Advances in Virtual Reality-Based Physical Rehabilitation for Neurodegenerative Diseases: A Systematic Review
Abstract
1. Introduction
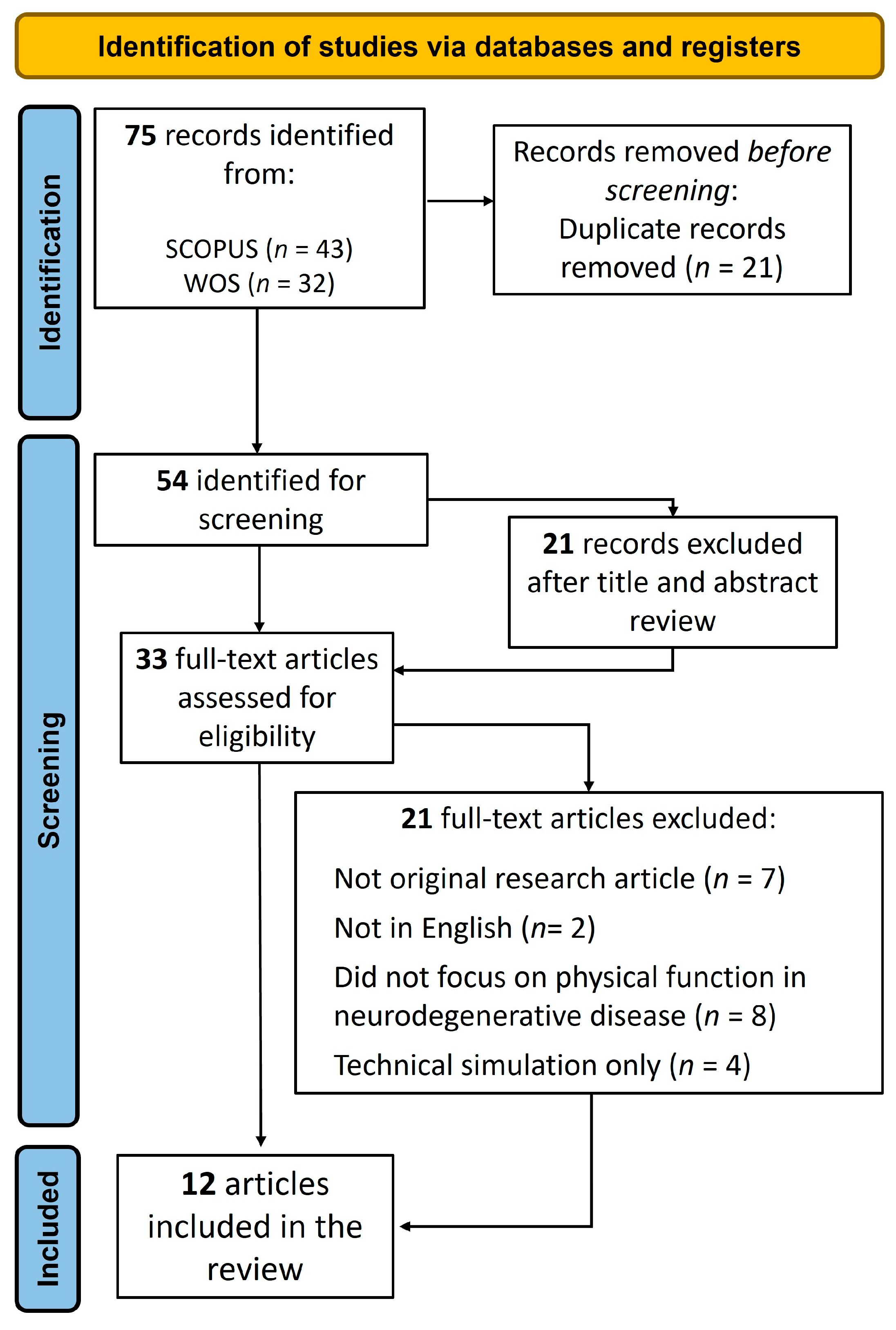
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Study Selection
3. Results
3.1. Study Characteristics
| Author(s), Year | VR * | Sample | Intervention | Assessment | Main Findings |
|---|---|---|---|---|---|
| Formica et al., 2023 [34] | Semi-immersive VR (CAREN * system) | Parkinson N = 31 (18 M *, 13 F *) No control group Age range = 18–73 | Duration: 2 months (8 weeks) Frequency: 3/week Session length: 50 min | Gait and balance: 10MWT *, BBS * General cognitive functioning: MoCA * Executive functioning: FAB * Coping strategies: COPE * Fear of falling: FES-I * Depression: HRS-D * | Cognitive and emotional benefits (executive function, anxiety, depression); greater physical and cognitive effort in response to stress. |
| Honzíková et al., 2025 [37] | Immersive VR (Meta Quest 2) | Parkinson N = 19 (sex not specified) No control group Mean age = 64.2 ± 12.8 | Duration: 1 month (4 weeks) Frequency: 2/week Session length: 20 min | Gait and balance: 10MWT, BBS Functional mobility: TUG * + dual task QoL *: PDQ-39 * | Improvements in stability, mobility, and quality of life. |
| Imbimbo et al., 2021 [35] | Semi-immersive VR (Nirvana system) | Parkinson N = 26 (22 M, 4 F) No control group Age range = 66.25–75.75 | Duration: 6 weeks Frequency: 2/week Session length: 45 min | Gait and balance: 6MWT *, BBS Cognitive reserve: CRI-q * | Improved balance and gait in patients with higher cognitive reserve. |
| Kashif et al., 2022 [29] | Non-immersive VR (wall-mounted display, Wii controllers and Wii Fit board) | Parkinson N = 44 (25 M, 19 F) Experimental group: n = 22 Control group: n = 22 Age range: 50–80 | Duration: 12 weeks Frequency: 3/week Session length: 60 min (40 min session + 20 min walking and cycling) | Motor function and balance: UPDRS *-III, BBS, ABC * ADLs *: UPDRS-II | Improved motor function, balance, and ADLs. |
| Kashif et al., 2024 [30] | Non-immersive VR (wall-mounted display, Wii controllers and Wii Fit board) | Parkinson N = 60 (33 M, 27 F) VR group: n = 20 MI * group: n = 20 PT * group: n = 20 | Duration: 12 weeks Frequency: 3/week Session length: 60 min (20 min VR/MI + 40 min PT) | Motor function and balance: UPDRS-III, BBS, ABC ADLs: UPDRS-II | VR showed better outcomes in balance and motor function vs. MI and PT. Best results were obtained for the VR + PT combination. |
| Malisky et al., 2024 [31] | Non-immersive VR (wall-mounted display, Wii controllers and Wii Fit board) | Spinocerebellar ataxia N = 28 (20 M, 8 F) No control group Age range: 15–70 | Duration: 10 weeks Frequency: 2/week Session length: 50 min | Balance: ABC ADLs: VADL * | Improved balance and gait, and reduced fall frequency. |
