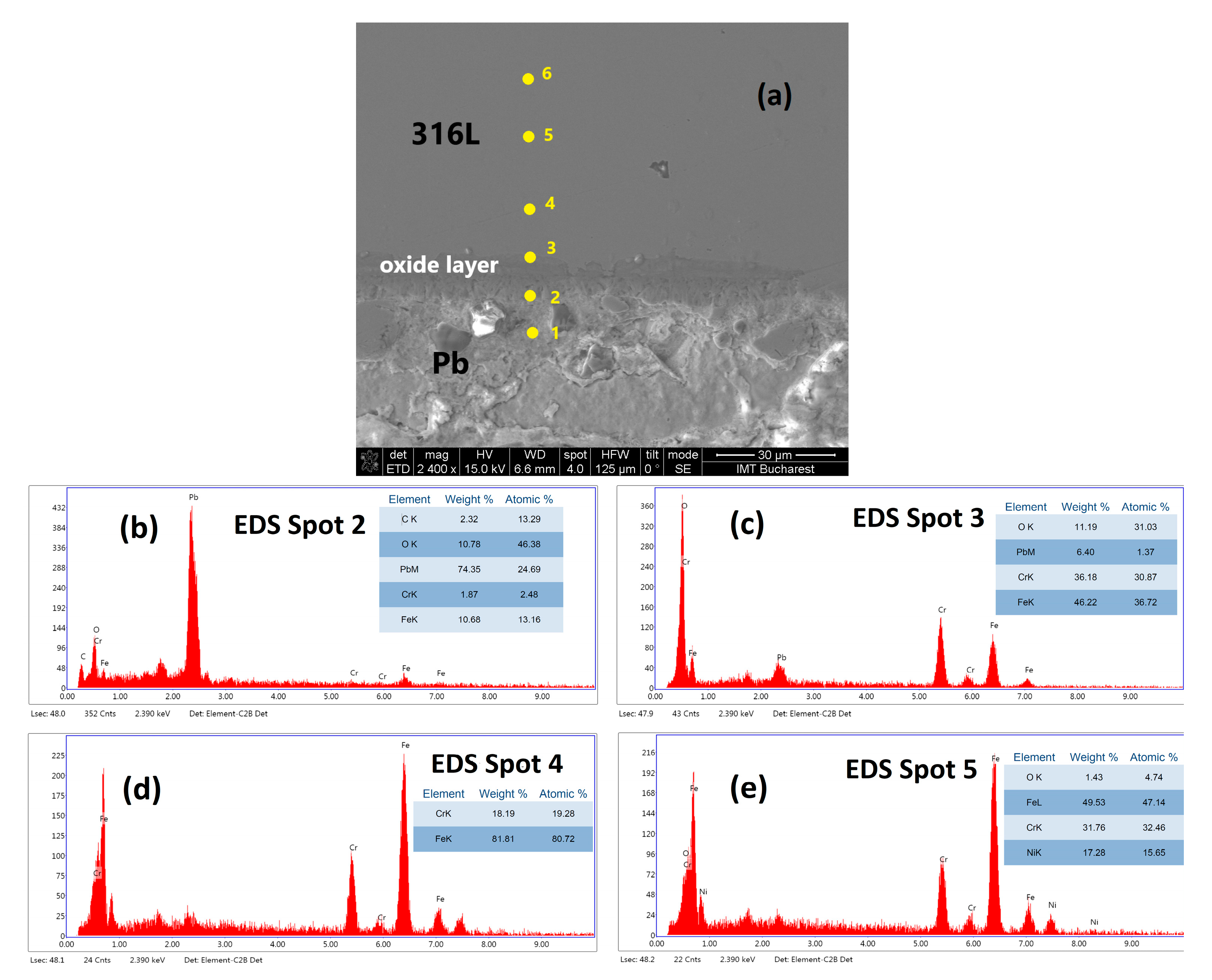
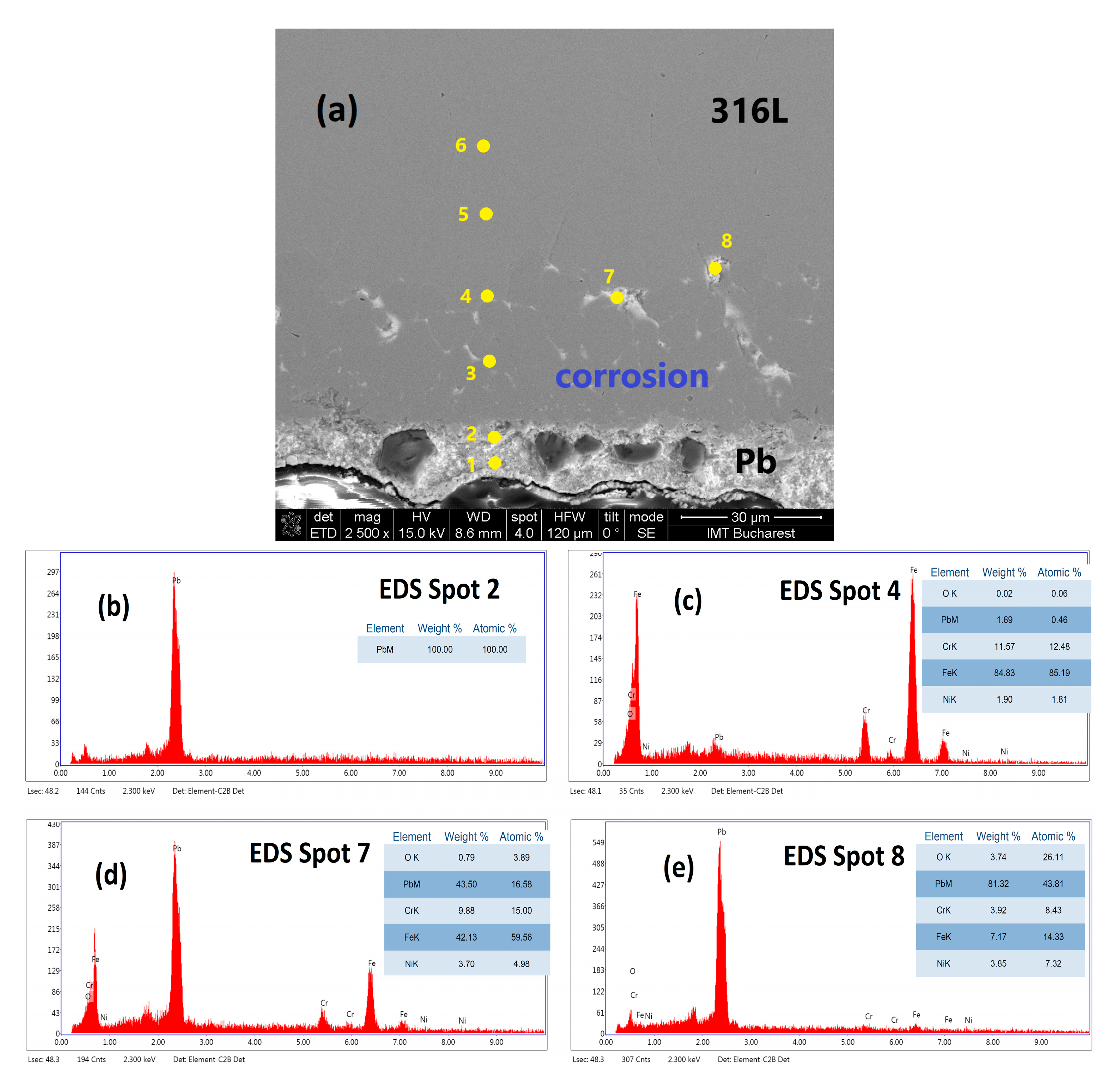
3.3. Characterisation Using Scanning Electron Microscopy (SEM)
3.4. Vickers Microhardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucan, D.; Jinescu, G. Materials and Specific Processes for Heat Transfer Equipment in Generation IV Fission Reactors; Eikon: Bucuresti, Romania, 2023; p. 19. [Google Scholar]
- Pioro, I.L. Handbook of Generation IV Nuclear Reactors; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Kelly, J.E. Generation IV International Forum: A decade of progress through international cooperation. Progr. Nucl. Energy 2014, 77, 240–246. [Google Scholar] [CrossRef]
- Stanculescu, A. GIF R&D outlook for generation IV nuclear energy systems: 2018 update. In Proceedings of the Generation IV International Forum, Paris, France, 18–19 March 2018; pp. 16–18. [Google Scholar]
- Tudose, A.E.; Golgovici, F.; Anghel, A.; Fulger, M.; Demetrescu, I. Corrosion Testing of CrNx-Coated 310 H Stainless Steel under Simulated Supercritical Water Conditions. Materials 2022, 15, 5489. [Google Scholar] [CrossRef] [PubMed]
- Trubcheninova, A.I.; Abramov, A.V.; Alimgulov, R.R.; Polovov, I.B.; Volkovich, V.A. The Effect of Microstructural Changes in Nickel-Based Alloys on Their Corrosion Resistance in Molten Halides: A Consideration of Prospective Structural Materials for Molten Salt Reactors. Appl. Sci. 2025, 15, 4753. [Google Scholar] [CrossRef]
- Ding, H.; Tong, J.; Wang, Y.; Zhang, L. Development of emergency planning zone for high temperature gas-cooled reactor. Ann. Nucl. Energy 2018, 111, 347–353. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, R. Molten-Pool Mobility. In Safety of Sodium-Cooled Fast Reactors; Springer: Singapore, 2021; p. 32. ISBN 978-981-16-6115-0. [Google Scholar]
