Enhanced Sensitivity of 17-α-Ethinylestradiol (EE2) Detection Using Carbon Quantum Dots-Integrated Tapered Optical Fiber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
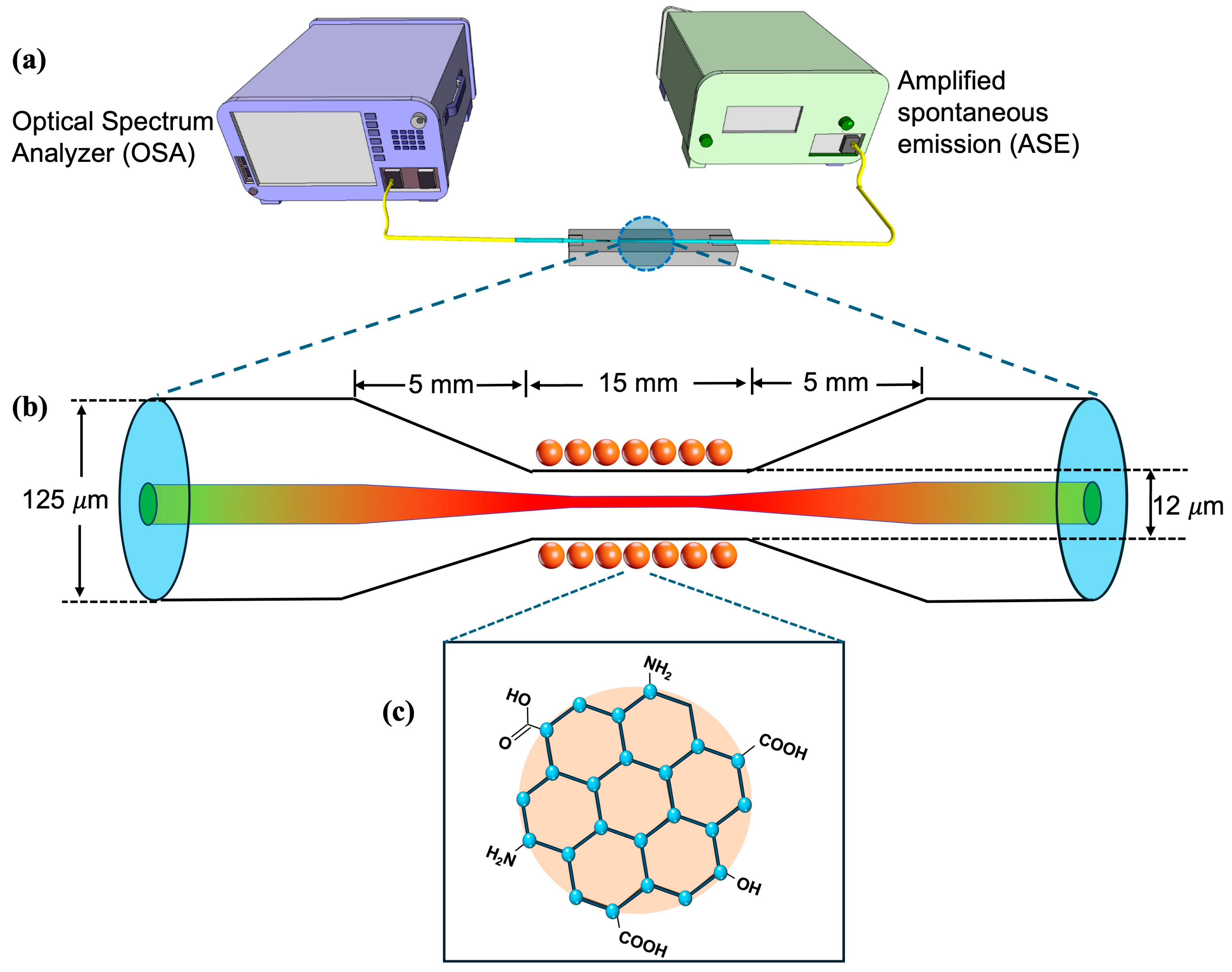
2.2. Experimental Setup and Fabrication of TOF
2.3. Deposition of CQDs on TOF
2.4. Surface Modification for EE2 Detection Using CQD-TOF
3. Results
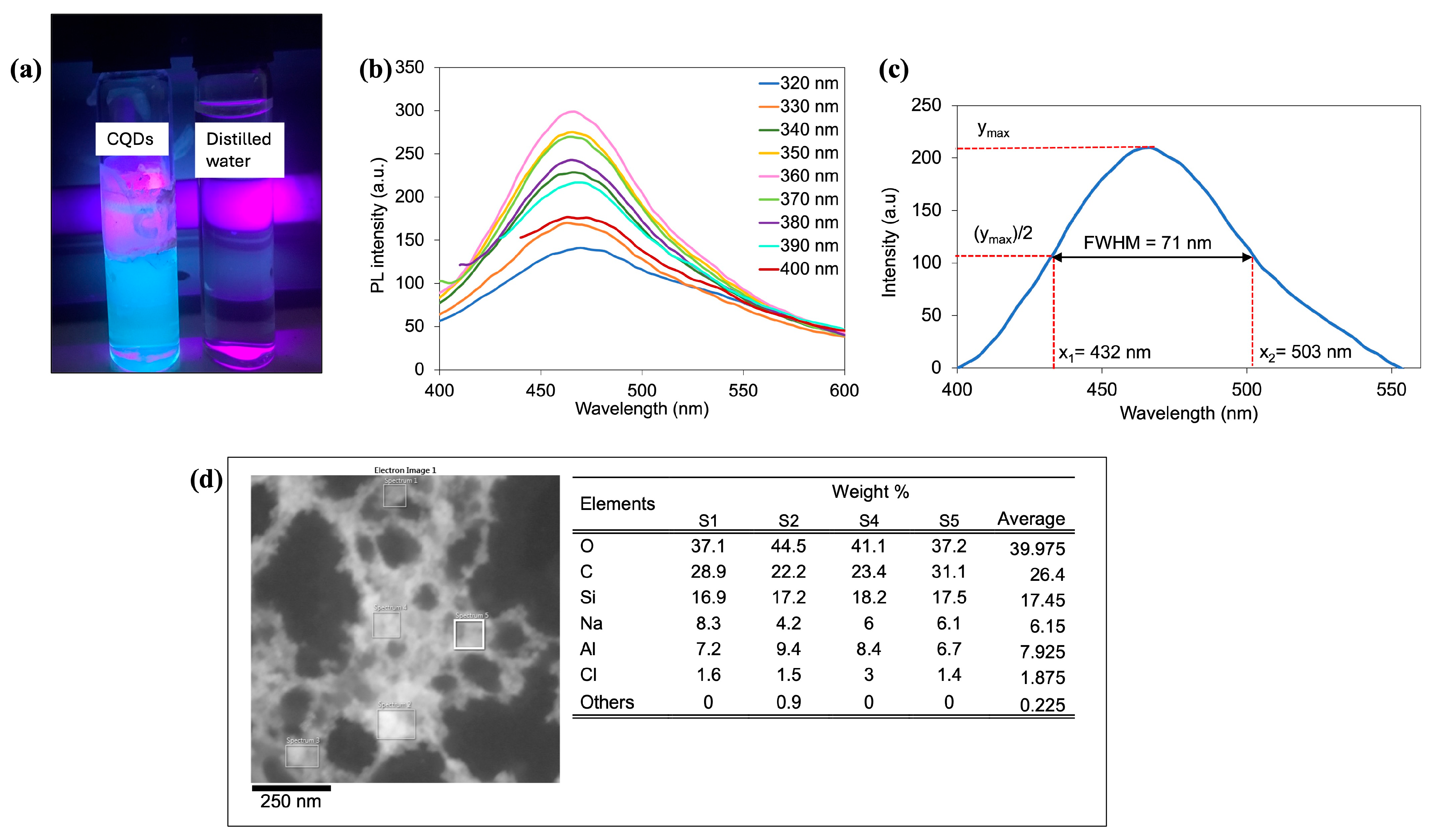
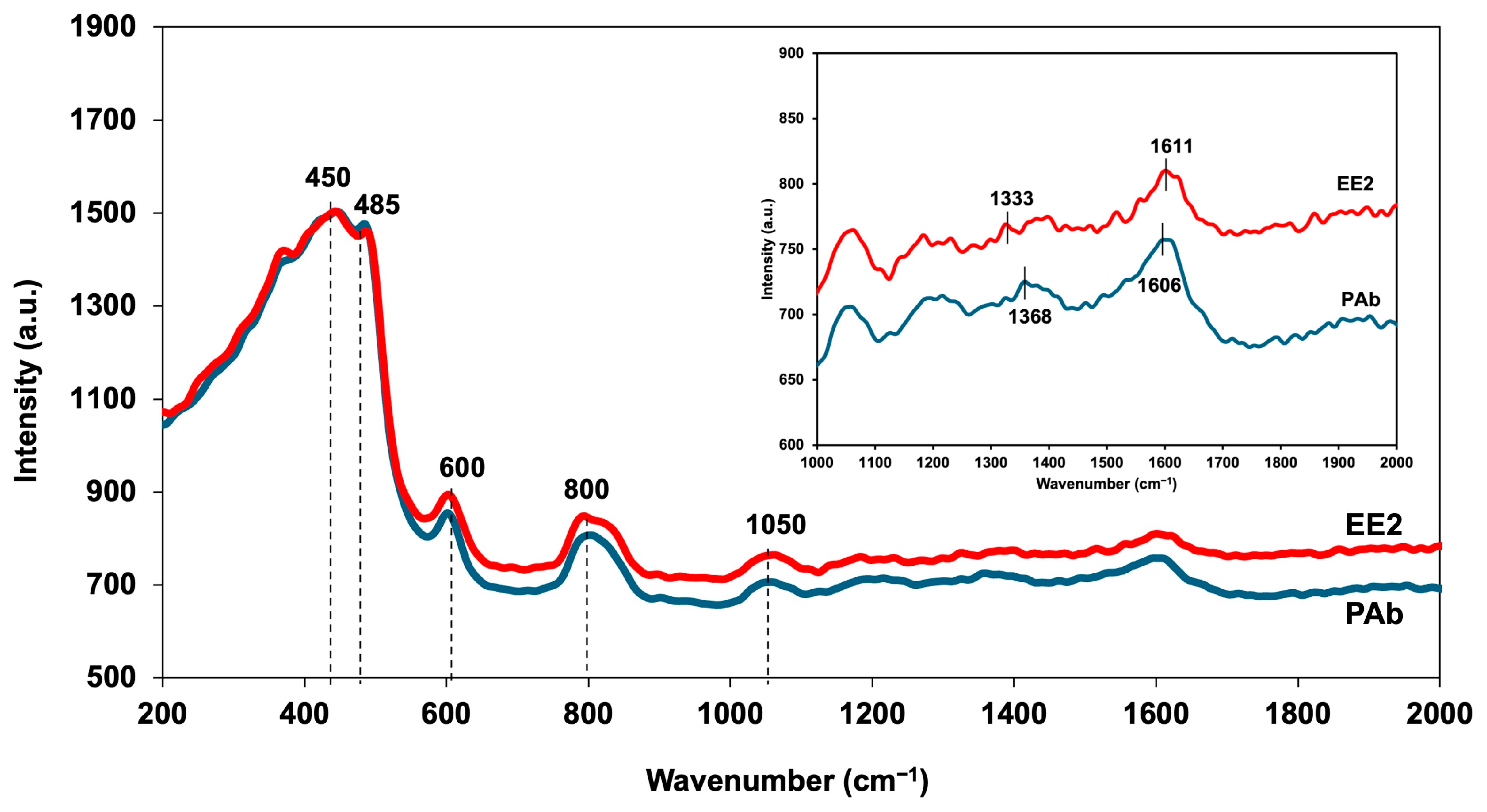
