Antifungal and Cytocompatible Properties of Juglans regia Extract for Dental Applications: A Novel Approach Against Oral Candida Infections
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Equipment
2.3. Plant Extract
2.4. Fungal Strains
2.5. Antimicrobial Activity in Planktonic Cultures
2.6. Synthesis of Biofilm Matrix
2.7. Crystal Violet (CV) Staining
2.8. Metabolic Activity of Microorganisms by MTT Assay
2.9. Cell Viability by Resazurin Assay
2.10. Statistical Analysis
3. Results
3.1. Antifungal Activity in Planktonic Cultures
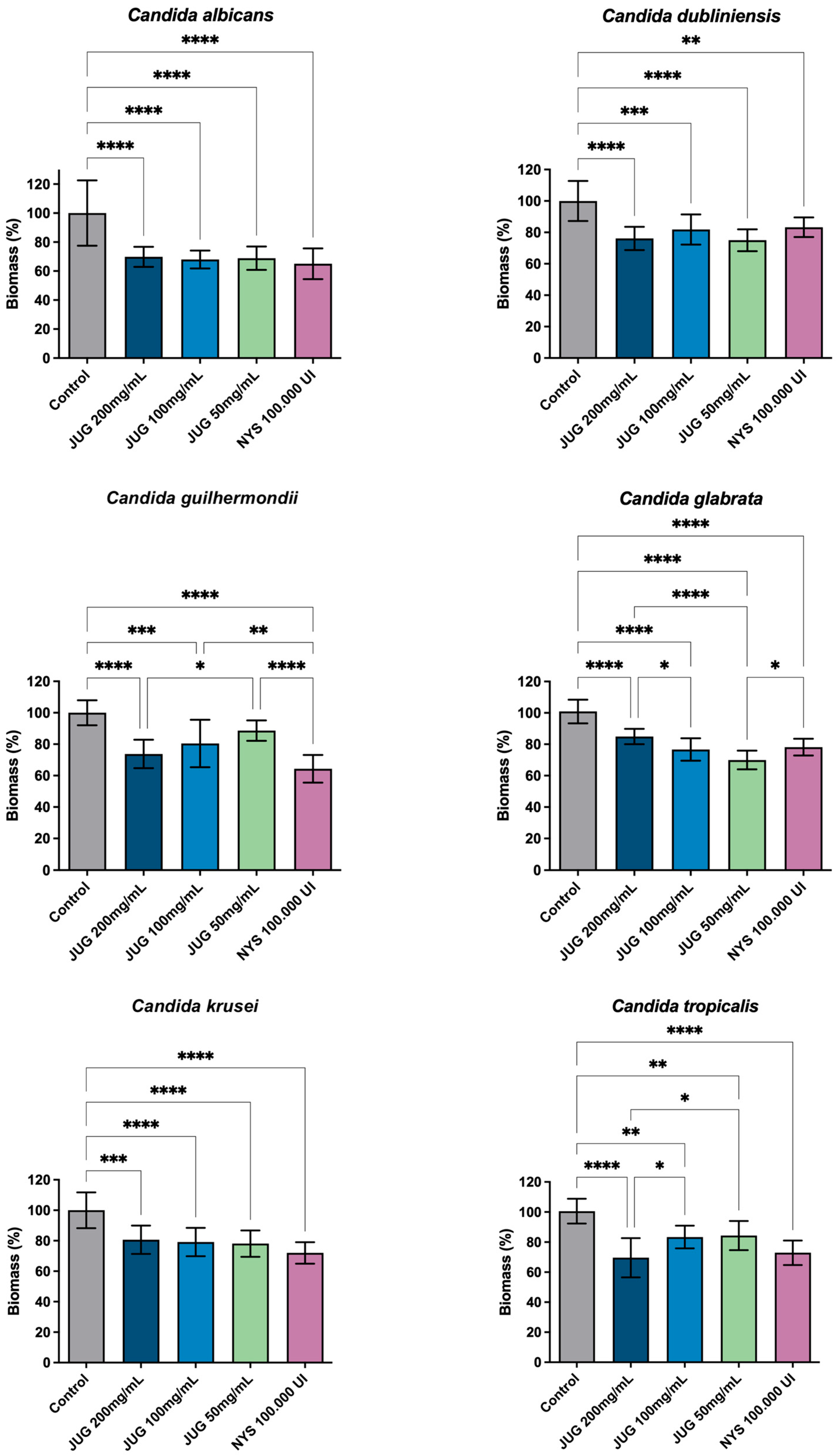
3.2. Anti-Biofilm Action—Biomass
3.3. Anti-Biofilm Action—MTT (Metabolic Activity)
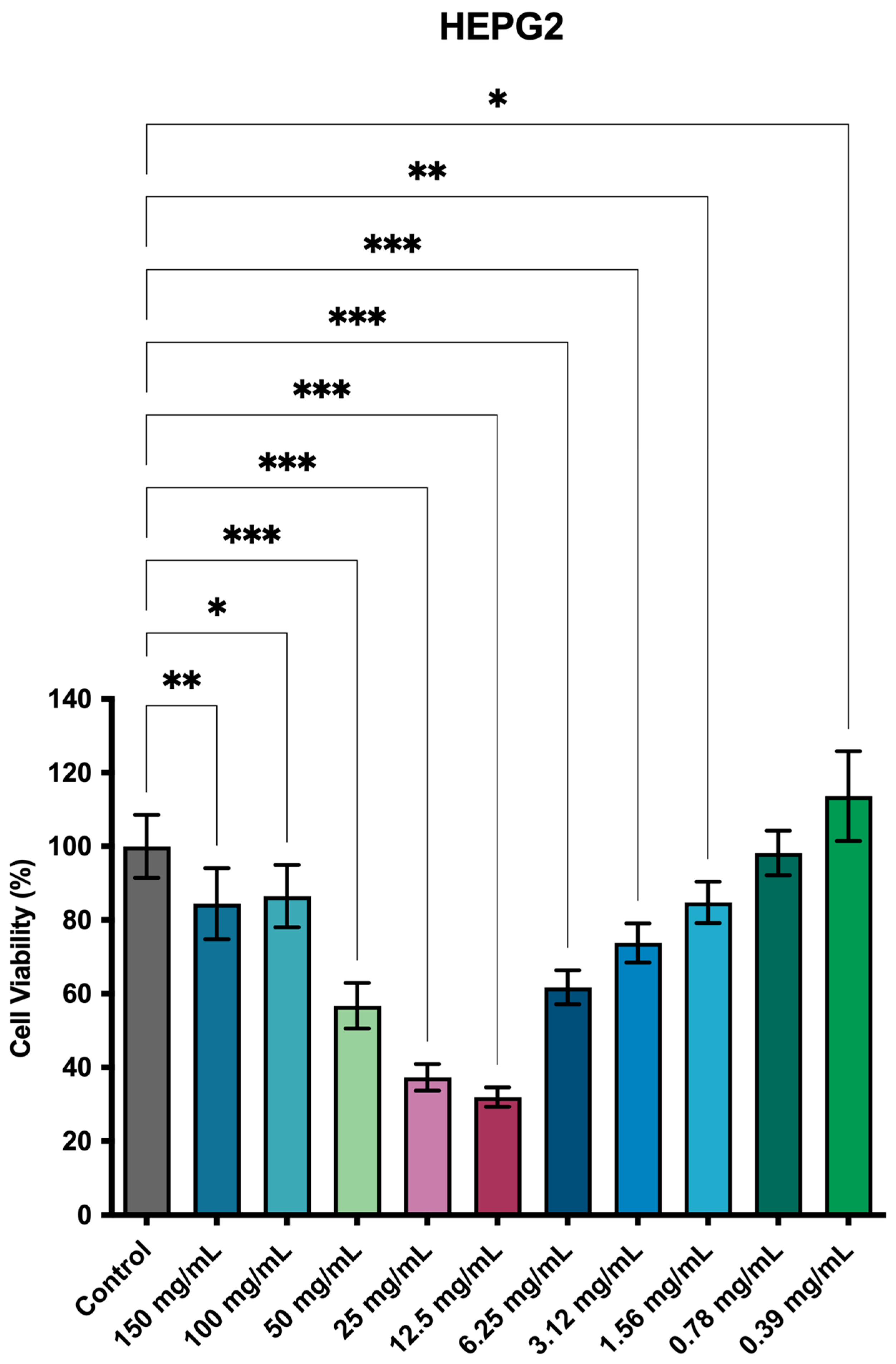
3.4. Cell Viability by Resazurin Essay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M. Antifungal resistance in superficial mycoses. J. Dermatol. Treat. 2022, 33, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, N.; Rhodes, J. Evolution of antifungal resistance in the environment. Nat. Microbiol. 2025, 10, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Di Stasio, D.; Romano, A.; Fiori, F.; Della Vella, F.; Rupe, C.; Lajolo, C.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral Candidiasis and Novel Therapeutic Strategies: Antifungals, Phytotherapy, Probiotics, and Photodynamic Therapy. Curr. Drug Deliv. 2023, 20, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Pfaller, M.A.; Bustamante, B.; Canton, E.; Fothergill, A.; Fuller, J.; Gonzalez, G.M.; Lass-Flörl, C.; Lockhart, S.R.; Martin-Mazuelos, E.; et al. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 2014, 58, 2006–2012. [Google Scholar] [CrossRef]
- Lima, S.M.F.; Sousa, M.G.A.C.; Freire, M.S.; Almeida, J.A.; Cantuaria, A.P.C.; Silva, T.A.M. Immune Response Profile against Persistent Endodontic Pathogens Candida albicans and Enterococcus faecalis In Vitro. J. Endod. 2015, 41, 1061–1065. [Google Scholar] [CrossRef]
