1. Introduction
Unaccustomed eccentric exercise, particularly when performed using isokinetic protocols, is known to cause exercise-induced muscle damage (EIMD), which is typically characterized by transient reductions in muscle strength, delayed onset muscle soreness (DOMS), and reductions in free pain range of motion [
1,
2]. Moreover, unaccustomed eccentric exercise has been found to cause disturbances in proprioception as assessed by position sense [
3,
4,
5,
6,
7]. These responses are a hallmark of the muscle’s adaptation process and are influenced by factors such as the type of contraction, training status, and the specific muscle group involved [
8,
9,
10]. Among these, eccentric contractions produce greater mechanical strain on muscle fibers, making them especially potent in provoking EIMD [
10].
It has been observed that the musculature of the lower limbs tends to be more resistant to EIMD compared to that of the upper limbs [
9]. It could be hypothesized that this difference could be attributed to the frequent engagement of lower limb muscles in daily weight-bearing and locomotor activities, which may confer a greater baseline level of neuromuscular adaptation and protection. In contrast, the upper limb muscles, particularly those around the elbow joint, are less routinely exposed to such high mechanical loading in everyday tasks, making them more susceptible to damage following unfamiliar eccentric loading.
Another factor that has been shown to influence the muscle damage response is the phenomenon of cross-education—whereby unilateral training can confer performance or protective benefits to the contralateral, untrained limb [
11,
12,
13,
14,
15,
16,
17,
18]. This effect has been demonstrated in the upper limbs, especially when training sessions are performed within a two-week interval [
11,
13,
14,
15,
16,
17]. However, such a cross-limb transfer appears to be absent or significantly attenuated in the lower limbs [
12,
14,
16,
17,
18,
19,
20]. Furthermore, the cross-education effect seems to diminish when the time between sessions is extended to four weeks [
14], highlighting its transient nature and potential dependency on neural or systemic mechanisms that decay over time.
Limb dominance has also been explored as a possible modulator of the EIMD response [
11,
21]. In upper limb muscles, no significant difference has been noted between dominant and non-dominant limbs following eccentric exercise [
11]. Regarding knee extensors, no dominance-related differences were observed in response to eccentric exercise, while an effect of exercise order was observed, which may influence the protective adaptations conferred to the second limb trained [
21]. It is important to note that these findings are based on muscle damage indices measured only 24 h post-exercise, a time point that may not capture the peak of EIMD-related alterations, which typically occur around 48 h post-exercise [
6]. Therefore, the purpose of this pilot investigation was to compare the alterations in muscle damage indices 48 h after eccentric isokinetic exercise between the dominant and non-dominant limb in order to better understand the role of limb dominance in muscle damage susceptibility.
Assessing the delayed-onset muscle soreness that follows eccentric exercise is clinically valuable because pain and tenderness can impair joint motion, alter proprioception, and limit functional performance. Understanding how such exercise-induced pain develops and whether it differs between dominant and non-dominant limbs helps practitioners design rehabilitation and injury-prevention programs that manage discomfort, preserve bilateral training balance, and reduce the risk of re-injury.
2. Materials and Methods
2.1. Participants
Eighteen (N = 18) physically active young female volunteers participated in the study (age: 23 ± 2 years; body mass: 58 ± 10 kg; height: 1.63 ± 0.06 m; body mass index: 21.8 ± 3.1 kg•m2). The sample size was determined based on peak torque data to achieve 85% statistical power (effect size 0.90) using G*Power software (ver. 3.1.9.6). Participants had not engaged in activities involving significant resistance exercise components or muscle-damaging activities for at least six months prior to the study. Exclusion criteria included any musculoskeletal injuries in the lower limbs within the past 6 months, use of contraceptives, and irregular menstrual cycles. Smoking, consumption of protein, antioxidant supplements, and potential inflammatory conditions were additional exclusion criteria. Given the modest sample size, the study was designed as an exploratory investigation to generate preliminary data on the role of limb dominance.
All participants had a regular menstrual cycle (24–31 days), and measurements were conducted during the early follicular phase (2nd–9th day after the onset of menstruation) to minimize hormonal fluctuations [
22,
23]. Verification of the menstrual cycle phase was based on self-report of cycle length and day of onset, while no hormonal assays were performed. Males were excluded to obtain a homogeneous sample and to minimize hormonal variability. Participants were instructed to abstain from strenuous exercise, except for the experimental sessions, throughout the study.
