Compressive, Dimensional, and Antimicrobial Characteristics of 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Following Dental Disinfection
Abstract
1. Introduction
2. Materials and Methods
- Fabrication of ABS cubes for in vitro assessment
- Pre-Immersion Assessments
- The Immersion Procedure
- Post Immersion Assessments
- Statistical Analyses
2.1. Fabrication of ABS Cubes for In Vitro Assessment
2.2. Pre-Immersion Assessments
2.2.1. Linear Measurements for Dimensional Stability
- ri: ideal value derived from CAD software
- oi: obtained physical value
2.2.2. Mass Analysis
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. The Immersion Procedure
2.4. Post Immersion Assessments
2.4.1. Compressive Strength Analysis
2.4.2. Microbial Accumulation Test
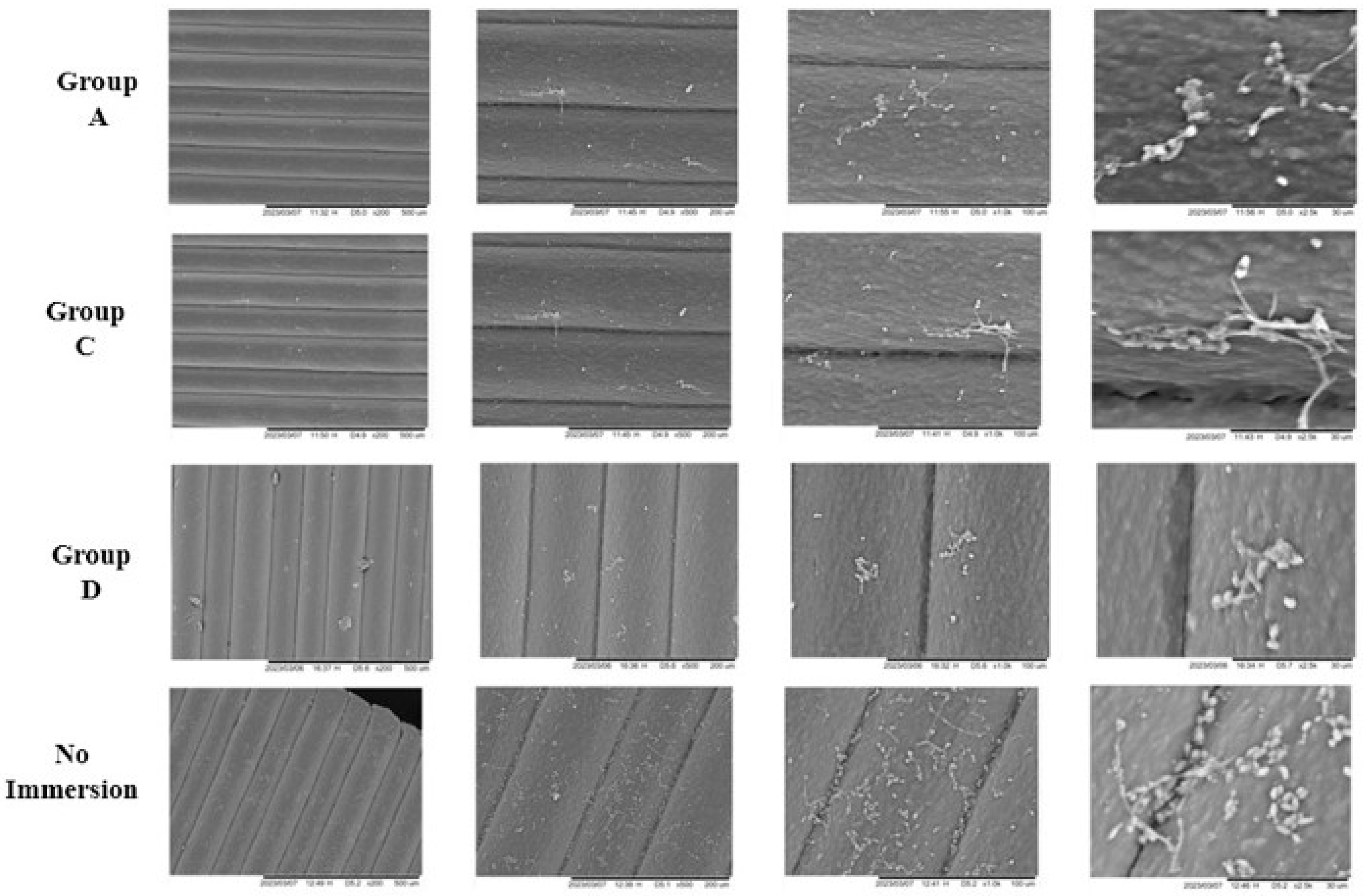
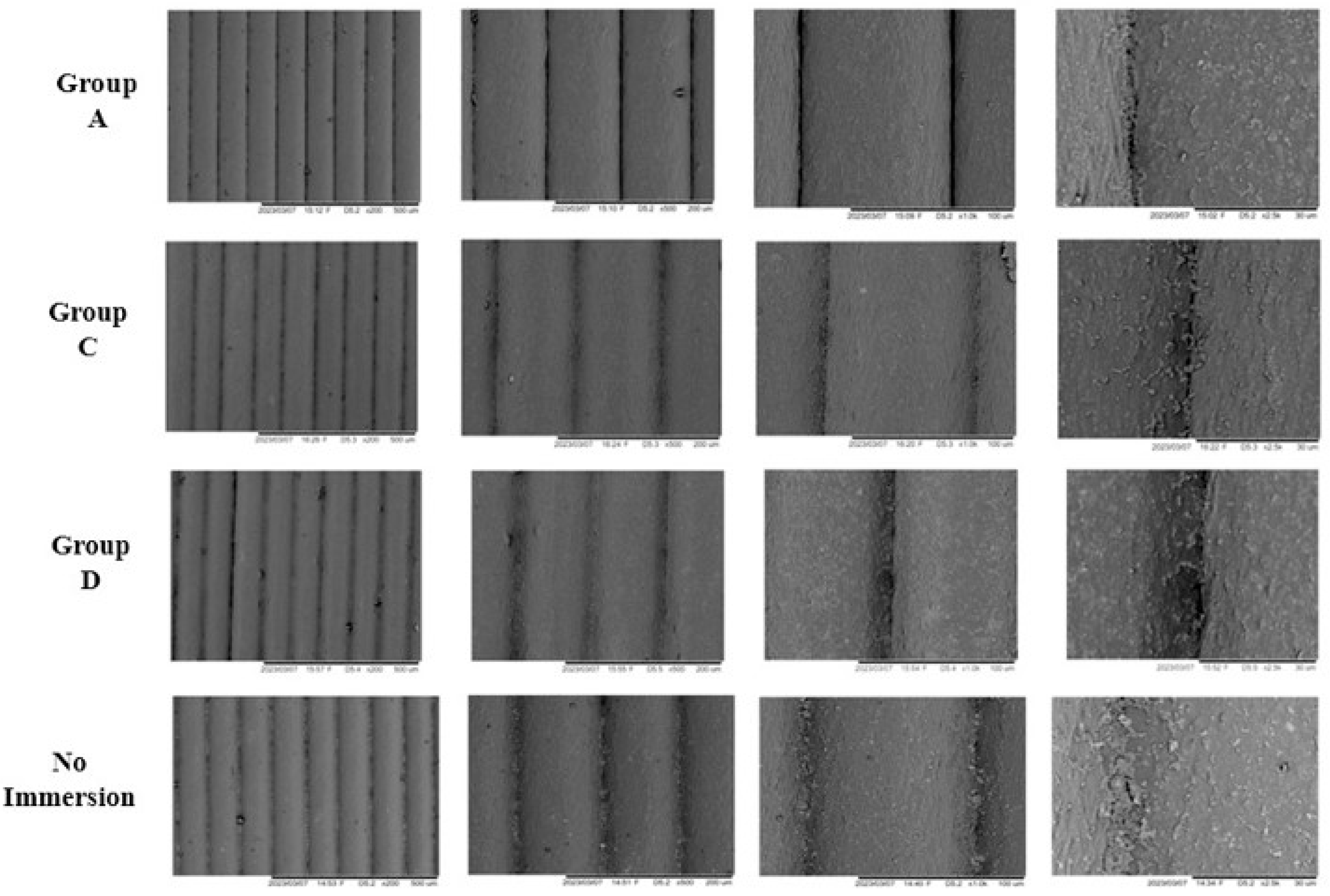
2.4.3. Scanning Electron Microscopy (SEM)
2.5. Statistical Analyses
3. Results
3.1. Linear Measurements (OM, IM, and RMS)
3.2. Mass Analysis
3.3. Infrared Spectroscopy
3.4. Compressive Strength Analysis
3.5. Scanning Electron Microscopy (SEM)
4. Discussion
4.1. Linear Measurements (OM, IM, and RMS)
4.2. Mass Analysis
4.3. Infrared Spectroscopy
4.4. Compressive Strength Analysis
4.5. Scanning Electron Microscopy (SEM)
4.6. Research Limitations and Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babu, R.D.; Devaprakasam, D. A Review on Additive Manufacturing Techniques in Medical Applications. Int. J. Adv. Sci. Eng. 2019, 5, 988–997. [Google Scholar] [CrossRef]
- Dodziuk, H. Applications of 3D printing in healthcare. Kardiochir. Torakochirurgia Pol. 2016, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D printing and digital processing techniques in dentistry: A review of literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar] [CrossRef]