- Rodríguez-Leguizamón, G.; Fiori, A.; Lagrou, K.; Gaona, M.A.; Ibáñez, M.; Patarroyo, M.A.; Van Dijck, P.; Gómez-López, A. New echinocandin susceptibility patterns for nosocomial Candida albicans in Bogotá, Colombia, in ten tertiary care centres: An observational study. BMC Infect. Dis. 2015, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Antifungal susceptibility patterns of a global collection of fungal isolates: Results of the SENTRY Antifungal Surveillance Program (2013). Diagn. Microbiol. Infect. Dis. 2016, 85, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Andalib, S.; Abed, A.; Rafieian-Kopaei, M.; Vaseghi, G. Neuroprotective, antimicrobial, antioxidant, chemotherapeutic, and antidiabetic properties of Salvia Reuterana: A mini review. Avicenna J. Phytomed. 2015, 5, 10–16. [Google Scholar] [PubMed]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.F.C.; Costa, P.C.; Bahia, M.F. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008, 106, 1014–1020. [Google Scholar] [CrossRef]
- Fernández-Agullóa, A.; Pereira, E.; Freire, M.S.; Valentão, P.; Andradec, P.B.; González-Álvareza, J. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Joshan, D.S.; Singhb, S.K. Investigational study of Juglans regia extract and quercetin against photoaging. Biomed. Aging Pathol. 2013, 3, 193–200. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic Antimicrobial Activity of Camellia sinensis and Juglans regia against Multidrug-Resistant Bacteria. PLoS ONE 2015, 10, e0118431. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.G.; Carrouel, F.; Attik, N.; Araujo, G.F.; Dos Santos Lopes, N.F.; Marcucci, M.C.; Rodrigues, F.P.; Caires, G.A.; Vigerelli, H.; Godoi, B.H.; et al. Juglans regia and Pfaffia paniculata extracts: Implications for periodontal disease treatment and correlation with Alzheimer’s risk. Front. Cell Infect. Microbiol. 2025, 15, 1585438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Angeli, F.; Malfa, G.A.; Garozzo, A.; Li Volti, G.; Genovese, C.; Stivala, A.; Nicolosi, D.; Attanasio, F.; Bellia, F.; Ronsisvalle, S.; et al. Antimicrobial, Antioxidant, and Cytotoxic Activities of Juglans regia L. Pellicle Extract. Antibiotics 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noumi, E.; Snoussi, M.; Hajlaoui, H.; Valentin, E.; Bakhrouf, A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 29, 81–88. [Google Scholar] [CrossRef]