3.1. Characterization of CQDs Solution
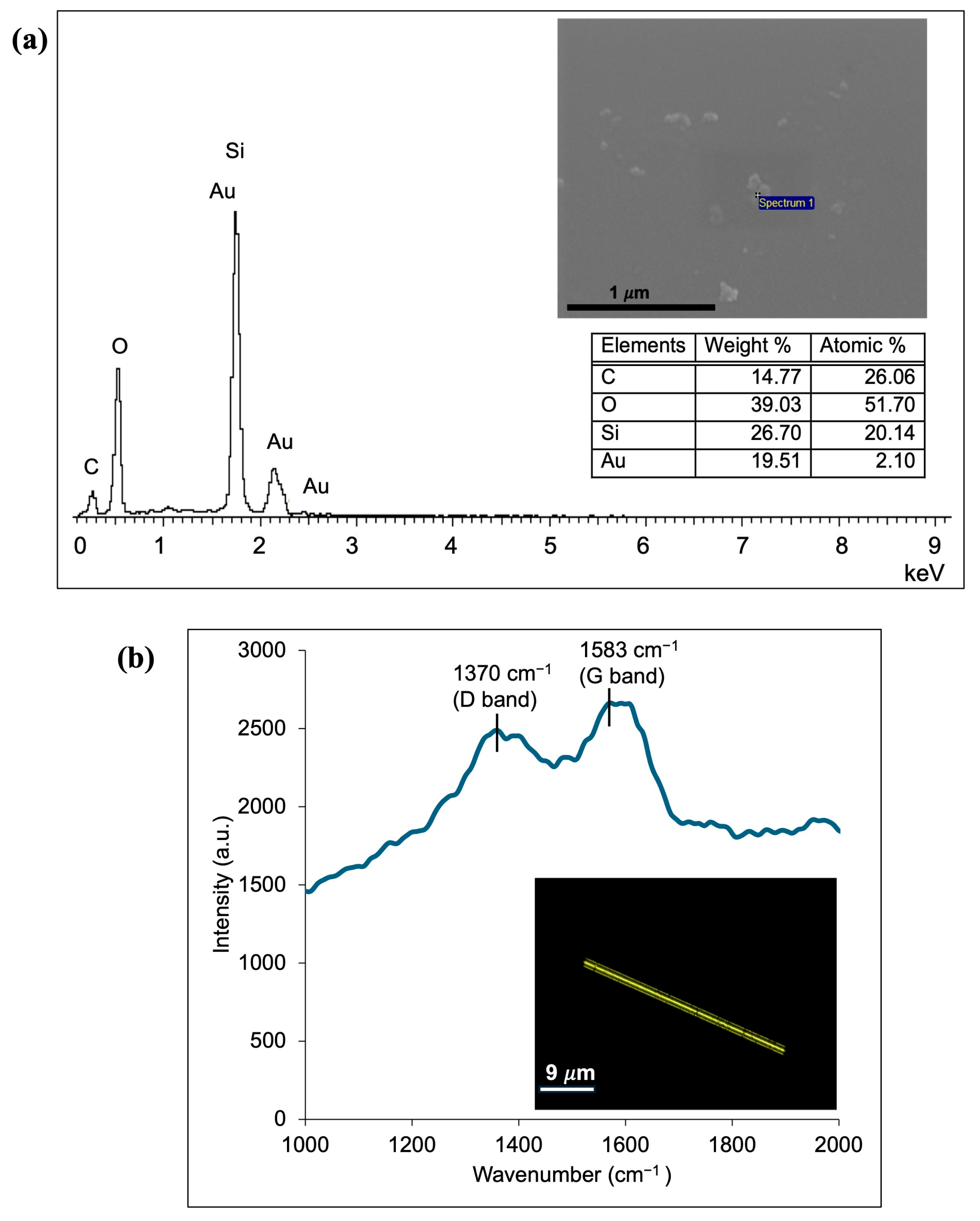
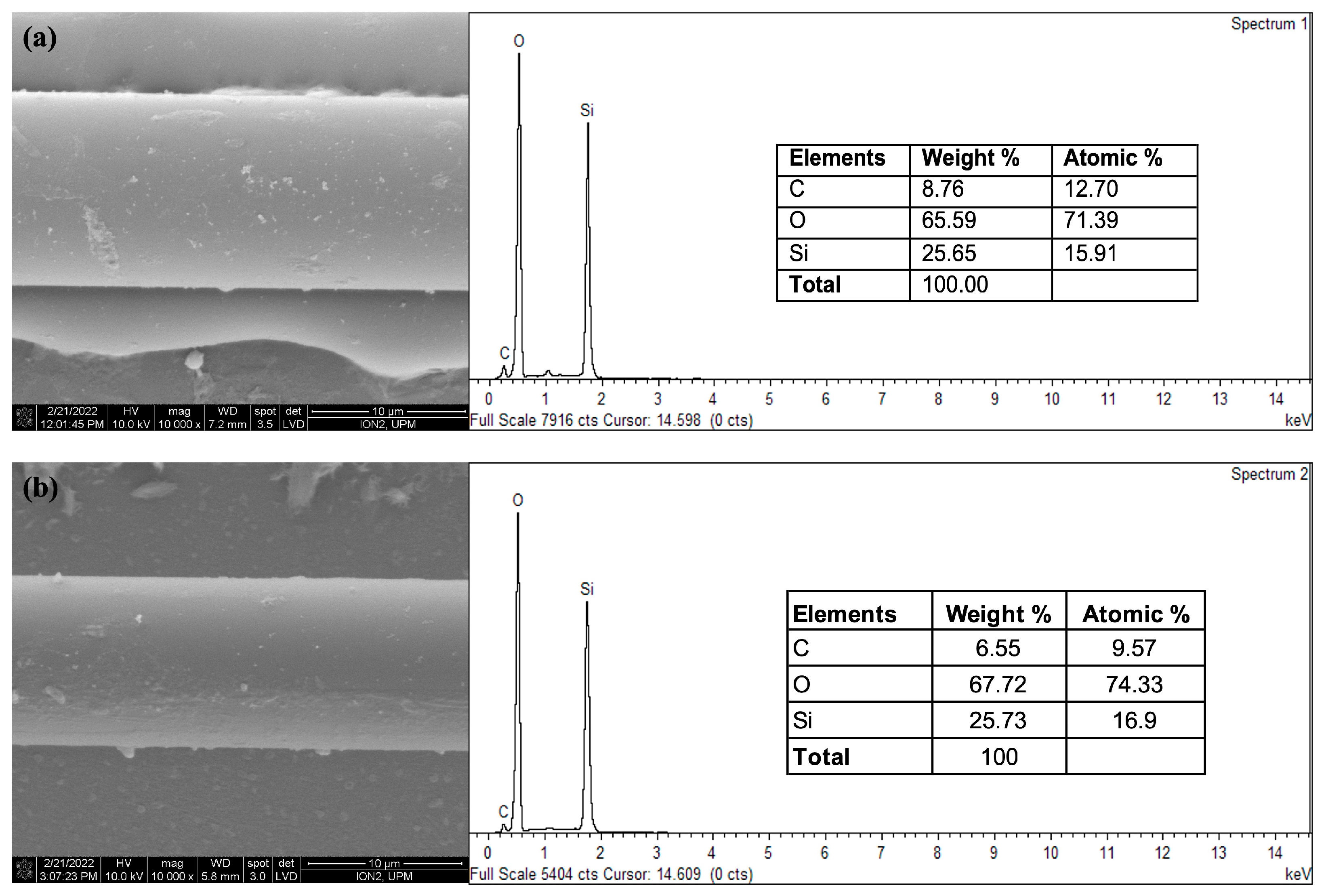
3.2. Characterization of CQD-TOF
3.3. EE2 Detection Using CQD-TOF
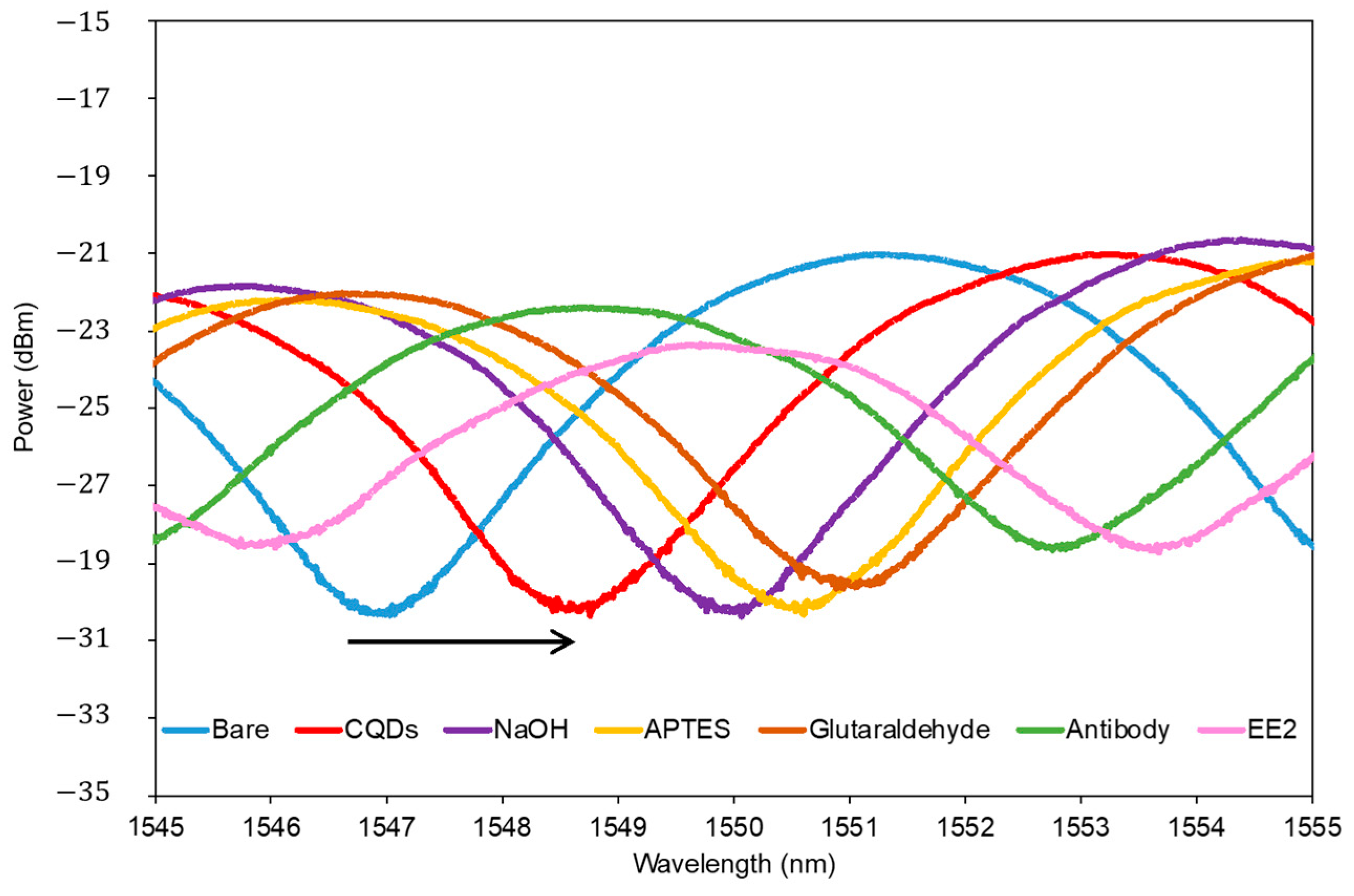
3.4. Selectivity of CQD-TOF
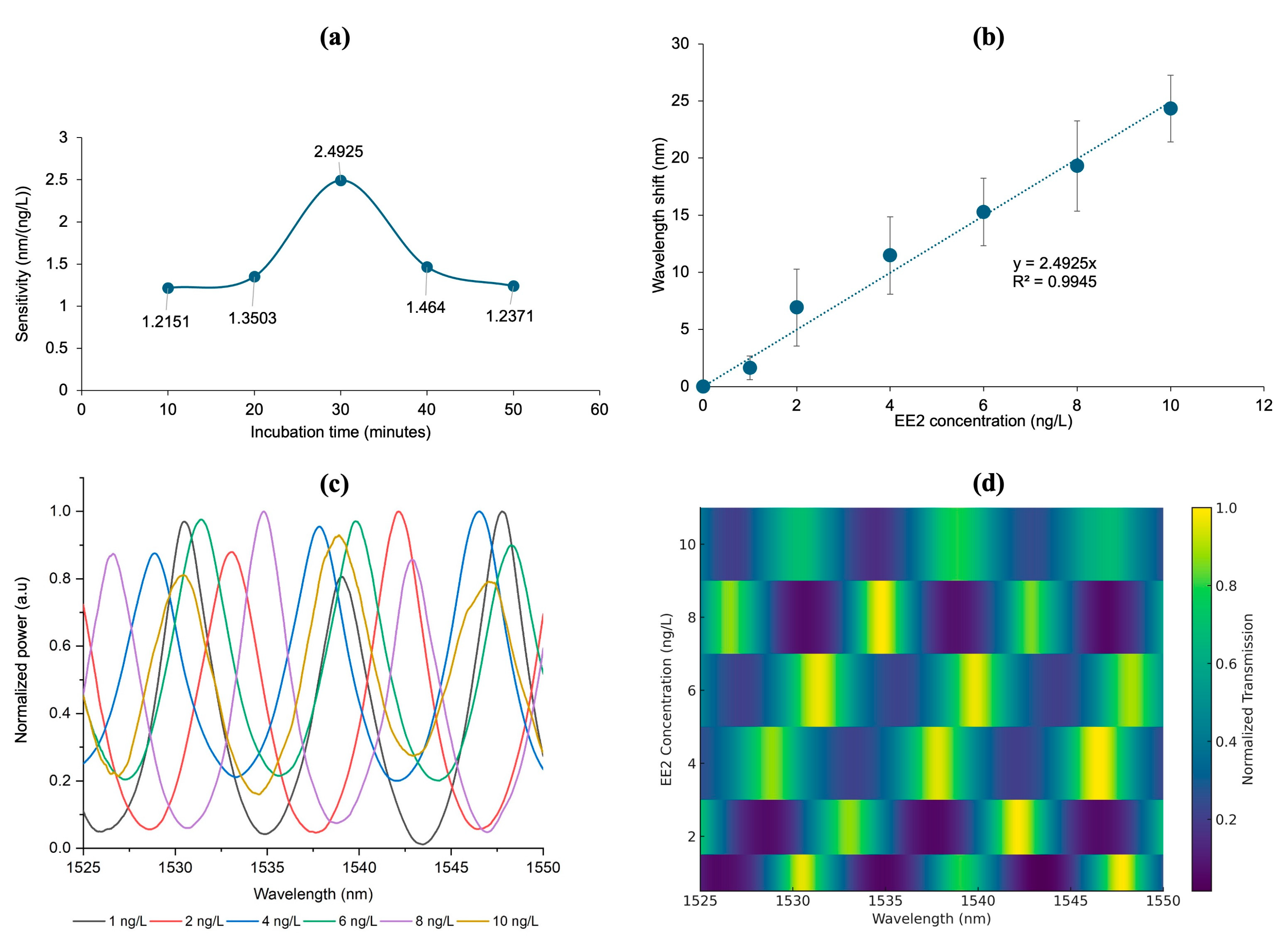
3.5. Sensitivity of and Limit of Detection of CQD-TOF Sensor
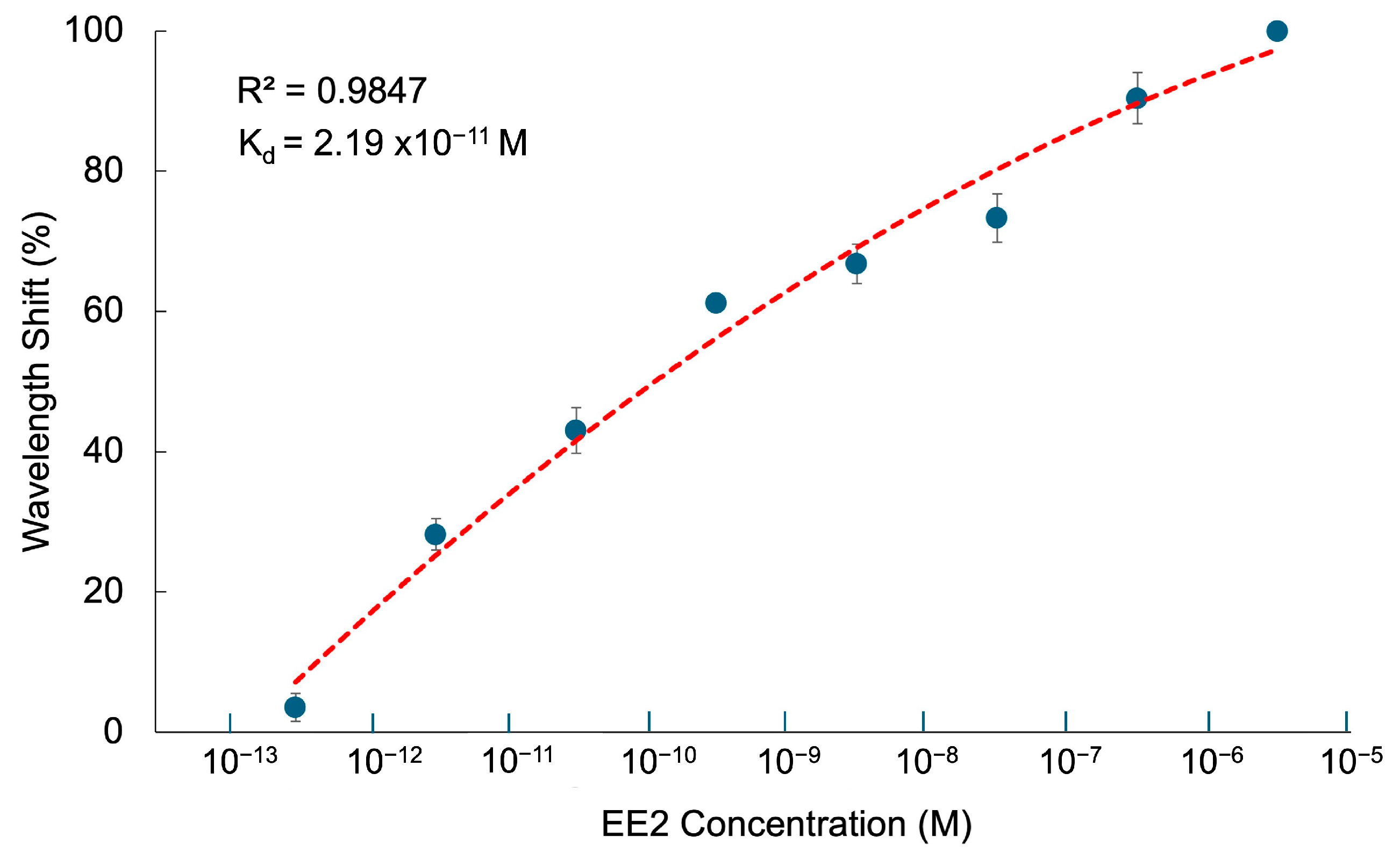
3.6. Binding Affinity of