Abstract
This study evaluated the microbiological quality, free amino acid profile (FAAs), and the biogenic amine (BA) accumulation in beef steaks during extended storage under 0–4 °C in modified atmosphere (MAP O2/CO2/N2): MAP80/20/0, MAP70/20/10, MAP60/20/20, and vacuum (VP). The VP meat had significantly higher Enterobacteriaceae counts than MAP meat, influencing BA accumulation. The total plate count (TPC) exceeded the acceptable fresh meat limit (107 cfu/g) on day 28 of beef storage, regardless of packaging method. The dynamics of the changes in the FAAs differed between VP and MAP beef throughout storage, which affected the BAs’ formation. From day 14 of storage, VP beef steaks had lower or significantly lower content of (p ≤ 0.05) FAAs such as histidine, lysine, ornithine, phenylalanine, tryptophan, and tyrosine, and significantly (p ≤ 0.05) higher content of BAs such as histamine, cadaverine, putrescine, 2-phenylethylamine, tryptamine and tyramine compared to MAP beef. Based on BAI values, VP beef was spoiled on day 14, which was two weeks earlier than MAP beef, demonstrating that vacuum packaging promotes faster BA accumulation, due to the growth of Enterobacteriaceae under low-oxygen conditions. MAP provided more stable microbiological quality and lower BAI values of beef during storage than VP. No differences in shelf life between various MAP gas mixtures were observed; however MAP 80/20/0 slowed down the formation of biogenic amines, which was reflected by lower BAI values.
1. Introduction
Optimizing shelf life prediction and date labelling of different food products can significantly contribute to reducing food waste. This is especially important for demanding, highly perishable food like meat [1]. According to the FAO, one-third of its production is wasted, and half could have been avoided with more accurate shelf life estimations [2,3].
The shelf life of highly perishable food is typically assessed based on sensory and microbiological characteristics [4,5]. Food is considered unfit for consumption when it shows degraded sensory attributes or poses a risk to health due to the presence of pathogens and/or toxins [6]. In the case of meat, spoilage caused by the growth and metabolic activity of specific spoilage organisms is a major concern [7]. As an alternative to microbiological testing, researchers have proposed the use of chemical indicators, such as biogenic amines (BAs), for evaluating food safety and quality. BAs are produced by the decarboxylation of free amino acids (FAAs) through the action of microbial enzymes, reductive amination and transamination of aldehydes and ketones, or as a result of the activity of tissues [8,9]. Elevated FAA levels can lead to increased BA formation, depending on the amino acid content. Because FAAs serve as precursors to BAs, some researchers [10] have suggested that analyzing amino acid composition can be a useful tool for monitoring BA levels, especially those with known toxic activity. During meat storage, changes occur in the amino acid profile due to proteolysis, various enzymatic and microbial activities. These changes can impact the flavor, texture, and nutritional value of the meat, and consequently its shelf life [11,12].
The formation of BAs in meat is affected by different factors related not only to raw material or microbiological quality but also to processing and preservation technologies, including packaging methods [8,13]. During storage, BA levels in meat typically increase, raising safety concerns due to their potential toxicity. For instance, cadaverine is a compound that tends to accumulate in meat during storage and is often correlated with increased bacterial counts [14,15]. To extend shelf life and inhibit microbial growth in meat, and consequently BA formation, various packaging methods are used. These methods include modified atmosphere packaging (MAP) and vacuum packaging (VP), both of which are commonly used in the meat industry [1]. In the case of MAP, a gas mixture composition plays a crucial role in extending meat shelf life [16]. Beef and pork are commonly packaged under high-oxygen MAP (typically 80% O2 and 20% CO2) to enhance myoglobin oxygenation and improve meat color. However, high oxygen levels can accelerate lipid and protein oxidation, leading to increased FAA levels and subsequently higher BA content [17,18]. Therefore, reducing oxygen concentration while maintaining CO2 levels in the MAP gas mixture may offer a viable solution for improving meat quality by limiting microbial growth, oxidation, and BA formation [19]. Liang et al. [20] reported that the oxidative stability of pork was improved when 50% of O2 (instead of 80%) in the MAP gas mixture was used. In other studies, with the storage time, the content of putrescine, and to a lesser extent histamine, also increased in chicken fillets. The Biogenic Amine Index (BAI) was lowest in meat packed under high-oxygen MAP (75% O2 and 25% CO2) compared to samples packaged in air (on tray) or vacuum [21].