- Burakovsky, L.; Preston, D.L.; Green, A.A. Analytic Model for U-Nb Liquidus and U-6Nb Melting Curve. Appl. Sci. 2025, 15, 3763. [Google Scholar] [CrossRef]
- Teng, F.; Copeland-Johnson, T.M.; Tucker, J.D.; Cao, G. Accelerated corrosion of Ni-based alloys in molten chloride salts, due to Ni2Cr phase formation. Materialia 2023, 31, 101875. [Google Scholar] [CrossRef]
- Petrescu, D.; Fulger, M.; Golgovici, F.; Demetrescu, I. Addressing some issues encountered in liquid lead corrosion tests of candidate materials for future nuclear reactors. U.P.B. Sci. Bull. Ser. B 2022, 84, 89–97. [Google Scholar]
- Zhang, C.; Chen, L. Advancements and Development Trends in Lead-Cooled Fast Reactor Core Design. Processes 2025, 13, 1773. [Google Scholar] [CrossRef]
- NEA. Handbook on Lead-Bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-Hydraulics and Technologies—2015 Edition; OECD Publishing: Paris, France.
- García Ferré, F.; Mairov, A.; Iadiciccoa, D.; Vanazzi, M.; Bassini, S. Corrosion and radiation resistant nanoceramic coatings for lead fast reactors. Corros. Sci. 2017, 124, 80–92. [Google Scholar] [CrossRef]
- Zhang, J. A review of steel corrosion by liquid lead and lead-bismuth. Corros. Sci. 2009, 51, 1207–1227. [Google Scholar] [CrossRef]
- Weisenburger, A.; Schroer, C.; Jianu, A. Long term corrosion on T91 and AISI 316L steel in flowing lead alloy and corrosion protection barrier development: Experiments and models. J. Nucl. Mater. 2011, 415, 260–269. [Google Scholar] [CrossRef]