- Sytykiewicz, H.; Chrzanowski, G.; Czerniewicz, P.; Leszczyński, B.; Sprawka, I.; Krzyżanowski, R.; Matok, H. Antifungal Activity of Juglans regia (L.) Leaf Extracts Against Candida albicans Isolates. Pol. J. Environ. Stud. 2015, 24, 1339–1348. [Google Scholar] [CrossRef]
- Raja, V.; Ahmad, S.; Irshad, M.; Wani, W.; Siddiqi, W.; Shreaz, S. Anticandidal activity of ethanolic root extract of Juglans regia (L.): Effect on growth, cell morphology, and key virulence factors. J. Med. Mycol. 2017, 27, 476–486. [Google Scholar] [CrossRef]
- Jafer, F.N.; A Naser, L. The Biological Activity of Aqueous and Methanolic Extracts of Juglans regia on Yeasts and Pathologic Bacteria. Arch. Clin. Microbiol. 2020, 11, 2405–2410. [Google Scholar] [CrossRef]
- de Sá Assis, M.A.; de Paula Ramos, L.; Abu Hasna, A.; de Queiroz, T.S.; Pereira, T.C.; Nagai de Lima, P.M.; Berretta, A.A.; Marcucci, M.C.; Talge Carvalho, C.A.; de Oliveira, L.D. Antimicrobial and Antibiofilm Effect of Brazilian Green Propolis Aqueous Extract against Dental Anaerobic Bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miranda, D.G.; Carrouel, F.; Silva, T.C.A.; Rozzatto, M.C.; Hasna, A.A.; Santos, C.E.R.; Morais, F.V.; de Oliveira, L.D.; de Paula Ramos, L. New Insights into Cutaneous Asepsis: Synergism between Pfaffia and Rosemary Extracts. Antibiotics 2024, 13, 226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Ibrahim Bhardwaj, N.; Puri, A.; Wadhwan, V.; Nangia, R.; Jahan, I. Antimicrobial effect of Juglans regia bark with commonly used antibiotics against initial colonizers of Plaque and Caries: A comparative study. J. Oral Maxillofac. Pathol. 2023, 27, 443–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitális, E.; Nagy, F.; Tóth, Z.; Forgács, L.; Bozó, A.; Kardos, G.; Majoros, L.; Kovács, R. Candida biofilm production is associated with higher mortality in patients with candidaemia. Mycoses 2020, 63, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis mediates acquired resilience: Using plant-derived chemicals to enhance health. Annu. Rev. Food Sci. Technol. 2021, 12, 355–381. [Google Scholar] [CrossRef]