The available studies on the influence of extended storage time of beef packed using various methods regarding microbiological spoilage, FAAs content and BA accumulation have not yet been fully documented. Moreover, the influence of varying oxygen levels in MAP on beef quality during prolonged storage was not assessed in detail. This study provides an integrated assessment of FAAs, BAs, and microbial quality in beef steaks stored under VP and MAP with different oxygen levels, delivering new insights into spoilage dynamics and the optimization of beef storage strategies. Therefore, this study aimed to determine the microbiological quality, FAA profile, and BA accumulation of beef during extended refrigerated storage for 28 days under various packaging methods (three MAP gas mixtures and vacuum).
2. Materials and Methods
2.1. Beef Preparation and Packaging Conditions
The research material consisted of culinary beef (top round—m. semimembranosus). The beef was prepared according to standard procedures in the meat processing plant for culinary meat intended for commercial distribution. It was obtained in three independent experimental series, each from a separate production batch. In each experimental series, 10 muscles with appropriate quality characteristics (pH of 5.7–5.8) and an average weight of approximately 4 kg were selected and cut into steaks weighing approximately 350 g. Steaks were randomly assigned to one of three different MAP gas mixtures, each containing the same CO2 content set up at 20%, but with different O2 and N2 content:
(1) MAP80/20/0: 80% O2, 20% CO2 and 0% N2,
(2) MAP70/20/10: 70% O2, 20% CO2 and 10% N2,
(3) MAP60/20/20: 60% O2, 20% CO2 and 20% N2.
In addition, some steaks were vacuum-packed (VP). For each experimental series, 8 packages were prepared for each of the packaging variants (MAP80/20/0, MAP70/20/10, MAP60/20/20, and VP), resulting in a total of 24 packages per variant across all three series. In each series, 2 packages of each packaging variant were used at four time points, which allowed for the usage of 24 prepared packages of each packaging variant.
Beef steaks intended for MAP were placed on rigid PET trays (187 × 137 × 36 mm) (Bagstar, Marki, Poland) and packed using a Multivac T250 tray-sealer (Multivac, Natalin, Poland) packaging machine. First, MAP packages were flushed with an appropriate gas mixture (Air Products, Siewierz, Poland) mixed with a MAP gas mixer (Mix 9001 ME-3/200B, Mocon Europe A/S, Ringsted, Denmark), and then the packages were closed with the top foil (OPALEN B62, Bemis, Aylesbury, Buckinghamshire, United Kingdom). Beef steaks intended for VP were placed in PA/PE bags (170 × 200 mm, 0.070 mm thick) and closed in a Multivac C200 (Multivac, Natalin, Poland) chamber vacuum machine.
2.2. Beef Storage and Experimental Schedule
The packed beef steaks were stored under cooling conditions (0–4 °C) without light exposure for 28 days. The initial meat quality was assessed on the packaging day (day 0) and subsequently at four additional time points, i.e., every seven days, up to day 28. On the packaging day, the quality of the beef was evaluated prior to packaging. On days 7, 14, 21, and 28, two packages of each packaging variant were randomly selected for the analyses. The meat from the first package was used for microbiological analyses, and the meat from the second package was used for free amino acid profile and biogenic amine determination.
2.3. Methods
2.3.1. Determination of O2 and CO2 Content in MAP Packages
The O2 and CO2 content in prepared gas mixtures in MAP packages was determined, as described by Chmiel et al. [22], using a CheckMate 3 gas analyzer (Mocon Europe A/S, Ringsted, Denmark).
