Computed Tomography Evaluation of the Renal Blood Vessels in the Omani Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
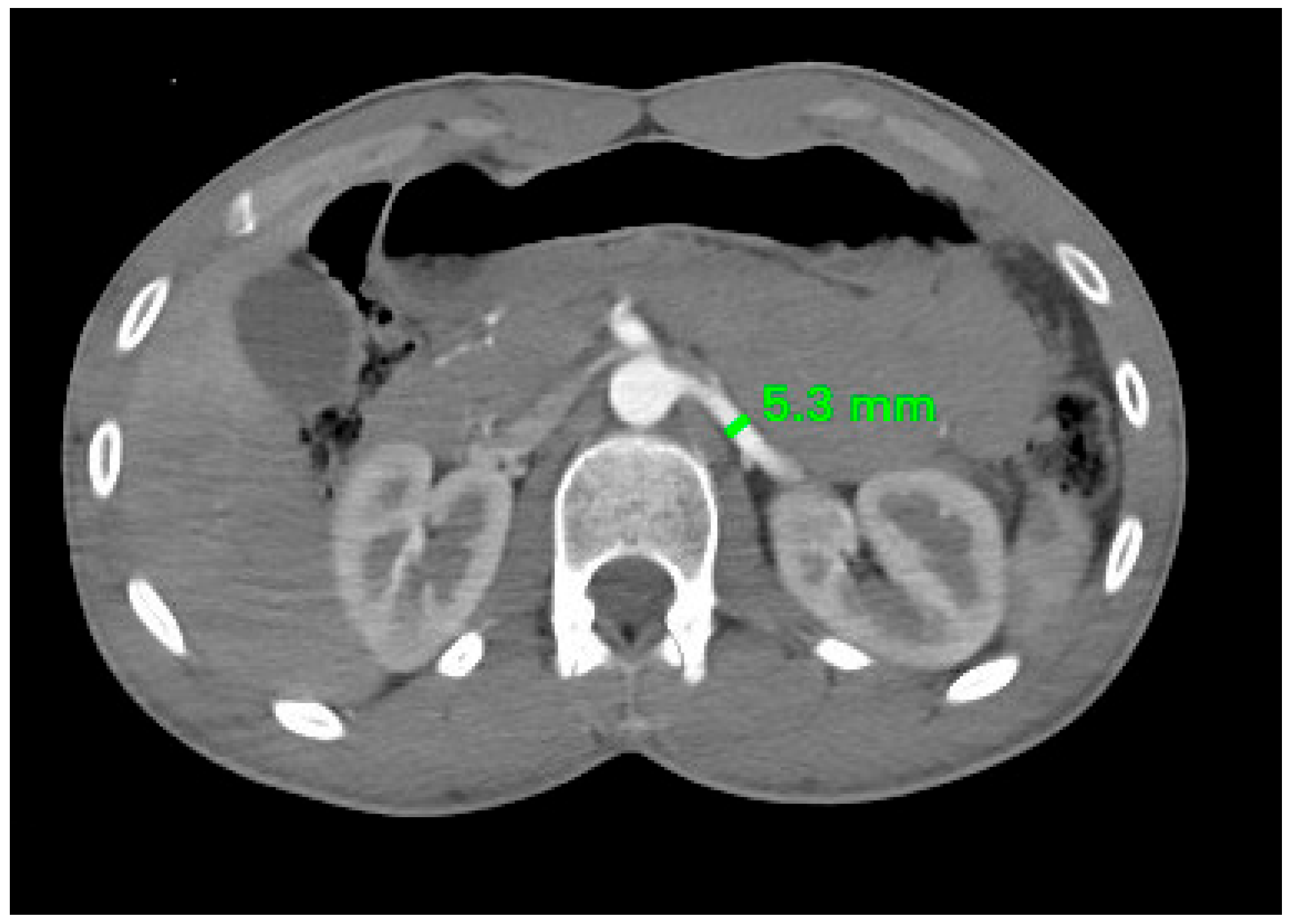
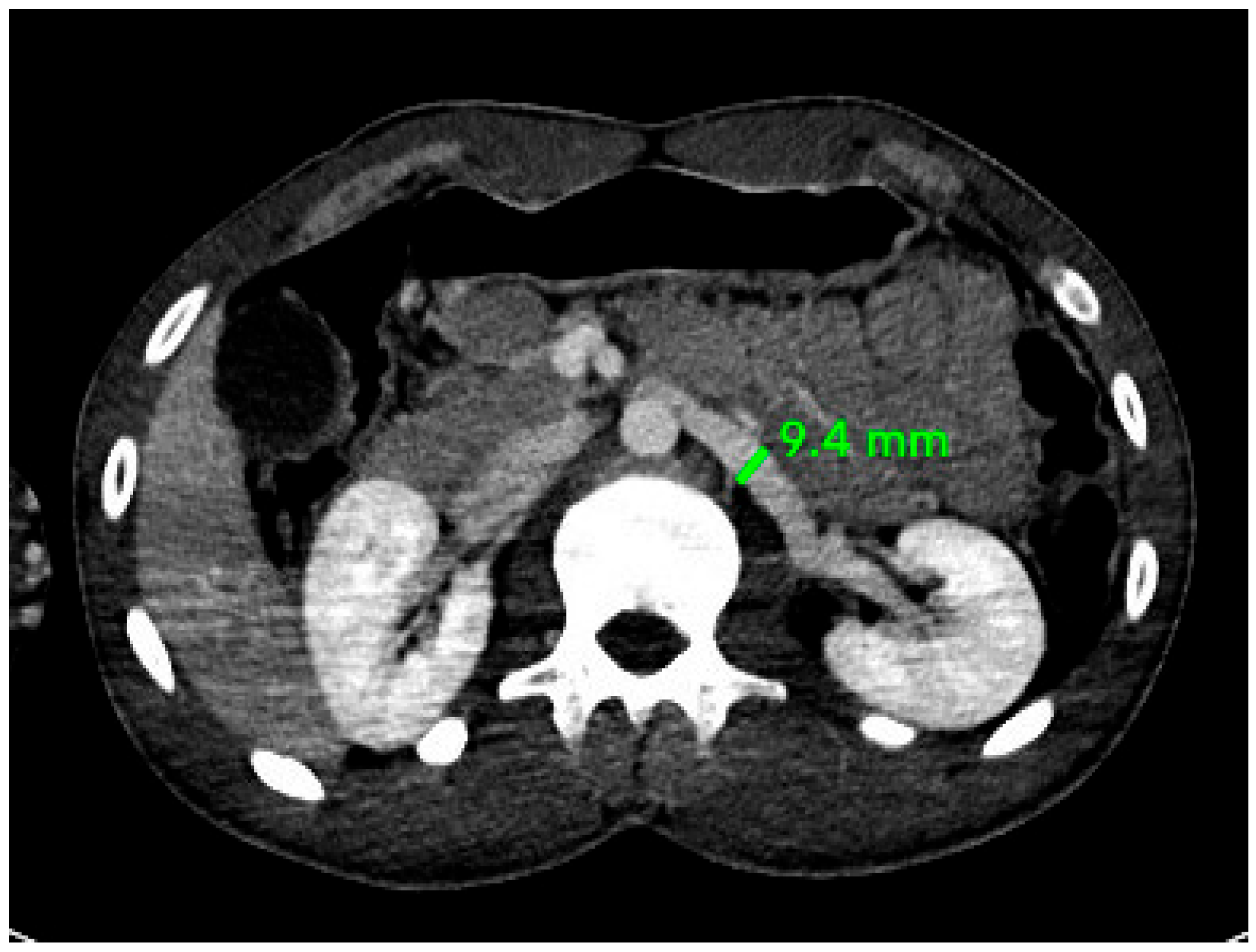
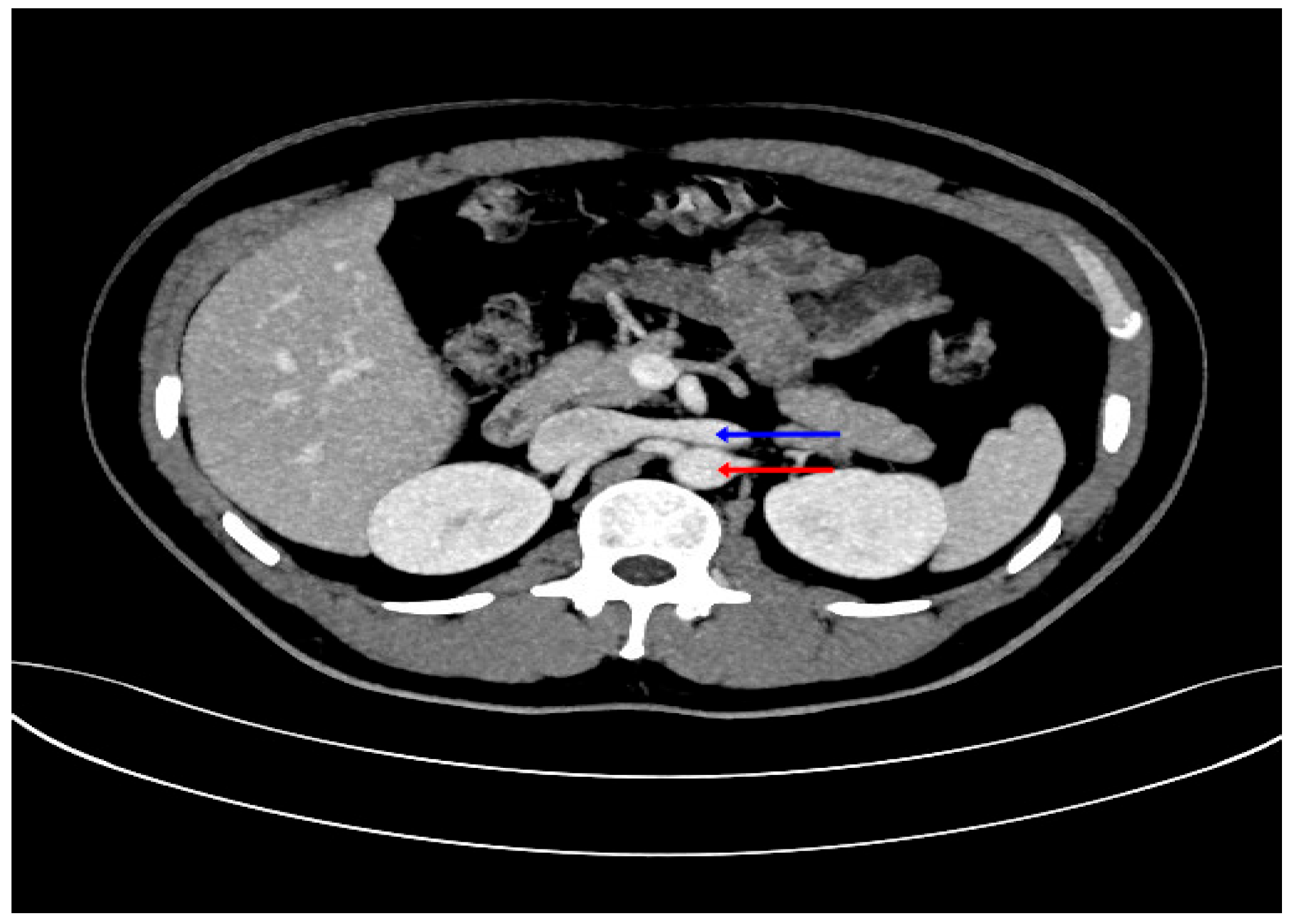
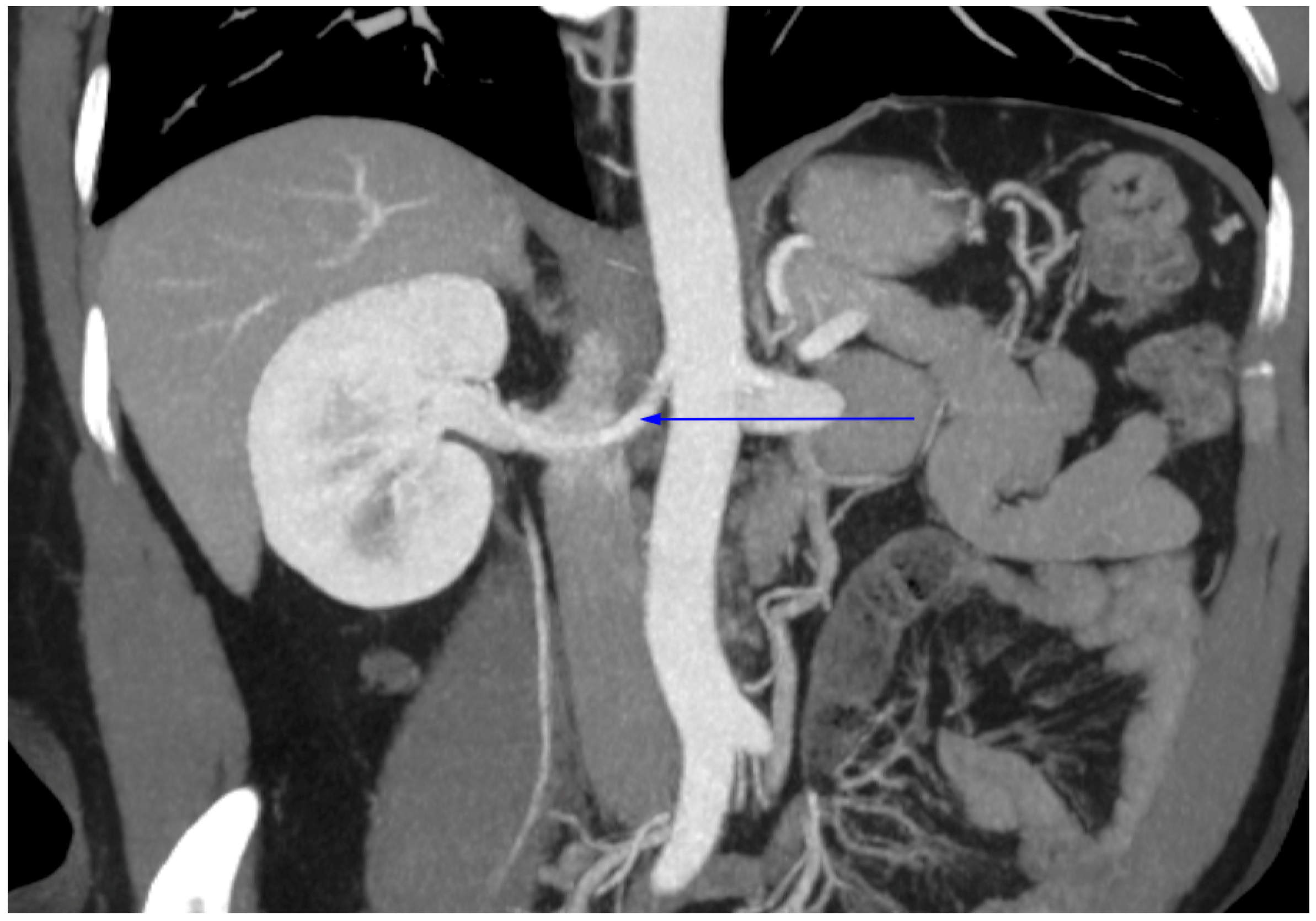
2.3. Radiological Study
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| ESKD | End-Stage Kidney Disease |
| MDCT | Multidetector Computed Tomography |
| NCS | Nutcracker Syndrome |
| RA | Renal Artery |
| RV | Renal Vein |
| SPSS | Statistical Package for Social Sciences |
| SQUH | Sultan Qaboos University Hospital |
| VEGF | Vascular Endothelial Growth Factor |
References
- Stephen, W.L. Anatomy, Abdomen and Pelvis, Renal Artery. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459158/ (accessed on 20 April 2025).
- Cole, S.B. Anatomy, Abdomen and Pelvis, Renal Veins. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538298/ (accessed on 20 April 2025).
- Satyapal, K.S.; Haffejee, A.A.; Singh, B.; Ramsaroop, L.; Robbs, J.V.; Kalideen, J.M. Additional renal arteries incidence and morphometry. Surg. Radiol. Anat. 2001, 23, 33–38. [Google Scholar] [CrossRef]
- Satyapal, K.S.; Kalideen, J.M.; Haffejee, A.A.; Singh, B.; Robbs, J.V. Left renal vein variations. Surg. Radiol. Anat. 1999, 21, 77–81. [Google Scholar] [CrossRef]
- Karayağız, A.H.; Cenal, U.; Ertürk, T.; Özdemir, E.; Polatkan, S.A.V.; Yılmaz, G.; Çakır, Ü.; Berber, İ. Renal arterial and venous system variations in 1073 kidney donors in Turkey. Istanb. Med. J. 2021, 22, 257–260. [Google Scholar] [CrossRef]