- Konys, J.; Krauss, W. Corrosion of structural materials in liquid lead alloys for nuclear applications. Corrosion 2007, 63, 1124–1137. [Google Scholar] [CrossRef]
- Schroer, C.; Wedemeyer, O. Aspects of minimizing steel corrosion in liquid lead-alloys by addition of oxygen. Nucl. Eng. Des. 2011, 241, 4913–4923. [Google Scholar] [CrossRef]
- Caro, M.; Woloshun, K. Heavy liquid metal corrosion of structural materials in advanced nuclear materials. JOM 2013, 65, 1057–1066. [Google Scholar] [CrossRef]
- Hosemann, P.; Dickerson, R. Transmission electron microscopy (TEM) on oxide layers formed on D9 stainless steel in lead bismuth eutectic (LBE). Corros. Sci. 2013, 66, 196–202. [Google Scholar] [CrossRef]
- Gong, X.; Short, M.P.; Auger, T. Environmental degradation of structural materials in liquid lead-and lead-bismuth eutectic-cooled reactors. Prog. Mater. Sci. 2022, 126, 100920. [Google Scholar] [CrossRef]
- Qin, B.; Fu, X.G. Preliminary experiment study on control of oxygen concentration via gas phase in liquid lead-bismuth alloy. Mater. Rep. 2019, 33, 1821–1824. [Google Scholar]
- Liu, Y.; Qin, B.; Fu, X. Influence of Surface State on the Corrosion Behavior of Si-Reinforced F/M Steels under Solid-Phase Oxygen-Controlled Static Liquid LBE Environment. Metals 2024, 14, 810. [Google Scholar] [CrossRef]
- Petrescu, D.; Nitu, A.; Golgovici, F.; Demetrescu, I. Behaviour Aspects of an EB-PVD Alumina (Al2O3) Film with an Interlayer (NiCrAlY) Deposited on AISI 316L Steel Investigated in Liquid Lead. Metals 2023, 13, 616. [Google Scholar] [CrossRef]
- Weisenburger, A.; Mansani, L.; Schumacher, G. Oxygen for protective oxide scale formation on pins and structural material surfaces in lead-alloy cooled reactors. Nucl. Eng. Des. 2014, 273, 584–594. [Google Scholar] [CrossRef]
