Life Cycle Assessment of Portland Cement Alternatives in Mine Paste Backfill
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Data Source and CPB Sample Preparation
2.2. Life Cycle Impact Assessment of Alkaline Activators
2.3. Regional-Scale Study Background
2.4. PC8 Environmental Footprint Modeling
2.5. Regional-Scale CPB Environmental Footprint Modeling
3. Results and Discussion
3.1. Review of Life Cycle Assessment Studies for MgO, OPC, Na2SiO3, and NaOH Activator
3.2. Activators LCIA Using Commercial Software (SimaPro) and Database (Ecoinvent)
3.3. Cemented Paste Backfill LCA and Economic Assessment (Regional Scale)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Global Material Resources Outlook to 2060; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Upadhyay, A.; Laing, T.; Kumar, V.; Dora, M. Exploring barriers and drivers to the implementation of circular economy practices in the mining industry. Resour. Policy 2021, 72, 102037. [Google Scholar] [CrossRef]
- Zhou, Y. Natural resources and green economic growth: A pathway to innovation and digital transformation in the mining industry. Resour. Policy 2024, 90, 104667. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. The Twelve Principles of Circular Hydrometallurgy. J. Sustain. Metall. 2023, 9, 1–25. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A review of circular economy strategies for mine tailings. Cleaner Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Baker, E.; Davies, M.; Fourie, A.; Mudd, G.; Thygesen, K. Chapter II: Mine Tailings Facilities: Overview and Industry Trends. In Towards Zero Harm—A Compendium of Papers Prepared for the Global Tailings Review; Global Tailings Review: London, UK, 2019. [Google Scholar]
- Franks, D.M.; Stringer, M.; Torres-Cruz, L.A.; Baker, E.; Valenta, R.; Thygesen, K.; Matthews, A.; Howchin, J.; Barrie, S. Tailings facility disclosures reveal stability risks. Sci. Rep. 2021, 11, 5353. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Di Maria, A.; Khoshkhoo, M.; Sand, A.; Van Acker, K. Towards sustainable resource valorization: A life cycle sustainability assessment of metals recovery from sulfidic mining residues in Sweden. Resour. Conserv. Recycl. 2024, 204, 107513. [Google Scholar] [CrossRef]
- Rosario-Beltré, A.J.; Sánchez-España, J.; Rodríguez-Gómez, V.; Fernández-Naranjo, F.J.; Bellido-Martín, E.; Adánez-Sanjuán, P.; Arranz-González, J.C. Critical Raw Materials recovery potential from Spanish mine wastes: A national-scale preliminary assessment. J. Clean. Prod. 2023, 407, 137163. [Google Scholar] [CrossRef]
- Adrianto, L.R.; Ciacci, L.; Pfister, S.; Hellweg, S. Toward sustainable reprocessing and valorization of sulfidic copper tailings: Scenarios and prospective LCA. Sci. Total Environ. 2023, 871, 162038. [Google Scholar] [CrossRef]
- Sarker, S.K.; Haque, N.; Bhuiyan, M.; Bruckard, W.; Pramanik, B.K. Recovery of strategically important critical minerals from mine tailings. J. Environ. Chem. Eng. 2022, 10, 107622. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burked, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Capasso, I.; Lirer, S.; Flora, A.; Ferone, C.; Cioffi, R.; Caputo, D.; Liguori, B. Reuse of mining waste as aggregates in fly ash-based geopolymers. J. Clean. Prod. 2019, 220, 65–73. [Google Scholar] [CrossRef]
- Kiventerä, J.; Golek, L.; Yliniemi, J.; Ferreira, V.; Deja, J.; Illikainen, M. Utilization of sulphidic tailings from gold mine as a raw material in geopolymerization. Int. J. Miner. Process. 2016, 149, 104–110. [Google Scholar] [CrossRef]
- He, X.; Yuhua, Z.; Qaidi, S.; Isleem, H.F.; Zaid, O.; Althoey, F.; Ahmad, J. Mine tailings-based geopolymers: A comprehensive review. Ceram. Int. 2022, 48, 24192–24212. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishna, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects. Process Saf. Environ. Prot. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- Thejas, H.K.; Hossiney, N. Alkali-activated bricks made with mining waste iron ore tailings. Case Stud. Constr. Mater. 2022, 16, e00973. [Google Scholar] [CrossRef]
