Abstract
Fusel oil is a fermentation by-product composed of a complex mixture of alcohols (ethanol, isoamyl, propanol, and butanol isomers) and water. The primary challenges lie in water separation and the recovery of the valuable component, isoamyl alcohol. In this work, we demonstrate an efficient separation process using a non-polar, non-toxic, water-immiscible solvent, namely hexane, to reduce the water content of fusel oil from an initial 14 wt.% to 1.46 wt.% at a solvent to fusel oil ratio of 1:1 and to 0.55 wt.% at a 4:1 ratio. The proposed separation process was designed with a 1:1 ratio to minimize equipment size. In the first step, a decanter vessel enabled phase separation, followed by two distillation columns. The bottom product from the second column achieved a purity of 99.29 wt.% isoamyl alcohol (97.91 wt.% isomers and 1.38 wt.% hexanol) with a recovery rate of 97.33%. The distillate flows were directed to the second decanter vessel, recovering 99.665% of hexane. This study confirms the effectiveness of the proposed process in separation of highly valuable isoamyl alcohol from fusel oil via a hybrid decanter–distillation scheme. The proposed process attains a specific energy consumption in the reboilers of 0.65 kWh per kilogram of product (equivalent to 1.21 kg of steam per kilogram of product). This represents a notable improvement compared to the configuration reported by other authors for the separation of isoamyl alcohol using divided-wall columns (DWC), which requires 2785 kJ per kilogram of product (i.e., 0.774 kWh per kilogram of product). An economic analysis was performed to compare the process of separating isoamyl alcohol from fusel oil using the minimum hexane ratio (1:1) and the maximum ratio (4:1). All cost values increased significantly with higher solvent ratio. Remaining challenges include the purification of waste aqueous streams and future valorization of the hexane–alcoholic mixture.
1. Introduction
Fusel oil (FO), until recently considered a waste product resulting from alcoholic fermentation, has gradually become a target for industrial waste recovery. In a circular economy, desirable for sustainable development, it can become a raw material for use in value-added products, while its recovery also contributes to reducing impact on the environment [1].
Those countries that rank highly in production of alcoholic beverages or bioethanol are also those that generate fusel oil on a large scale. In 2020, the USA was the global leader in the field of bio-ethanol (53% of world production), followed by Brazil (30%), European Union (5%), China (3%), India, Canada and Thailand (each with 2%) [2]. In the sugar fermentation stage of ethanol production, a considerable volume of fusel oil results as a by-product, making it available in significant industrial quantities. Between 1 and 11 L of fusel oil can be generated per 1000 L of ethanol, depending on the feedstock, fermentation conditions and engineering technology applied [3,4,5]. Worldwide, the most popular raw materials for fermentation are sugar cane, barley, corn, rice and sugar beet.
FO is a mixture of alcohols (isoamyl alcohol, ethanol, butanols and propanols) and water, the composition of which depends on the raw material used in the fermentation process of alcohol production, the yeast strain used, and the efficiency of its separation from the resulting mixture after fermentation.
The composition of a fusel oil can be as follows: 41–87 wt.% isoamyl alcohol (3-methyl-1-butanol), 1–20 wt.% water, 1–15 wt.% ethanol, 0–6 wt.% isobutyl alcohol, and 0–6 wt.% other C3–C5 alcohols and other minor compounds [6]. A highly detailed report on the composition of fusel oil was presented by Massa et al. [4]. For the beverage industry, fuel oil is an undesirable fraction due to its aggressive odor, dark color, and possible toxicity at high concentrations; however, in many industrial applications, it could be a valuable raw material, and potentially a renewable resource. Fusel oil has become a valuable resource with multiple applications in the chemical, cosmetic, energy, pharmaceutical, and agricultural industries [4].
Isoamyl alcohol is the major component in FO, and there is great interest in its separation from FO as an additional raw material for obtaining acetate esters, and primarily isoamyl acetate, the aroma of which (banana or pear) gives it great applicability in the perfumery, food, and pharmaceutical industries [7,8]. The composition of FO makes it favorable in biodiesel production, FO playing the role of an acyl acceptor in transesterification and esterification reactions for the production of esters. The literature reports [9] that the testing of fatty acid fusel esters (FAFE) in diesel engines confirms their applicability. Another potential use is the obtaining of bio-lubricants through esterification of FO and oleic acid and transesterification of FO and palm kernel oil, with favorable results to date [10,11].
The introduction of a percentage of oxygenated compounds in automotive gasolines became mandatory in Europe after 2009 [12]. Numerous studies have shown the potential of lower alcohols (methanol, ethanol, butanols) and ethers (ethyl-tert-butil-ether and ethyl-tert-amyl-ether) as bio-components in ecologically friendly automotive gasoline, providing benefits in both combustion performance and reducing the concentration of pollutants in exhaust gases. An obvious consequence is the use of this alcoholic mixture of FO as a fuel additive in spark-ignition engines. Much research has shown that it has a high octane number (RON 106 and MON 103), high oxygen content (30.23 wt.%), and a unique boiling point, making it favorable as a bio-component in gasoline [13,14,15,16]. Some research has shown that blending diesel with FO up to a certain percentage leads to better combustion performance and reduced emissions, proving the feasibility of fuel oil as an alternative fuel [17,18].
The herbicidal and fungicidal potential of some compounds (C5–C8 alcohols) has also received attention [4].