| Manuli et al., 2020 [32] | Semi-immersive VR (Nirvana system, CAREN system) Non-immersive VR (VRRS *) | Multiple sclerosis N = 84 (47 M, 37 F) No control group Age range: 18–75 | Duration: 8 weeks Frequency: 3–5/week Session length: 60 min | System usability: SUS * Goal achievement: GAS * Well-being perception: MSQOL * | Improved perceived QoL (physical and mental) and achievement of therapeutic goals. High satisfaction and usability. |
| Mazzari et al., 2025 [33] | Non-immersive VR (Technobody devices) | Parkinson N = 18 (sex not specified) Patients: n = 9 Controls: n = 9 Age range: 55–85 | Duration: 8 weeks Frequency: 2/week Session length: 60 min | Motor function and balance: TUG, BBS | Improvements in balance, gait, trunk flexion, pain threshold, and erector spinae displacement. |
| Pullia et al., 2023 [36] | Semi-immersive VR (C-Mill system) | Parkinson N = 20 (13 M, 7 F) Experimental group: n = 10 Control group: n = 10 Age range: 50–70 | Duration: 5 weeks Frequency: 4/week Session length: 45 min | Motor function, gait, and balance: 10MWT, 6MWT, TUG, UPDRS-III, TS *, BBS Fear of falling: FES-I ADLs: FIM * | Both conventional and VR training improved motor function; treadmill + VR improved endurance and postural control. |
| Rodríguez-Fuentes et al., 2024 [38] | Immersive VR (Meta Quest 3) | Multiple sclerosis N = 18 (5 M, 13 F) Control group: n = 10 Experimental group: n = 8 Age range: 18–65 | Duration: 5 weeks Frequency: 3/week Session length: 30 min | Motor function, gait, and balance: TS, TUG Functional mobility and strength: TUG + dual task, FTSST *, JHD * Fatigue: FSS * Reaction time: Rezzil software (1.9.0 version) VR-related assessments: SSQ *, SUS, GEQ, * ad hoc satisfaction questionnaire Perceived effort: Borg scale | High usability and satisfaction. Improved lower-limb endurance, functional mobility, and reduced fall risk. |
| Sánchez-Herrera-Baeza et al., 2020 [39] | Immersive VR (Oculus Rift 2, OR2-LMC) | Parkinson N = 6 (5 M, 1 F) No control group Age range: 69–80 | Duration: 6 weeks Frequency: 3/week Session length: 30 min | Strength: JHD Manual dexterity, coordination, fine motor speed: BBT *, PPT * Upper-limb performance: ARAT * Global satisfaction: CSQ-8 * | Improved strength, gross/fine coordination, and speed of movement on the affected side. |
| Schuch et al., 2020 [40] | Immersive VR (VR Box) | Parkinson N = 23 (16 M, 7 F) Experimental group: n = 11 (mean age: 63 ± 2.80) Control group: n = 12 (mean age: 69 ± 2.3) | Duration: 5 weeks Frequency: 2/week Session length: 28 min (8 min warm-up + 20 min VR) | Motor function, gait, and balance: UPDRS-III, 10MWT, TUG. Physical activity: IPAQ * General cognition: MMSE * Memory functioning: RBMT-3 * Anxiety: STAI * | No significant improvements in balance, mobility, or cognition. |