- Veiga Simão, F.; Chambart, H.; Vandemeulebroeke, L.; Nielsen, P.; Adrianto, L.R.; Pfister, S.; Cappuyns, V. Mine waste as a sustainable resource for facing bricks. J. Clean. Prod. 2022, 368, 133118. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012, 29, 323–331. [Google Scholar] [CrossRef]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine wastes based geopolymers: A critical review. Clean. Eng. Technol. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Veiga Simão, F.; Chambart, H.; Vandemeulebroeke, L.; Cappuyns, V. Incorporation of sulphidic mining waste material in ceramic roof tiles and blocks. J. Geochem. Explor. 2021, 225, 106741. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Yliniemi, J.; Nguyen, H.; Adesanya, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Utilisation of glass wool waste and mine tailings in high performance building ceramics. J. Build. Eng. 2020, 31, 101383. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, W.; Wu, M.; Shen, B.; Li, M.; Xu, G.; Zhang, B.; Ding, Q.; Chen, X. Experimental study on the utilization of copper tailing as micronized sand to prepare high performance concrete. Constr. Build. Mater. 2020, 244, 118312. [Google Scholar] [CrossRef]
- Benahsina, A.; El Haloui, Y.; Taha, Y.; Elomari, M.; Bennouna, M.A. Natural sand substitution by copper mine waste rocks for concrete manufacturing. J. Build. Eng. 2022, 47, 103817. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, Ö. Reusing copper tailings in concrete: Corrosion performance and socioeconomic implications for the Lefke-Xeros area of Cyprus. J. Clean. Prod. 2016, 112, 420–429. [Google Scholar] [CrossRef]
- Adiguzel, D.; Tuylu, S.; Eker, H. Utilization of tailings in concrete products: A review. Constr. Build. Mater. 2022, 360, 129574. [Google Scholar] [CrossRef]
- Arunachalam, K.P.; Avudaiappan, S.; Maureira, N.; Da Costa Garcia Filho, F.; Monteiro, S.N.; Batista, I.D.; de Azevedo, A.R.G. Innovative use of copper mine tailing as an additive in cement mortar. J. Mater. Res. Technol. 2023, 25, 2261–2274. [Google Scholar] [CrossRef]
- Shettima, A.U.; Hussin, M.W.; Ahmad, Y.; Mirza, J. Evaluation of iron ore tailings as replacement for fine aggregate in concrete. Constr. Build. Mater. 2016, 120, 72–79. [Google Scholar] [CrossRef]
- Vargas, F.; Lopez, M.; Rigamonti, L. Environmental impacts evaluation of treated copper tailings as supplementary cementitious materials. Resour. Conserv. Recycl. 2020, 160, 104890. [Google Scholar] [CrossRef]
- Vargas, F.; Lopez, M. Development of a new supplementary cementitious material from the activation of copper tailings: Mechanical performance and analysis of factors. J. Clean. Prod. 2018, 182, 427–436. [Google Scholar] [CrossRef]
- Araujo, F.S.M.; Taborda-Llano, I.; Nunes, E.B.; Santos, R.M. Recycling and Reuse of Mine Tailings: A Review of Advancements and Their Implications. Geosciences 2022, 12, 319. [Google Scholar] [CrossRef]
- Sivakugan, N.; Veenstra, R.; Naguleswaran, N. Underground Mine Backfilling in Australia Using Paste Fills and Hydraulic Fills. Int. J. Geosynth. Ground Eng. 2015, 1, 18. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Xin, L.; Wang, K.; Pan, H. Monitoring and Assessment of Cemented Paste Backfill Containing Coal Gangue and Fly Ash in an Underground Mine. Adv. Mater. Sci. Eng. 2021, 2021, 5946148. [Google Scholar] [CrossRef]
- Raffaldi, M.J.; Seymour, J.B.; Richardson, J.; Zahl, E.; Board, M. Cemented Paste Backfill Geomechanics at a Narrow-Vein Underhand Cut-and-Fill Mine. Rock Mech. Rock Eng. 2019, 52, 4925–4940. [Google Scholar] [CrossRef]
- Helinski, M.; Fahey, M.; Fourie, A. Behavior of Cemented Paste Backfill in Two Mine Stopes: Measurements and Modeling. J. Geotech. Geoenviron. Eng. 2011, 137, 171–182. [Google Scholar] [CrossRef]
- Qi, C.; Fourie, A. Cemented paste backfill for mineral tailings management: Review and future perspectives. Miner. Eng. 2019, 144, 106025. [Google Scholar] [CrossRef]
- Tariq, A.; Yanful, E.K. A review of binders used in cemented paste tailings for underground and surface disposal practices. J. Environ. Manag. 2013, 131, 138–149. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Belem, T.; Benzaazoua, M. Design and application of underground mine paste backfill technology. Geotech. Geol. Eng. 2008, 26, 147–174. [Google Scholar] [CrossRef]