The presence of a significant water content in fusel oil, along with a complex mixture of alcohols, is a major disadvantage from the point of view of thermodynamic behavior. All alcohols when combined with water form binary homogeneous azeotropes (ethanol/water; iso-propanol/water; 1-propanol/water) or binary heteroazeotropes (iso-butanol/water; 1-butanol/water; 3-methyl-1-butanol/water; 2-methyl-1-butanol/water; 1-hexanol/water) and ternary heteroazeotropes (1-butanol/3-methyl-1-butanol/water). All binary and ternary azeotropes/heteroazeotropes have minimum boiling point temperatures in the temperature range reported by [3,6]. Separation of isoamyl alcohol from this mixture is a real challenge for researchers. According to theory, simple distillation in several columns could be an option, and more individual alcohols would be obtained, but the number of columns would be large, the process would be energy-intensive, and the products obtained would be expensive. Other advanced technologies for the separation of azeotropic alcohol/water mixtures are the following: vacuum distillation, azeotropic distillation in the presence of various entrainers [19,20] or extractive distillation with selected extractive agents [21], liquid–liquid extraction including salting-out extraction [22], pervaporation [23] and adsorption. Only a few results of distillation-based studies for the separation of alcohols from FO and especially isoamyl alcohol have been presented. Mendoza-Pedroza et al. [6] developed a process for separation of isoamyl alcohol from the fusel oil resulting from Colombian sugar mills. The FO composition was 12.6 wt.% ethanol, 14.3 wt.% water, 70.4 wt.% isoamyl alcohol, 1.4 wt.% 1-butanol and 1.3 wt.% iso-butanol. The authors proposed an intensified configuration compared to the conventional scheme, namely a flowsheet based on a divided wall column (DWC). The conventional configuration consists of three simple distillation columns. The column (C-I) separates, as distillate, a mixture of ethanol and water, while the bottom product is a stream containing C4–C5 alcohols and some water. The distillate product of the column (C-II) is an ethanol–water azeotropic mixture, and the bottom product is excess water. Column (C-III) is fed with the bottom product from C-I. Column C-III is connected with a decanter (at the top), and the aqueous phase of the decanter removes C4–C5 alcohols and water from the system. The bottom product of the C-III column is isoamyl alcohol with 99 wt.% purity. In the intensified process using the divided wall column (DWC), the C-II column and C-III column are merged into one unit. The DWC is thermally coupled with column C-I. Isoamyl alcohol is obtained as the bottom product from the DWC, while the ethanol–water azeotropic mixture is recovered as the top product. Water and C4 –alcohols are removed as a side stream from the decanter connected to the intermediate sections of the DWC. The intensified alternative ensures a reduction of around 20% in energy consumption and a decrease of about 72% in the total annual cost (TAC).
Missyurin et al. [3] aimed to develop a technological process for separating most of the constituent components of fuel oil, not only isoamyl alcohol. Moreover, the composition of fusel oil is complex, containing ethanol, iso-propanol, 1-propanol, iso-butanol, and 1-butanol, in addition to water. The method excludes the introduction of potentially toxic components into the process, such as entrainers or extractive-distillation solvents, supporting the idea that the separated alcohols can be labeled as natural products. The separation process is complex and hybrid, combining liquid–liquid extraction with continuous distillation, and also includes a batch distillation stage. It is divided into three sections: a continuous liquid–liquid extraction section, coupled with the separation of excess water from the extract stream by distillation; a continuous distillation section which includes a column for ethanol recovery, two columns for the separation of isoamyl alcohol, and a column for the recovery of propanols and butanols; and a batch distillation section for the separation of iso-propanol, 1-propanol, iso-butanol and 1-butanol. The final products were obtained at the following concentrations: 96.4 wt.% ethanol, 98.6 wt.% isoamyl alcohols (sum of isomers), 99.4 wt.% 1-butanol, 70.9 wt.% iso-propanol (with 16.4 wt.% ethanol), 61.7 wt.% 1-propanol (with 38% water) and 81.8 wt.% iso-butanol (with 18.2 wt.% water). Further purification is required for iso-propanol, 1-propanol, and iso-butanol. In contrast, isoamyl alcohol can be directly used for esterification by acetic acid to synthesize valuable isoamyl acetate [3].
As previously shown, fusel oil is a complex mixture of alcohols in which a valuable component, isoamyl alcohol, predominates. However, its separation is challenging because the mixture also contains water, which forms both homogeneous and heteroazeotropes with all alcohols. In this paper, we propose a novel approach to the separation of isoamyl alcohol from fusel oil. For our study, we selected a fusel oil containing ethanol, propanols, butanols, isoamyl alcohol (mixture of isomers), hexanol, and water. First, we aim to demonstrate that hexane can extract all alcohols and separate water from fusel oil. We chose hexane because it is a non-polar and non-toxic component, which is why it is also accepted in the food industry as an extractive agent [24,25]. Hexane is valued in industry for its chemical stability, suitable boiling point, low corrosiveness, high extraction efficiency, and low cost [26]. However, its use in vegetable oil extraction raises health and environmental concerns. Prolonged exposure to hexane vapors can impact the nervous system [27]. Additionally, hexane is an atmospheric pollutant contributing to smog formation and can affect aqueous ecosystems [28,29]. For these reasons, researchers are seeking more eco-friendly alternatives for extraction [26,30,31].
Experimentally, we demonstrate that, at various hexane to fusel oil ratios, the water content remaining in the organic phase is very low (thousands of ppm). Next, from this organic phase, through a process combining distillation with decanter vessels, we separate isoamyl alcohol (mixture of isomers: 3-methyl-1-butanol of 95.18 wt.% and 2-methyl-1-butanol of 2.73 wt.%) and obtain a mixture of alcohols and hexane with less than 1000 ppm water. The proposed technological process for the separation of isoamyl alcohol was simulated using AVEVA PRO/II v.2024 software. At the end of the study, we conducted an economic analysis that included calculation of the fixed capital investment (FCI, updated to 2023 by CEPCI), the annual capital charge (ACC), the annual cost of production (COP) and total annual cost of production (TCOP) for a hexane to fusel oil ratio of 1:1 and 4:1.
To date, hexane has not been employed as a separating agent on its own, but rather as a constituent of mixed entrainers for ethanol dehydration. For example, Gomis et al. [32] utilized a gasoline fraction—where hexane represents one of the hydrocarbons present in conventional gasoline, together with cyclohexane, isooctane, and toluene—whereas Stratula et al. [33] employed a hexane-rich fraction.
2. Materials and Methods
2.1. Experimental Procedure
The fusel oil used in this work is the same as that employed in our previous study [3], and it was supplied by the company Natural Ingredients RO; its chemical composition is presented in Table 1 and is supported by the chromatogram shown in Figure S1 of the Supplementary Materials.

Table 1.
Composition of fusel oil (FO) [3].
Hexane, purchased from Merck (CAS number 110-54-3), has a purity of ≥99.5% (water content ≤ 0.005%) and was used without further purification.
In four glass vials with stoppers, 1 mL of fusel oil was introduced into each vial. Then, quantities of 1, 2, 3, and 4 mL of hexane were added to the four vials, respectively, thus establishing different hexane to fusel oil ratios. The vials were shaken vigorously by hand and subsequently left to stand for 24 h at room temperature.
Figure 1 depicts the glass vials after that 24 h period, clearly illustrating the formation of two distinct phases: an upper organic phase (composed of alcohols and hexane) and a lower aqueous phase (containing water, trace amounts of alcohols, and trace amounts of hexane).
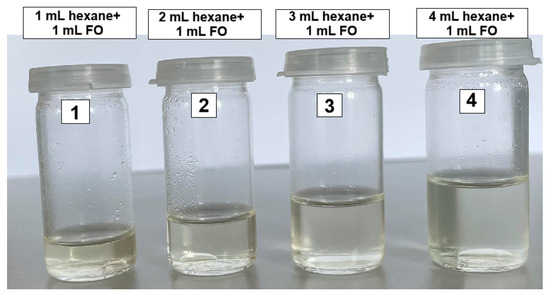
Figure 1.
