Abstract
Precast concrete culverts are commonly used as an essential part of the road network of a country. However, frequent site inspections, repair, and replacement of culverts in the remote area require road closure, high cost, and logistical challenges. Thus, a comprehensive understanding of the exposure conditions is essential to manufacture durable reinforced concrete culverts, especially for remote country regions. This paper investigates the influence of exposure conditions on the durability of precast reinforced concrete culverts by inspecting 14 selected culverts showing deterioration in the remote Wheatbelt Region of Western Australia. Soil and water samples collected from the sites were analyzed in the laboratory, and the results were used to assess the level of damage observed in the culverts during site inspection. The results revealed that 85% of the inspected culverts were situated in highly saline soil, and 65% of the deteriorated culverts were exposed to an environment rich in magnesium sulphate and chloride. In total, 9 out of 14 inspected sites were classified as exposure C under AS 5100.5. Overall, the investigation assessed the deterioration of culverts considering the influence of soil and water chemistry, offering a realistic perspective on culvert deteriorations and providing valuable insights for developing sustainable infrastructure in this regional context. The findings establish a baseline for enhancing the corrosion resistance of precast culverts and emphasize the importance of considering high salinity as a critical parameter for durability design.
1. Introduction
Western Australia (WA) is the largest state of Australia in terms of area, comprising 32% of the landmass and surrounded by coastal environments. Concrete structures are exposed to aggressive marine and saline environments. In addition, when the groundwater table rises, dissolved salts such as sodium and magnesium sulphates release ions (Na+, Mg2+, SO42−) into the concrete pores, which may deteriorate concrete structures [1]. Chloride ions enter the concrete matrix along with water from the flowing sources or moisture from the soil. After ingress, it breaks the passive oxide layer on the steel reinforcement. Once the concentration exceeds the threshold value, corrosion is initiated, which leads to cracking, spalling, and load-bearing loss [2]. Similarly, sulphate ions after entering the concrete pores react with hydration products such as tricalcium aluminate and portlandite, forming ettringite and gypsum, which occupy more space than the original compounds. It then develops internal stresses, leading to expansion, cracking, and concrete spalling, ultimately resulting in loss of strength and sacrificed clear cover [3]. Sulphate salts also occur in soil and water. Some fertilizers used in farming contain sulphate, which impacts concrete durability [4,5]. Under combined chloride–sulphate exposure, chloride ions diffuse faster than sulphates and react with aluminates to form Friedel’s salt, a non-expansive chloroaluminate. This reduces the availability of C3A for ettringite formation and suppresses gypsum precipitation, thereby mitigating sulphate-induced expansion and delaying surface cracking [6]. Due to these harsh environments where the structure is exposed, they may deteriorate sooner than their expected design life. Most culverts used in infrastructure projects are precast, usually with a design life of 50 to 100 years. It is noted that there are a range of standards that govern highway assets in Australia, and more specifically precast concrete culverts.
Culverts are structural components that are extensively used in road construction. Culverts are used when the water channel intersects the road and railway networks. They are also used in fauna and pedestrian underpasses. Box culverts are also used in mining locations for air circulation between the mine and the environment [7]. Precast culverts are preferred due to their quick manufacture and ability to achieve the required quality. Cast in situ culverts require a skilled workforce to facilitate the construction. WA has about 149,000 km of state and local road network, and among that, nearly 19,000 km is directly managed by Main Roads Western Australia (MRWA) [8]. Many culverts are in use by MRWA and other organizations in WA. There are nearly 28,000 culverts controlled by MRWA. Out of these, about 89% are precast reinforced concrete culverts, 1% are in situ reinforced concrete culverts, and the rest are made of other materials like plastic, aluminum, steel, masonry, timber, and asbestos [4]. About 26% of precast reinforced concrete culverts are box culverts, which hold 23% of total culverts controlled by MRWA. Figure 1 shows the locations of culverts in Western Australia that come under MRWA management.
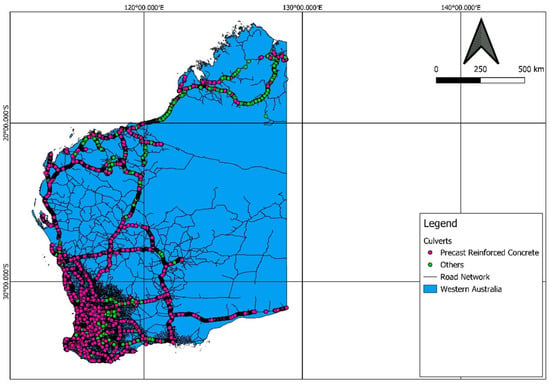
Figure 1.
Culverts in Western Australia controlled by MRWA.
The guidelines for the design, construction, and installation of precast culverts are provided in AS 1597.1 [9], and AS1597.2 [10], which are Australian standards for small (span 1200 and height 1200 mm) and large (span and height > 1200 and up to 4200 mm) precast reinforced concrete box culverts, respectively. MRWA specifications are followed for the construction of culverts on state roads and national highways of WA. The current standards aim to achieve a design service life of 100 years. To achieve a service life of 100 years, the specification and standards give basic guidelines, such as using blended cement, setting limits on wet and dry strength variation in aggregates, and their water absorption. Restriction on admixtures containing chlorides and sulphates and setting limits for acid-soluble chloride and sulphate ion in concrete can also be considered as the steps taken to enhance the performance of precast concrete culverts against deterioration. The defects in precast concrete culverts are classified into various types based on the width of cracks and diameter of surface blowholes, the extensiveness of spalling and corrosion, and other deterioration parameters [11]. This classification aids in the condition assessment of structures and undercover defects, which are then used for repair and maintenance. The Australian culvert standards have taken various loading values, exposure conditions, and concrete specifications from AS 5100.5 [12], the Australian Bridge design standard.
In a state like Western Australia, where in 2024, 92% of the population lived in the southwest region, and 79% of the state’s population was concentrated in the Greater Perth area of the southwest region only [13]. MRWA prescribes detailed visual inspection of culverts in every seven to ten years with recommendations for frequent inspections of high-risk culverts, showing advanced signs of deterioration [11]. Visiting the sites to inspect, repair, or replace culverts frequently exhibits expensive and logistic challenges. Factors like concrete mix design, transportation of precast culverts to the site, installation, and maintenance regime affect the ability of culverts to serve until their designed service life. The environment in which the culverts are exposed to in WA must be understood more thoroughly so that the precast culverts can be manufactured to withstand the prevailing exposure conditions.
This study considered the precast culverts in the Wheatbelt Region of WA facing deterioration issues. Fourteen culverts were inspected, and soil and water samples were collected. Deterioration patterns were recorded and related to environmental conditions. The results provide a baseline that directly links field-measured data with observed deterioration, thereby defining practical exposure conditions and durability requirements for achieving desired service life in saline regions.