- Van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, L.; Yong, R.; Ranjitkar, S.; Hughes, T.; Quayle, M.; McMenamin, P.G.; Kaidonis, J.; Townsend, G.C.; Adams, J.W. The use of 3D printing in dental anthropology collections. Am. J. Phys. Anthr. 2018, 167, 400–406. [Google Scholar] [CrossRef]
- He, Y.; Xue, G.; Fu, J. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci. Rep. 2014, 4, 6973. [Google Scholar] [CrossRef]
- Samykano, M.; Selvamani, S.K.; Kadirgama, K.; Ngui, W.K.; Kanagaraj, G.; Sudhakar, K. Mechanical property of FDM printed ABS: Influence of printing parameters. Int. J. Adv. Manuf. Technol. 2019, 102, 2779–2796. [Google Scholar] [CrossRef]
- Jamayet, N.B.; Farook, T.H.; Ayman, A.O.; Johari, Y.; Patil, P.G. Digital workflow and virtual validation of a 3D-printed definitive hollow obturator for a large palatal defect. J. Prosthet. Dent. 2023, 129, 798–804. [Google Scholar] [CrossRef]
- Sequeira, A.L.; Narayan, A.I.; George, V.T. Effects of nonaldehyde immersion disinfection on the mechanical properties of flexible denture materials. J. Prosthet. Dent. 2019, 121, 843–847. [Google Scholar] [CrossRef]
- Raut, A.; Kar, A.K.; Mohanty, A.K.; Hota, S.; Bhushan, P.; Priyadarshini, S. Influence of disinfectants Use on removable oral prosthesis: An in Vitro Study. World 2022, 13, 166–171. [Google Scholar]
- Zhang, K.; Zhang, S.; Shi, Y.; Zhang, L.; Fu, B. Effects of disinfectants on physical properties of denture base resins: A systematic review and meta-analysis. J. Prosthet. Dent. 2024, 131, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef]
- ISO (1994) 5725-1; Accuracy (Trueness and Precision) of Measurement Methods and Results-Part 1: General Principles and Definitions. International Organization for Standardization: Geneva, Switzerland, 1994.