3.2. Study Quality and Validity
3.3. Intervention Effectiveness
3.3.1. Motor Function and Balance
3.3.2. Cognitive and Emotional Function
3.3.3. Activities of Daily Living and Quality of Life
3.3.4. Subjective Assessment and Usability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vyawhare, P.G.; Ahire, G.M.; Yadav, N.M.; Tasgaonkar, R.R. Neurodegenerative Disorder. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 824–828. [Google Scholar] [CrossRef]
- Adlakha, S.; Chhabra, D.; Shukla, P. Effectiveness of gamification for the rehabilitation of neurodegenerative disorders. Chaos Solitons Fractals 2020, 140, 110192. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer’s or vascular type: A review. Alzheimers Res. Ther. 2013, 5, 35. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Dahlberg, C.; Kalmar, K.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Felicetti, T.; Giacino, J.T.; Harley, J.P.; Harrington, D.E.; et al. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch. Phys. Med. Rehabil. 2000, 81, 1596–1615. [Google Scholar] [CrossRef]
- Zuschnegg, J.; Schoberer, D.; Häussl, A.; Lassacher, D.; Lohrmann, C.; Eglseer, D. Effectiveness of computer-based interventions for community-dwelling people with cognitive decline: A systematic review with meta-analyses. BMC Geriatr. 2023, 23, 229. [Google Scholar] [CrossRef]
- Rizzo, A.A. Virtual reality and disability: Emergence and challenge. Disabil. Rehabil. 2002, 24, 567–569. [Google Scholar] [CrossRef]
- Weiss, P.L.; Kizony, R.; Feintuch, U.; Katz, N. Virtual reality in neurorehabilitation. In Textbook of Neural Repair and Rehabilitation: Medical Neurorehabilitation; Selzer, M., Clarke, S., Cohen, L., Duncan, P., Gage, F., Eds.; Cambridge University Press: Cambridge, UK, 2006; Volume 2, pp. 182–197. [Google Scholar]
- Mancuso, V.; Sarcinella, E.D.; Bruni, F.; Arlati, S.; Di Santo, S.G.; Cavallo, M.; Cipresso, P.; Pedroli, E. Systematic review of memory assessment in virtual reality: Evaluating convergent and divergent validity with traditional neuropsychological measures. Front. Hum. Neurosci. 2024, 18, 1380575. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.A.; Kim, G.J. A SWOT Analysis of the Field of Virtual Reality Rehabilitation and Therapy. Presence 2005, 14, 119–146. [Google Scholar] [CrossRef]
- Quan, W.; Liu, S.; Cao, M.; Zhao, J. A Comprehensive Review of Virtual Reality Technology for Cognitive Rehabilitation in Patients with Neurological Conditions. Appl. Sci. 2024, 14, 6285. [Google Scholar] [CrossRef]
- Høeg, E.R.; Povlsen, T.M.; Bruun-Pedersen, J.; Lange, B.; Nilsson, N.C.; Haugaard, K.; Faber, S.M.; Hansen, S.W.; Kimby, C.K.; Serafin, S. System Immersion in Virtual Reality-Based Rehabilitation of Motor Function in Older Adults: A Systematic Review and Meta-Analysis. Front. Virtual Real. 2021, 2, 647993. [Google Scholar] [CrossRef]