Written informed consent was obtained from all participants after they were informed about the procedures of the study. The experimental procedures adhered to the latest version of the Helsinki Declaration (October 2013), which was the latest version available at the time of ethics approval. The study protocol conformed to standard publishing practices in physiology and was reviewed and approved by the local University Ethics Committee (#1631/13 March 2024).
2.2. Study Design
All measurements were conducted between 08:00 and 11:00 a.m. Body mass was recorded to the nearest 0.1 kg using a calibrated beam balance (Seca, Model 710, Birmingham, UK), and standing height was measured to the nearest 1 cm using a stadiometer (Seca, Stadiometer 208, Birmingham, UK). Each participant performed two eccentric exercise sessions, one for each leg, involving their knee extensors. The two isokinetic exercise sessions were separated by 24–30 days, depending on the duration of the participants’ menstrual cycles. The assignment of which limb (dominant or non-dominant) performed first the eccentric exercise protocol was randomized and counterbalanced across participants to control for any potential effects of limb dominance. In all cases, when a limb was used in the eccentric exercise session, the other limb served as the control, and vice versa. Dependent variables were assessed at baseline (pre-exercise) and at 48 h post-exercise.
2.3. Exercise Protocol
The two eccentric exercise sessions were performed on an isokinetic dynamometer (Biodex System 4 PRO, Biodex Medical Systems Inc., Shirley NY, USA). During each eccentric exercise session, participants had to complete a moderate exercise volume consisting of 5 sets of 15 eccentric contractions (75 contractions in total) at maximal voluntary intensity with the knee extensors at an angular velocity of 60°/s [
24]. The range of motion was set to be 90° (0°–90°, where 0° indicates full extension). Verbal encouragement was provided to maximize effort. Participants underwent a three-day familiarization protocol a week before the experimental sessions, which included 8–10 low-intensity eccentric contractions to avoid learning effects and minimize the risk of muscle damage. A 1 min rest interval was provided between sets. Prior to each exercise session, participants performed an 8 min cycling warm-up at 50 W (i.e., 50 rpm with 1 kg resistance on the wheel) (Monark, Vansbro, Sweden).
The isokinetic dynamometer was calibrated according to the manufacturer’s guidelines. Both exercise protocols were undertaken from the seated position [60° hip flexion angle (with 0° representing the anatomical position of the hip joint)]. Detailed positioning was recorded for consistency in follow-up measurements. Gravitational corrections were applied to account for the passive torque produced by the limb’s weight when at rest. These corrections ensured that only active muscle-generated torque was analyzed during the contraction phases. Participants were allowed to continuously monitor the display screen, receiving dynamometer-automated real-time visual feedback regarding the intensity and duration of concentric and eccentric exercise modes.
2.4. Muscle Damage Indices
We measured a set of reliable and valid indirect markers of exercise-induced muscle damage [
25,
26]. After each eccentric exercise session, these markers were assessed in both limbs—one serving as the experimental limb and the other as the control.
The isometric peak torque was assessed at 90° knee flexion, while the eccentric peak torque was assessed at the angular velocity of 60°/s (knee range, 0° [full extension] to 90° flexion) [
26,
27]. A 2 min rest interval was provided between the assessments. In both isometric and eccentric assessments, the average of three maximal voluntary contractions was recorded. To ensure that the subjects provided their maximal effort, the measurements were repeated if the difference between the lower and the higher torque values exceeded 10%.
The assessment of ROM was performed using the isokinetic dynamometer. Specifically, the limb from the full extended position (knee angle 0°) was slowly flexed by the investigator, and the position where the participant felt any discomfort was recorded as the free pain range of motion [
28].
Delayed onset muscle soreness was assessed in each participant using two tests. The first test included palpation (DOMS from now on) of the belly muscle, and the perceived soreness was rated on a scale ranging from 1 (normal) to 10 (very sore) [
29]. The second test assessed the pain pressure threshold (4PPT), with a load of 4 kg applied for 3 s on predetermined anatomical sites using a force gauge with an external load cell (SAUTER FH 1KN, Balingen, Germany). Specifically, the external load cell was placed on the vastus lateralis muscle at a point located 12 cm proximal to the superior border of the patella and 3 cm lateral to this line, corresponding to the mid-belly region of the muscle. This method was considered a reliable indicator of DOMS [
30]. The use of both procedures was intended to provide complementary information on muscle soreness. The DOMS assessment using palpation of the muscle belly allows for a more individualized assessment of tenderness, capturing subjective pain sensitivity and the pressure threshold unique to each participant. Additionally, the application of the 4PPT assessment offers the advantage of standardized resistance across all participants, allowing for objective comparisons between conditions and time points. In each case, participants rated their perceived soreness using a visual analog scale ranging from 1 to 10 (1 = “no pain” and 10 = “unbearable pain”). Both procedures were performed twice, and the average value was recorded.