Phase separation in FO/hexane samples.
From each sample, the upper organic phase was carefully withdrawn to analyze the water content by Karl-Fisher (KF) titration. The KF instrument used in our investigations was the KF titrator K90365 (Koehler, New York, NY, USA). Table 2 displays the calculated relative error after measuring the water content of the organic phase three times for each sample.

Table 2.
Statistical errors of the experimental data.
2.2. Methodology of Simulation and Process Description
In this section of the paper, we describe the design methodology of the process for removing water from fusel oil using hexane, followed by the separation of isoamyl alcohol from the organic phase. To configure the process and select the most suitable operating parameters for the equipment, we employ simulations performed in AVEVA PRO/II v.2024 software. Thermodynamic properties and vapor–liquid as well as liquid–liquid phase equilibria were modeled using the NRTL (Non-Random Two-Liquid) thermodynamic framework, complemented by the UNIFAC group-contribution model.
The NRTL (Non-Random Two-Liquid) model is recommended for application to strongly non-ideal mixtures and partially miscible systems [34]. Since fusel oil is a mixture of eight alcohols and water, with the addition of hexane, the resulting thermodynamic system comprises 45 binary mixtures. To accurately represent the vapor–liquid and liquid–liquid phase equilibria for each binary mixture, the corresponding interaction parameters must be known. The selected NRTL model is a liquid-activity model characterized by eight binary interaction parameters aij, aji, bij, bji, cij, cji, where i, j denote each of the ten components considered. The ability of this activity-coefficient model to correctly and comprehensively describe the thermodynamic behavior of the binary systems involved in the mixture—namely, vapor–liquid equilibrium (VLE), liquid–liquid equilibrium (LLE), and/or vapor–liquid–liquid equilibrium (VLLE)—depends largely on the availability of binary interaction parameters. The PRO/II software package includes a database of interaction parameters obtained from experimental data, which is frequently updated. For most of the binary systems in the mixture, VLE, LLE, and VLLE interaction parameters for the NRTL model are available in the AVEVA PRO/II v.2024 database. For the remaining systems, the missing interaction parameters were estimated within the NRTL framework using the “fill” option with the temperature-dependent UNIFAC-Lyngby group-contribution model, which predicts interaction parameters based on the functional group contributions of the components [35]. The Supplementary Materials provide tabulated values of the binary interaction parameters for the thermodynamic systems considered in this study. The interaction parameters were estimated for 12 binary mixtures using the UNIFAC group-contribution method.
To remove almost all water from fusel oil, a non-polar and non-toxic solvent was chosen, namely hexane. It remains in the organic phase along with the alcohols, although a small amount of alcohol is lost in the aqueous phase. We chose a 1:1 hexane to fusel oil ratio to minimize the circulation of hexane flow in the plant and to avoid increasing the size of the equipment.
In the decanter Dc1, at 40 °C and 1.2 barg, an aqueous phase (AP 1) and an organic phase (OP 1) are separated. The organic phase feeds the distillation column C1. The distillate D1 comprises primarily ethanol and hexane (with minor amounts of other alcohols). The bottom product of column C1, denoted B1, is practically water-free; it is a mixture of alcohols in which isoamyl alcohol predominates and serves as feed to column C2. Distillate D2 contains ethanol, iso-propanol, 1-propanol, iso-butanol, 1-butanol, hexane, and trace losses of 2-methyl-1-butanol and 3-methyl-1-butanol. The purpose of column C2 is to separate isoamyl alcohol as the bottom product (B2) with a minimum concentration of 99 wt.%. The operating conditions of columns C1 and C2 are detailed in Table 3.

Table 3.
The operating conditions of columns C1 and C2.
Distillates D1 and D2 are combined in a mixer and subsequently routed into decanter Dc2. In the organic phase (OP 2), nearly all hexane and alcohols (ethanol, iso-propanol, 1-propanol, iso-butanol, 1-butanol) are recovered. This will be the subject of a separate investigation, including experimental blending of gasoline at various ratios. The aqueous phases AP 1 and AP 2 are currently designated as waste; a future study will examine their treatment, as their present composition excludes discharge to wastewater treatment facilities. The proposed flowsheet of isoamyl alcohol separation from fusel oil is depicted in Figure 2.
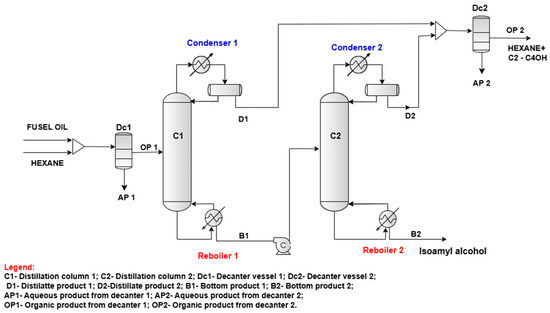
Figure 2.
The proposed flowsheet for isoamyl alcohol separation from fusel oil.
Simulations of the proposed technological process for the separation of isoamyl alcohol were conducted in steady state mode, using the Chemdist algorithm for mass and heat balances and phase equilibria calculations of both columns within the AVEVA PRO/II v.2024 software package.
2.3. Methodology of Cost Estimation
In this work, the fixed capital investment (FCI) and total cost of production (TCOP) estimations are performed based on data from Towler and Sinnott [36]. The fixed capital investment is made up of the following:
where ISBL is the inside battery limits investment (including direct field costs, such as cost of major equipment, bulk items, civil work, installation labor and supervision, and indirect field costs, such as temporary construction costs, field expenses and services, construction insurance, labor benefits and burdens, and miscellaneous overhead items); OSBL is the offsite cost; EC is the engineering cost (referring to the contractor charges, detailed design and other engineering services); and CO is contingency charges.
FCI = ISBL + OSBL + EC + CO
In this work, the inside battery limits (ISBL) investment included only the direct field cost. Hence, the ISBL is reduced to a fixed capital cost. Fixed capital cost (C) is based on an estimate of the purchase cost of the major equipment items required for the process, and the other costs (such as equipment erection including foundations and minor structural work; piping, including insulation and painting; electrical power and lighting; instruments and automatic process control systems; process buildings and structures; ancillary buildings, offices, laboratory buildings, storage for raw materials; site preparation) are estimated as factors of each equipment cost.
The Hand factor method proposes that the C fixed capital cost of a plant is given as a function of the total purchased equipment cost by the following equation:
where C is the total capital cost of the plant; Ce is the purchase cost of each major item of equipment (i = 1 … M); F are Hand installation factors specified for each major equipment type. The factors comprising F are presented in Supplementary Materials (Table S4) as a selection from Towler and Sinnott’s work. Equation (2) can be used to make a preliminary estimation once the flowsheet has been drawn up and the main plant equipment has been sized.