- Murray, K.O.; Clanton, T.L.; Horowitz, M. Epigenetic responses to heat: From adaptation to maladaptation. Exp. Physiol. 2022, 107, 1144–1158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Candida spp. | MIC | MMC |
|---|---|---|
| C. albicans | 25 mg/mL | 50 mg/mL |
| C. dubliniensis | 12.5 mg/mL | 50 mg/mL |
| C. guilhermondii | 12.5 mg/mL | 25 mg/mL |
| C. glabrata | 25 mg/mL | 25 mg/mL |
| C. krusei | 25 mg/mL | 25 mg/mL |
| C. tropicalis | 12.5 mg/mL | 25 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, D.G.; Ramos, L.d.P.; Morais, F.V.; Nascimento, L.S.; Ferreira, I.A.; Guimarães, B.M.; Tomé, F.M.; Rodrigues, F.P.; Carrouel, F. Antifungal and Cytocompatible Properties of Juglans regia Extract for Dental Applications: A Novel Approach Against Oral Candida Infections. Appl. Sci. 2025, 15, 10531. https://doi.org/10.3390/app151910531
Miranda DG, Ramos LdP, Morais FV, Nascimento LS, Ferreira IA, Guimarães BM, Tomé FM, Rodrigues FP, Carrouel F. Antifungal and Cytocompatible Properties of Juglans regia Extract for Dental Applications: A Novel Approach Against Oral Candida Infections. Applied Sciences. 2025; 15(19):10531. https://doi.org/10.3390/app151910531
Chicago/Turabian StyleMiranda, Diego Garcia, Lucas de Paula Ramos, Flavia Villaça Morais, Letícia Silva Nascimento, Isadora Abdalla Ferreira, Bruno Martini Guimarães, Fernanda Malagutti Tomé, Flavia Pires Rodrigues, and Florence Carrouel. 2025. "Antifungal and Cytocompatible Properties of Juglans regia Extract for Dental Applications: A Novel Approach Against Oral Candida Infections" Applied Sciences 15, no. 19: 10531. https://doi.org/10.3390/app151910531
APA StyleMiranda, D. G., Ramos, L. d. P., Morais, F. V., Nascimento, L. S., Ferreira, I. A., Guimarães, B. M., Tomé, F. M., Rodrigues, F. P., & Carrouel, F. (2025). Antifungal and Cytocompatible Properties of Juglans regia Extract for Dental Applications: A Novel Approach Against Oral Candida Infections. Applied Sciences, 15(19), 10531. https://doi.org/10.3390/app151910531