2.3.2. Microbiological Quality Evaluation
Packages with beef steaks were opened under sterile conditions on appropriate days of analysis. Samples were prepared using a sterile scalpel, according to Polish Standard PN-EN ISO 6887–2:2017 [23]. The microbiological evaluation included the determination of the total plate count of aerobic mesophilic microorganisms—TPC [24], count of psychrotrophic bacteria—PBC [25], lactic acid bacteria—LAB [26], Enterobacteriaceae [27], Brochothrix thermosphacta [28], and Pseudomonas spp. [29]. A detailed description of the microbiological methods used was provided by Chmiel et al. [30] and Cegiełka et al. [31]. All bacterial counts were expressed as colony-forming units per gram of meat (cfu/g).
2.3.3. Determination of Free Amino Acid and Biogenic Amine Profiles
Biogenic amine (BA) and free amino acid (FAA) concentrations were analyzed according to the method described by Świder et al. [32]. However, minor modifications were introduced. In brief, a weighed amount of the sample (2 g) was placed into a 50 mL centrifuge tube and spiked with the internal standard solution (1,7-diaminoheptane, Merck, Darmstadt, Germany). Then, the extraction step was performed as follows: 40 mL of 5% trichloroacetic acid solution (Avantor Performance Materials, Gliwice, Poland) was added, the sample was shaken thoroughly and centrifuged at 10,000× g for 10 min. In order to derivatize analytes, 100 μL of the extract was mixed with 2.5 mL of 0.05 M sodium carbonate (Merck, Darmstadt, Germany; water solution) and 2.5 mL of 20 mM dansyl-chloride (abcr GmbH, Karlsruhe, Germany; acetonitrile solution), and shaken in a water bath at 40 °C for 1 h. Subsequently, 10 μL of formic acid (Avantor Performance Materials, Gliwice, Poland) was added, and the sample was filtered through a 0.22 μm syringe filter (Captiva Econofilters, Agilent, Santa Clara, CA, USA) into a chromatographic vial. Certified analytical standards of biogenic amines and amino acids were purchased from Merck (Darmstadt, Germany).
An ultra-high performance liquid chromatograph (UPLC) coupled with a high-resolution mass spectrometer Q Exactive Orbitrap Focus MS (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine BA and FAA concentration. Full MS followed by All Ion Fragmentation (AIF) scan modes were applied. Scan ranges and resolutions were 200–1200 m/z and 70,000 in Full MS, respectively, and 80–1000 m/z and 35,000 in AIF, respectively. Analyses were conducted in a positive polarisation mode. Heated electrospray ionization (HESI) source parameters were as follows: spray voltage: 3 kV, capillary temperature: 256 °C, sheath gas flow rate: 48, auxiliary gas flow rate: 11, sweep gas flow rate: 2, probe heater temperature: 413 °C, S-lens RF level: 50. 2.5 μL of the sample was injected into an LC-MS system. The analytes were separated on the Raptor C18, 1.8 µm, 100 × 2.1 mm HPLC column supplied by Restek (Bellefonte, PA, United States). Liquid phases consisted of water/ACN (90:10)/0.1% FA/5 mM ammonium formate (phase A) and ACN/water (80:20)/0.1% FA/5 mM ammonium formate (phase B). The flow rate was set at 0.3 mL/min. The following liquid phase gradient was applied: A:B (%) gradient 0–2 min.—90:10—waste, 2–22 min.—0:100, 22–25 min.—0:100, 25–26 min.—90:10, 26–28 min.—90:10. LC-MS grade water and acetonitrile were supplied by Witko (Łódź, Poland). Formic acid (98–100%) and ammonium formate (≥97%) for LC-MS were purchased from Chem-Lab (Zedelgem, Belgium). Xcalibure 4.2.47 software (Thermo Fisher Scientific, Waltham, MA, USA) was used for data acquisition and analysis.
The values of biogenic amine indexes (BAI) were calculated by summing cadaverine, histidine, putrescine, and tyramine levels according to Triki et al. [33], and beef characterized by BAI > 50 mg/kg was classified in our studies as spoiled [34,35].
2.4. Statistical Analysis
The whole experiment was performed in triplicate. The data were presented as the mean ± standard deviation (SD) and analyzed using the StatisticaTM software v. 13.3 PL (StatSoft Inc., Tulsa, OK, USA). A one-way ANOVA was used for the data analysis, and the significant differences between treatments were determined according to Tukey’s HSD test (significance level α = 0.05). Pearson’s correlation coefficients were used to determine the relationship between BAs and bacterial counts.