- Al Alawi, I.H.; Al Salmi, I.; Al Mawali, A.; Sayer, J.A. Kidney disease in Oman: A view of the current and future landscapes. Iran J. Kidney Dis. 2017, 11, 263–270. [Google Scholar]
- Ramulu, M.V.; Prasanna, L.C. Accessory renal arteries—Anatomical details with surgical perceptions. J. Anat. Soc. India 2016, 65, 162–167. [Google Scholar] [CrossRef]
- Graves, F.T. The aberrant renal artery. J. Anat. 1956, 90, 553–558. [Google Scholar] [PubMed] [PubMed Central]
- Gulas, E.; Wysiadecki, G.; Szymański, J.; Majos, A.; Stefańczyk, L.; Topol, M.; Polguj, M. Morphological and clinical aspects of the occurrence of accessory (multiple) renal arteries. Arch. Med. Sci. 2018, 14, 442–453. [Google Scholar] [CrossRef]
- Regmi, P.R.; Amatya, I.; Kayastha, P.; Paudel, S.; Suwal, S.; Ghimire, R.K. Normal anatomy and variants of renal vasculature with multidetector computed tomography in a tertiary care hospital: A descriptive cross-sectional study. JNMA J. Nepal Med. Assoc. 2020, 58, 911–914. [Google Scholar] [CrossRef]
- Osman, S.; AbdAlla, E.; Ali, L.; MohamedAli, H.; Taha, B.; Elmahdi, T.S.A.; Mohammed, S. Prevalence and patterns of renal vascular variations among potential kidney donors: A computed tomography angiography (CTA) study in Sudan. BMC Nephrol. 2025, 26, 167. [Google Scholar] [CrossRef]
- Aremu, A.; Igbokwe, M.; Olatise, O.; Lawal, A.; Maduadi, K. Anatomical variations of the renal artery: A computerized tomographic angiogram study in living kidney donors at a Nigerian Kidney Transplant Center. Afr. Health Sci. 2021, 21, 1155–1162. [Google Scholar] [CrossRef]