The equipment cost Ce is estimated by the following equation:
where Ce is the purchased equipment cost on Chemical Engineering Plant Cost Index CEPCI of 478.6 available for January 2006; a and b are cost constants; S is the size parameter; n is the exponent for that type of equipment. Constants a and b, parameter S, and exponent n are shown in Supplementary Materials (Table S5) as selections from Towler and Sinnott’s work. It should be mentioned that for the vessel of columns C1 and C2 and the vessel of decanters Dc1 and Dc2, the size parameter S is the mass of the vessels. The procedure is detailed in Supplementary Materials and results are presented in Table S5. For the decanters, the internal diameters and heights were estimated by our own calculations (in an Microsoft Excel sheet), based on the methodology from GSPA (chapter 7) [37]. The size parameter for all heat exchangers is area in m2, as can be seen in Table S6, and miscellaneous equipment (mixers and pumps) are measured by flow rate in liters/second (Table S7). With reference to the flowsheet from Figure 2, we have included in the cost calculation the storage vessels for raw materials and their circulation pumps.
The estimated cost Ce is for base equipment made from carbon steel. To calculate the purchased equipment cost with different materials, a correction material factor is used (fm = 1.3) as is recommended in the above-mentioned book [36]. The CEPCI from Chemical Engineering Magazine [36] is used to update the prices as of 2006, according to Equation (4).
where cost in year A relates to year 2023; cost in year B relates to year 2006; cost index in 2023 is 798.7. All monetary values are in USD.
Fixed Capital Investment (FCI) is converted into a constant series of payments for every year of the plant’s life. The annual capital cost (ACC) is calculated from Equation (5).
where i is the interest rate and n is the project life time. The parenthesis in the above equation is the annual capital charge ratio.
The annual cost of production COP is made up as follows:
where VCOP and FCOP are variable and fixed costs of production, respectively. Variable costs of production are the sum of all the variable costs of production that are proportional to the plant output or operation rate. The FCOP is the sum of all the fixed costs of production that are covered, regardless of the plant operation. The VCOP and FCOP are estimated as direct calculations based on operating plant data or as factors of current and/or previous calculations, as can be seen in Table 4. It is assumed that the plant operates for 8000 h/year.
COP = VCOP + FCOP

Table 4.
Components of COP estimation.
In this work, it is useful to calculate a total cost of production (TCOP) as the sum of the annual cost of production and the annual capital cost (Equation (7)).
TCOP = COP + ACC
3. Results and Discussion
As mentioned in the Introduction, the original objective of the research was to investigate the use of hexane for removing water from fusel oil, followed by the design and simulation of a process to separate isoamyl alcohol.
3.1. Experimental Results and Discussion
In Figure 1, the image clearly demonstrates that the addition of hexane leads to distinct separation into a lower aqueous phase and an upper organic phase. Karl–Fischer analysis of the water content remaining in the organic phase shows that, from an initial fusel oil water content of 14 wt.% (Table 1), the remaining water decreases with increasing hexane to fusel oil ratio: at a 1:1 ratio it is 14,630 ppmw (i.e., 1.46 wt.%), and at a 4:1 ratio it drops to only 5 493 ppmw (i.e., 0.5493 wt.%).
These results confirm the feasibility of using hexane to remove unwanted water from fusel oil, as explored in this study.
3.2. The Simulation Results and Discussion
The fusel oil feed flowrate of the flowsheet proposed in Figure 2 was assumed at 2000 kg/h, and its composition is indicated in Table 1. Experimental results indicate that the highest hexane to fusel oil ratio (4:1) is the most effective. However, using hexane flow at four times the rate of fusel oil would significantly increase equipment dimensions, particularly the column diameters, and also lead to very high energy consumption in the reboilers of these columns. For these reasons, we selected the lowest feasible hexane flow rate of 2000 kg/h. However, the entire proposed process was also simulated using a higher hexane flow rate of 8000 kg/h (hexane to fusel oil ratio of 4:1) in order to demonstrate its detrimental effects on the equipment sizing, energy consumption, and overall process cost, as shown in the economic analysis in Section 3.3.
Both streams are mixed and then fed into decanter 1, where separation occurs at 40 °C and 1.2 barg. Table 5 presents the mass flow rates of components in each separated phase, compared to their flow rate in the original fusel oil feed.

Table 5.
The mass flow rates of components in aqueous and organic phases from Dc1 compared to fusel oil.
As shown in Table 5, hexane remains almost entirely in the organic phase, which is expected given the immiscibility of nonpolar hexane and polar water. Conversely, ethanol tends to partition between the aqueous and organic phases, a behavior attributable to the strong hydrogen-bonding interactions it forms with water.
The organic phase is fed to distillation column C1 at a temperature of 40 °C and a pressure of 1.2 barg, on tray number 12. This column is designed to produce a distillate flow (D1) containing mainly ethanol and the majority of the hexane, while the bottom flow (B1) consists of the remaining ethanol, iso-propanol, 1-propanol, iso-butanol, 1-butanol, isoamyl alcohol (including its isomers 2-methyl-1-butanol and 3-methyl-1-butanol), and hexanol and the remaining hexane. By adjusting the reflux ratio, it was found that using a molar reflux ratio of 1.0 minimizes the loss of isoamyl alcohol in the distillate product. It is worth noting that the presence of water alongside hexane in the upper section of distillation column C1 causes the formation of two liquid phases on the first five trays of the column, as well as in the reflux drum. Table 6 presents the mass flow rates and mass concentrations of the components in both distillate (D1) and bottom (B1) products.

Table 6.
The mass flow rates and mass concentrations of components in distillate (D1) and bottom (B1) flows.
From the data presented in Table 6, it is evident that almost all of the isoamyl alcohol (including its isomers 2-methyl-1-butanol and 3-methyl-1-butanol) and hexanol in the C1 column feed are separated into the bottom product, as intended.
Therefore, the bottom product from column C1, at 102.16 °C and 1.10 barg, is pumped into the feed of distillation column C2. The purpose of column C2 is to separate all the isoamyl alcohol (including the isomers 2M1B and 3M1B) and hexanol into the bottom product (B2). By setting the reflux ratio to an optimum value of 3 (molar basis), a final bottom product with a mass concentration of 99.29% is achieved. The distilled product (D2) is an homogeneous mixture of the remaining alcohols and hexane. Table 7 presents the mass flow rate and mass concentrations of the components in both the distillate (D2) and the bottom (B2) products from column C2.

Table 7.