CQD-TOF Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, D.; Pisano, F.; Collard, L.; Balena, A.; Pisanello, M.; Spagnolo, B.; Mach-Batlle, R.; Tantussi, F.; Carbone, L.; De Angelis, F.; et al. Toward Plasmonic Neural Probes: SERS Detection of Neurotransmitters through Gold-Nanoislands-Decorated Tapered Optical Fibers with Sub-10 nm Gaps. Adv. Mater. 2023, 35, 2200902. [Google Scholar] [CrossRef]
- Zheng, D.; Kashif, M.F.; Piscopo, L.; Collard, L.; Ciracì, C.; De Vittorio, M.; Pisanello, F. Tunable Nanoislands Decorated Tapered Optical Fibers Reveal Concurrent Contributions in Through-Fiber SERS Detection. ACS Photonics 2024, 11, 3774–3783. [Google Scholar] [CrossRef]
- Polokhin, A.A.; Shaman, Y.P.; Itrin, P.A.; Panyaev, I.S.; Sysa, A.A.; Selishchev, S.V.; Kitsyuk, E.P.; Pavlov, A.A.; Gerasimenko, A.Y. Tapered Optical Fiber Sensor Coated with Single-Walled Carbon Nanotubes for Dye Sensing Application. Micromachines 2023, 14, 579. [Google Scholar] [CrossRef]
- Puspita, I.; Irawati, N.; Madurani, K.A.; Kurniawan, F.; Koentjoro, S.; Hatta, A.M. Graphene- and Multi-Walled Carbon Nanotubes-Coated Tapered Plastic Optical Fiber for Detection of Lard Adulteration in Olive Oil. Photonic Sens. 2022, 12, 220411. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. 2020. Available online: https://pubs.acs.org/doi/pdf/10.1021/acscentsci.0c01306?ref=article_openPDF (accessed on 1 November 2022).