- Wu, J.; Wu, R.; Wang, Y. Prediction of Dissolved Impurities and Movement of Oxide Particles in the Primary Circuit of LBE Fast Reactor. Coatings 2021, 11, 1263. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, Y. Microstructure, Mechanical Properties, and Lead–Bismuth Eutectic Corrosion Behaviors of FeCrAlY-Al2O3 Nanoceramic Composite Coatings. Coatings 2024, 14, 393. [Google Scholar] [CrossRef]
- Heinzel, A.; Weisenburger, A. Corrosion behavior of austenitic steels in liquid lead bismuth containing 10−6 wt% and 10−8 wt% oxygen at 400–500 °C. J. Nucl. Mater. 2014, 448, 163–171. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, Y. Screening of the FeCrAl LBE corrosion-resistant coatings: The effect of Cr and Al contents. Surf. Coat. Technol. 2023, 462, 129477. [Google Scholar] [CrossRef]
- ASTM International. Standard Specification for Chromium and Chromium-Nickel Stainless Steel Plate, Sheet, and Strip for Pressure Vessels and for General Applications (ASTM A240/A240M-17); ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM International. Standard Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels, ASTM A262; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar] [CrossRef]
- Inspection certificate no. 783863/08.08.2017. Product 316L Steel, Outokumpu Stainless Oy. Tornio, Finland, 2017. certificate.tornio@outokum.com.
- Golgovici, F.; Tudose, A.-E.; Mosinoiu, L.F.; Demetrescu, I. Characterization of NiCrAlY Layers Deposited on 310H Alloy Using the EB-PVD Method After Oxidation in Water at High Temperature and Pressure. Appl. Sci. 2025, 15, 2361. [Google Scholar] [CrossRef]