2. Methodology
2.1. Sampling Location
MRWA has subdivided WA into eight regions: Wheatbelt, Mid-West, Gascoyne, Southwest, Pilbara, Great Southern, Goldfields–Esperance, Kimberley, and Metropolitan. Among these regions, the Wheatbelt Region has the highest number of culverts, consisting of nearly 22% of the total culverts of WA and almost 18% of the total precast reinforced box concrete culverts of WA [14]. Initially, visual inspection summary data from more than 1400 culverts in the Wheatbelt Region was studied and analyzed. The major defects were reinforcement corrosion, delamination and spalling of concrete, erosion, and scaling of the concrete surface. From that preliminary data, the defect type, severity of the damage, and treatment provided, if any, were studied. MRWA’s detailed visual inspection guidelines for culverts determine the level of severity based on an index ranging from 1 to 5, with 1 meaning no deficiencies and 5 representing a critical condition requiring immediate attention [11].
After studying the preliminary data, 53 deteriorated culverts were selected, and their latest level 1 inspection reports were obtained from MRWA. Based on the severity of deterioration and location, 14 culverts were selected for visual inspection and site sample collection to cover various parts of the Wheatbelt Region. MRWA identifies each culvert uniquely by the road name and straight-line kilometre (SLK). SLK is the distance in kilometres of a point concerning a reference item or initial point of the road [11]. In this study, the 14 culverts have been designated as 1 to 14. The chosen culverts were in different locations in the Wheatbelt Region of WA. Soil samples were collected from all the culvert locations, and water samples were obtained only in culvert 2, as the inspection was conducted in the dry season. The topmost layer of soil was collected as samples, removing debris and organic matter. The soil was collected from the culvert’s inlet, outlet sides, and the silted soil inside the culvert, depending on access to either side and the presence of silted soil inside the culvert. Out of the 14 culverts, 5 had considerable soil siltation within the barrels, 3 showed slight siltation, and 6 were free of any siltation. These samples provide the baseline chemical exposure data required for designing reinforced concrete culverts in saline environments.
2.2. Visual Inspection of Culverts
A visual inspection of each culvert site was conducted to understand if any indications in the surrounding environment could be correlated with the observed deterioration mechanism of the culvert. The units of culverts were observed for defects. The specific purpose of installing the precast box culvert at that location was analyzed, considering the surrounding area and land features. For instance, the culvert placed in a floodway, or a waterway may be subjected to different deterioration mechanisms than a relief culvert. Pictures were taken of the deterioration seen in the culverts and the vicinity of the culvert location. These observations provide reference for the durability requirements that should be considered in designing reinforced concrete culverts for aggressive environments.
2.3. pH and Electrical Conductivity Test on Samples
The pH of soil is a measure of its acidity or alkalinity. Culverts are in prolonged contact with soil. The pH of the soil can give insight into the probable deterioration method occurring in the culvert side with which it is in direct contact. The pH of the soil sample collected was tested as per AS 1289.4.3.1 [15]. The soil was sieved through a 2.36 mm size sieve, and components above this size were removed. Then, 30 g of the remaining soil sample was placed in a beaker, and 75 g of distilled water was added. The soil and distilled water mixture were stirred for 30 min using a magnetic stirrer. The beaker was then covered with a glass lid for at least an hour, and the pH of the soil sample was measured using a pH metre. Multiple readings were taken such that the difference in three consecutive readings was less than 0.05 pH. For water samples, the electrode of the pH metre was dipped into a beaker containing the sample, and the same process was performed to determine the pH.
The electrical conductivity (EC) of soil is the measure of its ability to conduct the electrical current. It can be correlated with the total soluble salt in the soil. High soil EC may indicate high salinity. Higher concentrations of these salts can be detrimental to the culverts. The soil EC was obtained using the soil survey standard test method of the New South Wales government [16]. A 1:5 weight-to-volume ratio of soil to water suspension was prepared using 20 g of soil and 100 mL of deionized water. The soil–water mixture was mixed using a magnetic stirrer for 1 h to dissolve the soluble salt, and the conductivity was measured using a conductivity probe (Figure 2). These pH and EC measurements establish the chemical environment that reinforced concrete culverts must withstand in saline and sulphate-rich soils.
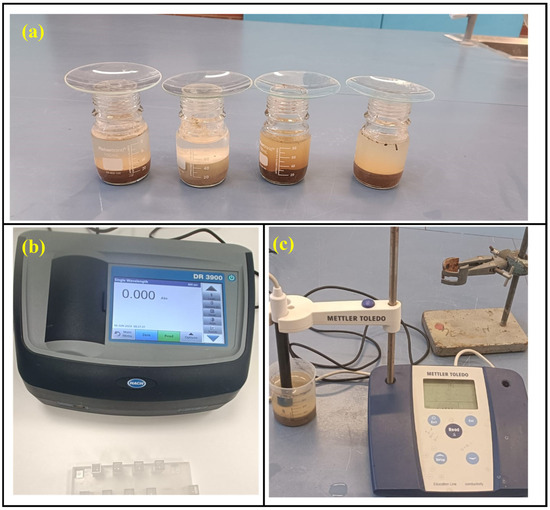
Figure 2.
(a) Soil suspension settling, (b) spectrophotometer, (c) conductivity metre.
2.4. Sulphate (SO42−) Concentration of Samples
The aggressive action of sulphate in the environment is responsible for many destructive and corrosive processes in concrete structures. These deteriorations are primarily seen in marshy and saline soils, and marine environments [17]. Sulphate attack in concrete occurs typically due to the presence of calcium, sodium, and magnesium sulphate.
A Hach spectrophotometer was used to determine the sulphate concentration of soil samples (Figure 2). The soil sample was initially centrifuged in deionized water as per AS 1289.4.2.1 [18] to obtain the sulphate concentration in the soil, which describes the extraction process to determine soluble sulphate concentration in the soil. The soluble sulphate extract was tested as per APHA 4500 SO42− [19]. The buffer solution was prepared by dissolving 30 g of magnesium chloride with 5 g of sodium acetate, 1 g of potassium nitrate and 20 mL of acetic acid. The mixture was made up to 1000 mL. The standard sulphate solution was prepared by dissolving 0.1479 g of anhydrous sodium sulphate into distilled water and diluted up to 1000 mL. It gives 100 ppm of sulphate solution, which was diluted to form standard solutions with a range of 0 ppm to 40 ppm at the interval of 10 ppm to make a calibration chart. The 0 ppm was chosen as a blank solution. A total of 100 mL of standard solutions were prepared, added with 20 mL of buffer solution and barium chloride, stirred, and tested at 420 nm wavelength. Standard solutions with a concentration less than 40 ppm were prepared because at the sulphate concentration above 40 ppm, the barium sulphate loses stability and the accuracy decreases. The filtrated water extract from the soil was kept in a spectrophotometer by mixing with buffer solution and barium chloride, in the same way as for the standard solution. For water extracts containing colour and turbidity even after filtration, the correction was made by running the samples without mixing barium chloride. The water extract was diluted to gain the result between 10 ppm and 40 ppm by testing the result once and diluting again to gain an accurate result if required.