The mass flow rates and mass concentrations of components in distillate (D2) and bottom (B2) flows.
The results presented in Table 7 demonstrate that the separation carried out in column C2, under the operating conditions specified in Table 3, achieves the objective proposed in this study: obtaining a bottom product with a mass purity of 99.29% (comprising 97.91 wt.% of isoamyl alcohol isomers and 1.38 wt.% hexanol). Furthermore, from the 1440 kg/h of isoamyl alcohols in the initial fusel oil, 1401.55 kg/h is recovered, corresponding to a recovery rate of 97.33%.
Distillates D1 and D2 are combined and directed to the decanter vessel Dc2. At a temperature of 40 °C, phase separation occurs, yielding an aqueous phase (AP2) and an organic phase (OP2). In OP2, almost all hexane is recovered (i.e., 99.665%). The mass flow rates of AP2 and OP2 are presented in Table 8.

Table 8.
The mass flow rates of components in aqueous and organic phases from Dc2.
As discussed in Section 2.2, the aqueous phases AP1 and AP2 are residual streams, and their separation will be addressed in a future study. Regarding the organic phase OP2, it contains the C2–C4 alcohols (with very little loss of C5 alcohols) and the recovered hexane. We intend to continue the investigations in this study in two future directions: first, to separate the hexane for recycling into the feed of the separation process proposed in Figure 2 and to utilize the alcohols as bio-components in eco-friendly gasoline; and second, to employ the entire organic phase as a component in automotive gasoline. Concerning the two aqueous streams (AP1 and AP2), which are complex mixtures of alcohols with a total flow rate of 467.68 kg/h (60% water, 28.14% ethanol, 10.43% a mixture of other alcohols, and 1.43% hexane), our primary economic interest lies in the recovery of ethanol and the other alcohols. Beyond their potential economic value, these aqueous streams cannot be discharged, as this would violate environmental regulations concerning the maximum allowable discharge concentrations for pollutants in municipal and industrial wastewaters released into natural waters [38]. At this stage, based on an extensive literature review [39,40,41,42,43,44], we propose to experimentally investigate two approaches: adsorption of alcohols on mesoporous activated carbon and extraction of alcohols with environmentally friendly solvents. In addition, we intend to evaluate, through simulation, the separation of alcohols by pervaporation using membranes, stripping with carbon dioxide, and extractive distillation with environmentally friendly solvents.
AVEVA PRO/II v.2024 includes sizing capabilities for packed beds. Therefore, this function was used to calculate the diameter of the column vessels and Sulzer’s structured packing, Mellapak 350X, was selected in both columns. The Mellapak type 350X packing has a high separation efficiency (which it makes suitable for challenging separations such as those encountered in this study), and a high specific surface area, which leads to lower pressure drop across the packing and a lower height equivalent theoretical plate (HETP) [45]. The HETP was calculated according to the Sulzer methodology depending on the F-factor and the pressure drop per unit of height. Next, in the dedicated section of the AVEVA PRO/II simulator we set the HETP values, and the Capacity% was set to a value of 50%. By setting this value for Capacity%, the columns C1 and C2 were designed to operate at a fraction of the flooding velocity, thereby avoiding liquid entrainment, hydraulic instability, and degraded separation performance [46]. The software calculated the diameter of each section, the total height of each section, and provided detailed values of the F-factor and pressure drop (mbar/m of packing) for each stage. Further details on the evolution of these parameters for each section of the two columns are presented in the Supplementary Materials (Table S8).
For the distillation–decanter process at a 1:1 ratio of hexane to FO, each column contains two packing sections, separated by a space of 0.4 m. In distillation column C1, the upper packing section is 3 m high and has a calculated diameter of 0.579 m, while the lower section is 4 m high with a diameter of 0.841 m. In distillation column C2, the upper section is 4 m high, and the lower section is 5 m high; both sections share the same diameter of 0.687 m.
In the final part of this section, we address some aspects related to energy consumption and utility requirements of the proposed process. In both distillation columns, vapor condensation is achieved by shell-and-tube heat exchangers. The heat input at the bottom of the columns is provided by means of Kettle reboilers. The overall heat transfer coefficient and the logarithmic mean temperature differences in each heat exchanger were used to calculate the heat transfer area. The overall heat transfer coefficients (U) were taken from Luyben [47], while the logarithmic mean temperature differences (LMTD) were calculated with the PRO/II facilities for each heat exchanger. Recycled water is employed as the cooling agent in the condensers, entering at 25 °C and leaving at 52 °C, whereas saturated steam at 14 barg is used in the reboilers. The results concerning the function of the heat exchangers, their surface areas, and the utility requirements are summarized in Table 9.

Table 9.
The heat exchangers’ duties, surface areas and utility requirements.
Based on the data regarding utility consumption in the proposed installation and the product flow rate at the bottom of column 2 (B2), the specific consumptions are calculated as follows: the recycled water consumption is 16.47 kg water/kg B2, and the saturated steam consumption is 1.21 kg steam/kg B2. With respect to the economic comparison with previous studies, only the specific energy consumption in the reboilers can be directly contrasted with the work of Mendoza-Pedroza et al. [6], who proposed a configuration based on divided-wall columns (DWC) for the separation of isoamyl alcohol, reporting a specific energy consumption of 2785 kJ per kilogram of product (i.e., 0.774 kWh per kilogram of product). In contrast, the configuration developed in the present study attains a lower specific energy consumption of 0.65 kWh per kilogram of product. Although the literature [19,20,21,22,23] describes several alternative approaches for the separation of azeotropic alcohol/water mixtures, none of these have been validated for complex mixtures such as fusel oil. Consequently, a direct comparison between the process proposed herein and those alternative methods is not feasible.
3.3. The Cost Estimation Results and Discussion
As mentioned in Section 2.3, in this work the fixed capital investment (FCI) and total cost of production (TCOP) are performed according to the methodology developed by Towler and Sinnott [36]. For all the equipment necessary in the decanter–distillation process at hexane to FO ratios of 1:1 and 4:1, the equipment purchased cost Ce and fixed capital cost (C or ISBL) are presented in Table 10. From the point of view of hexane and fusel oil storage, additional measures must be taken to avoid emissions into the atmosphere that would lead to the exposure of workers to a toxic working atmosphere, and to avoid their impact on atmospheric pollution. We chose to store the raw materials in floating roof tanks to minimize evaporation losses, both equipped with vapor recovery units (VRU), inerting systems (N2) for filling and maintenance operations, low explosive level (LEL) detectors, anti-spark control, and compliance with ATEX procedures. The price of such a system is very expensive and is not included in the methodology proposed by Towler and Sinnott. Therefore, in the order of magnitude proposed by other sources (USD 15,000–75,000) [48], we choose a price of USD 50,000 for a storage tank of 50 m3 (for the separation process of the decanter–distillation process at a 1:1 ratio of hexane to FO) and a price of USD 85,000 for a storage tank of 150 m3 (for the separation process of the decanter–distillation process at a 4:1 ratio of hexane to FO).