- Serag, E. Corrosion behavior of steels in oxygenated liquid lead environments. Crystals 2022, 12, 968. [Google Scholar]
- Weisenburger, A.; Müller, G.; Schroer, C.; Heinzel, A.; Fazio, C. Corrosion and protection of steels in liquid lead and LBE: Mechanisms and oxygen control. J. Nucl. Mater. 2011, 415, 260–269. [Google Scholar] [CrossRef]
- Charalampopoulou, E.; Müller, G.; Weisenburger, A.; Heinzel, A. Selective leaching and ferritization of 316L stainless steel in LBE at low oxygen. J. Nucl. Mater. 2019, 516, 165–174. [Google Scholar]
- Yamaki, T.; Konishi, T.; Takahashi, M.; Kasahara, K. Dissolution kinetics of 316L stainless steel in low-oxygen LBE. J. Nucl. Mater. 2015, 456, 231–240. [Google Scholar]
- Hosemann, P.; Kondo, S.; Weisenburger, A.; Heinzel, A.; Müller, G.; Pereiro, A.; Gröschel, F.; Schroer, C.; Abe, H. Ferritization of austenitic steels in LBE under low oxygen conditions. Acta Mater. 2010, 58, 2813–2821. [Google Scholar]
- Di Gabriele, F.; Amore, S.; Scaiola, C. Corrosion behavior of 12Cr-ODS steel in molten lead. Nucl. Eng. Des. 2014, 280, 69–75. [Google Scholar] [CrossRef]
- Navas, M.; Hernandez, R. Compatibility of Structural Materials with Lead and Lead Bismuth Eutectic for CSP Applications. AIP Conf. Proc. 2018, 2033, 230009. [Google Scholar] [CrossRef]
- Vogt, J.-B.; Serre, I.P. A Review of the Surface Modifications for Corrosion Mitigation of Steels in Lead and LBE. Coatings 2021, 11, 53. [Google Scholar] [CrossRef]
- Cionea, C.; Abad, M.D.; Aussat, Y.; Frazer, D. Oxide scale formation on 316L and FeCrAl steels exposed to oxygen controlled static LBE at temperatures up to 800 °C. Sol. Energy Mater. Sol. Cells 2016, 144, 235–246. [Google Scholar] [CrossRef]