2.5. Magnesium (Mg2+) Concentration in Samples
Knowing the magnesium concentration in the samples (soil and water) is important as magnesium sulphate is the most aggressive compared to other sulphates [5,20].
The magnesium concentration in the sample was obtained using the SHIMADZU AA-6300 atomic absorption spectrophotometer, at 285.2 nm using a hollow cathode lamp. Absorbance at this wavelength was recorded and correlated with concentration. First, magnesium ions are extracted from the soil to find the magnesium concentration of the soil. The neutral normal ammonium acetate extraction method was used, complying with the process mentioned in the Soil Analysis Handbook of Reference Methods [21]. This method uses a neutral salt solution to replace magnesium ions present in soil. An amount of 77.1 g ammonium acetate (NH4CH3CO2) was thoroughly mixed with 900 mL of deionized water. The pH of the solution was maintained at 7. If the pH was higher than 7, a few drops of glacial acetic acid were added to the solution, and if the pH was less than 7, a few drops of ammonium hydroxide were added to make the pH 7. An amount of 10 g of sample soil were taken and mixed with 50 mL of extraction liquid and mixed for 5 min in a reciprocating shaker in 1:5 W/V ratio as in the handbook. The standard preparation and test were performed as mentioned in APHA 3111 B using the direct air–acetylene flame method [22]. A standard was prepared to dissolve 0.1658 g of MgO in a 20 mL of (1 + 1) HNO3. Then, 10 mL of concentrated HNO3 was added and diluted to 1000 mL using deionized water. This gives 100 ppm of solution. This 100 ppm solution was diluted to make 10, 20, 30, and 40 ppm solutions. Lanthanum of 10 mL was added to 100 mL of every standard and sample solution to increase the accuracy by reducing the interference. For finding the magnesium concentration in the water extract, the EPA 3050 [23] method was used, as mentioned in APHA 3111 B [22].
2.6. Chloride (Cl−) Concentration in Samples
The precast reinforced concrete culvert may be exposed to a chloride environment due to soil or water containing chloride ions. A HACH spectrophotometer was used to find the chloride concentration of soil and water samples. The EPA 325.2 method using mercuric thiocyanate was used to find chloride concentration. This similar method is also enlisted in the method 8113 reagent solution in the guidelines of HACH. To start with the process, a mercuric thiocyanate solution was prepared by mixing 4.17 g of mercuric thiocyanate in 500 mL of methanol and diluting it into 1.0 L. Subsequently, the ferric nitric solution was prepared by dissolving 202 g of ferric nitrate in 500 mL of deionized water. Then, 31.5 mL of concentrated nitric acid was added, and the mix was diluted to 1.0 L. The colouring reagents were prepared by mixing 150 mL of mercuric thiocyanate and ferric nitrate solution and then diluting to 1.0 L of deionized water. After that, stock solutions were prepared by dissolving 0.8241 g of pre-dried (140 °C) NaCl in distilled water for 1.0 L. It gives a solution of 500 ppm chloride. The solution was subsequently diluted to form standard solutions of 20 ppm, 40 ppm, 75 ppm, 100 ppm, 150 ppm, and 200 ppm. A zero ppm of solution was prepared using deionized water. The 100 mL standard solution of zero ppm was mixed with 10 mL of colour reagent, and the absorbance at 480 nm was set to zero. Similarly, 100 mL of other standard solutions were also mixed with 10 mL of colour reagent, and the absorbance was noted to make a calibration chart. Then, the soil was mixed with deionized water to obtain the chloride extract. The leaching of chloride ions from soil were performed complying with the sample preparation method described in TEX-620-J [24]. The extract was filtrated to remove suspended particles, if any. Then, 100 mL of sample extract was mixed with 10 mL of colour reagents, and the cuvette was kept in the spectrophotometer. The extract was diluted based on the initial result to obtain the ppm within the range of 200 ppm for accurate results.
3. Results and Discussion
3.1. Visual Inspection
A careful assessment of the culvert unit and the surrounding environment was conducted during the visual inspection, complying with MRWA guidelines [11]. It was conducted to detect and quantify the damage based on the severity rating and to assess viable culvert sites for further special investigations. The environment the culvert was exposed to was also observed so that the likely cause of the damage could be investigated. The key findings of the visual inspection included the presence of relief culverts, which may experience greater permeability issues compared to waterway or floodway culverts, as well as the presence of salt-tolerant plants and construction defects such as clear cover and rounded aggregates, which will be discussed in detail.
The visual inspection summary is shown in Table 1. Among the culvert sites visited, many culverts were built either in a waterway or as relief culverts (Figure 3). Waterway culverts direct floodwaters and prevent erosion, but accumulation of debris at inlets was observed, which can create hydraulic blockage (Figure 3) [25]. Waterway culverts can face the problem of severe erosion, as the flow velocity of water may be comparatively higher than in the relief culverts. Relief culverts are the culverts that prevent the accumulation of water in roadways and railways. They stay in contact with water for a long time as the logged water needs to dry off. Therefore, the permeability of the relief culverts should be given more focus for long-term durability.

Table 1.
Summary of visual inspection findings for culverts.

Figure 3.
(a) Accumulation of debris and wood logs at the inlet of the waterway culvert, (b–d) potential waterlogging areas around the relief culvert.
The presence of salt-tolerant plant beaded glasswort (Sarcocornia quinqueflora), also known as samphire, was seen near some culvert sites (3, 4, 11, 12, 13, and 14). This is a significant environmental indicator (Figure 4 and Figure 5). They are halophyte plants, i.e., plants that can grow in highly saline soil or water, salt marshes, and brackish water [26,27]. These plants can grow in areas where the salt concentration is detrimental to other plants. The Queensland government has kept this plant as a wetland indicator species [28]. As these plants grow in highly saline soil and waterlogged conditions, it is understood that these culverts are in highly saline areas, and there is a higher probability of water accumulation.

Figure 4.
Salt tolerant plant (beaded glasswort) in the vicinity of culvert sites: (a,d) site 4, (b) site 14, (c) site 13, and (e) site 3.

Figure 5.
(a) Salt leaching out in culvert vicinity, (b,c) salt tolerant plant (beaded glasswort) in the vicinity of culvert site.