- Khalaf, A.L.; Abdallah, A.; Shabaneh, A. Carbon Nanotubes and Graphene Oxide Applications in Optochemical Sensors; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Hui, S.; Yap, K.; Chan, K.K.; Zhang, G.; Tjin, S.C.; Yong, K. Carbon Dot-functionalized Interferometric Optical Fiber Sensor for Detection of Ferric Ions in Biological Samples. ACS Appl. Mater. Interfaces 2019, 11, 28546–28553. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, U.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E. A Review of Carbon Quantum Dots and Their Applications in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Irmawati, R.; Hashim, H.S.; Ramdzan, N.S.M.; Fauzi, N.I.M. A Review on Carbon Dots: Synthesis, Characterization and Its Application in Optical Sensor for Environmental Monitoring. Nanomaterials 2022, 12, 2365. [Google Scholar] [CrossRef] [PubMed]
- Pudza, M.Y.; Abidin, Z.Z.; Abdul-Rashid, S.; Yassin, F.M.; Noor, A.S.; Abdullah, M. Synthesis and Characterization of Fluorescent Carbon Dots from Tapioca. ChemistrySelect 2019, 4, 4140–4146. [Google Scholar] [CrossRef]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. C 2018, 4, 67. [Google Scholar] [CrossRef]
- Pramanik, A.; Biswas, S.; Kumbhakar, P. Solvatochromism in highly luminescent environmental friendly carbon quantum dots for sensing applications: Conversion of bio-waste into bio-asset. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 191, 498–512. [Google Scholar] [CrossRef]
- Nazri, N.A.; Azeman, N.H.; Bakar, M.H.; Mobarak, N.N.; Masran, A.S.; Zain, A.R.; Mahdi, M.A.; Saputro, A.G.; Wung, T.D.; Luo, Y.; et al. Polymeric carbon quantum dots as efficient chlorophyll sensor-analysis based on experimental and computational investigation. Opt. Laser Technol. 2024, 170, 110259. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Chee, H.Y.; Rashid, S.A.; Ahmad, M.Z.; Bakar, M.H.; Mahdi, M.A.; Yaacob, M.H. Carbon quantum dots functionalized tapered optical fiber for highly sensitive and specific detection of Leptospira DNA. Opt. Laser Technol. 2023, 157, 108696. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Chee, H.Y.; Ahmad, M.Z.; Mahdi, M.A.; Bakar, M.H.A.; Yaacob, M.H. Sensitive Leptospira DNA detection using tapered optical fiber sensor. J. Biophotonics 2018, 11, e201700363. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Huang, B.; Lin, C.; Zhou, J.; Pan, X. Biological effects and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in high-back crucian carp exposed to wastewater treatment plant effluents. Environ. Pollut. 2012, 162, 325–331. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, C.; Liu, J. Capture-SELEX of DNA Aptamers for Estradiol Specifically and Estrogenic Compounds Collectively. Environ. Sci. Technol. 2022, 56, 17702–17711. [Google Scholar] [CrossRef]
- Jackson, L.M.; Klerks, P.L. Impact of Long-Term Exposure to 17A-Ethinylestradiol in the Live-Bearing Fish Heterandria formosa. Arch Environ. Contam. Toxicol. 2019, 77, 51–61. [Google Scholar] [CrossRef]
- Caldwell, D.J.; Mastrocco, F.; Anderson, P.D.; Länge, R.; Sumpter, J.P. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem. 2012, 31, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.; Ribeiro, A.R.; Barbosa, M.O.; Ribeiro, C.; Tiritan, M.E.; Pereira, M.F.; Silva, A.M. Monitoring of the 17 EU Watch List contaminants of emerging concern in the Ave and the Sousa Rivers. Sci. Total Environ. 2019, 649, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Klaic, M.; Jirsa, F. 17α-Ethinylestradiol (EE2): Concentrations in the environment and methods for wastewater treatment—An update. R. Soc. Chem. 2022, 12, 12794–12805. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.; Cai, W. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1–59. [Google Scholar] [CrossRef]
- Patrícia, C.; Lima, D.L.D.; Schneider, R.J.; Otero, M.; Esteves, V.I. Development of ELISA methodologies for the direct determination of 17 b-estradiol and 17 a-ethinylestradiol in complex aqueous matrices. J. Environ. Manag. 2013, 124, 121–127. [Google Scholar] [CrossRef]
- Uraipong, C.; Allan, R.D.; Li, C.; Kennedy, I.R.; Wong, V.; Alice, N. A survey of 17α-ethinylestradiol and mestranol residues in Hawkesbury River, Australia, using a highly specific enzyme-linked immunosorbent assay (ELISA) demonstrates the levels of potential biological significance. Ecotoxicol Environ. Saf. 2017, 144, 585–592. [Google Scholar] [CrossRef]
- Schneider, C.; Schöler, H.F.; Schneider, R.J. Direct sub-ppt detection of the endocrine disruptor ethinylestradiol in water with a chemiluminescence enzyme-linked immunosorbent assay. Anal Chim Acta 2005, 551, 92–97. [Google Scholar] [CrossRef]
- Schneider, C.; Schöler, H.F.; Schneider, R.J. A novel enzyme-linked immunosorbent assay for ethynylestradiol using a long-chain biotinylated EE2 derivative. Steroids 2004, 69, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Gusmaroli, L.; Insa, S.; Petrovic, M. Development of an online SPE-UHPLC-MS/MS method for the multiresidue analysis of the 17 compounds from the EU B Watch list. Anal Bioanal. Chem. 2018, 410, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, C.; Cukrowska, E.; Chimuka, L. Determination of estrogen hormones in raw and treated water samples by reverse phase ultra-fast liquid chromatography mass spectrometry—A case study in Johannesburg South, South Africa. Water SA 2018, 44, 111–117. [Google Scholar]