- Lo, H.H.M.; Fong, P.Y.H.; Wang, B.; Fung, C.L.; Wong, S.Y.; Sit, R.W.S. Clinical Efficacy of Virtual Reality Cave Automatic Virtual Environments (CAVE) for Chronic Musculoskeletal Pain in Older Adults: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2025, 26, 105344. [Google Scholar] [CrossRef]
- Kim, W.S.; Cho, S.; Ku, J.; Kim, Y.; Lee, K.; Hwang, H.J.; Paik, N.J. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J. Clin. Med. 2020, 9, 3369. [Google Scholar] [CrossRef] [PubMed]
- Cicerone, K.D.; Goldin, Y.; Ganci, K.; Rosenbaum, A.; Wethe, J.V.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Kingsley, K.; Nagele, D.; et al. Evidence-Based Cognitive Rehabilitation: Systematic Review of the Literature From 2009 Through 2014. Arch. Phys. Med. Rehabil. 2019, 100, 1515–1533. [Google Scholar] [CrossRef]
- Martirosov, S.; Bureš, M.; Zítka, T. Cyber sickness in low-immersive, semi-immersive, and fully immersive virtual reality. Virtual Real. 2022, 26, 15–32. [Google Scholar] [CrossRef]
- Kourtesis, P.; Collina, S.; Doumas, L.A.A.; MacPherson, S.E. Validation of the Virtual Reality Neuroscience Questionnaire: Maximum Duration of Immersive Virtual Reality Sessions Without the Presence of Pertinent Adverse Symptomatology. Front. Hum. Neurosci. 2019, 13, 417. [Google Scholar] [CrossRef]
- Buele, J.; Palacios-Navarro, G. Cognitive-motor interventions based on virtual reality and instrumental activities of daily living (iADL): An overview. Front. Aging Neurosci. 2023, 15, 1191729. [Google Scholar] [CrossRef]
- Maggio, M.G.; De Bartolo, D.; Calabrò, R.S.; Ciancarelli, I.; Cerasa, A.; Tonin, P.; Di Iulio, F.; Paolucci, S.; Antonucci, G.; Morone, G.; et al. Computer-assisted cognitive rehabilitation in neurological patients: State-of-art and future perspectives. Front. Neurol. 2023, 14, 1255319. [Google Scholar] [CrossRef]
- Geraets, C.N.W.; van der Stouwe, E.C.D.; Pot-Kolder, R.; Veling, W. Advances in immersive virtual reality interventions for mental disorders: A new reality? Curr. Opin. Psychol. 2021, 41, 40–45. [Google Scholar] [CrossRef]
- Tuena, C.; Borghesi, F.; Bruni, F.; Cavedoni, S.; Maestri, S.; Riva, G.; Tettamanti, M.; Liperoti, R.; Rossi, L.; Ferrarin, M.; et al. Technology-Assisted Cognitive Motor Dual-Task Rehabilitation in Chronic Age-Related Conditions: Systematic Review. J. Med. Internet Res. 2023, 25, e44484. [Google Scholar] [CrossRef]
- Bergó, E.; Lombardi, G.; Pambuku, A.; Della Puppa, A.; Bellu, L.; D’Avella, D.; Zagonel, V. Cognitive Rehabilitation in Patients with Gliomas and Other Brain Tumors: State of the Art. Biomed. Res. Int. 2016, 2016, 3041824. [Google Scholar] [CrossRef]