- Hostiuc, S.; Rusu, M.C.; Negoi, I.; Dorobanțu, B.; Grigoriu, M. Anatomical variants of renal veins: A meta-analysis of prevalence. Sci. Rep. 2019, 9, 10802. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Manzoor, A.; Ali, M.; Hassan, N. Analysis of renal artery morphometery in adults: A study conducted by using multidetector computed tomography angiography. Pak. J. Med. Sci. 2017, 33, 943–947. [Google Scholar] [CrossRef]
- Hasan, A.A.; Hassan, S.A.; Mubarak, W.A.; Maklad, S.M. A study of evaluation of renal artery measurements using computed tomography angiography in adult Aswanian population. Aswan Univ. Med. J. 2021, 1, 10–18. [Google Scholar] [CrossRef]
- Abd Elrahim, E. Computed tomography evaluation of renal artery morphometry in adults: The impact of age and gender. Saudi Med. J. 2020, 41, 34–37. [Google Scholar] [CrossRef]
- Majos, M.; Stefańczyk, L.; Szemraj-Rogucka, Z.; Elgalal, M.; De Caro, R.; Macchi, V.; Polguj, M. Does the type of renal artery anatomic variant determine the diameter of the main vessel supplying a kidney? Surg. Radiol. Anat. 2018, 40, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Shaukat, A.; Jamil, M.N. Morphometry of renal veins in a cadaver study. Khyber J. Med. Sci. 2018, 11, 208–211. [Google Scholar]
- Poyraz, A.K.; Firdolas, F.; Onur, M.R.; Kocakoc, E. Evaluation of left renal vein entrapment using multidetector computed tomography. Acta Radiol. 2013, 54, 144–148. [Google Scholar] [CrossRef]