- Curylofo, P.A.; Raile, P.N.; Vasconcellos, G.L.L.; Macedo, A.P.; Pagnano, V.O. Effect of Denture Cleansers on Cobalt-Chromium Alloy Surface: A Simulated Period of 5 Years’ Use. J. Prosthodont. 2020, 29, 142–150. [Google Scholar] [CrossRef]
- Sutula, J.; Coulthwaite, L.; Thomas, L.; Verran, J. The effect of a commercial probiotic drink on oral microbiota in healthy complete denture wearers. Microb. Ecol. Health Dis. 2012, 23, 18404. [Google Scholar] [CrossRef]
- Hipólito, A.C.; Barão, V.A.; Faverani, L.P.; Ferreira, M.B.; Assunção, W.G. Color degradation of acrylic resin denture teeth as a function of liquid diet: Ultraviolet-visible reflection analysis. J. Biomed. Opt. 2013, 18, 105005. [Google Scholar] [CrossRef]
- Kürkcüoğlu, I.; Özkir, S.E.; Köroğlu, A.; Sahin, O.; Yilmaz, B. Effect of denture cleansing solutions on different retentive attachments. J. Prosthet. Dent. 2016, 115, 606–610. [Google Scholar] [CrossRef]
- Buergers, R.; Rosentritt, M.; Schneider-Brachert, W.; Behr, M.; Handel, G.; Hahnel, S. Efficacy of denture disinfection methods in controlling Candida albicans colonization in vitro. Acta Odontol. Scand. 2008, 66, 174–180. [Google Scholar] [CrossRef]
- de Sousa, F.A.C.G.; Paradella, T.C.; Koga-Ito, C.Y.; Jorge, A.O.C. Effect of sodium bicarbonate on Candida albicans adherence to thermally activated acrylic resin. Braz. Oral. Res. 2009, 23, 381–385. [Google Scholar] [CrossRef]
- Porwal, A.; Khandelwal, M.; Punia, V.; Sharma, V. Effect of denture cleansers on color stability, surface roughness, and hardness of different denture base resins. J. Indian Prosthodont. Soc. 2017, 17, 61. [Google Scholar] [CrossRef]
- Stanford, B.D.; Pisarenko, A.N.; Snyder, S.A.; Gordon, G. Perchlorate, bromate, and chlorate in hypochlorite solutions: Guidelines for utilities. J.-Am. Water Work. Assoc. 2011, 103, 71–83. [Google Scholar] [CrossRef]