- Georgiev, D.; Georgieva, I.; Gong, Z.; Nanjappan, V.; Georgiev, G. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sci. 2021, 11, 221. [Google Scholar] [CrossRef]
- Bonanno, M.; De Pasquale, P.; De Marchis, C.; Lombardo Facciale, A.; Paladina, G.; Fonti, B.; Quartarone, A.; Calabrò, R.S. Might patients with cerebellar ataxia benefit from the Computer Assisted Rehabilitation ENvironment (CAREN)? A pilot study focusing on gait and balance. Front. Bioeng. Biotechnol. 2024, 12, 1385280. [Google Scholar] [CrossRef]
- Da Silva, K.G.; Nuvolini, R.A.; Bacha, J.M.; De Freitas, T.B.; Doná, F.; Torriani-Pasin, C.; Pompeu, J.E. Comparison of the Effects of an Exergame-Based Program with Conventional Physiotherapy Protocol Based on Core Areas of the European Guideline on Postural Control, Functional Mobility, and Quality of Life in Patients with Parkinson’s Disease: Randomized Clinical Trial. Games Health J. 2023, 12, 228–241. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef]
- Janeh, O.; Steinicke, F. A Review of the Potential of Virtual Walking Techniques for Gait Rehabilitation. Front. Hum. Neurosci. 2021, 15, 717291. [Google Scholar] [CrossRef]
- Agostini, F.; Conti, M.; Morone, G.; Iudicelli, G.; Fisicaro, A.; Savina, A.; Mangone, M.; Paoloni, M. The Role of Virtual Reality in Postural Rehabilitation for Patients with Parkinson’s Disease: A Scoping Review. Brain Sci. 2025, 15, 23. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Kashif, M.; Ahmad, A.; Bandpei, M.A.M.; Gilani, S.A.; Hanif, A.; Iram, H. Combined effects of virtual reality techniques and motor imagery on balance, motor function and activities of daily living in patients with Parkinson’s disease: A randomized controlled trial. BMC Geriatr. 2022, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Albalwi, A.A.; Zulfiqar, A.; Bashir, K.; Alharbi, A.A.; Zaidi, S. Effects of virtual reality versus motor imagery versus routine physical therapy in patients with parkinson’s disease: A randomized controlled trial. BMC Geriatr. 2024, 24, 229. [Google Scholar] [CrossRef] [PubMed]
- Malisky, J.S.; Cavalcante-Leão, B.L.; Severiano, M.I.; Santos, G.J.B.D.; Teive, H.A.G.; José, M.R.; De Araújo, C.M.; Zeigelboim, B.S. Evaluation of Quality of Life After Use the Virtual Reality in Patients with Neurodegenerative Disease. Int. Arch. Otorhinolaryngol. 2024, 28, e523–e529. [Google Scholar] [CrossRef]
- Manuli, A.; Maggio, M.G.; Tripoli, D.; Gullì, M.; Cannavò, A.; La Rosa, G.; Sciarrone, F.; Avena, G.; Calabrò, R.S. Patients’ perspective and usability of innovation technology in a new rehabilitation pathway: An exploratory study in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 44, 102312. [Google Scholar] [CrossRef]