- Satyapal, K.S.; Rambiritch, V.; Pillai, G. Morphometric analysis of the renal veins. Anat. Rec. 1995, 241, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.J.; Petroianu, A.; Silva, L.C.; Andrade, L.M.; Alberti, L.R. Study of arterial pattern of 200 renal pedicle through angiotomography. Rev. Col. Bras. Cir. 2011, 38, 116–121. [Google Scholar] [CrossRef]
- Gupta, M.; Kaul, N.V.; Shukla, A.K. Evaluation of diameter of main hilar renal artery to predict the presence of supplementary renal artery by contrast-enhanced MDCT: A retrospective study in Northern India. J. Clin. Diagn. Res. 2022, 16, AC10–AC13. [Google Scholar] [CrossRef]
- Radiopaedia.Org. Renal Vein. Available online: https://radiopaedia.org/articles/renal-vein-1 (accessed on 8 June 2025).
- Singh, R.P.; Jamal, A. A study of normal renal dimensions at ultrasonography and their influencing factors in an Indian population. Cureus 2023, 15, e40748. [Google Scholar] [CrossRef]
- Moorthy, H.K.; Venugopal, P. Measurement of renal dimensions in vivo: A critical appraisal. Indian J. Urol. 2011, 27, 169–175. [Google Scholar] [CrossRef]
- Tryggestad, J.B.; Thompson, D.M.; Copeland, K.C.; Short, K.R. Sex differences in vascular compliance in normal-weight but not obese boys and girls: The effect of body composition. Int. J. Pediatr. 2012, 2012, 607895. [Google Scholar] [CrossRef]
- Ichii, T.; Morimoto, R.; Okumura, T.; Ishii, H.; Tatami, Y.; Yamamoto, D.; Aoki, S.; Hiraiwa, H.; Furusawa, K.; Kondo, T.; et al. Impact of renal functional/morphological dynamics on the calcification of coronary and abdominal arteries in patients with chronic kidney disease. J. Atheroscler. Thromb. 2017, 24, 1092–1104. [Google Scholar] [CrossRef]