Table 10.
The fixed capital cost C (or the inside battery limits investment ISBL) estimation for the decanter–distillation process at a 1:1 ratio of hexane to FO and a 4:1 ratio of hexane to FO.
According to Equation (1), the fixed capital investment (FCI) includes the ISBL cost, the offsite cost (OSBL), the engineering costs (EC), and contingency (CO). As recommended by Towler and Sinnott [36], the OSBL is assumed to be 30% of ISBL, and CO is assumed to be 10% of ISBL plus OSBL. The overall FCI is shown in Table 11. The FCI is actualized to 2023 prices in line with Equation (4).

Table 11.
The fixed capital investment (FCI) estimation for distillation–decanter process at ratios of hexane to FO of 1:1 and 4:1.
Fixed Capital Investment (FCI) is converted into a constant series of payments for every year of the plant life, assuming that the plant life is 20 years and the interest rate is about 15%. For this interest rate and recovery period, the annual capital charge ratio is 0.160, so the annual capital charge (ACC) is 0.160 × USD 3,991,512 = 638,612 USD/year for a distillation–decanter process at a 1:1 ratio of hexane to fusel oil (FO), or 0.160 × USD 7,656,894 = 1,225,103 USD/year for a distillation–decanter process at a 4:1 ratio of hexane to fusel oil (FO). The above calculations were performed using Equation (5).
In order to complete the comparison between separation processes at ratios of 1:1 and 4:1, the annual costs of production COP are estimated in the following step. The variable and fixed costs of production are calculated for the components from Table 4. The operating labor cost is calculated for five shift position operators. The steam medium pressure cost does not include its production (fuel fired at boilers, etc.). Process water consumption was assumed in the plant’s operation. It can be assumed that all the process water needs are supported by internal recycling. The electrical energy consumption in the distillation–decanter plant was assumed as electrical energy for all pumps (i.e., recycling pump, reflux pump, water pumps), electrical energy for control devices, electricity for lighting, etc. It is obvious that electrical energy consumption is very difficult to estimate. In our opinion, 500–550 kWh/h electrical energy is a reasonable assumption. Table 12 shows the annual costs of production COP.

Table 12.
Annual costs of production COP estimation for the distillation–decanter process at a ratio of 1:1 hexane to FO and at a ratio of 4:1 hexane to FO.
Finally, the total cost of production (TCOP) as the sum of the annual cost of production (COP) from Table 12 and the annual capital charge (ACC) previously estimated, is calculated via Equation (7). The TCOP for a distillation–decanter process at a ratio of 1:1 hexane to fusel oil is 3,620,435 USD/year and the TCOP for (FO) and a process at a ratio of 4:1 hexane to fusel oil is 4,976,552 USD/year. As a quick check, it can be seen that ACC is roughly 18% of the total cost of production (at a 1:1 ratio of hexane to fusel oil) and roughly 25% of the total cost of production (at a 4:1 ratio of hexane to fusel oil), which is typical for chemical processes, according Towler and Sinnott [36].
The results of the economic calculation presented in Table 10, Table 11 and Table 12, along with the conclusions drawn from them, indicated that increasing the hexane to fusel oil ratio leads only to economic disadvantages: fixed capital investment (FCI) and annual capital charge (ACC) both increase by 92%, the annual cost of production (COP) rises by 26%, and the total annual cost of production (TCOP) increases by 36%. We note that isoamyl alcohol production (flowrate of B2) remains unchanged regardless of the solvent ratio.
4. Conclusions
In this work, we demonstrated that water can be separated from fusel oil using a non-polar, non-toxic, and water-immiscible solvent, namely hexane. Preliminary experimental studies showed that, from an initial water content of 14 wt.%, the concentration decreases to 1.46 wt.% at a 1:1 hexane to fusel oil ratio and to 0.5493 wt.% at a 4:1 ratio. At the industrial level, this stage requires a decanter vessel that separates an organic phase containing the alcohols from fusel oil and hexane from a residual aqueous phase. This first decanter is provided in the scheme proposed by us. For the process design, we selected a 1:1 ratio in order to avoid unnecessarily increasing equipment size and the entire cost of the process. The next step was the design of a process consisting of two distillation columns aimed at separating the isoamyl alcohol from the organic phase. As a bottom product from column C2 (for process performed at a 1:1 ratio), we obtained a bottom product with purity of 99.29 wt.% (comprising 97.91 wt.% isoamyl alcohol isomers and 1.38 wt.% hexanol) and a recovery rate of 97.33%. The distillates from the two columns are directed to the second decanter vessel, where an organic phase is separated, in which 99.665% of hexane is recovered together with C2–C4 alcohols. The calculated dimensions of all equipment and the entire operating expenses indicate that the capital investment, annual capital charge, annual cost of production, and total annual cost of production could be reasonable. However, to conduct a comprehensive economic analysis and process comparison, we also simulated a hexane to fusel oil ratio of 4:1. These simulations were performed to obtain a B2 product with the same quality and recovery rate as the bottom product from column C2. The results of the economic assessment indicated that increasing the hexane to fusel oil ratio leads exclusively to economic disadvantages.
The research conducted in this work achieved the expected results; however, further efforts are needed to purify the aqueous phases from the two decanters and to identify practical applications for the mixture of hexane and alcohols.
Our study represents fundamental research with potential industrial applications, yet the transition from the simulation of a separation process to its industrial-scale implementation still requires many additional steps.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15189954/s1, Table S1: Binary interaction parameters of VLE for NRTL thermodynamic systems; Table S2: Binary interaction parameters of LLE for NRTL thermodynamic systems; Table S3: Binary interaction parameters of VLLE for NRTL thermodynamic systems; Table S4: The installation F factors for Hand method; Table S5: Cost of the vertical vessels and structured packing for the decanter–distillation process at a ratio of 1:1 of hexane to FO (price at year 2006); Table S6: The heat exchanger areas calculations and equipment cost for the decanter–distillation process at a 1:1 ratio of hexane to FO; Table S7: Cost of the miscellaneous items (price at year 2006) of the decanter–distillation process at a 1:1 ratio of hexane to FO; Table S8: Packed columns data details (for distillation–decanter process at a 1:1 ratio of hexane to FO); Figure S1: Chromatogram of raw fusel oil.