- Serag, E.; Caers, B.; Schuurmans, P.; Lucas, S.; Haye, E. Challenges and coating solutions for wear and corrosion inside lead–bismuth eutectic: A review. Surf. Coat. Technol. 2022, 441, 128542. [Google Scholar] [CrossRef]
- Popovic, M.; Chen, K.; Shen, H.; Stan, C.; Olmsted, D.; Tamura, N.; Asta, M.; Abad, M.; Hosemann, P. A study of deformation and strain induced in bulk by the oxide layer formation on a Fe–Cr–Al alloy in high-temperature liquid Pb–Bi eutectic. Acta Mater. 2018, 151, 301–309. [Google Scholar] [CrossRef]
- Ma, Z.; Shen, T.; Zhou, T.; Wang, Z. Performance of the new ferritic/martensitic steel SIMP against liquid lead–bismuth eutectic corrosion: Comparison with T91 and 316L steels. Mater. Corros. 2023, 74, 221–232. [Google Scholar]
- Tian, S.; Pang, Y.; Jiang, Z.; Luo, L.; Xiao, Z. Corrosion characteristics of T91 steel in lead–bismuth eutectic with different oxygen concentrations at 500 °C. Mater. Corros. 2022, 73, 134–142. [Google Scholar]
- Lee, S.G.; Shin, Y.-H.; Park, J.; Hwang, I.S. High-temperature corrosion behaviors of structural materials for lead-alloy-cooled fast reactor application. Appl. Sci. 2021, 11, 2349. [Google Scholar] [CrossRef]
- Orlov, S.N.; Bogachev, N.A.; Mereshchenko, A.S.; Zmitrodan, A.A.; Skripkin, M.Y. Electrochemical sensors for controlling oxygen content and corrosion processes in lead–bismuth eutectic coolant—State of the art. Sensors 2023, 23, 812. [Google Scholar] [CrossRef] [PubMed]
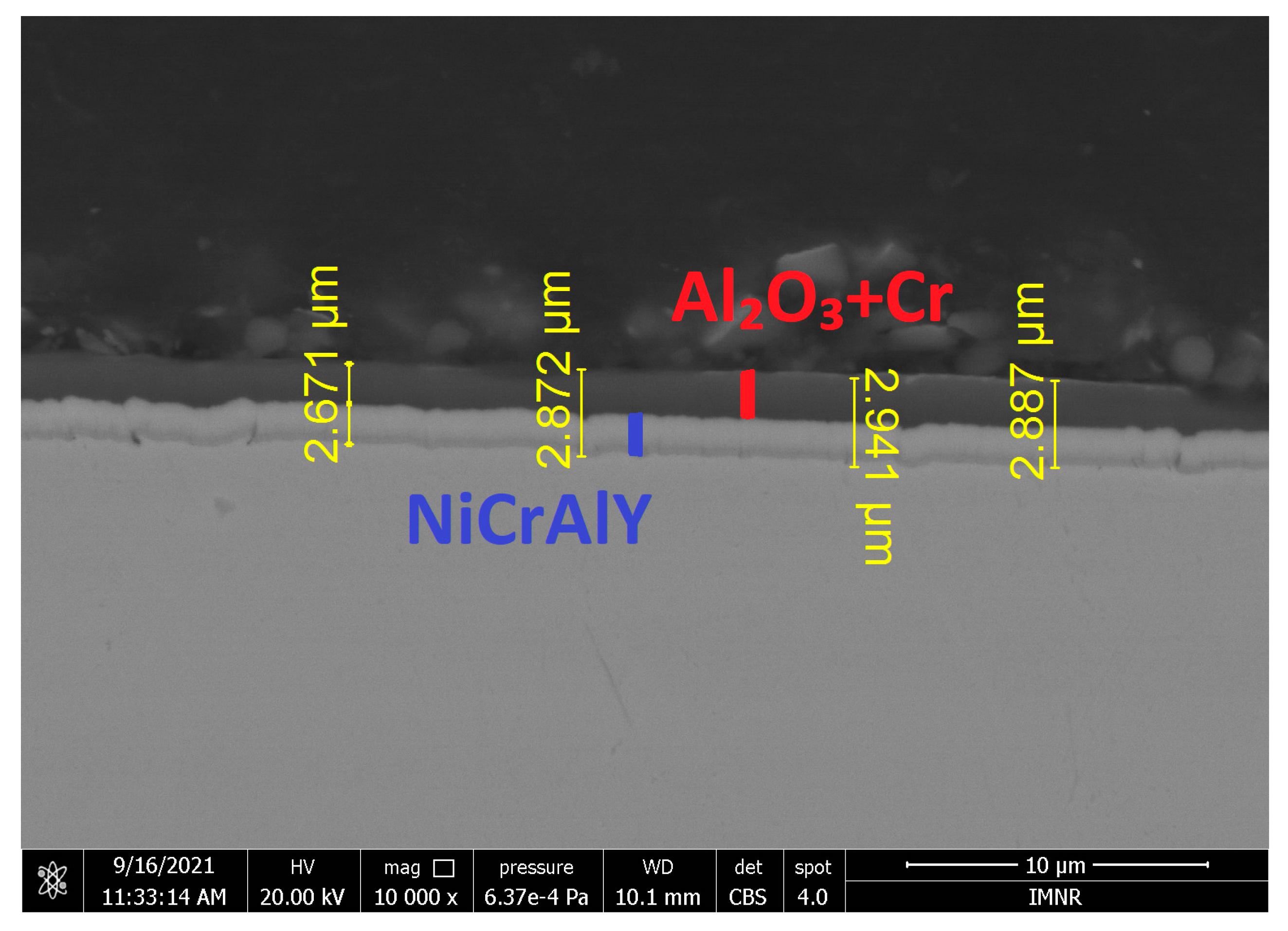
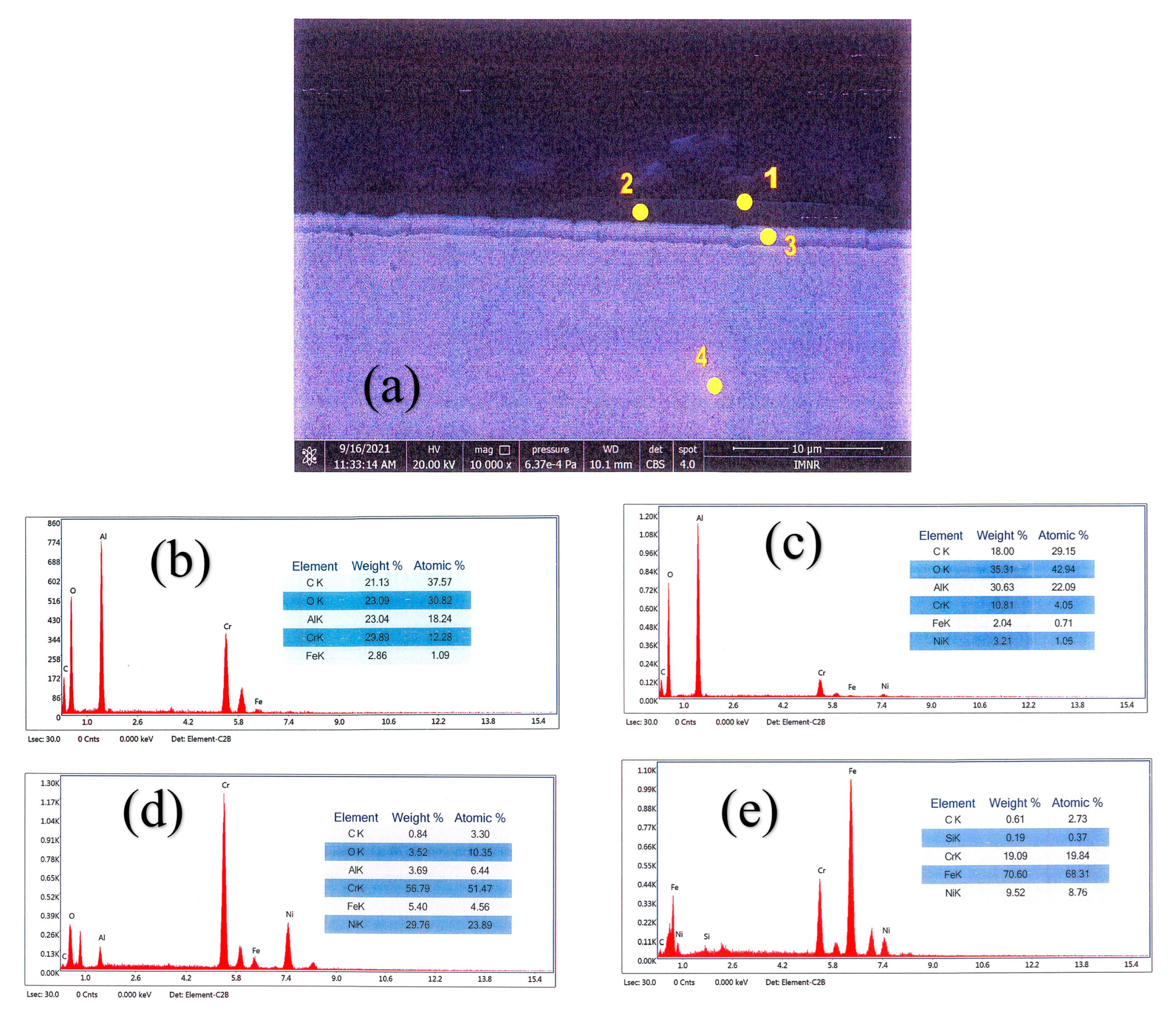



| Elements % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe | C | Cr | Ni | Mo | Mn | Si | P | S |