- Lee, H.-E.; Li, C.-Y.; Chang, H.-W.; Yang, Y.-H.; Wu, J.-H. Effects of different denture cleaning methods to remove Candida albicans from acrylic resin denture based material. J. Dent. Sci. 2011, 6, 216–220. [Google Scholar] [CrossRef]
- Felipucci, D.N.B.; Davi, L.R.; Paranhos, H.F.O.; Bezzon, O.L.; Silva, R.F.; Pagnano, V.O. Effect of different cleansers on the surface of removable partial denture. Braz. Dent. J. 2011, 22, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Valentini-Mioso, F.; Maske, T.T.; Cenci, M.S.; Boscato, N.; Pereira-Cenci, T. Chemical hygiene protocols for complete dentures: A crossover randomized clinical trial. J. Prosthet. Dent. 2019, 121, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Fahmy, T.; Sarhan, A.; Elsayed, I.A.; Abdelwahed, H.G. Optical properties of poly (vinyl chloride-co-vinyl acetate-co-2-hydroxypropyl acrylate)/(acrylonitrile-butadiene-styrene) blends. Int. J. Eng. Res. 2018, 11, 1405–1415. [Google Scholar]
- Oliveira Paranhos, H.F.; Silva-Lovato, C.H.; De Souza, R.F.; Cruz, P.C.; De Freitas-Pontes, K.M.; Watanabe, E.; Ito, I.Y. Effect of three methods for cleaning dentures on biofilms formed in vitro on acrylic resin. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2009, 18, 427–431. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning electron microscopy. Curr. Protoc. Microbiol. 2012. Chapter 2: Unit2B. 2-2B. 2. [Google Scholar] [CrossRef]
- Mangore, R.V.; Ahankari, S.S.; Verma, K.D.; Kar, K.K. Fly ash-reinforced acrylonitrile butadiene styrene composites. In Handbook of Fly Ash; Butterworth-Heinemann: Oxford, UK, 2022; p. 301. [Google Scholar]
- Lee, D.; Lee, S.Y.; Kim, H.; Park, C. A Hybrid Dental Model Concept Utilizing Fused Deposition Modeling and Digital Light Processing 3D Printing. Int. J. Prosthodont. 2020, 33, 229–231. [Google Scholar] [CrossRef]