From the inspection, it was observed that 3 precast culverts (7, 8 and 10) needed more clear cover (Figure 6). The minimum clear cover required as per AS 1597.1 [9] and AS 1597.2 [10] for 50 MPa concrete at exposure class B1 is 25 mm, which increases to 50 mm for exposure class C. The lack of clear cover indicates the risk of early corrosion initiation time in concrete. Rasheeduzzafar et al. [29] reported that a 50 mm clear cover is twelve and six times more protective than 12.5 mm and 19 mm clear cover, respectively, in concrete with a water–cement ratio of 0.45. In contrast, at the water–cement ratio of 0.65, a 50 mm clear cover is considered six and three times more protective than a 12.5 mm and 19 mm clear cover, respectively [12]. Similarly, circular-shaped aggregates were observed inside the culvert unit in Culvert 1 (Figure 6). Angular aggregates improve the interlocking property of concrete and produce more compressive strength. The rough texture and irregular shapes of angular aggregates result in better bond strength. Due to the increase in bond strength, there is usually less erosion. Therefore, as shown in Figure 6, round-shaped aggregates may also be one reason for surface erosion.

Figure 6.
(a) Low clear cover and (b) round-shaped aggregates observed in some cases.
The deterioration issues seen on site included cracking, delamination, spalling, and exposed reinforcement in the culvert barrel (Figure 7 and Figure 8). Corrosion of reinforcement was also observed in the deteriorated culverts. There were minor issues of erosion, a process in which the concrete surface of culverts is gradually worn away by action of flowing fluids and action of waterborne abrasives as per detailed visual culvert inspection guidelines from MRWA [11]. Due to the water velocity and debris present in the water, the cover concrete starts peeling, initiating aggregate plucking underneath [30]. Australian Bridge design standard AS 5100.5 [12] emphasizes increasing the compressive strength required to resist erosion. The investigation conducted by Atis [31] also emphasizes the significance of compressive strength to improve the erosion resistance concrete, mentioning that concrete curing conditions did not show significant influence in increasing the property. However, Liu et al. [32] reported erosion as a surface property of concrete, and surface treatments, curing methods, and finishing techniques influence it. It even mentions strength as a poor parameter to determine the quality of concrete against erosive force. During steam curing of the precast culvert, its surface is exposed to high temperatures, making the culvert leg more vulnerable to erosion due to potential heat damage to the concrete surface. Shi et al. [33] reported higher surface permeability and coarser pores on the concrete surface of steam cured concrete. These coarser pores can contribute to removing surface concrete under erosive forces.

Figure 7.
(a,b) Corrosion of reinforcing steel and salt mark observed in the culvert units.

Figure 8.
(a,b) Delamination observed in culvert unit.
To understand the probable reason behind delamination, spalling, and corrosion in culverts, soil samples were taken for investigation from all culvert sites, and water samples were taken as per availability. The samples were tested to determine pH, conductivity, and concentration of Cl−, SO42−, and Mg2+ ions. This analysis aimed to identify the causes of the defects and their correlation with the observed effects. The findings highlight the influence of saline environments, inadequate cover, and aggregate quality, providing baseline requirements for reinforced concrete culverts designed for aggressive exposures.
3.2. pH and Electrical Conductivity (EC)
As the culvert structures are in contact with the soil, the pH and EC of soil will help to understand the possible cause of deterioration of the culverts. Notably, the five culverts with considerable soil siltation offer more concrete insight on defects compared to those with minor or no siltation. The pH of the soil gives information about the soil’s acidity, neutrality, or alkalinity. Chemical degradation of cement is mainly due to decalcification and the dissolution and leaching of cement components. Soil pH may influence this process, as prolonged contact allows exchange between the pore solution of concrete and the surrounding soil environment. The changes in the pH of concrete are one of the primary reasons behind this chemical degradation. Jacques et al. [34] outlined four stages of cement degradation based on pH, wherein first, the cement pore water had a pH higher than 12.5. Second, the pH was 12.5, controlled by the dissolution of calcium hydroxide. Third, after the leaching of all calcium hydroxide, pH drops from 12.5 to 10, and pore water composition was altered by calcium silicate hydrate (C-S-H) and another cement phase. In the last stage, the pH is less than 10, when the pore water composition is mainly influenced by the composition of intruding water [34].
Similarly, salinity is measured through electrical conductivity. The department of water and environmental regulation estimates every stream and river in the southwest part of WA is to be affected by salinity [35]. Highly conductive soil may be detrimental due to more salts that can act as aggressive agents to deteriorate concrete. In this way, the EC of soil can be related to the speed of corrosion in concrete structures. The department of primary industries and regional development recognizes electrical conductivity as the most common measure of soil salinity. They have divided soil into 6 different salinity classes based on the electrical conductivity, as shown in Table 2.

Table 2.
Salinity classes in EC1:5 for sandy soil texture as per government of WA [36].
The pH and EC of the collected samples from the inspected culvert sites are given in Table 3.

Table 3.
pH and EC of samples at culvert locations.
The pH and EC of the water sample collected at culvert 2 were 6.51 and, 8660 ms/m, respectively. From the pH and EC results of the collected soil samples, it was observed that the pH of the soil in majority of the culvert sites was alkaline in nature. Caritat et al. [37] reported similar results from the national survey they conducted to find the pH of Australian soils. Soil samples from culverts 7, 10, and 14 were mildly acidic (Table 2), but it is not significantly high to cause harm to the culvert.
As per this classification, seven out of the fourteen visited sites fall in the extremely saline range, three in the severely saline and one in the highly saline category. The salt encroachment in the Wheatbelt region of WA is likely a result of valley water logging, from a shallow water table, only 1.5–2 m from the ground surface. The salinity of underground water here varies from 17,000 ppm to 68,000 ppm [38]. High salinity may be due to salt seepage in the ground or due to dry land salt. Halse et al. [39] mentioned a 20-fold increase in the salinity trend in the southwest region of WA. WA consists of nearly 14 million hectares of salt marshes, salt lakes, and salt flats. The precast culvert near that vicinity will be affected severely by the extreme salinity. George et al. [40] reported that sodium chloride is the most common salt deposited in the coastal vicinity of WA. Though Wheatbelt is not in the coastal vicinity, the widespread land clearing of native vegetation for food (cropping and livestock) has resulted in rising groundwater levels and salinisation [41,42,43,44]. The pH and EC results confirm that many culverts are exposed to highly saline and alkaline soils, providing baseline conditions for reinforced concrete culverts designed for aggressive environments.