| Bal. | 0.02 | 17.1 | 10.1 | 2.03 | 0.9 | 0.47 | 0.033 | <0.001 |
| Yield strength | Rp0.2 = 306 MPa |
| Tensile strength | Rm = 604 MPa |
| Elongation | A5 = 59% |
| Hardness | 166 HBW |
| Material | Coating (EB-PVD) | Oxygen Concentration (wt.%) | Time (h) | Temperature (°C) |
|---|---|---|---|---|
| 316L | - | ~10−3 | 1000 | 550 |
| - | ~10−3 | 2000 | ||
| - | ~10−8 | 1000 | ||
| NiCrAlY (~1 µm) | ~10−8 | 1000 | ||
| NiCrAlY + Al2O3 + Cr (~3 µm) | ~10−8 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrescu, D.; Golgovici, F.; Corban, M.; Brincoveanu, O.; Demetrescu, I. Effect of Oxygen Concentration on the Corrosion Behaviour of Coated and Uncoated 316L Stainless Steel in Liquid Lead. Appl. Sci. 2025, 15, 10572. https://doi.org/10.3390/app151910572
Petrescu D, Golgovici F, Corban M, Brincoveanu O, Demetrescu I. Effect of Oxygen Concentration on the Corrosion Behaviour of Coated and Uncoated 316L Stainless Steel in Liquid Lead. Applied Sciences. 2025; 15(19):10572. https://doi.org/10.3390/app151910572
Chicago/Turabian StylePetrescu, Daniel, Florentina Golgovici, Mircea Corban, Oana Brincoveanu, and Ioana Demetrescu. 2025. "Effect of Oxygen Concentration on the Corrosion Behaviour of Coated and Uncoated 316L Stainless Steel in Liquid Lead" Applied Sciences 15, no. 19: 10572. https://doi.org/10.3390/app151910572
APA StylePetrescu, D., Golgovici, F., Corban, M., Brincoveanu, O., & Demetrescu, I. (2025). Effect of Oxygen Concentration on the Corrosion Behaviour of Coated and Uncoated 316L Stainless Steel in Liquid Lead. Applied Sciences, 15(19), 10572. https://doi.org/10.3390/app151910572