- Tshentu, Z.R.; Togo, C.; Walmsley, R.S. Polymer-anchored oxovanadium (IV) complex for the oxidation of thioanisole, styrene and ethylbenzene. J. Mol. Catal. A Chem. 2010, 318, 30–35. [Google Scholar] [CrossRef]
- Alkaltham, N.S.; Aldhafiri, R.A.; Al-Thobity, A.M.; Alramadan, H.; Aljubran, H.; Ateeq, I.S.; Khan, S.Q.; Akhtar, S.; Gad, M.M. Effect of denture disinfectants on the mechanical performance of 3D-printed denture base materials. Polymers 2023, 15, 1175. [Google Scholar] [CrossRef] [PubMed]
- Dundar, M.A.; Dhaliwal, G.S.; Ayorinde, E.; Al-Zubi, M. Tensile, compression, and flexural characteristics of acrylonitrile–butadiene–styrene at low strain rates: Experimental and numerical investigation. Polym. Polym. Compos. 2021, 29, 331–342. [Google Scholar] [CrossRef]
- de Sousa Porta, S.R.; de Lucena-Ferreira, S.C.; da Silva, W.J.; Del Bel Cury, A.A. Evaluation of sodium hypochlorite as a denture cleanser: A clinical study. Gerodontology 2015, 32, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Machado de Andrade, I.; Cruz, P.C.; Silva-Lovato, C.H.; de Souza, R.F.; Souza-Gugelmin, M.C.M.; Paranhos, H.d.F.O. Effect of chlorhexidine on denture biofilm accumulation. Journal of Prosthodontics: Implant, Esthetic and Reconstructive Dentistry. J. Prosthodont. 2012, 21, 2–6. [Google Scholar] [CrossRef]
- Drake, D.; Wells, J.; Ettinger, R. Efficacy of denture cleansing agents in an in vitro bacteria-yeast colonization model. Int. J. Prosthodont. 1992, 5, 214–220. [Google Scholar]