- Mohamed, T.; Sequeira-Lopez, M.L.S. Development of the renal vasculature. Semin. Cell Dev. Biol. 2019, 91, 132–146. [Google Scholar] [CrossRef]
- García-Barrios, A.; Cisneros-Gimeno, A.I.; Celma-Pitarch, A.; Whyte-Orozco, J. Anatomical study about the variations in renal vasculature. Folia Morphol. 2023, 83, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Damaskos, C.; Georgakopoulou, V.E.; Garmpis, N.; Garmpi, A.; Dimitroulis, D. Triple renal arteries in a cadaveric kidney donor: A case report. Cureus 2020, 12, e11639. [Google Scholar] [CrossRef]
- Lv, J.-C.; Zhang, L.-X. Prevalence and disease burden of chronic kidney disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Moreno-Alarcón, C.; Server-Pastor, G.; López-González, P.Á.; López-Cubillana, P.; Ruiz-Morcillo, J.C.; Doñate-Iñíguez, G.; Olarte-Barragán, E.H.; Gómez-Gómez, G.A. Must we still be worried about multiple arteries in kidney transplantation? Nephro-Urol. Mon. 2013, 5, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, V.; Baccarani, U.; Ventin, M.; Pravisani, R.; Puggioni, A.; Zanini, V.; Lorenzin, D.; Vetrugno, L.; Risaliti, A.; Terrosu, G.; et al. Current practice with grafts with multiple renal arteries in kidney transplantation: Role of the methylene blue in the lower pole. Acta Biomed. 2022, 93, e2022006. [Google Scholar] [CrossRef]
- Sevmis, M.; Demir, M.E.; Merhametsiz, O.; Aktas, S.; Sevmis, S.; Uyar, M. Grafts with multiple renal arteries in kidney transplantation. Transplant. Proc. 2021, 53, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela Fuenzalida, J.J.; Vera-Tapia, K.; Urzúa-Márquez, C.; Yáñez-Castillo, J.; Trujillo-Riveros, M.; Koscina, Z.