2.5. Assessment of Muscle Fatigue
Assessment of muscle fatigue was performed using the same isokinetic dynamometer. All measurements were performed in a seated position at 90° flexion in the knee joint. The fatigue index was calculated during a 30 s maximal isometric effort. The subjects were encouraged verbally and visually to achieve maximal effort, as the percent decrease in torque by the following formula [
31]:
Fatigue index = 100 − (average torque of the last five seconds)/(average torque of the first five seconds) × 100
Subjects received verbal encouragement to achieve maximal effort while they visually monitored their attempts on the computer screen.
2.6. Position Sense
Position sense was evaluated according to previous reports [
4,
5,
6,
7,
9,
32]. Specifically, the subjects were seated [60° hip angle (with 0° representing the anatomical position of the hip joint)] on the isokinetic dynamometer. The angles were automatically recorded by the dynamometer. The investigator positioned the lower limb at 45° knee flexion and maintained it for 10 s [knee range, 0° (full extension) to 90° flexion]. The movement for the placement of the limb was performed manually, but the position was fixed by the dynamometer. After the investigator placed participants’ lower limbs in a 90° knee angle position, the participants were asked to recall the reference position after the limb had been placed in that position [
4,
5,
6,
7,
9,
32]. Then participants actively moved their limb concentrically to the target angle, and when they were satisfied with the selected angle, held the lever arm for about 2 s while the investigator blocked (held the button) the dynamometer. The value of the angle that was chosen by the participant appeared on the monitor of the dynamometer and was recorded. The degrees deviating from the reference angle were calculated and recorded for further analysis [
4,
5,
6,
7,
9,
32]. Four efforts were performed, and the mean of the two closest to the reference angle was recorded and used for the statistical analysis. The leg moved from 0° to 90° to the target position and then back to 0° prior to each of the four efforts.
2.7. Statistical Analysis
Normality of data was verified using the Shapiro–Wilk test and homogeneity of variance with Levene’s test. A three-way repeated-measures ANOVA [conditions (control and experimental) × limb dominance (dominant and non-dominant) × time (pre and 48 h)] was used to analyze muscle damage indices. Pairwise comparisons were performed using the Sidak test when significant interactions were found. Greenhouse–Geisser corrections were applied when sphericity was violated. Pearson’s product-moment correlation coefficient was also used to reveal the impact of eccentric exercise on the two limbs. The magnitude of Pearson’s correlation was interpreted as strong when r was between 0.50 and 1.0, moderate when between 0.30 and 0.49, and weak when below 0.29 or close to 0, with values approaching 1.0 considered perfect. Data are presented as mean ± standard deviation (SD), and significance was set at a = 0.05. Data processing and statistical analyses were performed using IBM SPSS Statistics software, version 25.0 (IBM Corp., Armonk, NY, USA).
3. Results
Participants exerted maximal voluntary effort during the eccentric exercise, as indicated by the rate of perceived exertion (RPE) values, which were 18.8 ± 1.7 for the dominant limb and 18.6 ± 1.4 for the non-dominant limb at the end of the 5th set. The RPE did not differ significantly between exercise types at the end of the 5th set (p = 0.601).