- Dills, S.S.; Olshan, A.M.; Goldner, S.; Brogdon, C. Comparison of the antimicrobial capability of an abrasive paste and chemical-soak denture cleaners. J. Prosthet. Dent. 1988, 60, 467–470. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.; Cai, Y. Effects of two peroxide enzymatic denture cleaners on Candida albicans biofilms and denture surface. BMC Oral Health 2020, 20, 193. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Abbott, P.V. The properties and applications of chlorhexidine in endodontics. Int. Endod. J. 2009, 42, 288–302. [Google Scholar] [CrossRef]
- Nabi, D.; Carmona, E.; Menger, F.; Römerscheid, M.; Lips, S.; Beck, A.J.; Böhme, A.; Joerss, H.; Jahnke, A.; Tasdemir, D.; et al. Uv Weathering Alters Toxicity and Chemical Composition of Consumer Plastic Leachates. SSRN Electron. J. 2025, 29. [Google Scholar] [CrossRef]
- Bin Jamayet, N.; Barman, A.; Rashid, F.; Eusufzai, S.Z.; Özcan, M.; Dudley, J.; Farook, T.H. In Vitro Characterisation of 3D Printable Filaments Subjected to Edible Liquids: An Analysis of Fused Deposition Modelling for Intraoral Applicability. Int. J. Dent. 2024, 2024, 2118412. [Google Scholar] [CrossRef]
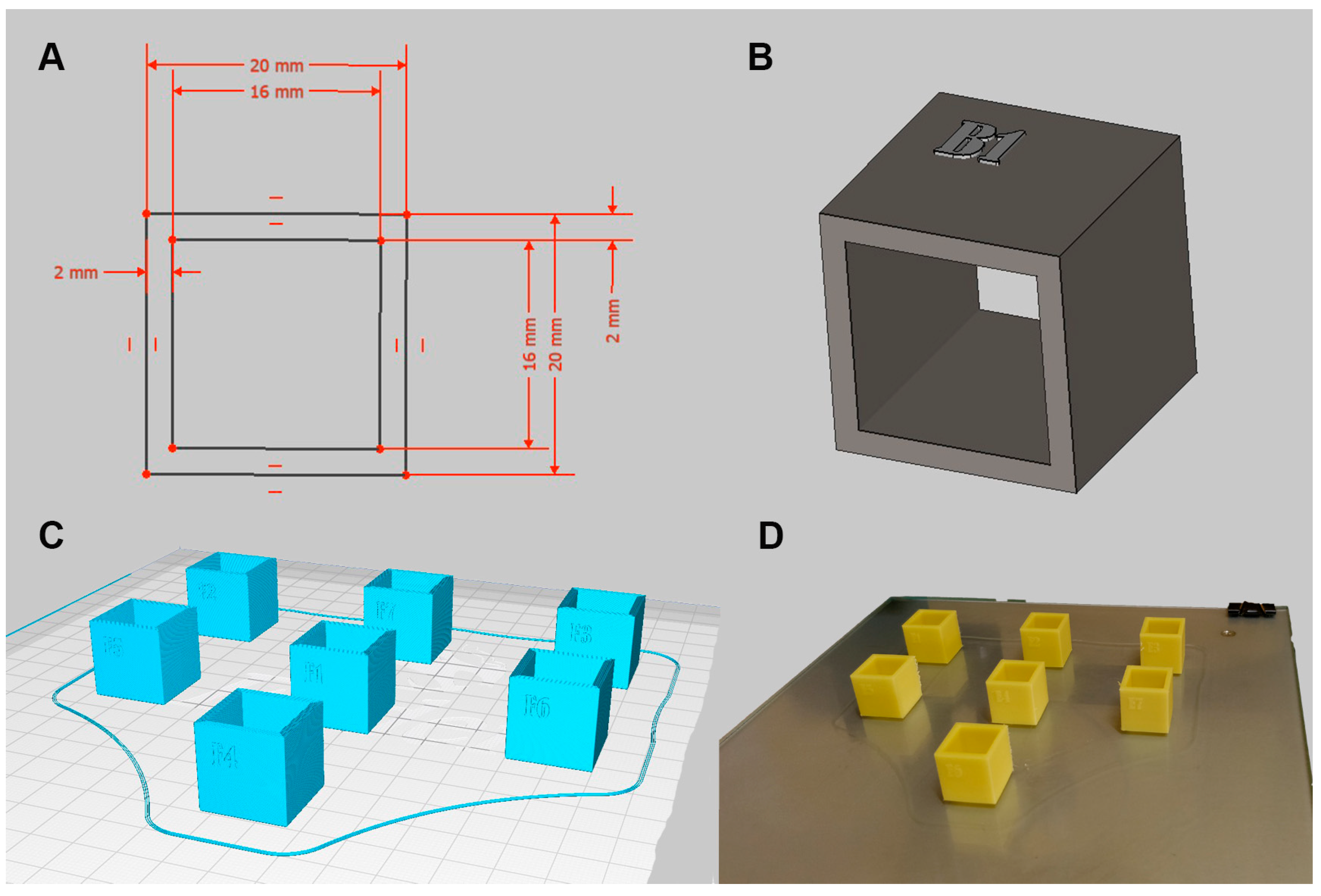
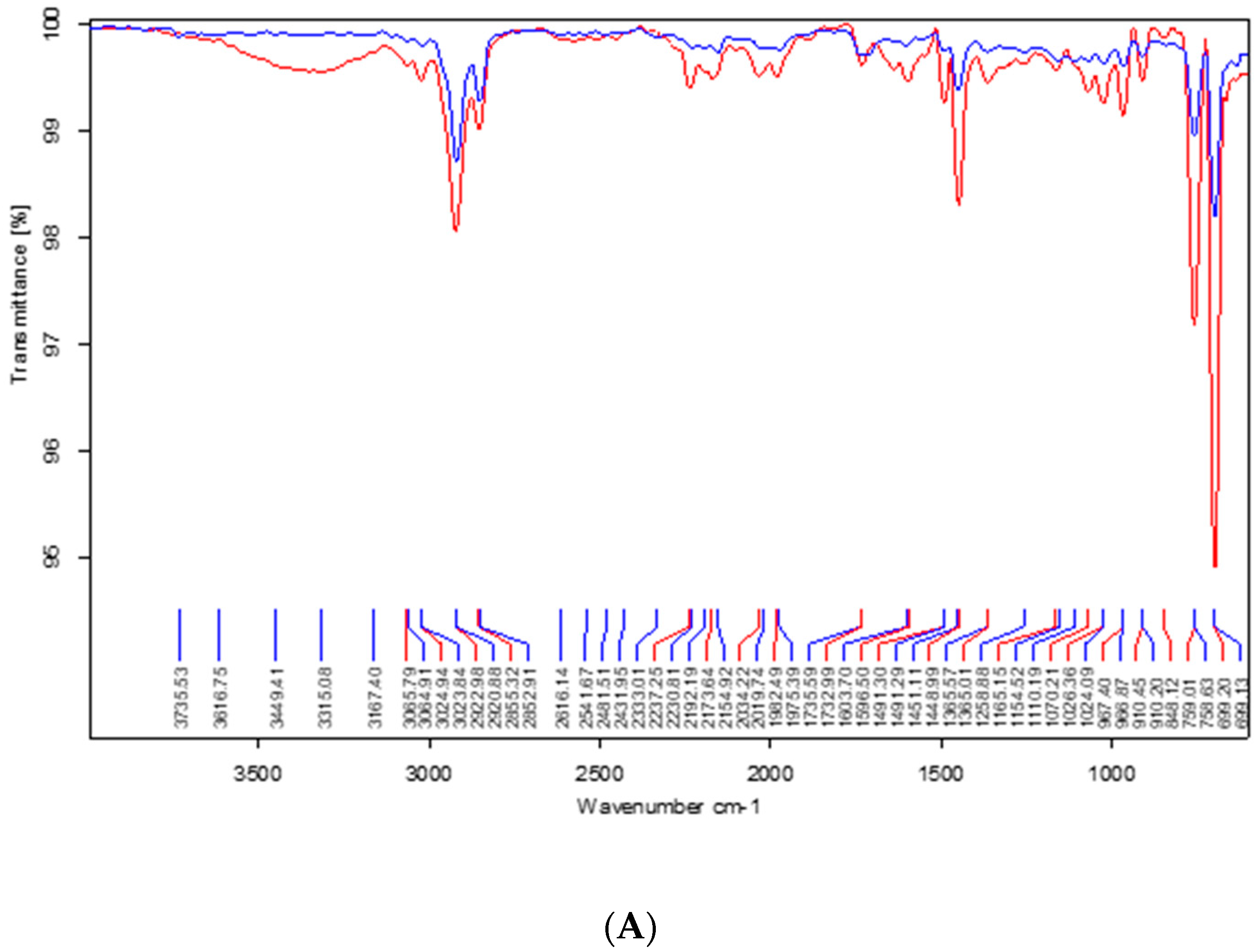

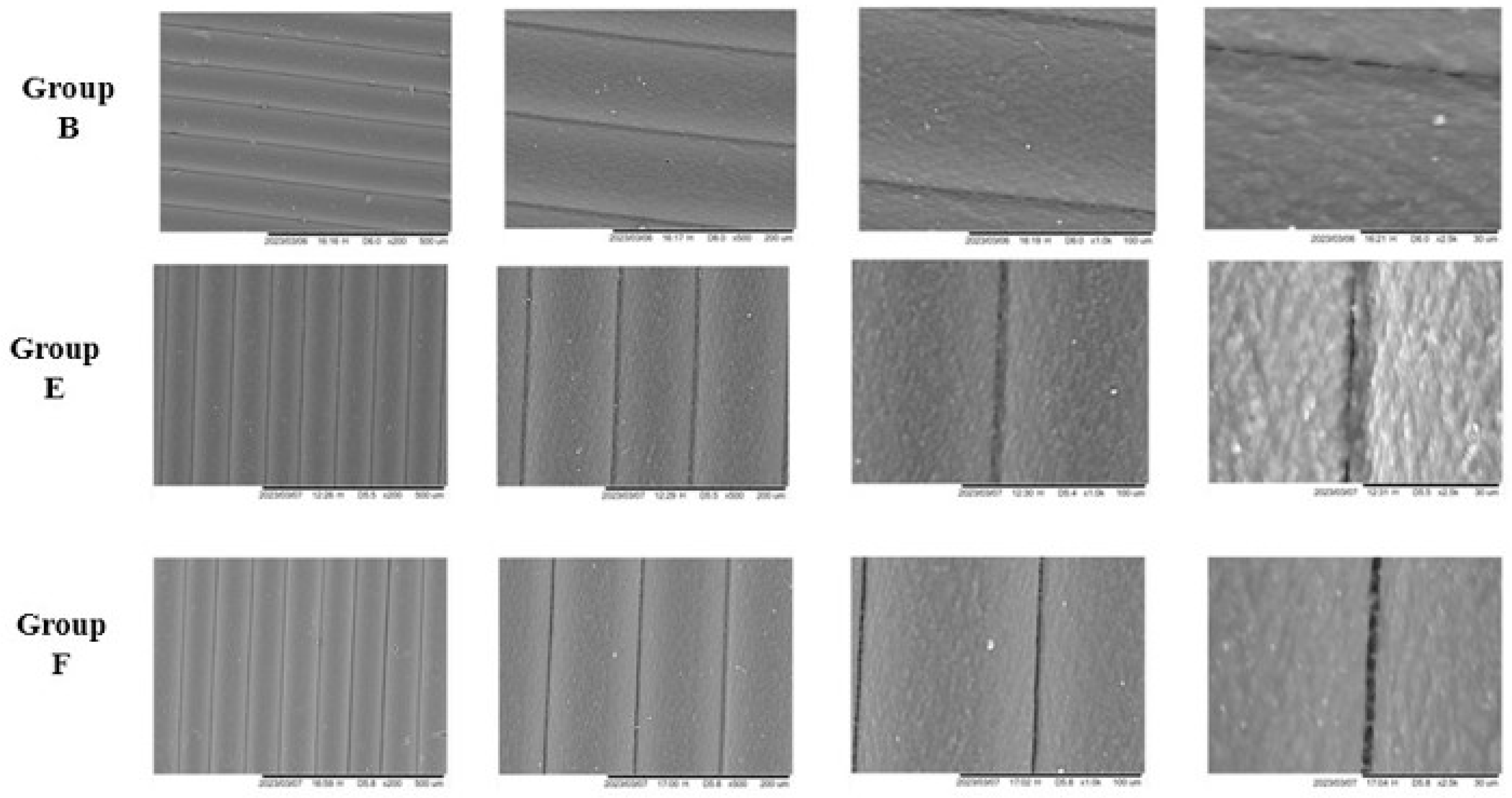
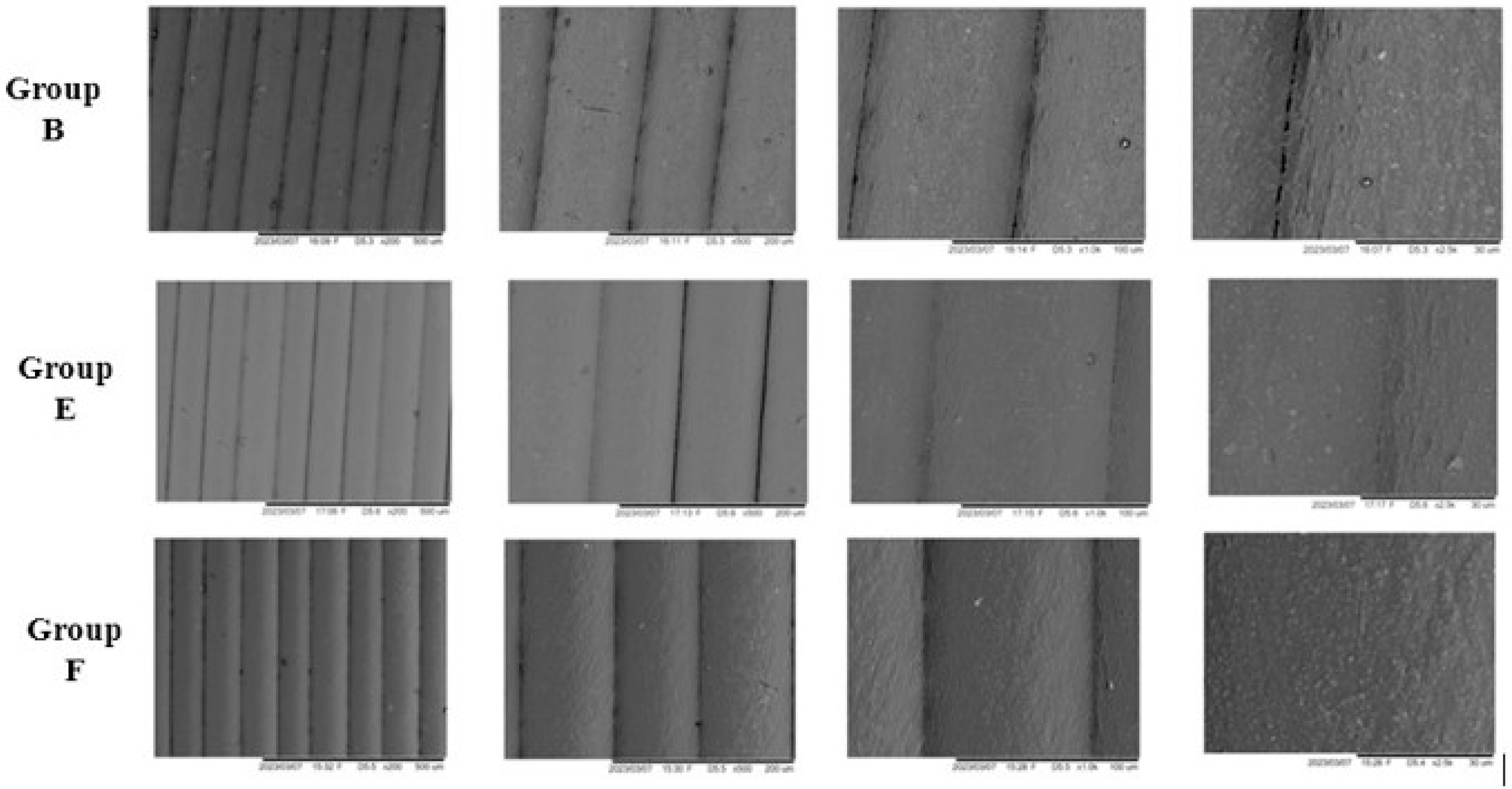
| Pre-Immersion | |||
|---|---|---|---|
| Group | Median (IQR) | ꭓ2 (df) | p-Value * |
| A | 232.05 (0.96) | 1.232 (5) | 0.942 |
| B | 232.72 (2.14) | ||
| C | 231.90 (1.35) | ||
| D | 232.33 (1.75) | ||
| E | 231.73 (1.71) | ||
| F | 232.09 (1.50) | ||
| 7 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 231.01 (1.23) | 8.607 (5) | 0.126 |
| B | 231.85 (1.00) | ||
| C | 231.15 (1.72) | ||
| D | 232.04 (2.17) | ||
| E | 231.54 (1.39) | ||
| F | 232.29 (1.12) | ||
| 14 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 231.16 (1.09) | 4.613 (5) | 0.465 |
| B | 231.59 (1.38) | ||
| C | 231.06 (1.71) | ||
| D | 230.78 (1.32) | ||
| E | 231.18 (2.17) | ||