3.3. Sulphate Ion (SO42−) Concentration
Sulphate ions can cause deterioration of concrete culverts by destabilizing the cement paste and creating an expansive effect [45]. In the culverts, the sulphate ions can penetrate through exposure to the soil adjacent to concrete structures, fertilizers used in the soil, and water flowing in the culverts. In the concrete matrix, the hydration of alite and belite compounds in cement is mainly responsible for developing the initial and later strengths in concrete by forming calcium silicate hydrate C-S-H gel. The third component of cement, aluminate, helps with the flash-setting of concrete. Gypsum is also present in a binder, which reacts with aluminate to form long needle-shaped crystals called ettringite, responsible for cracking hardened cement or concrete, as explained in Equation (1) [46]:
3CaO·Al2O3 + 3CaSO4 + 32 H2O → 3CaO·Al2O3·3CaSO4·32H2O
(aluminate) (gypsum) (ettringite)
(aluminate) (gypsum) (ettringite)
Ettringite is an unstable material, and after complete depletion of gypsum, the internally formed gypsum converts to mono-sulphate aluminate hydrate, which is twice as light as ettringite and surrounds the cement paste, preventing the reformation of ettringite. However, there is also the ingress of sulphate ions from the external environment due to concrete exposure to a sulphate-containing environment. Then, the mono-sulphate aluminate hydrates absorb sulphate from the external environment and release calcium ions, reconverting themselves to gypsum and ettringite [47]. The size of ettringite formed is 2.5 times larger than mono-sulphate aluminate hydrate, leading to an expansion effect.
The results of sulphate concentrations in the collected soil samples are shown in Table 4. The sulphate concentration of soil determines the exposure classification, which represents the severity of the environment the culvert is exposed to. As per AS 5100.5 [12], sulphate concentration in soil less than 1000 ppm is classified as exposure classification B1, 1000–3000 ppm falls in classification B2, 3001–20,000 ppm in exposure classification C1, and more than 20,000 ppm in C2. The soil samples were highly permeable sandy soils. For the water sample, sulphate concentrations of more than 1500 ppm and less than 10,001 ppm lie in exposure classification C1. The sulphate concentration of the water sample from culvert 2 was measured at 6500 ppm. Testing sulphate concentration of the soil sample in culvert site 2, it is exposed to the environmental classification of B2 while testing the water sample, it lies in classification C1. The later classification is considered, as the standard dictates, that if a site exhibits two different exposure classifications based on separate tests, the more severe classification should be considered. The sulphate concentration of water was greater than the sulphate concentration of soil. It can be attributed to the rise in the groundwater table. In the Wheatbelt Region affected by dry land salinity, the rising water table during the rainy season brings the salt closer to the surface. This salt leaches into the surface water bodies or shallow aquifers and remains in the surface soil when the water evaporates. As the water evaporates, the salts are left behind and washed away by subsequent rainfall.

Table 4.
Concentrations of SO42−, Mg2+, and Cl− ions in the soil samples of culvert sites.
The high concentration of sulphate and alkaline soil restricts the risk of acid sulphate soil, which was expected to occur in the culvert sites of WA. Government documents have reported that some soil areas of WA, including the Wheatbelt, have acid sulphate soils. However, this study did not observe an acid sulphate soil scenario in the culvert areas that were visited. The high alkalinity of the soils and the presence of beaded glasswort, a species unable to survive in acidic conditions, support this observation, which can be attributed to the dominance of alkaline salts.
3.4. Magnesium Ion (Mg2+) Concentration
The aggressiveness of sulphate attack depends strongly on the cation with which sulphate is associated. Calcium sulphate is the least aggressive, sodium sulphate is moderately aggressive, while magnesium sulphate is the most destructive. It penetrates concrete and reacts with calcium hydroxide (portlandite) to form calcium sulphate (gypsum) and magnesium hydroxide (brucite), as explained in Equations (2) and (3) [48]:
Ca(OH)2 + MgSO4 + 2H2O → CaSO4·2H2O + Mg(OH)2
3CaO·2SiO2.3H2O + 3MgSO4 + 2H2O → 3MgO·2SiO2·3H2O + 3CaSO4·2H2O.
Calcium hydroxide is a hard hexagonal material in concrete, and the formation of gypsum from calcium hydroxide causes some strength loss in concrete. Brucite is insoluble. The brucite layer, which forms at the surface, acts like a protective cover for some time, disrupting further sulphate penetration, but it will tend to remove calcium hydroxide continuously. It keeps promoting the forward reaction in the dissolution of calcium hydroxide. The dissolution can be up to the extent that completely removing calcium hydroxide may occur in the concrete matrix. More dissolution of calcium hydroxide causes more reduction in the pH of concrete. Due to the reduction in pH, the C-S-H gel starts to decalcify. This decalcifying of C-S-H is because calcium leeches out from the gel in a low pH environment to compensate for the pH loss and maintains the alkalinity of the concrete matrix. So, decalcification of C-S-H leads to the formation of silica hydrate, which does not have a binding capacity like C-S-H (Equation (3)). Then, magnesium hydroxide reacts with silicate hydrates, which results in progressive loss of stability of C-S-H and forms magnesium silicate hydrate (M-S-H) [5,17,49,50].
The magnesium concentration results of the collected soil samples are given in Table 3. When the magnesium ions in the soil exceed 1000 ppm along with 1000 ppm of sulphate ions in soil or 400 ppm of sulphate ions in water, then AS 5100.5 [12] recommends that the aggressivity classification of one higher class be adopted along with the other additional protective measures. Therefore, one higher exposure classification was adopted in culvert sites having a magnesium concentration of more than 1000 ppm. The magnesium concentration of the water sample from culvert 2 was obtained as 3060 ppm. The magnesium concentration in culvert site 7 is less. That site is mildly acidic based on the pH test, and the EC of the soil and the sulphate concentration of the soil in that culvert site is also lower compared to other sites. The high concentration of magnesium ions in the culvert sites indicates the risk of magnesium sulphate attack on the precast culvert. It may be linked with the spalling of concrete and scaling while magnesium sulphate decalcifies the calcium ion in the C-S-H gel; the sulphate attack can complement the erosion effect occurring in the culvert because magnesium sulphate weakens the bond in the surface of the concrete and sodium sulphate can result in a physical salt attack, which aids the occurrence of surface erosion in concrete even on less water velocity and small debris accumulation.
3.5. Chloride Ion (Cl−) Concentration
The major problem seen in culverts from the data from MRWA and field inspections is corrosion, particularly due to chloride ingress in high saline environments. Therefore, the chloride concentrations in the soil and water of the culvert sites were determined. Chloride ions in the environment (soil, water, airborne chlorides) can enter the concrete matrix by diffusion, capillary action, and hydrostatic pressure.
The reinforcement in the concrete matrix is protected by the highly alkaline passivity layer of oxide film [51]. These chloride ions break the passivity layer of concrete, triggering reinforcement corrosion by forming an electrochemical cell. The rebars used in reinforced concrete come from iron ore, and everything naturally tends to return to its ground energy state or basic energy level. Therefore, the rebar also tends to return to its natural stable of iron oxide in a suitable environment. For that, it starts corroding. Corrosion is an electrochemical process making a galvanic cell, where the steel reinforcement behaves like both anode and cathode due to differences in chloride concentration. Steel dissolves at the anode, creating a supply of electrons, which are consumed at the cathode (Equations (4)–(8)). Rust will form at the anode. The volume of corrosion products will be greater than the volume of iron consumed, leading to the creation of internal expansion stress. When the stress formed is greater than the concrete strength, cracking and spalling occur [52].