- Mazzari, L.; Zambon, E.; Tonzar, S.; Martini, M.; Sabot, R.; Galmonte, A.; Manganotti, P. Exergaming-Based Rehabilitation for Lateral Trunk Flexion in Parkinson’s Disease: A Pilot Study. Appl. Sci. 2025, 15, 1745. [Google Scholar] [CrossRef]
- Formica, C.; Bonanno, L.; Latella, D.; Ferrera, M.C.; Maresca, G.; Logiudice, A.L.; Sorbera, C.; Brigandì, A.; Di Lorenzo, G.; Marino, S. The effect of Computer Assisted Rehabilitation Environment (CAREN) in cognitive impairment and coping strategies in Parkinson’s disease: A preliminary study. Sci. Rep. 2023, 13, 2214. [Google Scholar] [CrossRef]
- Imbimbo, I.; Coraci, D.; Santilli, C.; Loreti, C.; Piccinini, G.; Ricciardi, D.; Castelli, L.; Fusco, A.; Bentivoglio, A.R.; Padua, L. Parkinson’s disease and virtual reality rehabilitation: Cognitive reserve influences the walking and balance outcome. Neurol. Sci. 2021, 42, 4615–4621. [Google Scholar] [CrossRef]
- Pullia, M.; Ciatto, L.; Andronaco, G.; Donato, C.; Aliotta, R.E.; Quartarone, A.; De Cola, M.C.; Bonanno, M.; Calabrò, R.S.; Cellini, R. Treadmill Training Plus SemiImmersive Virtual Reality in Parkinson’s Disease: Results from a Pilot Study. Brain Sci. 2023, 13, 1312. [Google Scholar] [CrossRef]
- Honzíková, L.; Dąbrowská, M.; Skřinařová, I.; Mullerová, K.; Čecháčková, R.; Augste, E.; Trdá, J.; Baníková, Š.; Filip, M.; Školoudík, D.; et al. Immersive Virtual Reality as Computer-Assisted Cognitive–Motor Dual-Task Training in Patients with Parkinson’s Disease. Medicina 2025, 61, 248. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, G.; Ferreiro-Gómez, E.; Campo-Prieto, P.; Cancela-Carral, J.M. Exergames and Immersive Virtual Reality as a Novel Therapy Approach in Multiple Sclerosis: Randomised Feasibility Study. J. Clin. Med. 2024, 13, 5845. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Herrera-Baeza, P.; Cano-De-La-Cuerda, R.; Oña-Simbaña, E.D.; Palacios-Ceña, D.; Pérez-Corrales, J.; Cuenca-Zaldivar, J.N.; Gueita-Rodriguez, J.; De Quirós, C.B.; Jardón-Huete, A.; Cuesta-Gomez, A. The Impact of a Novel Immersive Virtual Reality Technology Associated with Serious Games in Parkinson’s Disease Patients on Upper Limb Rehabilitation: A Mixed Methods Intervention Study. Sensors 2020, 20, 2168. [Google Scholar] [CrossRef]
- Schuch, C.P.; Balbinot, G.; Bonilla, M.N.; Machado, A.G.; De Oliveira, A.A. Feasibility of a Short-Term Virtual Reality Balance Intervention to Improve Mobility Smoothness in Parkinson’s Disease. Front. Virtual Real. 2020, 1, 7. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; pp. 219–272. [Google Scholar] [CrossRef]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias in Quasi-Experimental Studies. JBI Evid. Synth. 2024, 22, 378–388. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Randomized Controlled Trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Lockwood, C.; Munn, Z.; Porritt, K. Qualitative research synthesis: Methodological guidance for systematic reviewers utilizing meta-aggregation. Int. J. Evid. Based Healthc. 2015, 13, 179–187. [Google Scholar] [CrossRef]