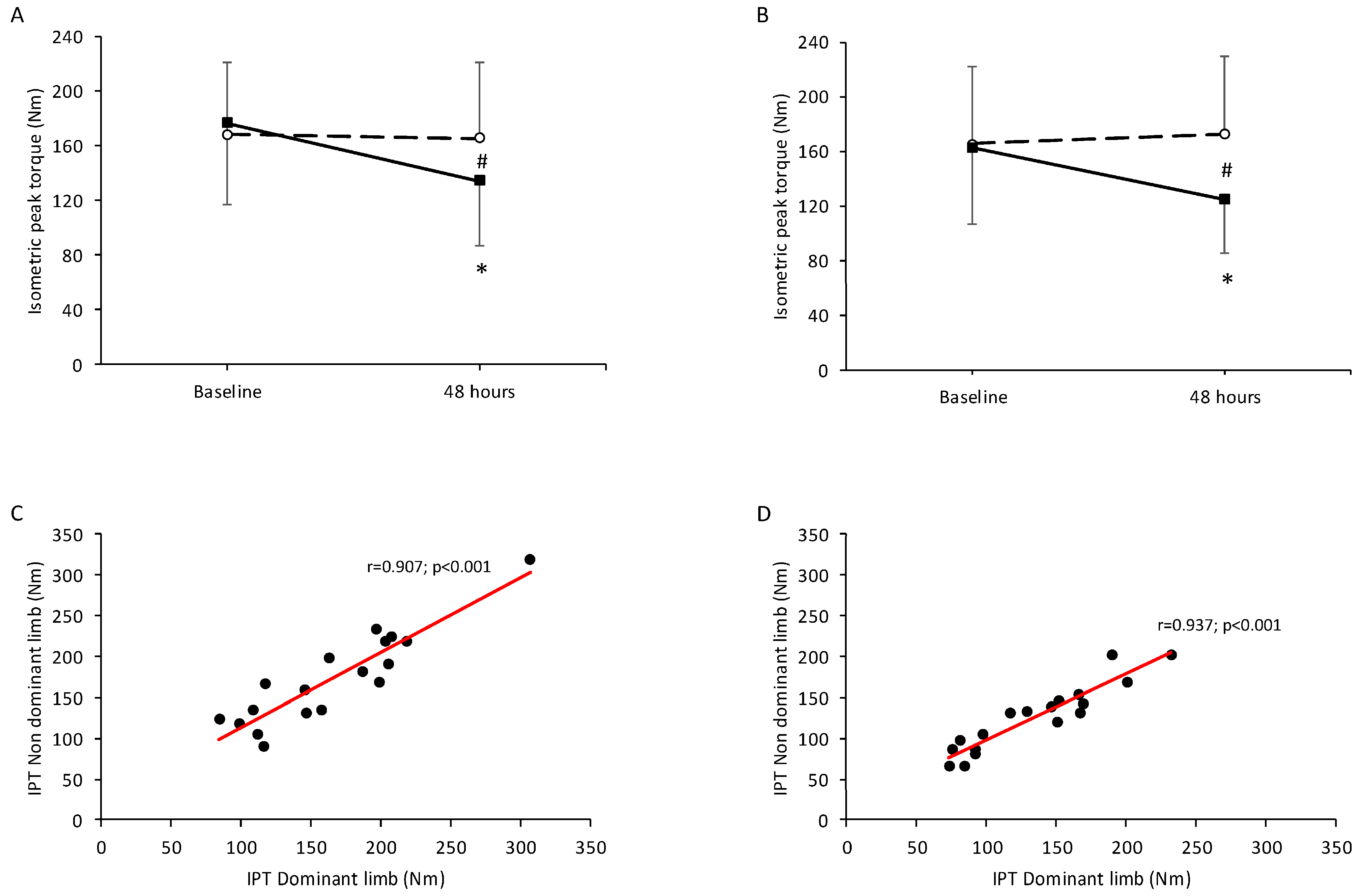
For isometric peak torque, there was a significant main effect of time (
p < 0.001) and a significant time × condition (i.e., control and experimental limb) (
p < 0.001), but no significant main effect of dominance (
p = 0.716). Isometric peak torque declined significantly 48 h post-eccentric exercise only in the experimental condition for both the dominant (
Figure 1A) and non-dominant limbs (
Figure 1B) (
p < 0.001 for both). A strong correlation was observed between the dominant and non-dominant limbs in the control condition 48 h post-eccentric exercise (r = 0.907;
p < 0.001;
Figure 1C), and also in the experimental condition at the same time point (r = 0.937;
p < 0.001;
Figure 1D).
In eccentric peak torque, there was a significant main effect of time (
p < 0.001) and a significant time × condition interaction (
p < 0.001), but no significant main effect of dominance (
p = 0.886). Eccentric peak torque declined significantly 48 h post-eccentric exercise only in the experimental condition for both the dominant (
Figure 2A) and non-dominant limbs (
Figure 2B) (
p < 0.001 for both). A strong correlation was found between the dominant and the non-dominant limbs in the control condition 48 h post-eccentric exercise (r = 0.917;
p < 0.001;
Figure 2C), and in the experimental condition (r = 0.752;
p < 0.001;
Figure 2D) at the same time point.