Anodic reaction:
Fe → Fe2+ + 2e−
Cathodic reaction:
2e− + H2O + 1/2O2 →2OH−
Formation of Iron (II) Hydroxide:
Fe2+ + 2OH− → Fe (OH)2
Formation of Iron (III) hydroxide by oxidization of Iron (II) hydroxide:
4Fe (OH)2 + O2 + 2H2O → 4Fe (OH)3
Dehydration of Iron (III) Hydroxide to Iron (II) oxide-Hydroxide (rust):
2Fe (OH)3 → Fe2O3·H2O + 2H2O
The chloride concentration results of the soil samples are given in Table 3. The chloride concentration in the water sample of culvert site 2 is 83,700 ppm, which is more than four times the seawater chloride content. For literature comparison, the chloride concentration of seawater is generally taken as 19,000–19,500 ppm [53,54]. Figure 9 shows evaporated salt deposited in silted soil inside the culverts. It is an indication of moisture evaporation, leaving crystalline salt deposits. High EC, sulphate, and chloride content in culverts are likely due to the presence of high salt in the soil, as shown in Figure 9. Pasupathy et al. [1] reported chloride concentrations of 150,080 ppm and 468,390 ppm in lake water in WA, where a precast culvert was placed. The chloride concentrations in other soil samples were also high. The sulphate and chloride concentrations of water were much higher than that of soil in culvert site 2. All the culverts would be facing numerous wet and dry cycles that might accelerate the deterioration process. Due to water logging, relief culverts will typically remain in contact with saline water for a long time.

Figure 9.
(a) Scaling and corrosion stain in culvert 2, (b–d) evaporated salt deposits on silted soil inside the culverts 3, 13, and 14.
The combination of visual observations and the chemistry of surrounding soil and water indicates that high salinity significantly contributes to the deterioration of culverts in the Wheatbelt region. Delamination and spalling of the concrete are likely to be caused by corrosion of the reinforcement, external sulphate attack, or both. Sulphate ions react with hydration products inside the concrete matrix to form expansive ettringite, leading to tensile cracks in the concrete. Similarly, the rust formed after steel corrosion will have a higher volume than the reinforcement, which induces tensile cracks in the concrete. These cracks aid the entry of aggressive ions to accelerate the deterioration. High EC further escalates this ionic process, increasing the rate of deterioration, ultimately resulting in delamination and eventually spalling of the concrete. The corrosion here may likely be chloride-induced, as salt-tolerant plants and high soil and water chloride concentrations were seen in visual inspection. The primary ways to prevent these mechanisms are to reduce the permeability of concrete matrix, increase the concrete cover, or both. However, during steam curing of the precast concrete, unevenly distributed and coarse hydration products may form, leading to higher permeability and reduced resistance to ion ingress [55]. The adverse effects in concrete properties due to steam curing are termed ‘heat damage’. Therefore, to increase the durability of precast culverts in WA, the heat damage effect in culverts may be reduced, making the culvert less porous so the sulphate and chloride ions’ penetration to the concrete matrix is reduced. It can be achieved by optimizing the steam curing regime, providing subsequent curing, using multiple transmission media to reduce heat damage, and/or using mineral additives.
4. Conclusions
The exposure condition and factors contributing to the durability failures of the precast concrete box culverts were investigated by visual inspection of 14 deteriorated precast culvert sites in various locations within the Wheatbelt region of WA. Soil and water samples were collected from the sites and pH, electrical conductivity, and the chloride, sulphate, and magnesium concentrations were determined from laboratory tests. The following conclusions are drawn from the findings.
- The soil samples from the majority of the inspected culvert sites were alkaline in nature, while few were mildly acidic.
- The electrical conductivity of soil and water was observed to be very high in most of the culvert sites, with 12 out of 14 inspected culverts to be in highly saline areas.
- A significant concentration of sulphate ions was observed in the soil of the culvert site vicinity, with the highest value reaching up to 10,000 ppm.
- There was also a significant presence of magnesium ions, which increased the likelihood of a magnesium sulphate attack. The exposure classification of one class higher should be adopted to incorporate the risk of magnesium sulphate attack where magnesium concentration is more than 1000 ppm in soil.
- The chloride concentration in most of the culvert site soil samples was very high. The chloride concentration in the one water sample was 83,700 ppm, which is more than four times the chloride concentration in seawater.
- The reinforcement corrosion, delamination, and concrete spalling damage observed in the visual inspections can likely be attributed to simultaneous presence of high concentrations of sulphate, magnesium, and chloride ions. Poor quality concrete with low cover accelerates the time for deterioration to occur.
- Thus, considerations of the high sulphate and chloride concentrations in the site soil and water are recommended as important durability design parameters of precast concrete culverts to be used in the remote country regions.
5. Direction of Future Study
A detailed inspection is proposed to be conducted at selective culvert sites, where non-destructive tests such as electrical resistivity and corrosion rate measurements will be carried out as in accordance with ASTM C876 [56], concrete cores will be extracted for laboratory testing, and water samples will be collected for analysis. The carbonation depth will be measured as per WA 620.1 [57], and chloride profile will be determined in accordance with ASTM C1552 [58] to confirm the reasons for corrosion. The sulphate ions concentration in concrete can be measured as per AS 1012.20.1 [59]. Since the chloride, sulphate, and magnesium contents in the site soils were found to be very high, most of the visited sites were classified as exposure C in accordance with the Australian standard AS 5100.5 [12] and with highly saline soil as per the electrical conductivity results. The following research phase will focus on developing reinforced concrete culverts designed and tested under these extreme environmental conditions. Reducing the permeability of steam-cured concrete through optimized curing regimes, use of supplementary cementitious materials, and advanced mix design will be a key strategy to extend the durability and service life of precast culverts in aggressive saline environment of Western Australia.