Principal Component Analysis (PCA) was performed to reduce the dimensionality of the dataset and to identify patterns of variability between samples depending on packaging variant and storage time. The analysis was conducted using Python (v3.11) using the following libraries: pandas (v2.0.3), scikit-learn (v1.3.0), matplotlib (v3.7.1), and seaborn (v0.12.2). The input dataset consisted of 22 biochemical and microbiological variables. Categorical variables (Packaging variant and Day of storage) were excluded from the PCA modelling and used only for visual grouping on the plots. All numerical variables were standardized using Z-score normalization with the StandardScaler class from scikit-learn, transforming them to have a mean of 0 and a standard deviation of 1. Standardization was necessary due to differences in units and scales across the variables.
3. Results and Discussion
3.1. Changes in Gas Composition in MAP Packages During Beef Storage
During long-term storage, the levels of O2 within MAP80/20/0, MAP70/20/10 and MAP60/20/20 at day 28 significantly (p ≤ 0.05) decreased when compared to day 0 and 7 and were accompanied by a significant (p ≤ 0.05) increase in CO2 levels, especially after 21 days of storage (Figure 1). No significant (p > 0.05) differences in N2 levels (calculated as a supplement to the O2/CO2 gas mixture of 100%) between storage time days were observed (Figure 1).
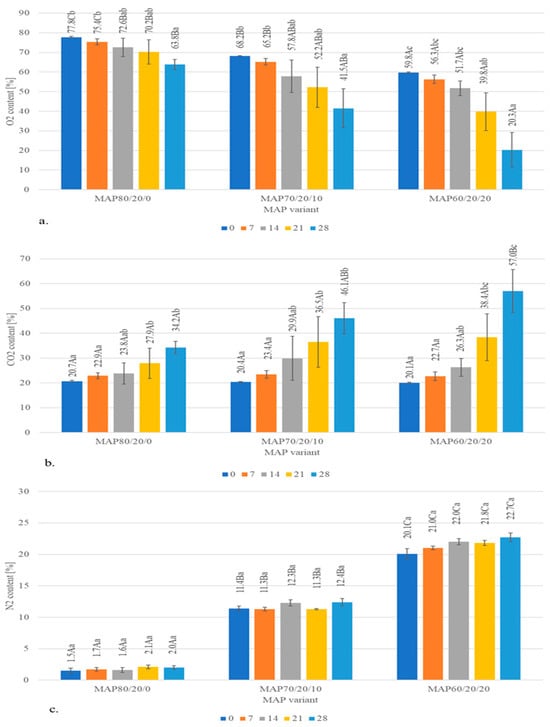
Figure 1.
Changes in O2 (a), CO2 (b) and N2 (c) content in MAP packages with beef steaks during long-term storage (mean values). The symbols identifying significant differences are as follows: A–C—means with different letters differ significantly at p ≤ 0.05 within MAP variants at particular day of storage; a–c—means with different letters differ significantly at p ≤ 0.05 within storage days for particular packaging variant.
Changes in the gas composition in MAP packages with meat were also observed by Yang et al. [36], who reported significant changes in O2 (decrease) and CO2 (increase) concentration in high-oxygen MAP during beef steaks storage time. The authors observed that this change was due to the increased bacterial respiratory metabolism and microbial outgrowth from day 10. This tendency could be related to the dominant bacteria in the refrigerated meat packed in MAP. The Pseudomonas sp. consume available O2 in the headspace of the MAP packages; facultative anaerobic bacteria, such as Brochothrix thermosphacta and LAB, produce CO2 as a metabolic product, and as a result, a reduction of O2 and the emission of CO2 into packages during the storage period is observed [37,38]. Such changes in gas mixture composition were also observed in our study (Figure 1). The combined effects of O2 depletion and CO2 inhibition create a selective environment in packages. Pseudomonas, an obligate aerobic bacterium, exhibits limited growth capacity under conditions of reduced O2 availability. With Pseudomonas suppressed, other microorganisms that are anaerobic or more tolerant to high CO2 may proliferate, leading to shifts in the dominant microbial population and potential spoilage [36].