No significant correlation was found between the percentage decline in isometric peak torque and eccentric peak torque in the experimental condition 48 h post-eccentric exercise, either in the control condition (r = 0.155;
p = 0.589;
Figure 3A) or in the experimental condition (r = −0.068;
p = 0.788;
Figure 3B).
For DOMS assessed by palpation, there was a significant main effect of time (
p < 0.001) and a significant time × condition interaction (
p < 0.001), but no significant main effect of dominance (
p = 0.798). DOMS increased significantly 48 h post-eccentric exercise only in the experimental condition for both the dominant (
Figure 4A) and non-dominant limb (
Figure 4B) (
p < 0.001 for both). No correlation was found between the dominant and non-dominant limbs in the control condition (r = 0.000;
p = 1.000;
Figure 4C), whereas in the experimental condition, the correlation was significant (r = 0.718;
p < 0.001;
Figure 4D).
In DOMS assessed by pain pressure threshold of 4 kg (4PPT), there was a significant main effect of time (
p < 0.001) and a significant time × condition interaction (
p < 0.001), but no significant main effect of dominance (
p = 0.253). DOMS increased significantly 48 h post-eccentric exercise only in the experimental condition for both the dominant (
Figure 5A) and non-dominant limb (
Figure 5B) (
p < 0.001 for both). No correlation was observed between dominant and non-dominant limbs in the control condition 48 h post-eccentric exercise (r = 0.138;
p = 0.584;
Figure 5C), whereas in the experimental condition, the correlation was marginally non-significant (r = 0.454;
p = 0.059;
Figure 5D).
For fatigue index, there was a significant main effect of time (
p < 0.001) and a significant time × condition interaction (
p < 0.001), but no significant main effect of dominance (
p = 0.886). Fatigue index declined significantly 48 h post-eccentric exercise in the experimental condition for both the dominant (
Figure 6A) and non-dominant limb (
Figure 6B) (
p < 0.001 for both). At baseline, a significant difference between conditions was found for the non-dominant limb (
p = 0.023). In the control condition of the non-dominant limb, the fatigue index increased significantly 48 h post-eccentric exercise (
p = 0.026). No correlation was observed between the dominant and non-dominant limbs in either the control condition 48 h post-eccentric exercise (r = 0.331;
p = 0.179;
Figure 6C) or the experimental condition (r = 0.240;
p = 0.624;
Figure 6D) at the same time point.
For position sense, there was a significant main effect of time (
p < 0.001) and a significant time × condition interaction (
p < 0.001), but no significant main effect of dominance (
p = 0.798). Position sense declined significantly 48 h post-eccentric exercise only in the experimental condition for both the dominant (
Figure 7A) and non-dominant limb (
Figure 7B) (
p < 0.001 for both). No correlation was found between the dominant and non-dominant limbs in either the control condition 48 h post-eccentric exercise (r = 0.260;
p = 0.298;
Figure 7C) or the experimental condition (r = −0.171;
p = 0.4984;
Figure 7D) at the same time point.
4. Discussion
The purpose of the present investigation was to compare alterations in muscle damage indices 48 h after eccentric isokinetic exercise between the dominant and non-dominant limb. The main finding of this study is that eccentric exercise-induced muscle damage (EIMD) was not influenced by limb dominance, as no significant differences were found between dominant and non-dominant limbs across all muscle damage indices 48 h post-eccentric exercise. These results suggest that the susceptibility of the knee extensors to EIMD is independent of habitual limb preference, despite potential differences in motor control and functional use between limbs.
Previous research investigating the role of limb dominance in the elbow flexors [
11,
33] and knee extensors [
21] similarly reported no differences between dominant and non-dominant limbs in the magnitude of alterations in muscle damage indices. In an earlier study of our research group, it was found that the elbow flexors are more prone to eccentric exercise-induced muscle damage compared to the knee extensors [
34]. Based on these findings and considering limb dominance, we hypothesized that both quadriceps are regularly exposed to high mechanical loads during locomotor and postural activities. The present findings align with the body of evidence, further supporting the notion that limb dominance does not confer protection against EIMD in the lower limbs [
21].