- Honda, L.; Becerra-Herrera, M.; Richter, P. Liquid chromatography–time-of-flight high-resolution mass spectrometry study and determination of the dansylated products of estrogens and their hydroxylated metabolites in water and wastewater. Anal Bioanal. Chem. 2018, 410, 7909–7919. [Google Scholar] [CrossRef]
- Scala-Benuzzi, M.L.; Soler-Illia, G.J.; Raba, J.; Battaglini, F.; Schneider, R.J.; Pereira, S.V.; Messina, G. Immunosensor based on porous gold and reduced graphene platform for the determination of EE2 by electrochemical impedance spectroscopy. J. Electroanal. Chem. 2021, 897, 115604. [Google Scholar] [CrossRef]
- Barton, H.; Berbel-Filho, W.M.; Consuegra, S.; Francis, L.; Tizaoui, C.; Conlan, R.S.; Teixeira, S.R. Ultrasensitive environmental assessment of xeno-estrogens in water samples using label-free graphene immunosensors. Anal Biochem. 2018, 548, 102–108. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mercante, L.A.; Facure, M.H.; Pena, R.B.; Sanfelice, R.C.; Mattoso, L.H.; Correa, D.S. Ultrasensitive biosensor based on polyvinylpyrrolidone/chitosan/reduced graphene oxide electrospun nanofibers for 17α -ethinylestradiol electrochemical detection. Appl. Surf. Sci. 2018, 458, 431–437. [Google Scholar] [CrossRef]
- Kamil, Y.M.; Bakar, M.A.; Mustapa, M.A.; Yaacob, M.H.; Abidin, N.H.; Syahir, A.; Lee, H.J.; Mahdi, M.A. Label-free Dengue E protein detection using a functionalized tapered optical fiber sensor. Sens. Actuators B Chem. 2018, 257, 820–828. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Abdul-Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Fabrication, characterization and response surface method optimization for quantum efficiency of fluorescent nitrogen-doped carbon dots obtained from carboxymethylcellulose of oil palms empty fruit bunch. Chin. J. Chem. Eng. 2020, 28, 584–592. [Google Scholar] [CrossRef]
- Kamil, Y.M.; Bakar, M.H.; Yaacob, M.H.; Syahir, A.; Lim, H.N.; Mahdi, M.A. Dengue e Protein Detection Using a Graphene Oxide Integrated Tapered Optical Fiber Sensor. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Mustapha Kamil, Y.; Al-Rekabi, S.H.; Yaacob, M.H.; Syahir, A.; Chee, H.Y.; Mahdi, M.A.; Abu Bakar, M.H. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019, 9, 13483. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mohapatra, P.K.; Kalyanasundaram, D.; Kumar, S. Self-functionalized ultrastable water suspension of luminescent carbon quantum dots. Mater. Chem. Phys. 2019, 225, 23–27. [Google Scholar] [CrossRef]
- Aghamali, A.; Khosravi, M.; Hamishehkar, H.; Modirshahla, N.; Behnajady, M.A. Synthesis and characterization of high efficient photoluminescent sunlight driven photocatalyst of N-Carbon Quantum Dots. J. Lumin. 2018, 201, 265–274. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.A.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C Mater. 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Vasudevan, D.; Narayan, R.; Raju, K.V. Controllable synthesis of biosourced blue-green fluorescent carbon dots from camphor for the detection of heavy metal ions in water. RSC Adv. 2014, 4, 57137–57143. [Google Scholar] [CrossRef]
- Sun, R.; Liu, S. Synthesis of photoluminescent carbon dots and its effect on chondrocytes for knee joint therapy applications. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Saikia, B.K.; Dekaboruah, H.P.; Bordoloi, M.; Neog, D.; Bora, J.J.; Lahkar, J.; Narzary, B.; Roy, S.; Ramaiah, D. Blue-fluorescent and biocompatible carbon dots derived from abundant low-quality coals. J. Photochem. Photobiol. B 2019, 195, 1–11. [Google Scholar] [CrossRef]
- Mohsin, A.Z.; Sukor, R.; Mustapha-Kamil, Y.; Shatar, L.; Selamat, J.; Meor-Hussin, A.S.; Ismail, I.H.; Mahdi, M.A. Sensitive Detection of Goat αs1-Casein Using Tapered Optical Fiber Sensor. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Manghnani, M.H.; Hushur, A.; Sekine, T.; Wu, J.; Stebbins, J.F.; Williams, Q. Raman, Brillouin, and nuclear magnetic resonance spectroscopic studies on shocked borosilicate glass. J. Appl. Phys. 2011, 109, 2346. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, J.; Li, M.; He, F. Raman spectra of soda-lime-silicate glass doped with rare earth. Physica B Condens. Matter. 2011, 406, 3865–3869. [Google Scholar] [CrossRef]
- Fesenko, O.; Dovbeshko, G.; Dementjev, A.; Karpicz, R.; Kaplas, T.; Svirko, Y. Graphene-enhanced Raman spectroscopy of thymine adsorbed on single-layer graphene. Nanoscale Res. Lett. 2015, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Long, B.T.V.; Huege, F.R. The Laser-Raman Spectrum of Ferrocene. Chem. Commun. 1968, 20, 1239–1241. [Google Scholar] [CrossRef]
- Aksoy, C.; Severcan, F. Role of vibrational spectroscopy in stem cell research. Spectroscopy 2012, 27, 167–184. [Google Scholar] [CrossRef]
- Cheruku, R.; Bhaskaram, D.S.; Govindaraj, G. Variable range hopping and relaxation mechanism in graphene oxide sheets containing sp3 hybridization induced localization. J. Mater. Sci. Mater. Electron. 2018, 29, 9663–9672. [Google Scholar] [CrossRef]