Author Contributions
Conceptualization, S.H. and P.K.S.; methodology, S.H. and P.K.S.; validation, S.H. and P.K.S.; formal analysis, S.H.; investigation, S.H.; resources, P.K.S. and F.U.A.S.; data curation, S.H.; writing—original draft preparation, S.H.; writing—review and editing, S.H., P.K.S. and F.U.A.S.; visualization, S.H.; supervision, P.K.S. and F.U.A.S.; project administration, P.K.S. and F.U.A.S.; funding acquisition, P.K.S. and F.U.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SmartCrete CRC, grant number (22.PP.0150).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors gratefully acknowledge the support of CRC SmartCrete, BG&E Pty Limited and Main Roads Western Australia to conduct this study.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| AS | Standards Australia |
| APHA | American Public Health Association (Standard Methods) |
| AAS | Atomic absorption spectrophotometer |
| Cl− | Chloride ion |
| SO42− | Sulphate ion |
| Mg2+ | Magnesium ion |
| EC (or EC1:5) | Electrical conductivity of 1:5 soil: water extract |
| MRWA | Main Roads Western Australia |
| ppm | Parts per million |
| SCM | Supplementary cementitious material |
| SLK | Straight-line kilometre (MRWA road location identifier) |
Glossary
Magnesium (Mg2+). The divalent magnesium cation. Natural soils do not contain magnesium in pure form; magnesium occurs as Mg2+ in solution or bound in mineral/salt phases (e.g., MgSO4·nH2O) (n = 1…11).
3CaO·2SiO2·3H2O (C-S-H). Calcium silicate hydrate, the main binding phase in hydrated cement paste. Written here with a representative stoichiometric formula.
3MgO·2SiO2·3H2O (M-S-H). Magnesium silicate hydrate, weak binding gel formed by decalcification of C–S–H in the presence of Mg2+.
CaSO4·2H2O (Gypsum). Calcium sulphate dihydrate, formed during sulphate attack.
Fe(OH)2 (Iron (II) hydroxide, ferrous hydroxide). Precipitate formed during the early stages of steel corrosion.
Fe(OH)3 (Iron (III) hydroxide, ferric hydroxide). Oxidized form of Fe (OH)2 precursor of rust.
Fe2O3·H2O (Hydrated Iron (III) oxide, rust). Dehydration product of Fe (OH)3, expansive corrosion product that causes cracking and spalling.
References
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Han, S.-H. Influence of diffusion coefficient on chloride ion penetration of concrete structure. Constr. Build. Mater. 2007, 21, 370–378. [Google Scholar] [CrossRef]
- Wang, C.; Fang, Z.; Zhuang, X.; Chen, Q.; Han, K.; Zhou, S. External sulfate attack on cement-based materials in underground structures with the stray current. Constr. Build. Mater. 2025, 465, 140284. [Google Scholar] [CrossRef]
- Assaad Abdelmseeh, V.; Jofriet, J.; Hayward, G. Sulphate and sulphide corrosion in livestock buildings, Part I: Concrete deterioration. Biosyst. Eng. 2008, 99, 372–381. [Google Scholar] [CrossRef]
- Neville, A. Confused World Sulfate Attack concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G.; Wang, N.; Wei, Y.; Chen, Z.; Li, J.; Chen, B. Effect of chlorides on the deterioration of mechanical properties and microstructural evolution of cement-based materials subjected to sulphate attack. Case Stud. Constr. Mater. 2025, 22, e04235. [Google Scholar] [CrossRef]
- Tarigan, J.; Nursyamsi, N.; Farhan, M. Precast box culvert analysis with direct load. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1122, 012020. [Google Scholar] [CrossRef]
- MRWA. Road Facts Summary Sheet. 2024. Available online: https://annualreports.mainroads.wa.gov.au/AR-2024/downloads-and-appendices/road-facts-summary-sheet.html (accessed on 31 January 2025).
- AS 1597.1-2010; Precast Reinforced Concrete Box Culverts, Part 1: Small Culverts (Not Exceeding 1200 mm Span and 1200 mm Height). Standards Australia: Sydney, Australia, 2010.
- AS 1597.2-2013; Precast Reinforced Concrete Box Culverts, Part 2: Large Culverts (Exceeding 1200 mm Span or 1200 mm Height and Up to and Including 4200 mm Span and 4200 mm Height). Standards Australia: Sydney, Australia, 2013.
- MRWA. Detailed Visual Culvert Inspection Guidelines (Level 2 Inspections); MRWA: East Perth, Australia, 2012. [Google Scholar]
- AS 5100.5:2017; Bridge Design Part 5: Concrete. Standards Australia: Sydney, Australia, 2017.
- Australian Bureau of Statistics. Greater Perth. 2021 Census All Persons QuickStats. 2021. Available online: https://abs.gov.au/census/find-census-data/quickstats/2021/5GPER (accessed on 20 June 2024).
- MRWA. Culverts. 2021. Available online: https://portal-mainroads.opendata.arcgis.com/datasets/a5931669bdc643e4848c3e428226a1be_2/about (accessed on 17 May 2024).
- AS 1289.4.3.1:2021; Methods of Testing Soils for Engineering Purposes Soil Chemical Tests—Determination of the pH Value of a Soil—Electrometric Method. Standards Australia: Sydney, Australia, 2021.
- Department of Sustainable Natural Resource. Soil Survey Standard Test Method Electrical Conductivity; NSW Government: Sydney, Australia.
- Bonen, D.; Cohen, M.D. Magnesium sulfate attack on portland cement paste-I. Microstructural analysis. Cem. Concr. Res. 1992, 22, 169–180. [Google Scholar] [CrossRef]
- AS 1289.4.2.1; Methods of Testing Soils for Engineering Purposes Method 4.2.1: Soil Chemical Tests—Determination of the Sulfate Content of a Natural Soil and the Sulfate Content of the Groundwater—Normal Method. Standards Australia: Sydney, Australia, 2020.
- APHA. Standard Methods for the Examination of Water and Wastewater: Method 4500-SO42−; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Amin, D.M.; Jamaludin, S.B.; Pa, F.C.; Chuen, K.K. Effects of Magnesium Sulfate Attack on Ordinary Portland Cement (OPC) Mortars. Port. Electrochim. Acta 2007, 26, 235–242. [Google Scholar] [CrossRef]
- Soil and Plant Analysis Council Inc. Soil Analysis Handbook of Reference Methods; St. Lucie Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- APHA. APHA Method 3111: Standard Methods for the Examination of Water and Wastewater, in 3111B; American Public Health Association: Washington, DC, USA, 1992; p. APHA 3111B. [Google Scholar]
- U.S. EPA. Acid Digestion of Sediments, Sludges and Soils; EPA method 3050B (SW-846); U.S. Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- Texas Department of Transportation. Determining Chloride and Sulfate Contents in Soil; Texas Department of Transportation: Austin, TX, USA, 1999; p. TEX-620-J. [Google Scholar]
- French, R.; Jones, M. Design for culvert blockage: The ARR 2016 guidelines. Australas. J. Water Resour. 2018, 22, 84–87. [Google Scholar] [CrossRef]
- Jossop, J.P.; Toelken, H.R.; Black, J.M. Flora of South Australia; State Herbarium of South Australia: Adelaide, Australia, 1986. [Google Scholar]
- Harden, G.J. Flora of New South Wales; NSW University Press: Randwick, Australia, 1990. [Google Scholar]
- Species profile Sarcocornia quinqueflora. Environment, Land and Water. Available online: https://apps.des.qld.gov.au/species-search/details/?id=21957 (accessed on 24 June 2024).