- Behera, S.K.; Mishra, D.P.; Ghosh, C.N.; Prashant; Mandal, P.K.; Singh, K.M.P.; Buragohain, J.; Singh, P.K. Characterization of lead–zinc mill tailings, fly ash and their mixtures for paste backfilling in underground metalliferous mines. Environ. Earth Sci. 2019, 78, 394. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E.G. Mix proportioning of underground cemented tailings backfill. Tunn. Undergr. Space Technol. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Behera, S.K.; Mishra, D.P.; Singh, P.; Mishra, K.; Mandal, S.K.; Ghosh, C.N.; Kumar, R.; Mandal, P.K. Utilization of mill tailings, fly ash and slag as mine paste backfill material: Review and future perspective. Constr. Build. Mater. 2021, 309, 125120. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Belem, T.; Bussiè, B. Chemical factors that inf luence the performance of mine sulphidic paste backfill. Cem. Concr. Res. 2002, 32, 1133–1144. [Google Scholar] [CrossRef]
- Diosdado-Aragón, A.J.; Valenzuela-Díaz, M.J.; Dávila, J.M.; Becerra-Herrera, M.; Caraballo, M.A. Influence of mine tailings mineralogy and curing conditions in the cementation of pastes for mine galleries backfilling. Miner. Eng. 2025, 232, 109524. [Google Scholar] [CrossRef]
- Soomro, M.; Tam, V.W.Y.; Jorge Evangelista, A.C. Production of cement and its environmental impact. In Recycled Concrete: Technologies and Performance; Elsevier: Amsterdam, The Netherlands, 2022; pp. 11–46. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Saedi, A.; Jamshidi-Zanjani, A.; Darban, A.K. A review on different methods of activating tailings to improve their cementitious property as cemented paste and reusability. J. Environ. Manag. 2020, 270, 110881. [Google Scholar] [CrossRef]
- Sala, S.; Reale, F.; Cristobal-Garcia, J.; Marelli, L.; Rana, P. Life Cycle Assessment for the Impact Assessment of Policies; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Owsianiak, M.; Laurent, A.; Bjørn, A.; Hauschild, M.Z. IMPACT 2002+, ReCiPe 2008 and ILCD’s recommended practice for characterization modelling in life cycle impact assessment: A case study-based comparison. Int. J. Life Cycle Assess. 2014, 19, 1007–1021. [Google Scholar] [CrossRef]
- Rachid, S.; Taha, Y.; Benzaazoua, M. Environmental evaluation of metals and minerals production based on a life cycle assessment approach: A systematic review. Miner. Eng. 2023, 198, 108076. [Google Scholar] [CrossRef]
- Segura-Salazar, J.; Lima, F.M.; Tavares, L.M. Life Cycle Assessment in the minerals industry: Current practice, harmonization efforts, and potential improvement through the integration with process simulation. J. Clean. Prod. 2019, 232, 174–192. [Google Scholar] [CrossRef]
- Segura-Salazar, J.; Tavares, L.M. A life cycle-based, sustainability-driven innovation approach in the minerals industry: Application to a large-scale granitic quarry in Rio de Janeiro. Miner. Eng. 2021, 172, 107149. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P. Life cycle analysis of copper-gold-lead-silver-zinc beneficiation process. Sci. Total Environ. 2019, 659, 41–52. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P. Impacts of aluminum production: A cradle to gate investigation using life-cycle assessment. Sci. Total Environ. 2019, 663, 958–970. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P.; Lang, C. A global life cycle assessment of manganese mining processes based on EcoInvent database. Sci. Total Environ. 2019, 688, 1102–1111. [Google Scholar] [CrossRef]
- Reid, C.; Bécaert, V.; Aubertin, M.; Rosenbaum, R.K.; Deschênes, L. Life cycle assessment of mine tailings management in Canada. J. Clean. Prod. 2009, 17, 471–479. [Google Scholar] [CrossRef]
- Song, X.; Pettersen, J.B.; Pedersen, K.B.; Røberg, S. Comparative life cycle assessment of tailings management and energy scenarios for a copper ore mine: A case study in Northern Norway. J. Clean. Prod. 2017, 164, 892–904. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Parvez Mahmud, M.A.; Saidur, R. A review on the impact of mining and mineral processing industries through life cycle assessment. J. Clean. Prod. 2019, 231, 1200–1217. [Google Scholar] [CrossRef]
- Doka, G. A Model for Waste-Specific and Climate-Specific Life Cycle Inventories of Tailings Impoundments; Doka Life Cycle Assessments: Zurich, Switzerland, 2002. [Google Scholar]
- Adrianto, L.R.; Pfister, S.; Hellweg, S. Regionalized Life Cycle Inventories of Global Sulfidic Copper Tailings. Environ. Sci. Technol. 2022, 56, 4553–4564. [Google Scholar] [CrossRef] [PubMed]