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibañez, A.; Sanchis-Gimeno, J.; et al. Anatomical variants of the renal veins and their relationship with morphofunctional alterations of the kidney: A systematic review and meta-analysis of prevalence. J. Clin. Med. 2024, 13, 3689. [Google Scholar] [CrossRef]
- Nam, J.K.; Park, S.W.; Lee, S.D.; Chung, M.K. The clinical significance of a retroaortic left renal vein. Korean J. Urol. 2010, 51, 276–280. [Google Scholar] [CrossRef]
- Ananthan, K.; Onida, S.; Davies, A.H. Nutcracker syndrome: An update on current diagnostic criteria and management guidelines. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 886–894. [Google Scholar] [CrossRef]
- Çınar, C.; Türkvatan, A. Prevalence of Renal Vascular Variations: Evaluation with MDCT Angiography. Diagn. Interv. Imaging 2016, 97, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Hekimoglu, A.; Ergun, O. Evaluation of Renal Vascular Variations with Computed Tomography. Afr. J. Urol. 2022, 28, 21. [Google Scholar] [CrossRef]
- Sudre, C.H.; Moriconi, S.; Rehwald, R.; Smith, L.; Tillin, T.; Barnes, J.; Atkinson, D.; Ourselin, S.; Chaturvedi, N.; Hughes, A.D.; et al. Accelerated Vascular Aging: Ethnic Differences in Basilar Artery Length and Diameter, and Its Association with Cardiovascular Risk Factors and Cerebral Small Vessel Disease. Front. Cardiovasc. Med. 2022, 9, 939680. [Google Scholar] [CrossRef] [PubMed]