In the studies of Conte-Junior et al. [38], a significant reduction of O2 and an increase in CO2 levels were observed for all the MAP types, similar to other studies [37]. Despite a significant increase in CO2 levels noted in our studies in all MAP variants at day 14–21, the antibacterial effect of CO2 is attributed to its initial concentration in the headspace of MAP. It should be at the level of >20% [39].
3.2. Changes in Microbiological Quality During Beef Storage
The initial (determined on packaging day) TPC of beef steaks was 2.2 × 103 cfu/g (Table 1), indicating that the meat was of acceptable microbiological quality in accordance with the standards outlined by Latou et al. [40]. Over time, a significant (p ≤ 0.05) increase in TPC was observed in meat in all packaging conditions. On day 28 of storage, the highest TPC level was observed for MAP80/20/0, reaching 1.3 × 108 cfu/g, while other packaging variants also exhibited elevated levels exceeding 107 cfu/g (Table 1). The maximum TPC limit for fresh meat according to Lázaro et al. [41] and Kim et al. [42] is 107 cfu/g. In our study, this limit was exceeded on the last day (day 28) of storage. Beef steaks packaged under MAP with the highest oxygen content (80%) exhibited the most rapid microbial growth, reaching the highest TPC values compared to other packaging variants. This suggests that elevated oxygen levels may promote the proliferation of aerobic spoilage microorganisms, thereby accelerating the decline in microbiological quality, which is in accordance with other findings [43,44].

Table 1.
Changes in microbiological quality of beef steaks during long-term storage (mean ± standard deviation).
Psychrotrophic bacteria (PBC) were detected in meat from all packaging variants on day 7 (Table 1). Regardless of the packaging method, the number of these bacteria groups increased with storage time, reaching a significantly higher number (p ≤ 0.05) on the last day of storage (107–108 cfu/g). Beef steaks packaged under MAP with higher oxygen levels (70% and 80%) showed the fastest growth of psychrotrophic bacteria, and their levels on day 28 of storage were significantly different (p ≤ 0.05) from the other packaging variants. As indicated by Al-Mazrouei et al. [45], psychrotrophic bacteria often dominate spoilage processes of meat stored at low temperatures, contributing to sensory deterioration through the production of off-odors, slime, and discoloration. Monitoring PBC can provide early insights into meat spoilage potential, particularly under high-oxygen MAP conditions, where spoilage may progress more rapidly [45,46].
A significant (p ≤ 0.05) increase in LAB was observed during storage time in all beef treatments on day 21, compared to day 1 and 7, and for MAP70/20/10, MAP60/20/20 and VP beef steaks also on day 28, reaching on that day >106 cfu/g, regardless of packaging variant (Table 1). At such levels, LAB are known to contribute to sensory deterioration of meat, leading to spoilage manifested by off-flavors, slime formation, and the production of organic acids such as lactic and acetic acids [47]. In addition, some LAB, particularly Lactobacillus (L.) divergens and L. carni, are capable of producing BAs, with tyramine being most commonly associated with their proliferation [48]. In the study by Liang et al. [20], storage time affected LAB counts in pork patties packed under optimized O2 levels, and with its extension, an increase in LAB was observed, regardless of packaging method. Lactic acid bacteria can grow quickly in fresh meat and are not efficiently inhibited by the presence of CO2 [36]. Different gas mixtures in MAP did not differentiate the LAB level as observed by Bassey et al. [49]. A similar trend was observed in our study, as no significant (p > 0.05) differences in LAB were detected between different packaging variants (Table 1).