- Rasheeduzzafar; Al-Saadoun, S.S.; Al-Gahtani, A.S. Corrosion Cracking in Relation to Bar Diameter, Cover, and Concrete Quality. J. Mater. Civ. Eng. 1992, 4, 327–342. [Google Scholar] [CrossRef]
- Scott, B.; Safiuddin, M. Abrasion Resistance of Concrete—Design, Construction and Case Study. Concr. Res. Lett. 2015, 6, 136–148. [Google Scholar]
- Atiş Cengiz, D. High Volume Fly Ash Abrasion Resistant Concrete. J. Mater. Civ. Eng. 2002, 14, 274–277. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Pann, K.-S. Abrasion resistance of concrete containing surface cracks. J. Chin. Inst. Eng. 2011, 34, 683–694. [Google Scholar] [CrossRef]
- Shi, J.; Liu, B.; Wu, X.; Tan, J.; Dai, J.; Ji, R. Effect of steam curing on surface permeability of concrete: Multiple transmission media. J. Build. Eng. 2020, 32, 101475. [Google Scholar] [CrossRef]
- Jacques, D.; Wang, L.; Martens, E.; Mallants, D. Modelling chemical degradation of concrete during leaching with rain and soil water types. Cem. Concr. Res. 2010, 40, 1306–1313. [Google Scholar] [CrossRef]
- Sparks, T.; George, R.; Wallace, K.; Pannell, D.; Burnside, D.; Stelfox, L. Salinity Investement Framework Phase II; Department of Agriculture and Food: Washington, DC, USA, 2006. [Google Scholar]
- DPRID. Measuring Soil Salinity; Department of Primary Industries and Regional Development: South Perth, Australia; DPIRD Digital Library: Nedlands, Australia, 2024. [Google Scholar]
- de Caritat, P.; Cooper, M.; Wilford, J. The pH of Australian soils: Field results from a national survey. Soil Res. 2011, 49, 173–182. [Google Scholar] [CrossRef]
- Smith, S.T. Soil salinity in Western Australia. J. Dep. Agric. West. Australia Ser. 4 1961, 2, 757–760. [Google Scholar]
- Halse, S.; Ruprecht, J.; Pinder, A. Salinisation and prospects for biodiversity in rivers and wetlands of south-west Western Australia. Aust. J. Bot. 2003, 51, 673–688. [Google Scholar] [CrossRef]
- George, R.; McFarlane, D.; Nulsen, B. Salinity Threatens the Viability of Agriculture and Ecosystems in Western Australia. Hydrogeol. J. 1997, 5, 6–21. [Google Scholar] [CrossRef]
- Hatton, T.J.; Ruprecht, J.; George, R.J. Preclearing hydrology of the Western Australia wheatbelt: Target for the future. Plant Soil 2003, 257, 341–356. [Google Scholar] [CrossRef]
- Hobbs, R.J. Effects of landscape fragmentation on ecosystem processes in the Western Australian wheatbelt. Biol. Conserv. 1993, 64, 193–201. [Google Scholar] [CrossRef]
- George, R.; Clarke, J.; English, P. Modern and palaeogeographic trends in the salinisation of the Western Australian wheatbelt: A review. Soil Res. 2008, 46, 751–767. [Google Scholar] [CrossRef]
- Clarke, C.J.; George, R.J.; Bell, R.W.; Hatton, T.J. Dryland salinity in south-western Australia: Its origins, remedies, and future research directions. Soil Res. 2002, 40, 93–113. [Google Scholar] [CrossRef]
- Al-Jabari, M. Sulfate attack. In Integral Waterproofing of Concrete Structures; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ahmed, A. Elucidating Chemo-Mechanical Synthesis and Microstructural Study on the Performance of Partial Cement-Based Concrete Composites Against Sulphate Attack—A Review. Res. Dev. Mater. Sci. 2022, 18, 2065–2078. [Google Scholar] [CrossRef]
- Boudali, S.; Kerdal, D.; Ayed, K.; Abdulsalam, B.; Soliman, A. Performance of self-compacting concrete incorporating recycled concrete fines and aggregate exposed to sulphate attack. Constr. Build. Mater. 2016, 124, 705–713. [Google Scholar] [CrossRef]
- Nadir, H.M.; Ahmed, A. The mechanisms of sulphate attack in concrete—A review. Mod. Approaches Mater. Sci. 2022, 5, 658–670. [Google Scholar]
- Silva, Y.F.; Burbano-Garcia, C.; Rueda, E.J.; Reyes-Román, A.; Araya-Letelier, G. Short- and Long-Term Mechanical and Durability Performance of Concrete with Copper Slag and Recycled Coarse Aggregate Under Magnesium Sulfate Attack. Appl. Sci. 2025, 15, 8329. [Google Scholar] [CrossRef]
- Fernando Silva, Y.; Delvasto, S.; Valencia, W.; Araya-Letelier, G. Performance of Self-Compacting Concrete with Residue of Masonry and Recycled Aggregate under Sulfate Attack. J. Mater. Civ. Eng. 2024, 36, 04023491. [Google Scholar] [CrossRef]
- Pasupathy, K.; Sanjayan, J.; Rajeev, P.; Law, D.W. The effect of chloride ingress in reinforced geopolymer concrete exposed in the marine environment. J. Build. Eng. 2021, 39, 102281. [Google Scholar] [CrossRef]
- Hunkeler, F. Corrosion in reinforced concrete: Processes and mechanisms. In Corrosion in Reinforced Concrete Structures; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–45. [Google Scholar]
- Kohout, F.A. Cyclic flow of salt water in the Biscayne aquifer of southeastern Florida. J. Geophys. Res. 1960, 65, 2133–2141. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Jeong, S.-G.; Ju, C.-S. The effect of dominant ions on solvent extraction of lithium ion from aqueous solution. Korean J. Chem. Eng. 2014, 31, 828–833. [Google Scholar] [CrossRef]
- He, J.; Long, G.; Ma, K.; Xie, Y. Comprehensive study on the hydration kinetics, mechanical properties and autogenous shrinkage of cement pastes during steam curing. Cem. Concr. Res. 2023, 174, 107310. [Google Scholar] [CrossRef]
- ASTM C876-22b; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshohocken, PA, USA, 2022.
- MRWA. Carbonation of Concrete; Main Roads Western Australia: East Perth, Australia, 2022; p. WA 620.1. [Google Scholar]
- ASTM C1152/C1152M; Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- AS 1012.20.1; Methods of Testing Concrete Determination of Chloride and Sulfate in Hardened Concrete and Aggregates—Nitric Acid Extraction Method. Standards Australia: Sydney, Australia, 2016.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).