- Nonterah, E.A.; Crowther, N.J.; Klipstein-Grobusch, K.; Oduro, A.R.; Kavousi, M.; Agongo, G.; Anderson, T.J.; Asiki, G.; Boua, P.R.; Choma, S.S.R.; et al. Racial and Ethnic Differences in the Association Between Classical Cardiovascular Risk Factors and Common Carotid Intima-Media Thickness: An Individual Participant Data Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e023704. [Google Scholar] [CrossRef]
- Masuda, E.M.; Caps, M.T.; Singh, N.; Yorita, K.; Schneider, P.A.; Sato, D.T.; Eklof, B.; Nelken, N.A.; Kistner, R.L. Effect of Ethnicity on Access and Device Complications during Endovascular Aneurysm Repair. J. Vasc. Surg. 2004, 40, 24–29. [Google Scholar] [CrossRef][Green Version]


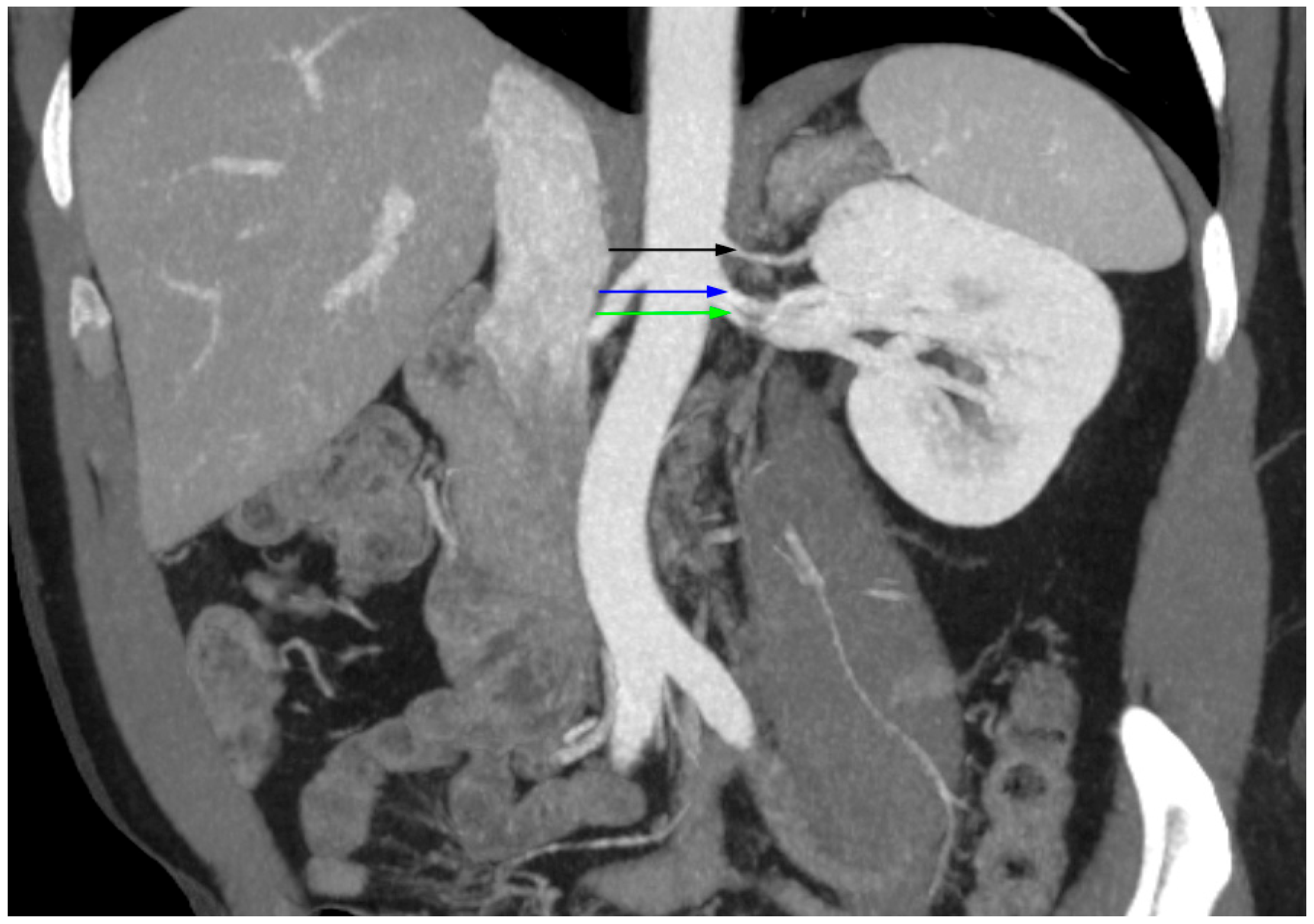
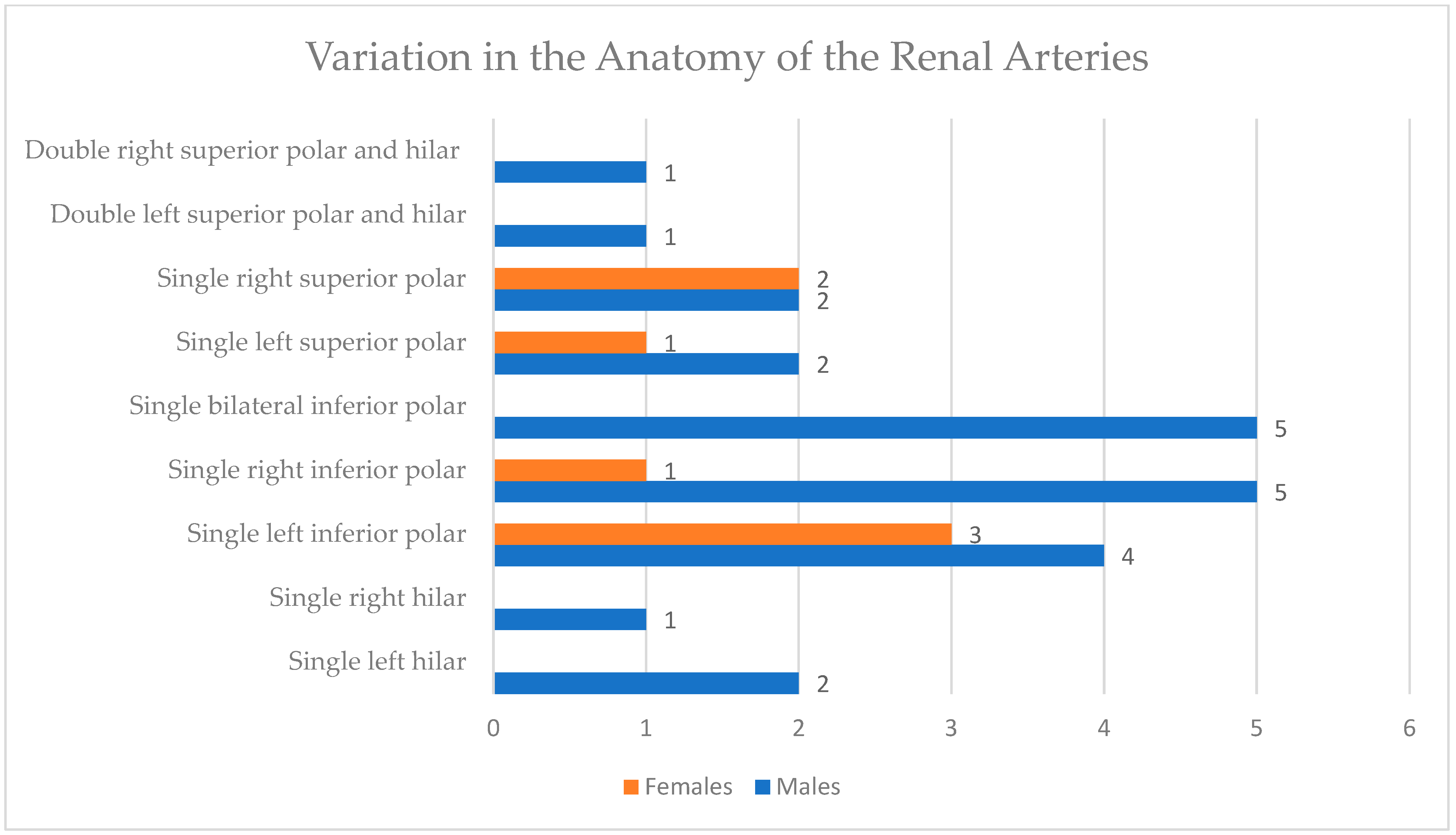
| Males | Females | p-Value | |
|---|---|---|---|
| Right RA | 4.62 ± 0.93 mm | 4.22 ± 0.79 mm | 0.020 |
| Left RA | 5.07 ± 1.04 mm | 4.64 ± 0.76 mm | 0.026 |
| Males | Females | Total | |
|---|---|---|---|
| Single left RV, anterior to aorta | 88 | 32 | 120 |
| Circumaortic with both trunks | 1 | - | 1 |
| Retroaortic | 3 | 4 | 7 |
| Setting | Year | Population (M/F) | Right Renal Artery Diameter (M/F) | Left Renal Artery Diameter (M/F) | Reference |
|---|---|---|---|---|---|
| Karachi, Pakistan | 2017 | 250 (129M, 121F) | 6.66 mm (M), 6.40 mm (F) | 6.79 mm (M), 6.54 mm (F) | [14] |
| Lodz, Poland | 2018 | 248 (126M, 122F) | No significant difference | No significant difference | [17] |
| Taif, Saudi Arabia | 2020 | 50 (34M, 16F) | 5.54 mm (M), 5.19 mm (F) | 5.48 mm (M), 5.29 mm (F) | [16] |
| Aswania, India | 2021 | 106 (53M, 53F) | 4.83 mm (M), 4.58 mm (F) | 5.04 mm (M), 4.79 mm (F) | [15] |
| Muscat, Oman (our study) | 2025 | 128 (92M, 36F) | 4.62 mm (M), 4.22 mm (F) | 5.07 mm (M), 4.64 mm (F) | This study |