- Qazi, H.H.; Memon, S.F.; Ali, M.M.; Irshad, M.S.; Ehsan, S.A.; Salim, M.R.; Mohammad, A.B.; Zulkifli, M.Z.; Idrees, M. Surface roughness and the sensitivity of D-shaped optical fibre sensors. J. Mod. Opt. 2019, 66, 1244–1251. [Google Scholar] [CrossRef]
- Zhong, N.; Zhu, X.; Liao, Q.; Wang, Y.; Chen, R.; Sun, Y. Effects of surface roughness on optical properties and sensitivity of fiber-optic evanescent wave sensors. Appl Opt. 2013, 52, 3937–3945. [Google Scholar] [CrossRef]
- Śmietana, M.; Dudek, M.; Koba, M.; Michalak, B. Influence of diamond-like carbon overlay properties on refractive index sensitivity of nano-coated optical fibres. Phys. Status Solidi (A) Appl. Mater. Sci. 2013, 210, 2100–2105. [Google Scholar] [CrossRef]
- Hamid, R.; Kamil, Y.M.; Aris, A.Z.; Bakar, M.H.; Suhailin, F.H.; Alresheedi, M.T.; Ng, E.K.; Mahdi, M.A. Detection of 17-α-Ethinylestradiol with Bio-Functionalized Tapered Optical Fiber Sensor. Measurement 2024, 238, 115305. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. Highly selective SPR-based fiber optic sensor for the detection of hydrogen peroxide. Sens. Actuators B Chem. 2021, 329, 129062. [Google Scholar] [CrossRef]
- Nodehi, M.; Baghayeri, M.; Ansari, R.; Veisi, H. Electrochemical quantification of 17 α–Ethinylestradiol in biological samples using a Au/Fe3O4 @ TA/MWNT/GCE sensor. Mater. Chem. Phys. 2020, 244, 122687. [Google Scholar] [CrossRef]
- Monteiro, M.D.; Sant’Anna, M.V.; Santos Junior, J.C.; Macedo, J.F.; Alves, A.A.; de Oliveira, S.; Silva, J.; Gimenez, I.F.; Midori Sussuchi, E. Reduced Graphene Oxide-based Sensor for 17α-Ehinylestradiol Voltammetric Determination in Wastewater, Tablets and Synthetic Urine Samples. Electroanalysis 2022, 34, 1422–1430. [Google Scholar] [CrossRef]
- Braga, G.B.; Oliveira, A.E.; Pereira, A.C. Total Determination of Estrogenic Phenolic Compounds in River Water Using a Sensor Based on Reduced Graphene Oxide and Molecularly Imprinted Polymer. Electroanalysis 2018, 30, 2176–2184. [Google Scholar] [CrossRef]
- Canevari, T.D.; Cincotto, F.H.; Nakamura, M.; Machado, S.A.; Toma, H.E. Efficient electrochemical biosensors for ethynylestradiol based on the laccase enzyme supported on single-walled carbon nanotubes decorated with nanocrystalline carbon quantum dots. Anal. Methods 2016, 39, 7254–7259. [Google Scholar] [CrossRef]
- Scala-Benuzzi, M.L.; Raba, J.; Soler-Illia, G.J.; Schneider, R.J.; Messina, G.A. Novel Electrochemical Paper-Based Immunocapture Assay for the Quantitative Determination of Ethinylestradiol in Water Samples. Anal. Chem. 2018, 90, 4104–4111. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.; Fen, Y.W.; Abdullah, J.; Mustapha Kamil, Y.; Daniyal, W.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive Detection of Dengue Virus Type 2 E-Proteins Signals Using Self-Assembled Monolayers/Reduced Graphene Oxide-PAMAM Dendrimer Thin Film-SPR Optical Sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef]


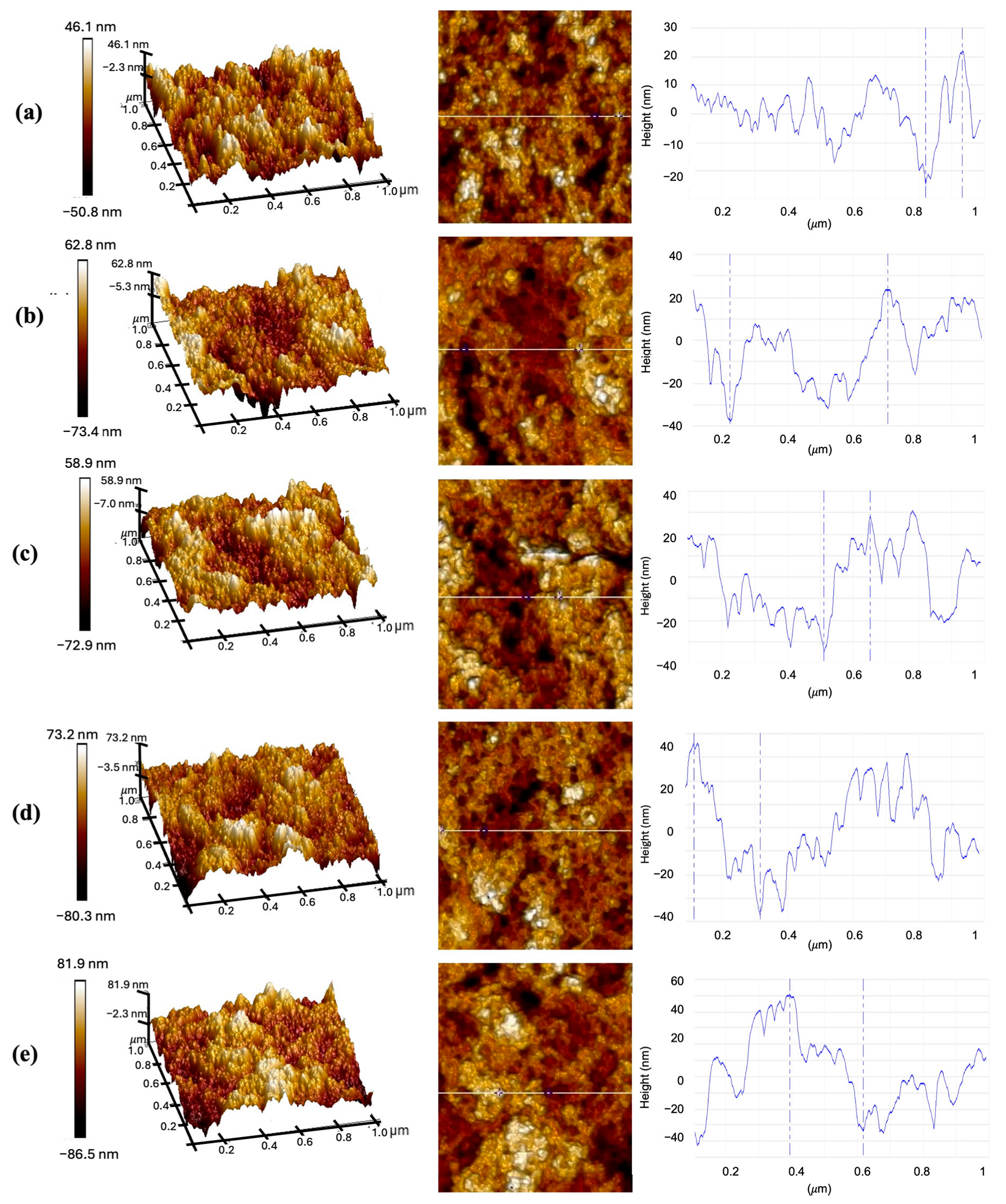

| Deposition Time (min) | Roughness (nm) | Thickness (nm) |
|---|---|---|
| 10 | 1.25 | 46.340 |
| 20 | 1.43 | 60.395 |
| 30 | 1.55 | 62.592 |
| 40 | 1.64 | 72.156 |
| 50 | 1.67 | 84.329 |
| Method | NPs Modifier | Recognition Mechanism | LOD | LDR | Ref. |
|---|---|---|---|---|---|
| EIS | Au/rGO | Antibody binding | 1.5 ng/L | 0–120 ng/L | [30] |
| EIS | GO | Antibody binding | 0.07 pg/L | 0.075–200 ng/L | [31] |
| Electrochemical DPV | Au/CNT | Direct oxidation | 0.987 µg/L | 0.00296–35.568 mg/L | [57] |
| Electrochemical DPV | rGO | Direct oxidation | 2.0126 µg/L | 0.0118–2.454 mg/L | [58] |
| Electrochemical DPV | rGO | MIP rebinding | 79.44 µg/L | 0.0474–4.446 mg/L | [59] |
| Electrochemical DPV | CNT/CQDs | Enzymatic oxidation | 1.1856 µg/L | 0.0474–4.446 mg/L | [60] |
| Electrochemical SWV | rGO | Antibody binding | 0.1 ng/L | 0.5–120 ng/L | [61] |
| Electrochemical amperometry | rGO | Enzymatic oxidation | 0.044 ng/L | 0.0741–5.928 ng/L | [32] |
| TOF | CQDs | Antibody binding | 0.0426 ng/L | 0–10 ng/L | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamid, R.; Mustapha Kamil, Y.; Aris, A.Z.; Abu Bakar, M.H.; Suhailin, F.H.; Alresheedi, M.T.; Ng, E.K.; Mahdi, M.A. Enhanced Sensitivity of 17-α-Ethinylestradiol (EE2) Detection Using Carbon Quantum Dots-Integrated Tapered Optical Fiber. Appl. Sci. 2025, 15, 9890. https://doi.org/10.3390/app15189890
Hamid R, Mustapha Kamil Y, Aris AZ, Abu Bakar MH, Suhailin FH, Alresheedi MT, Ng EK, Mahdi MA. Enhanced Sensitivity of 17-α-Ethinylestradiol (EE2) Detection Using Carbon Quantum Dots-Integrated Tapered Optical Fiber. Applied Sciences. 2025; 15(18):9890. https://doi.org/10.3390/app15189890
Chicago/Turabian StyleHamid, Rosyati, Yasmin Mustapha Kamil, Ahmad Zaharin Aris, Muhammad Hafiz Abu Bakar, Fariza Hanim Suhailin, Mohammed Thamer Alresheedi, Eng Khoon Ng, and Mohd Adzir Mahdi. 2025. "Enhanced Sensitivity of 17-α-Ethinylestradiol (EE2) Detection Using Carbon Quantum Dots-Integrated Tapered Optical Fiber" Applied Sciences 15, no. 18: 9890. https://doi.org/10.3390/app15189890
APA StyleHamid, R., Mustapha Kamil, Y., Aris, A. Z., Abu Bakar, M. H., Suhailin, F. H., Alresheedi, M. T., Ng, E. K., & Mahdi, M. A. (2025). Enhanced Sensitivity of 17-α-Ethinylestradiol (EE2) Detection Using Carbon Quantum Dots-Integrated Tapered Optical Fiber. Applied Sciences, 15(18), 9890. https://doi.org/10.3390/app15189890