| F | 231.54 (1.16) | ||
| 21 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 230.98 (0.61) | 7.740 (5) | 0.171 |
| B | 232.01 (1.96) | ||
| C | 230.83 (1.16) | ||
| D | 231.27 (1.48) | ||
| E | 231.64 (1.28) | ||
| F | 232.03 (0.81) | ||
| 28 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 230.93 (1.21) | 5.466 (5) | 0.362 |
| B | 231.75 (1.69) | ||
| C | 230.61 (1.48) | ||
| D | 230.88 (1.69) | ||
| E | 230.78 (1.52) | ||
| F | 231.02 (0.45) | ||
| Pre-Immersion | |||
|---|---|---|---|
| Group | Median (IQR) | ꭓ2 (df) | p-Value * |
| A | 122.51 (1.74) | 7.389 (5) | 0.193 |
| B | 122.70 (1.80) | ||
| C | 121.74 (2.25) | ||
| D | 121.86 (2.26) | ||
| E | 122.98 (1.68) | ||
| F | 123.19 (1.66) | ||
| 7 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 122.96 (1.80) | 3.675 (5) | 0.597 |
| B | 123.34 (1.61) | ||
| C | 122.94 (2.18) | ||
| D | 121.82 (2.11) | ||
| E | 123.07 (2.16) | ||
| F | 123.52 (2.09) | ||
| 14 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 123.38 (1.68) | 4.816 (5) | 0.439 |
| B | 123.84 (1.55) | ||
| C | 122.77 (2.42) | ||
| D | 121.82 (2.54) | ||
| E | 122.77 (2.00) | ||
| F | 123.81 (1.92) | ||
| 21 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 123.12 (1.94) | 3.977 (5) | 0.553 |
| B | 123.63 (2.05) | ||
| C | 122.54 (2.23) | ||
| D | 121.59 (2.48) | ||
| E | 122.60 (1.82) | ||
| F | 123.27 (1.64) | ||
| 28 days post immersion | |||
| Group | Median (IQR) | ꭓ2 (df) | p-value * |
| A | 122.87 (1.85) | 3.448 (5) | 0.631 |
| B | 123.28 (1.76) | ||
| C | 122.58 (1.73) | ||
| D | 121.80 (2.84) | ||
| E | 123.04 (2.19) | ||
| F | 123.71 (1.76) | ||
| Group | Mean ± SD | Correlation Coefficient | p Value * |
|---|---|---|---|
| A | 2.76 ± 0.04 | 0.165 | 0.342 |
| B | 2.83 ± 0.04 | 0.339 | 0.047 * |
| C | 2.82 ± 0.03 | 0.176 | 0.311 |