- Dandautiya, R.; Singh, A.P. Utilization potential of fly ash and copper tailings in concrete as partial replacement of cement along with life cycle assessment. Waste Manag. 2019, 99, 90–101. [Google Scholar] [CrossRef]
- Saldanha, R.B.; Caicedo, A.M.L.; de Araújo, M.T.; Scheuermann Filho, H.C.; Moncaleano, C.J.; Silva, J.P.S.; Consoli, N.C. Potential use of iron ore tailings for binder production: A life cycle assessment. Constr. Build. Mater. 2023, 365, 130008. [Google Scholar] [CrossRef]
- Munir, Q.; Abdulkareem, M.; Horttanainen, M.; Kärki, T. A comparative cradle-to-gate life cycle assessment of geopolymer concrete produced from industrial side streams in comparison with traditional concrete. Sci. Total Environ. 2023, 865, 161230. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Ghose, A. Can LCA be FAIR? Assessing the status quo and opportunities for FAIR data sharing. Int. J. Life Cycle Assess. 2024, 29, 733–744. [Google Scholar] [CrossRef]
- Diosdado-Aragón, A.J.; Valenzuela-Díaz, M.J.; Ruíz-Rodríguez, M.; Davila, J.M.; Becerra-Herrera, M.; Caraballo, M.A. Chemometric study of mine tailings cementation, using NaOH or Mg(OH)2, for mine galleries backfilling. Miner. Eng. 2025, 232, 109518. [Google Scholar] [CrossRef]
- An, J.; Xue, X. Life-cycle carbon footprint analysis of magnesia products. Resour. Conserv. Recycl. 2017, 119, 4–11. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Shao, S.; Zhang, S. Comparative life cycle assessment of conventional and new fused magnesia production. J. Clean. Prod. 2015, 91, 170–179. [Google Scholar] [CrossRef]
- Ren, W.; Xue, B.; Lu, C.; Zhang, Z.; Zhang, Y.; Jiang, L. Evaluation of GHG emissions from the production of magnesia refractory raw materials in Dashiqiao, China. J. Clean. Prod. 2016, 135, 214–222. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Influence of supplementary cementitious materials on the performance and environmental impacts of reactive magnesia cement concrete. J. Clean. Prod. 2017, 159, 62–73. [Google Scholar] [CrossRef]
- Shahbaz, F.; Singh, I.; Krishnan, P.; Celik, K. Life cycle assessment of brucite and synthetic MgO produced from reject brine using different alkalis. J. Clean. Prod. 2022, 380, 135071. [Google Scholar] [CrossRef]
- Shen, W.; Cao, L.; Li, Q.; Wen, Z.; Wang, J.; Liu, Y.; Dong, R.; Tan, Y.; Chen, R. Is magnesia cement low carbon? Life cycle carbon footprint comparing with Portland cement. J. Clean. Prod. 2016, 131, 20–27. [Google Scholar] [CrossRef]
- Trojer, M. Principles of Benchmarking Criteria for the European Magnesia Industry. Master’s Thesis, University of Leoben, Leoben, Austria, 2009. [Google Scholar]
- Zhao, L.; Feng, J.; Dong, H. Analysis of carbon footprint and reduction approach of magnesia production in China. J. Clean. Prod. 2022, 334, 130194. [Google Scholar] [CrossRef]
- Arguillarena, A.; Margallo, M.; Irabien, Á.; Urtiaga, A. Life cycle assessment of zinc and iron recovery from spent pickling acids by membrane-based solvent extraction and electrowinning. J. Environ. Manag. 2022, 318, 115567. [Google Scholar] [CrossRef]
- Boesch, M.E.; Hellweg, S. Identifying improvement potentials in cement production with life cycle assessment. Environ. Sci. Technol. 2010, 44, 9143–9149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.C.M.; Setunge, S.; Dissanayake, R. Life cycle assessment and cost analysis of fly ash–rice husk ash blended alkali-activated concrete. J. Environ. Manag. 2021, 295, 113140. [Google Scholar] [CrossRef]
- Moretti, L.; Caro, S. Critical analysis of the Life Cycle Assessment of the Italian cement industry. J. Clean. Prod. 2017, 152, 198–210. [Google Scholar] [CrossRef]
- Ramagiri, K.K.; Chintha, R.; Bandlamudi, R.K.; Kara De Maeijer, P.; Kar, A. Cradle-to-gate life cycle and economic assessment of sustainable concrete mixes—Alkali-activated concrete (Aac) and bacterial concrete (bc). Infrastructures 2021, 6, 104. [Google Scholar] [CrossRef]
- Teh, S.H.; Wiedmann, T.; Castel, A.; de Burgh, J. Hybrid life cycle assessment of greenhouse gas emissions from cement, concrete and geopolymer concrete in Australia. J. Clean. Prod. 2017, 152, 312–320. [Google Scholar] [CrossRef]
- Valderrama, C.; Granados, R.; Cortina, J.L.; Gasol, C.M.; Guillem, M.; Josa, A. Implementation of best available techniques in cement manufacturing: A life-cycle assessment study. J. Clean. Prod. 2012, 25, 60–67. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Havukainen, J.; Nuortila-Jokinen, J.; Horttanainen, M. Environmental and economic perspective of waste-derived activators on alkali-activated mortars. J. Clean. Prod. 2021, 280, 124651. [Google Scholar] [CrossRef]