- Lorca, M.; Araya, E.; Monrroy, M.; Enríquez, J.; Moscoso, P.; Montefusco, R.; Martín, M.S. Experience of the use and presence of cyber disease in inmmersive virtual reality exposure in urban community-dwelling older adults. Rev. Esp. Geriatr. Gerontol. 2025, 60, 101634. [Google Scholar] [CrossRef]
- Garcia-Navarra, S.; Llana, T.; Mendez, M. Spatial Memory Deficits in Parkinson’s Disease: Neural Mechanisms and Assessment. Am. J. Neurodegener. Dis. 2025, 14, 67–81. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, T.; Shang, H. The Impact of Motor-Cognitive Dual-Task Training on Physical and Cognitive Functions in Parkinson’s Disease. Brain Sci. 2023, 13, 437. [Google Scholar] [CrossRef]
- Kim, H.; Tobisawa, S.; Park, H.; Kim, J.; Lee, J.; Shin, D. Aging-induced degradation in tracking performance in three-dimensional movement. SICE J. Control Meas. Syst. Integr. 2024, 17, 239–246. [Google Scholar] [CrossRef]
- Schmidt, N.; Tödt, I.; Berg, D.; Schlenstedt, C.; Folkerts, A.; Ophey, A.; Dimenshteyn, K.; Elben, S.; Wojtecki, L.; Liepelt-Scarfone, I.; et al. Memory enhancement by multidomain group cognitive training in patients with Parkinson’s disease and mild cognitive impairment: Long-term effects of a multicenter randomized controlled trial. J. Neurol. 2021, 268, 4655–4666. [Google Scholar] [CrossRef]
- Van Balkom, T.D.; Van Den Heuvel, O.A.; Berendse, H.W.; Van Der Werf, Y.D.; Hagen, R.H.; Berk, T.; Vriend, C. Long-term effects of cognitive training in Parkinson’s disease: A randomized, controlled trial. Clin. Park. Relat. Disord. 2023, 9, 100204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solares, L.; Llana, T.; García-Navarra, S.; Mendez, M. Advances in Virtual Reality-Based Physical Rehabilitation for Neurodegenerative Diseases: A Systematic Review. Appl. Sci. 2025, 15, 9903. https://doi.org/10.3390/app15189903
Solares L, Llana T, García-Navarra S, Mendez M. Advances in Virtual Reality-Based Physical Rehabilitation for Neurodegenerative Diseases: A Systematic Review. Applied Sciences. 2025; 15(18):9903. https://doi.org/10.3390/app15189903
Chicago/Turabian StyleSolares, Lucía, Tania Llana, Sara García-Navarra, and Marta Mendez. 2025. "Advances in Virtual Reality-Based Physical Rehabilitation for Neurodegenerative Diseases: A Systematic Review" Applied Sciences 15, no. 18: 9903. https://doi.org/10.3390/app15189903
APA StyleSolares, L., Llana, T., García-Navarra, S., & Mendez, M. (2025). Advances in Virtual Reality-Based Physical Rehabilitation for Neurodegenerative Diseases: A Systematic Review. Applied Sciences, 15(18), 9903. https://doi.org/10.3390/app15189903