| D | 2.84 ± 0.05 | 0.178 | 0.306 |
| E | 2.79 ± 0.05 | 0.106 | 0.544 |
| F | 2.76 ± 0.04 | 0.117 | 0.504 |
| Immersion Media | Mean (SD) | F Stat (df) | p Value * |
|---|---|---|---|
| No Immersion | 23.617 (0.175) | 1.036 (6) | 0.443 |
| Set A | 26.110 (0.866) | ||
| Set B | 26.147 (2.894) | ||
| Set C | 26.152 (1.836) | ||
| Set D | 26.206 (1.074) | ||
| Set E | 24.991 (2.319) | ||
| Set F | 26.469 (1.435) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamayet, N.B.; Barman, A.; Yaw, C.T.; Xuan, K.Y.; Rashid, F.; Parolia, A.; Dudley, J.; Farook, T.H. Compressive, Dimensional, and Antimicrobial Characteristics of 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Following Dental Disinfection. Appl. Sci. 2025, 15, 10428. https://doi.org/10.3390/app151910428
Jamayet NB, Barman A, Yaw CT, Xuan KY, Rashid F, Parolia A, Dudley J, Farook TH. Compressive, Dimensional, and Antimicrobial Characteristics of 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Following Dental Disinfection. Applied Sciences. 2025; 15(19):10428. https://doi.org/10.3390/app151910428
Chicago/Turabian StyleJamayet, Nafij Bin, Aparna Barman, Chong Terng Yaw, Khoo Yi Xuan, Farah Rashid, Abhishek Parolia, James Dudley, and Taseef Hasan Farook. 2025. "Compressive, Dimensional, and Antimicrobial Characteristics of 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Following Dental Disinfection" Applied Sciences 15, no. 19: 10428. https://doi.org/10.3390/app151910428
APA StyleJamayet, N. B., Barman, A., Yaw, C. T., Xuan, K. Y., Rashid, F., Parolia, A., Dudley, J., & Farook, T. H. (2025). Compressive, Dimensional, and Antimicrobial Characteristics of 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Following Dental Disinfection. Applied Sciences, 15(19), 10428. https://doi.org/10.3390/app151910428