Regarding muscle performance, isometric and eccentric peak torque declined significantly 48 h after eccentric exercise. Significant correlations were observed between dominant and non-dominant limbs for both isometric and eccentric peak torque, indicating similar responses for both conditions. Interestingly, high correlations were also found for the non-exercised limbs, suggesting strength balance in the study participants. However, no significant correlation was found between the percentage declines in isometric and eccentric peak torque, highlighting that individuals may respond differently across indices when exposed to the same stressor [
10].
Delayed onset muscle soreness, assessed by palpation [DOMS(P)] and by pain pressure threshold of 4 kg (PPT4), was significantly increased in both dominant and non-dominant limbs 48 h post muscle-damaging eccentric exercise. DOMS(P) responses were significantly correlated between the two limbs, suggesting that palpation of the knee extensor musculature provides an accurate reflection of the pain experienced by participants. However, the correlation between limbs was marginally non-significant for the PPT4 assessment. One possible explanation is that the specific measurement site (12 cm proximal to the superior border of the patella and 3 cm lateral to this line, in the belly of the vastus lateralis) did not capture soreness consistently, even within the same subject.
Although the data suggested greater fatigue resistance 48 h after eccentric exercise, this is most likely a methodological artifact rather than a true adaptation. Reduced torque output caused by DOMS can underestimate the relative decline in performance, giving a false impression of improved fatigue resistance. Together with the variability in baseline torque, this indicates that fatigue index—and likewise joint-position sense—are not reliable predictors of exercise-induced muscle damage, a point of practical importance for researchers and practitioners.
This study further extends knowledge by showing that eccentric exercise caused comparable impairments in position sense in both limbs. Proprioceptive disturbances after EIMD are well-documented [
4,
5,
6,
7,
9,
32] and are attributed to muscle spindle damage and altered afferent feedback. The lack of dominance-related differences indicates that these neuromuscular consequences are systemic responses to eccentric-induced strain rather than being modulated by habitual limb use. No significant correlation was found between the dominant and the non-dominant limb, suggesting variability at the individual level. Thus, position sense may not be a reliable indirect marker of exercise-induced muscle damage.
4.1. Methodological Considerations
Because the sample size was relatively small, our findings should be interpreted as those of a pilot study, intended to inform and guide larger future investigations. This study has several notable strengths. First, all post-exercise assessments were performed 48 h after the eccentric exercise, which corresponds to the typical peak of EIMD markers [
35]. Previous work of a similar nature assessed muscle damage indices only at 24 h post-exercise [
21], potentially missing the peak responses. Second, a strict control of menstrual cycle phase ensured that all testing occurred during the early follicular phase, minimizing hormonal variability. Third, the counterbalanced design, in which the exercise order of the dominant and non-dominant limbs was randomized, reduced potential order effects. Finally, the exclusion of participants with recent training or other muscle-damaging activity further minimized confounding variables and enhanced the reliability of our findings.
Nevertheless, several limitations should be noted. Only young, physically active females were recruited, which limits the generalizability of the findings to males, older adults, or clinical populations. Although all participants were required to be physically active yet free of structured resistance training or eccentric-exercise exposure for at least six months, individual differences in everyday activity levels cannot be completely ruled out. Such variation in habitual conditioning may have contributed to some of the inter-individual variability observed and should be considered a potential source of bias. Given the potential influence of sex hormones on muscle damage and recovery [
36], future studies should explore whether similar results occur in other populations. Moreover, although a moderate eccentric workload was employed, higher volumes or intensities may reveal subtle dominance-related effects not detected in the present study. Finally, the unexpected finding of greater fatigue resistance post-eccentric exercise should be interpreted cautiously, as it reflects a methodological artifact related to reduced initial force capacity under painful conditions, rather than true adaptations in fatigue resistance.
4.2. Practical Implications
The present findings indicate that limb dominance does not affect the extent of muscle damage following eccentric loading of the knee extensors. Practitioners can therefore design eccentric training and rehabilitation programs without adjusting workload or recovery strategies based on dominance. Both limbs should be monitored equally for strength loss, soreness, fatigue, and proprioceptive impairments after eccentric exercise. This knowledge may aid in optimizing injury prevention, bilateral training balance, and return-to-play decisions. Importantly, these results also support rehabilitation and prevention strategies, based on which clinicians and therapists can apply identical eccentric-loading protocols to both limbs when addressing muscle imbalances or recovering from unilateral injuries, and coaches can incorporate symmetrical eccentric training to reduce the risk of overuse injuries and maintain balanced neuromuscular performance.