| Setting | Year | Population | Right Renal Vein Diameter | Left Renal Vein Diameter | Reference |
|---|---|---|---|---|---|
| South Africa | 1995 | 100 cadavers | 12 ± 2 mm | 12 ± 2 mm | [20] |
| Turkey | 2013 | 1000 patients | 8.8 ± 1.9 mm | 8.9 ± 1.8 mm | [19] |
| Lahore, Pakistan | 2018 | 50 male cadavers | 10.85 mm | 11.57 mm | [18] |
| Oman (our study) | 2025 | 128 patients (92M, 36F) | 9.31 mm (M), 10.46 mm (F) | 9.29 mm (M), 9.40 mm (F) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Lawati, A.; Abduwani, A.; Al Khudhuri, A.; Alhabsi, A.N.; Balushi, K.A.; Das, S.; Baawain, S. Computed Tomography Evaluation of the Renal Blood Vessels in the Omani Population. Appl. Sci. 2025, 15, 9967. https://doi.org/10.3390/app15189967
Al Lawati A, Abduwani A, Al Khudhuri A, Alhabsi AN, Balushi KA, Das S, Baawain S. Computed Tomography Evaluation of the Renal Blood Vessels in the Omani Population. Applied Sciences. 2025; 15(18):9967. https://doi.org/10.3390/app15189967
Chicago/Turabian StyleAl Lawati, Abdullah, Ali Abduwani, Ali Al Khudhuri, Ayman N. Alhabsi, Khalid Al Balushi, Srijit Das, and Saleh Baawain. 2025. "Computed Tomography Evaluation of the Renal Blood Vessels in the Omani Population" Applied Sciences 15, no. 18: 9967. https://doi.org/10.3390/app15189967
APA StyleAl Lawati, A., Abduwani, A., Al Khudhuri, A., Alhabsi, A. N., Balushi, K. A., Das, S., & Baawain, S. (2025). Computed Tomography Evaluation of the Renal Blood Vessels in the Omani Population. Applied Sciences, 15(18), 9967. https://doi.org/10.3390/app15189967