Author Contributions
Conceptualization, M.N. and O.G.; methodology, M.N.; software, M.N. and O.G.; validation, D.-L.C. and A.M.; formal analysis, A.M.; investigation, O.G.; resources, M.N. and A.M.; data curation, M.N.; writing—original draft preparation, M.N.; writing—review and editing, M.N. and D.-L.C.; visualization, M.N. and A.M.; supervision, M.N. and D.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FO | Fusel oil |
| 2M1B | 2-Methyl-1-butanol |
| 3M1B | 3-Methyl-1-butanol |
| LMTDs | Log-mean temperature difference |
| U | Overall heat transfer coefficient |
| NRTL | Non-random two liquid |
| UNIFAC | Universal quasi-chemical |
| Symbols | |
| C1 | Distillation column 1 |
| C2 | Distillation column 2 |
| Dc1 | Decanter vessel 1 |
| Dc2 | Decanter vessel 2 |
| D1 | Distillate product 1 |
| D2 | Distillate product 2 |
| B1 | Bottom product 1 |
| B2 | Bottom product 2 |
| AP1 | Aqueous phase from decanter 1 |
| AP2 | Aqueous phase from decanter 2 |
| OP1 | Organic phase from decanter 1 |
| OP2 | Organic phase from decanter 2 |
References
- Marinho, L.H.N.; Aragão, F.; de Genaro Chiroli, D.; Zola, F.C.; Tebcherani, S.M. A systematic review of fusel oil as a renewable biofuel: Challenges, opportunities, and circular economy integration. Fuel 2025, 402, 135924. [Google Scholar] [CrossRef]
- Hoang, T.-D.; Nghiem, N. Recent Developments and Current Status of Commercial Production of Fuel Ethanol. Fermentation 2021, 7, 314. [Google Scholar] [CrossRef]
- Missyurin, A.; Cursaru, D.-C.; Neagu, M.; Nicolae, M. Hybrid Process Flow Diagram for Separation of Fusel Oil into Valuable Components. Processes 2024, 12, 2888. [Google Scholar] [CrossRef]
- Massa, T.; Raspe, D.; Feiten, M.; Cardozo-Filho, L.; da Silva, C. Fusel Oil: Chemical Composition and an Overview of Its Potential Application. J. Braz. Chem. Soc. 2023, 34, 153–166. [Google Scholar] [CrossRef]
- Shenbagamuthuraman, V.; Patel, A.; Khanna, S.; Banerjee, E.; Parekh, S.; Karthick, C.; Ashok, B.; Velvizhi, G.; Nanthagopal, K.; Ong, H.C. State of art of valorising of diverse potential feedstocks for the production of alcohols and ethers: Current changes and perspective. Chemosphere 2022, 286, 131587. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pedroza, J.d.J.; Sánchez-Ramírez, E.; Segovia-Hernández, J.G.; Hernández, S.; Orjuela, A. Recovery of alcohol industry wastes: Revaluation of fusel oil through intensified processes. Chem. Eng. Process.—Process Intensif. 2021, 163, 108329. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Hatami, T.; Martínez, J.; Ciftci, O. Biocatalytic production of isoamyl acetate from fusel oil in supercritical CO2. J. Supercrit. Fluids 2020, 164, 104917. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Kongparakul, S.; Karnjanakom, S.; Reubroycharoen, P.; Guan, G.; Chanlek, N.; Samart, C. Selective production of green solvent (isoamyl acetate) from fusel oil using a sulfonic acid-functionalized KIT-6 catalyst. Mol. Catal. 2020, 484, 110724. [Google Scholar] [CrossRef]
- Monroe, E.; Shinde, S.; Carlson, J.S.; Eckles, T.P.; Liu, F.; Varman, A.M.; George, A.; Davis, R.W. Superior performance biodiesel from biomass-derived fusel alcohols and low grade oils: Fatty acid fusel esters (FAFE). Fuel 2020, 268, 117408. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Bredda, E.; de Castro, H.; Da Rós, P. Enzymatic catalysis: An environmentally friendly method to enhance the transesterification of microalgal oil with fusel oil for production of fatty acid esters with potential application as biolubricants. Fuel 2020, 273, 117786. [Google Scholar] [CrossRef]
- Cerón, A.A.; Boas, V.; Biaggio, F.C.; De Castro, H.F. Synthesis of biolubricant by transesterification of palm kernel oil with simulated fusel oil: Batch and continuous processes. Biomass Bioenergy 2018, 119, 166–172. [Google Scholar] [CrossRef]
- Neagu, M. The Potential Environmental Benefits of Utilising Oxy-Compounds as Additives In Gasoline, a Laboratory Based Study. In Environmental Health—Emerging Issues and Practice; Oosthuizen, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 147–176. [Google Scholar]
- Ardebili, S.M.S.; Solmaz, H.; Duygu İpci, D.; Calam, A.; Mostafaei, M. A review on higher alcohol of fusel oil as a renewable fuel for internal combustion engines: Applications, challenges, and global potential. Fuel 2020, 279, 118516. [Google Scholar] [CrossRef]
- Simsek, S.; Ozdalyan, B. Improvements to the Composition of Fusel Oil and Analysis of the Effects of Fusel Oil–Gasoline Blends on a Spark-Ignited (SI) Engine’s Performance and Emissions. Energies 2018, 11, 625. [Google Scholar]
- Ghazali, M.F.; Rosdi, S.M.; Erdiwansyah, E.; Mamat, R.B. Effect of the ethanol-fusel oil mixture on combustion stability, efficiency, and engine performance. Results Eng. 2025, 25, 104273. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ibrahim, T.K.; Kettner, M.; Kadirgama, K.; Leman, A.M.; Saiful, A.I.M. Effects of fusel oil water content reduction on fuel properties, performance and emissions of SI engine fueled with gasoline -fusel oil blends. Renew. Energy 2018, 118, 858–869. [Google Scholar] [CrossRef]
- Yılmaz, E. Investigation of the effects of diesel-fusel oil fuel blends on combustion, engine performance and exhaust emissions in a single cylinder compression ignition engine. Fuel 2020, 273, 117786. [Google Scholar] [CrossRef]
- Liu, J.; Dong, J.; Li, X.; Xu, T.; Li, Z.; Ampah, J.D.; Ikram, M.; Zhang, S.; Jin, C.; Geng, Z.; et al. Technical analysis of blending fusel to reduce carbon emission and pollution emission of diesel engine. Fuel Process. Technol. 2023, 241, 107560. [Google Scholar] [CrossRef]