- Habert, G.; D’Espinose De Lacaillerie, J.B.; Lanta, E.; Roussel, N. Environmental Evaluation for Cement Substitution with Geopolymers. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010. [Google Scholar]
- Habert, G.; Ouellet-Plamondon, C. Recent Update on the Environmental Impact of Geopolymers; RILEM: Paris, France, 2016; pp. 17–23. [Google Scholar]
- Komkova, A.; Habert, G. Environmental impact assessment of alkali-activated materials: Examining impacts of variability in constituent production processes and transportation. Constr. Build. Mater. 2023, 363, 129032. [Google Scholar] [CrossRef]
- Nikravan, M.; Firdous, R.; Stephan, D. Life cycle assessment of alkali-activated materials: A systematic literature review. Low-Carbon Mater. Green Constr. 2023, 1, 13. [Google Scholar] [CrossRef]
- Sandanayake, M.; Gunasekara, C.; Law, D.; Zhang, G.; Setunge, S. Greenhouse gas emissions of different fly ash based geopolymer concretes in building construction. J. Clean. Prod. 2018, 204, 399–408. [Google Scholar] [CrossRef]
- Alvarez-Gaitan, J.P.; Peters, G.M.; Rowley, H.V.; Moore, S.; Short, M.D. A hybrid life cycle assessment of water treatment chemicals: An Australian experience. Int. J. Life Cycle Assess. 2013, 18, 1291–1301. [Google Scholar] [CrossRef]
- Du, F.; Warsinger, D.M.; Urmi, T.I.; Thiel, G.P.; Kumar, A.; Lienhard, J.H. Sodium Hydroxide Production from Seawater Desalination Brine: Process Design and Energy Efficiency. Environ. Sci. Technol. 2018, 52, 5949–5958. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. Environmental challenges of the chlor-alkali production: Seeking answers from a life cycle approach. Sci. Total Environ. 2017, 580, 147–157. [Google Scholar] [CrossRef]
- Hong, J.; Chen, W.; Wang, Y.; Xu, C.; Xu, X. Life cycle assessment of caustic soda production: A case study in China. J. Clean. Prod. 2014, 66, 113–120. [Google Scholar] [CrossRef]
- Medina-Martos, E.; Gálvez-Martos, J.L.; Almarza, J.; Lirio, C.; Iribarren, D.; Valente, A.; Dufour, J. Environmental and economic performance of carbon capture with sodium hydroxide. J. CO2 Util. 2022, 60, 101991. [Google Scholar] [CrossRef]
- Raza, M.H.; Khan, M.; Zhong, R.Y. Strength, porosity and life cycle analysis of geopolymer and hybrid cement mortars for sustainable construction. Sci. Total Environ. 2024, 907, 167839. [Google Scholar] [CrossRef]
- Thiel, G.P.; Kumar, A.; Gómez-González, A.; Lienhard, J.H. Utilization of Desalination Brine for Sodium Hydroxide Production: Technologies, Engineering Principles, Recovery Limits, and Future Directions. ACS Sustain. Chem. Eng. 2017, 5, 11147–11162. [Google Scholar] [CrossRef]
- Sala, S.; Crenna, E. Global Normalisation Factors for the Environmental Footprint and Life Cycle Assessment; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Hou, C.; Yang, L.; Li, L.; Yan, B. Mechanical Characteristics and Stress Evolution of Cemented Paste Backfill: Effect of Curing Time, Solid Content, and Binder Content. Front. Mater. 2022, 8, 812402. [Google Scholar] [CrossRef]
- Muñoz, I.; Soto, A.; Maza, D.; Bayón, F. Life cycle assessment of refractory waste management in a Spanish steel works. Waste Manag. 2020, 111, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alibaba. Reference Price of OPC. 2025. Available online: https://www.alibaba.com/product-detail/Factory-Direct-Manufacturer-Fast-Hardening-Portland_10000002732544.html (accessed on 10 August 2025).
- Alibaba. Reference Price of NaOH. 2025. Available online: https://www.alibaba.com/product-detail/China-manufacturer-Caustic-Potash-Soda-Pearls_1601168174605.html (accessed on 10 August 2025).
- De Bruyn, S.; Bijleveld, M.; De Graaff, L.; Schep, E.; Schroten, A.; Vergeer, R.; Ahdour, S. Environmental Prices Handbook; CE Delft: Delft, The Netherlands, 2018. [Google Scholar]
- Gil Saenz, R. Treball Final de Grau. Design of a Lab-Scale System for the Study of Dry Desulfurization Process. Disseny D’un Sistema a Escala Laboratori per a L’estudi de Dessulfuració per via Seca. Raúl Gil Sáenz. 2018. Available online: https://diposit.ub.edu/dspace/bitstream/2445/141897/1/Gil_Saenz_Raul.pdf (accessed on 10 August 2025).
- Chimenos, J.M.; Fernández, A.I.; Hernández, A.; Haurie, L.; Espiell, F.; Ayora, C. Optimization of phosphate removal in anodizing aluminium wastewater. Water Res. 2006, 40, 137–143. [Google Scholar] [CrossRef] [PubMed]
- del Valle-Zermeño, R.; Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Low-grade magnesium oxide by-products for environmental solutions: Characterization and geochemical performance. J. Geochem. Explor. 2015, 152, 134–144. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Stabilization study of a contaminated soil with metal(loid)s adding different low-grade MgO degrees. Sustainability 2020, 12, 7340. [Google Scholar] [CrossRef]
- Seco, A.; del Castillo, J.M.; Espuelas, S.; Marcelino, S.; Echeverría, A.M. Assessment of the ability of MGO based binary binders for the substitution of Portland cement for mortars manufacturing. Constr. Build. Mater. 2022, 341, 127777. [Google Scholar] [CrossRef]
- Seco, A.; del Castillo, J.M.; Perlot, C.; Marcelino, S.; Espuelas, S. Recycled granulates manufacturing from spent refractory wastes and magnesium based binder. Constr. Build. Mater. 2023, 365, 130087. [Google Scholar] [CrossRef]
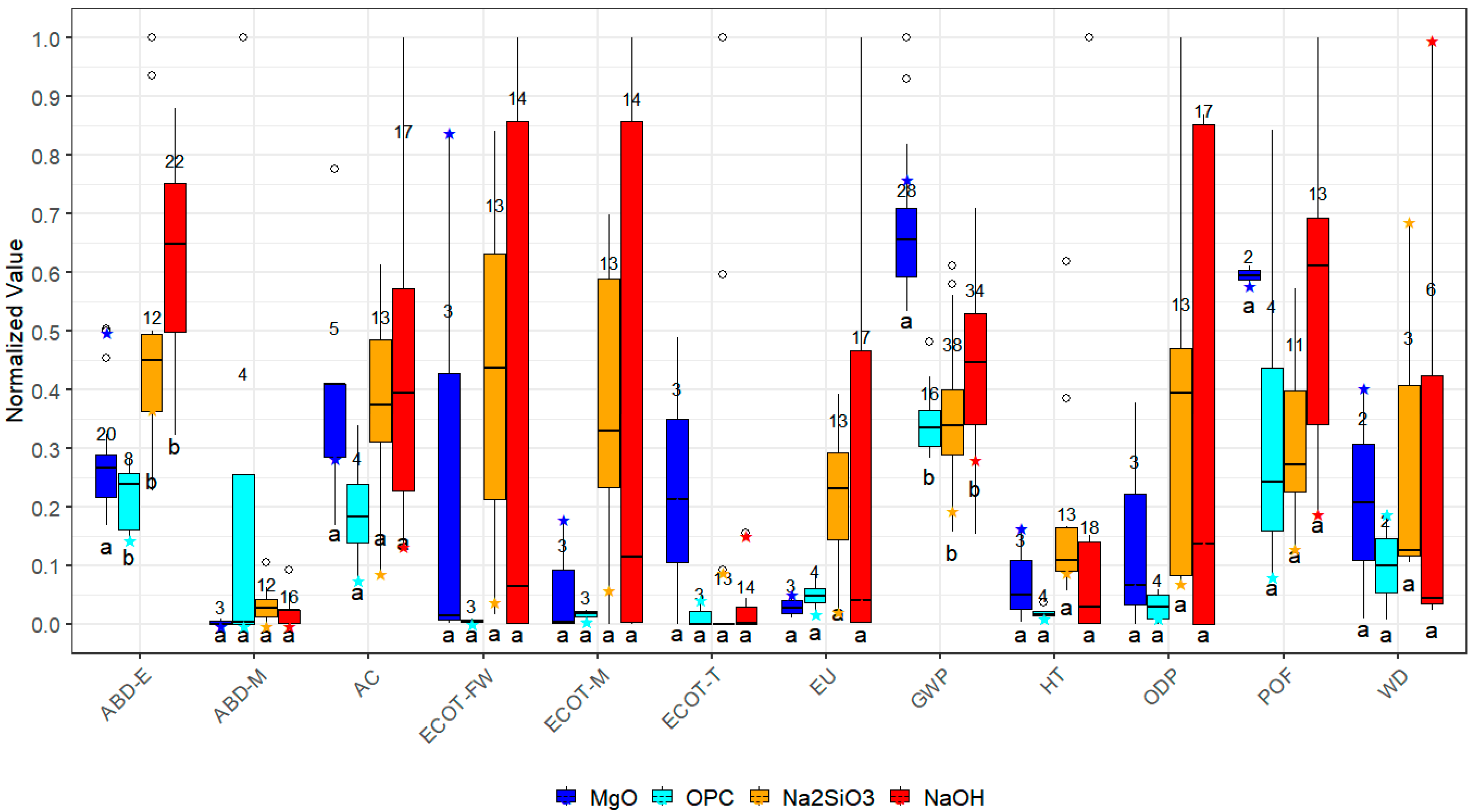
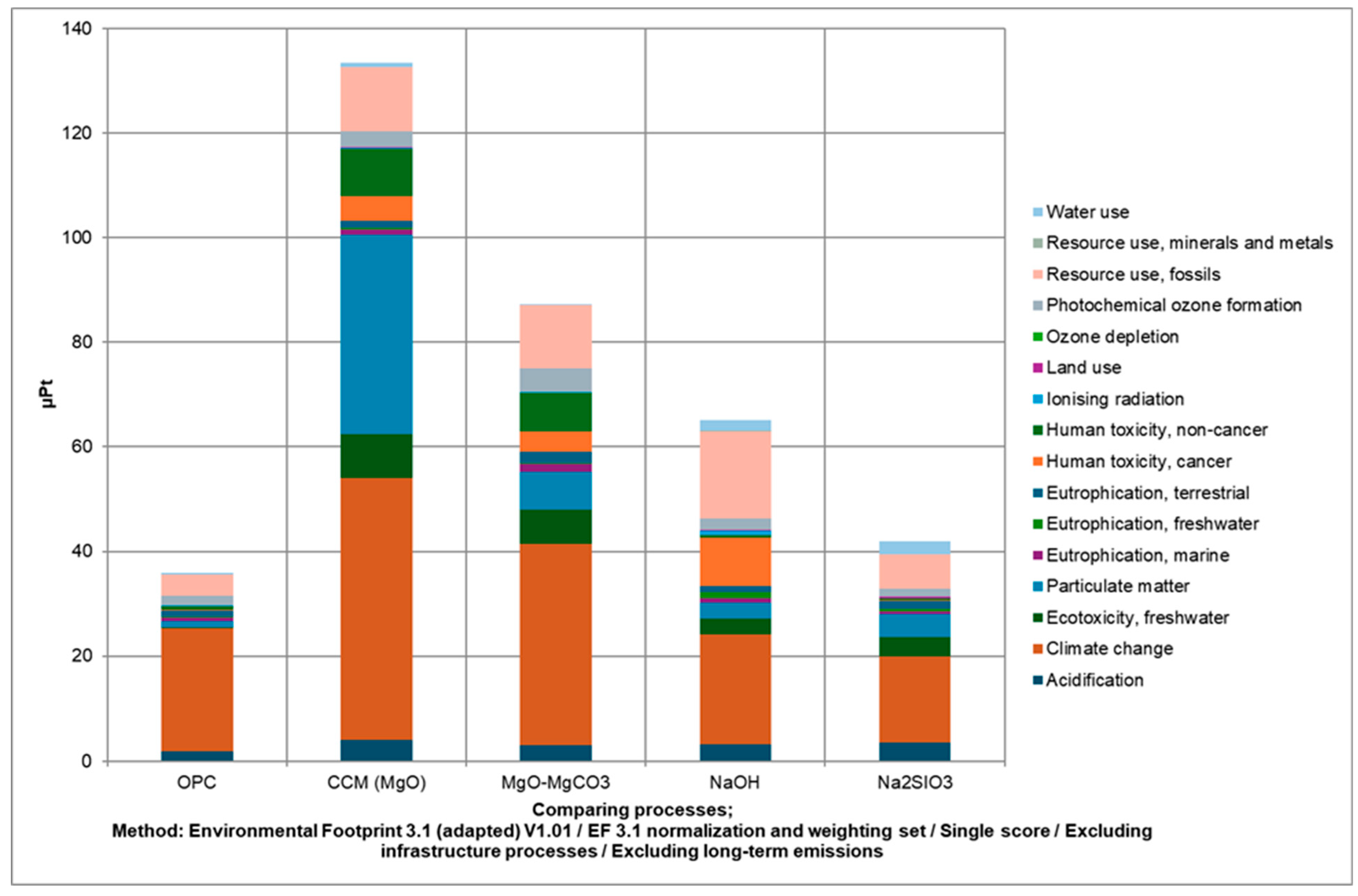
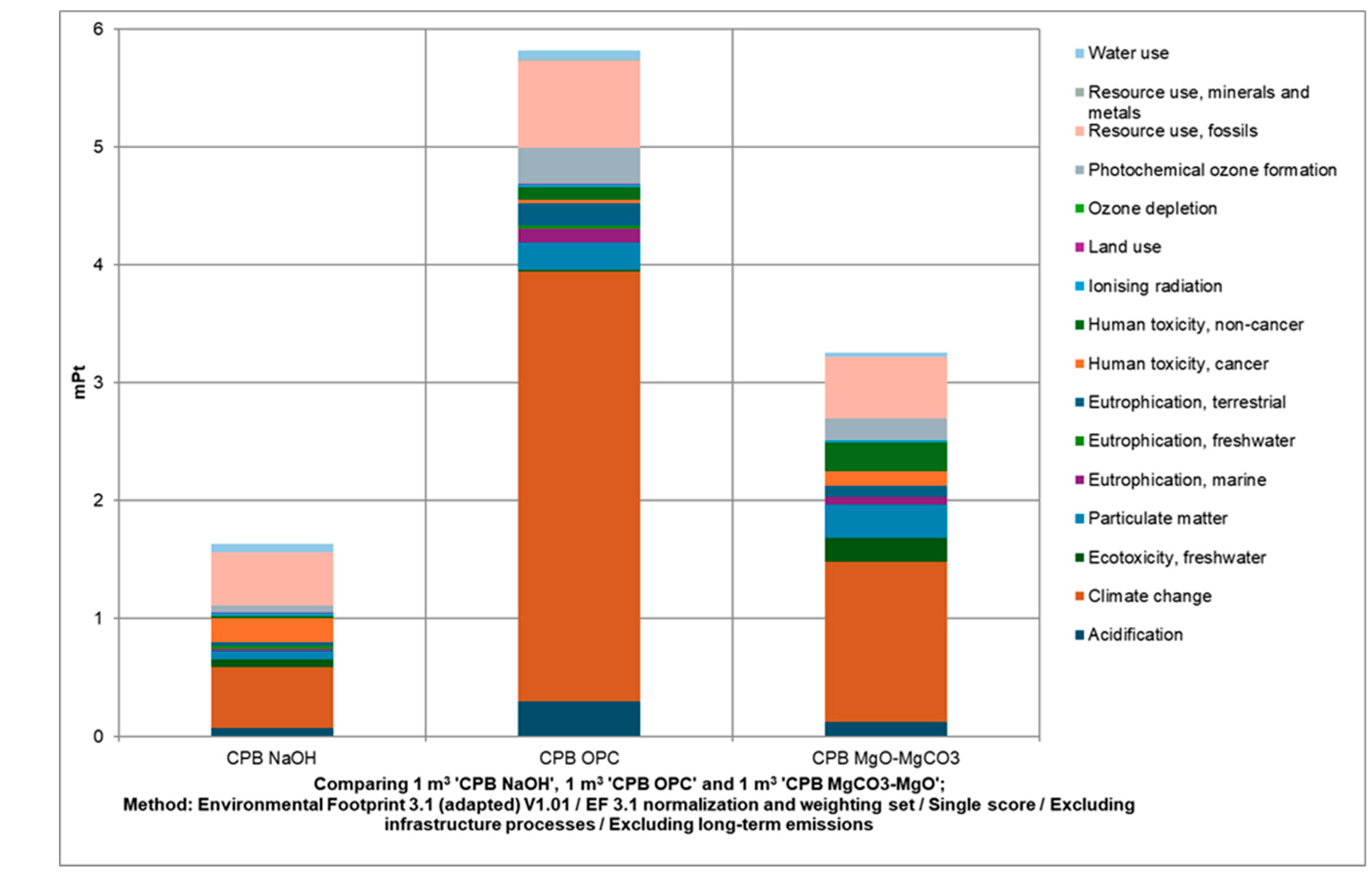
| Alk Agent | UCS [MPa] | SD [MPa] | N° Test |
|---|---|---|---|
| NaOH | 0.33 | 0.34 | 12 |
| PC8 | 1.55 | 0.33 | 20 |
| OPC | 2.92 | 1.49 | 38 |
| Binder | Reference |
|---|---|
| MgO | [68,69,70,71,72,73,74,75,76] |
| OPC | [47,71,74,77,78,79,80,81,82,83,84,85] |
| Na2SiO3 | [63,86,87,88,89,90,91] |
| NaOH | [77,85,86,87,88,90,91,92,93,94,95,96,97,98] |
| Item\Paste | NaOH | MgCO3-MgO | OPC |
|---|---|---|---|
| Cost [€/m3] | 13.83 | 5.01 | 16.61 |
| Binder (from purchase price) | 10.71 | 1.43 | 7.48 |
| Transport (1000 km) | 1.00 | 1.46 | 7.01 |
| Energy (from paste plant) | 2.12 | 2.12 | 2.12 |
| Environmental cost [€/m3] | 5.04 | 9.05 | 17.90 |
| Final cost [€/m3] | 18.87 | 14.06 | 34.5 |
| CO2 emissions [kg CO2-eq/m3] | 18.40 | 48.50 | 131 |
| UCS [MPa] | 0.33 ± 0.34 | 1.55 ± 0.33 | 2.92 ± 1.49 |