- Luyben, W.L.; Chien, I.-L. Design and Control of Distillation Systems for Separating Azeotropes; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- De Guido, G.; Monticelli, C.; Spatolisano, E.; Pellegrini, L.A. Separation of the Mixture 2-Propanol + Water by Heterogeneous Azeotropic Distillation with Isooctane as an Entrainer. Energies 2021, 14, 5471. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhu, K.; Li, S.; Li, M.; Fan, X.; Gao, J. An efficient heat-pump extractive distillation process for recovering lower alcohols from bioethanol fusel oil. Chem. Eng. Process.—Process Intensif. 2025, 213, 110291. [Google Scholar] [CrossRef]
- Fu, C.; Li, Z.; Sun, Z.; Xie, S. A review of salting-out effect and sugaring-out effect: Driving forces for novel liquid-liquid extraction of biofuels and biochemicals. Front. Chem. Sci. Eng. 2021, 15, 854–871. [Google Scholar] [CrossRef]
- Zheng, P.Y.; Zhang, W.H.; Chen, K.F.; Wang, N.X.; An, Q.F. Pervaporation dehydration of fusel oil with sulfated polyelectrolyte complex hollow fiber membrane. J. Taiwan Inst. Chem. E 2019, 95, 627–634. [Google Scholar] [CrossRef]
- Okajima, I.; Ly, L.T.T.; Yi, K.C.; Sako, T. Phosphorus-free oil extraction from rice bran using CO2-expanded hexane. Chem. Eng. Process.—Process Intensif. 2021, 166, 108502. [Google Scholar] [CrossRef]
- Gasparetto, H.; de Castilhos, F.; Gonçalves Salau, N.P. Recent advances in green soybean oil extraction: A review. J. Mol. Liq. 2022, 361, 119684. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, Y. Exploring alternative solvents to n-hexane for green extraction of lipid from camellia oil cakes. Food Chem. X. 2025, 27, 102443. [Google Scholar] [CrossRef] [PubMed]
- van Thriel, C.; Boyes, K.W. Chapter Five—Neurotoxicity of organic solvents: An update on mechanisms and effects. Adv. Neurotox. 2022, 7, 133–202. [Google Scholar]
- Tala, W.; Janta, R.; Kraisitnitikul, P.; Chantara, S. Patterns and impact of volatile organic compounds on ozone and secondary organic aerosol formation: Implications for air pollution in Upper Southeast Asia. J. Hazard. Mater. Adv. 2025, 18, 100762. [Google Scholar] [CrossRef]
- Kumar, P.; Pandey, K.S. Chapter 31—Exploring Hexane’s impact: Toxicological insights, challenges, and forward-looking perspectives. In Hazardous Chemicals: Overview, Toxicological Profile, Challenges, and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2025; pp. 453–465. [Google Scholar]
- Hernandez, C.; Kaucz, A.P.; Condoret, J.S.; Remigy, J.C.; Pantel, C.A.; Camy, S. Life cycle assessment of refined sunflower oil production for food industry: Exploring hexane-free alternative using supercritical CO2 for processing pressed cake. J. Supercrit. 2026, 227, 106735. [Google Scholar] [CrossRef]
- Oliveira, É.R.; Silva, R.F.; Santos, P.R.; Queiroz, F. Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod. Process. 2019, 11, 47–58. [Google Scholar] [CrossRef]
- Gomis, V.; Pedraza, R.; Saquete, D.M.; Font, A.; García-Cano, J. Ethanol dehydration via azeotropic distillation with gasoline fractions as entrainers: A pilot-scale study of the manufacture of an ethanol–hydrocarbon fuel blend. Fuel 2015, 139, 568–574. [Google Scholar] [CrossRef]
- Stratula, C.; Oprea, F.; Mihaescu, D. The obtaining of anhydrous ethanol by azeotropic distillation using a new entrainer. Rev. Chim. 2005, 56, 544–548. [Google Scholar]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Nicolae, M.; Oprea, F.; Fendu, E.M. Dipropylene glycol as a solvent for the extraction of aromatic hydrocarbons. Analysis and evaluation of the solvency properties and simulation of the extraction processes. Chem. Eng. Res. Des. 2015, 104, 287–295. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R. Chemical Engineering Design: Principle, Practice and Economics of Plant and Process Design; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2008. [Google Scholar]
- Section 7: Separation Equipment. In GPSA Engineering Data Book, 14th ed.; Gas Processors Suppliers Association: Tulsa, OK, USA, 2017.
- Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. Available online: https://eur-lex.europa.eu/eli/dir/1991/271/oj/eng (accessed on 6 September 2025).
- Gomis, A.; García-Cano, J.; Font, A.; Gomis, V. Operational Limits in Processes with Water, Salt, and Short-Chain Alcohol Mixtures as Aqueous Two-Phase Systems and Problems in Its Simulation. Ind. Eng. Chem. Res. 2021, 60, 2578–2587. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Paul, S.; Roy, S.; Harvey, G.B.; Mitra, S. Recovery of Isoamyl Alcohol by Graphene Oxide Immobilized Membrane and Air-Sparged Membrane Distillation. Membranes 2024, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Hughes, S.; Maddox, I.S.; Cotta, M.A. Energy-efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess Biosyst. Eng. 2005, 27, 215–222. [Google Scholar] [CrossRef]
- Sander, A.; Rogoši´c, M.; Frljak, L.; Vasiljevi´c, D.; Blaževi´c, I.; Parlov Vukovi´c, J. Separating 2-Propanol and Water: A Comparative Study of Extractive Distillation, Salting-Out, and Extraction. Separations 2025, 12, 196. [Google Scholar] [CrossRef]
- Janković, T.; Straathof, J.J.A.; Kiss, A.A. A perspective on downstream processing performance for recovery of bioalcohols. J. Chem. Technol. Biotechnol. 2024, 99, 1933–1940. [Google Scholar] [CrossRef]
- Lakshmy, K.S.; Lal, D.; Nair, A.; Babu, A.; Das, H.; Govind, N.; Dmitrenko, M.; Kuzminova, A.; Korniak, A.; Penkova, A.; et al. Pervaporation as a Successful Tool in the Treatment of Industrial Liquid Mixtures. Polymers 2022, 14, 1604. [Google Scholar] [CrossRef]
- Fendu, E.M.; Nicolae, M. Synthesis and simulation of a distillation columns system for the propylene glycols mixtures separation. Eng. Rep. 2021, 3, 12301. [Google Scholar] [CrossRef]
- McCarley, K. Finding the Capacity of a Distillation Column. 2021. Available online: https://www.aiche.org/resources/publications/cep/2021/december/finding-capacity-distillation-column (accessed on 6 September 2025).
- Luyben, W.L. Plantwide control of an isopropyl alcohol dehydration process. AIChE J. 2006, 52, 2290–2296. [Google Scholar] [CrossRef]
- Woods, D.R. Rules of Thumb in Engineering Practice; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).