| PF [kg CO2eq/m3/MPa] | 55.80 | 31.29 | 44.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Díaz, M.J.; Diosdado-Aragón, A.J.; Munizaga-Rosas, J.C.; Caraballo, M. Life Cycle Assessment of Portland Cement Alternatives in Mine Paste Backfill. Appl. Sci. 2025, 15, 9996. https://doi.org/10.3390/app15189996
Valenzuela-Díaz MJ, Diosdado-Aragón AJ, Munizaga-Rosas JC, Caraballo M. Life Cycle Assessment of Portland Cement Alternatives in Mine Paste Backfill. Applied Sciences. 2025; 15(18):9996. https://doi.org/10.3390/app15189996
Chicago/Turabian StyleValenzuela-Díaz, Martín J., Antonio J. Diosdado-Aragón, José Charango Munizaga-Rosas, and Manuel Caraballo. 2025. "Life Cycle Assessment of Portland Cement Alternatives in Mine Paste Backfill" Applied Sciences 15, no. 18: 9996. https://doi.org/10.3390/app15189996
APA StyleValenzuela-Díaz, M. J., Diosdado-Aragón, A. J., Munizaga-Rosas, J. C., & Caraballo, M. (2025). Life Cycle Assessment of Portland Cement Alternatives in Mine Paste Backfill. Applied Sciences, 15(18), 9996. https://doi.org/10.3390/app15189996