Enterobacteriaceae bacteria are considered to be indicators of microbiological quality and hygiene in food production. An intensive increase in their population leads to negative sensory changes such as off-flavors occurrence, discoloration, and other quality deterioration, ultimately reducing the shelf life of meat [50]. In our studies, no Enterobacteriaceae were detected in meat stored under modified atmosphere packaging (MAP) up to day 7 (Table 1). In vacuum-packed (VP) meat, the presence of Enterobacteriaceae bacteria was noted at the earliest stage, i.e., on day 7. However, regardless of the beef packaging method used, the number of Enterobacteriaceae gradually increased over time, with a significant increase (p ≤ 0.05) observed at the end of the storage time, i.e., on day 28. At the end of the storage time, beef steaks stored under VP had significantly higher (p ≤ 0.05) counts of Enterobacteriaceae compared to those stored in MAP (Table 1).
These findings are consistent with research by Stella et al. [51] and Li et al. [52], who also identified Enterobacteriaceae as major contributors to the spoilage of vacuum-packaged meat. MAP is more effective than VP in suppressing the growth of spoilage-related Enterobacteriaceae bacteria during cold storage of meat [53]. According to Hou et al. [54], this is due to CO2 presence in gas mixtures for MAP, which has an inhibitory effect on the growth of aerobic and facultative anaerobic microorganisms. In contrast, vacuum packaging, the absence of oxygen in vacuum packaging, may create a more favorable anaerobic environment for certain spoilage bacteria, including some Enterobacteriaceae [55].
Enterobacteriaceae is the most common group responsible for the production of biogenic amines (BAs) in meat, as these microorganisms exhibit high decarboxylase activity and therefore are associated with the formation of two specific BAs, such as putrescine and cadaverine [41]. In our studies, the growth of Enterobacteriaceae was faster in VP beef than in MAP beef, which was also associated with greater accumulation of BAs such as cadaverine and putrescine in VP beef (Table 2) Chmiel et al. [56] reported a strong correlation between the presence of Enterobacteriaceae and the levels of histidine, tyramine, 2-phenylethylamine, tryptamine, putrescine, and cadaverine.

Table 2.
Changes in the free amino acids—direct BA precursors—profile of beef steaks during long-term storage (mean ± standard deviation).
Brochothrix thermosphacta is one of the primary spoilage microorganisms of meat, and its spoilage-causing activity is most noticeable in an ambient with lower concentrations of oxygen and higher concentrations of CO2. It can, however, grow very well in the vacuum and other types of environment, and it can cause spoilage even at low population levels [57]. Species of Brochothrix are able to ferment glucose, generating by-products such as acetate, lactate, and formate. The accumulation of these compounds in meat can significantly impair its sensory quality, mainly odor [58]. On day 0, Brochothrix thermosphacta was not detected in beef samples. However, as storage time extended, the bacterial counts increased significantly (p ≤ 0.05), regardless of the packaging method. On the final day of storage, the counts exceeded 106–107 cfu/g. No significant differences (p > 0.05) were observed between the packaging methods applied, although slightly higher counts were noted in meat stored under MAP (Table 1). According to Yang et al. [36], Brochothrix bacteria gain an advantage in beef steaks packed and stored in MAP with 80% O2 or 50% O2 content.
With prolonged storage, the counts of Pseudomonas spp. increased, reaching the highest level (>107 cfu/g) on day 28 in all beef samples (Table 1). According to Yang et al. [59], Pseudomonas spp. counts in the range of 107–108 cfu/g are typically associated with undesirable alterations in meat quality, including color changes, slime formation and the development of unpleasant odors. No significant differences in Pseudomonas spp. counts were detected in meat from different packaging methods, including MAP with different gas mixture composition (Table 1), which can be due to different strains among Pseudomonas genus [59]. Pseudomonas bacteria are recognized for their ability to decarboxylate the amino acid lysine. This process is linked with the formation of biogenic amines such as cadaverine in meat [60]. Moreover, putrescine can be accumulated through a single-step decarboxylation pathway by ornithine decarboxylase, an enzyme common to Pseudomonas, Enterobacteriaceae or LAB. Pseudomonas can produce not only significant amounts of putrescine and cadaverine but also accumulate histidine [61].
3.3. Changes in Free Amino Acid Profile During Beef Storage
Beef is a rich source of nine essential amino acids (EAAs) and also contains various non-essential amino acids (NEAAs) that contribute to its overall nutritional value [62]. In this discussion, particular attention was paid to changes in the profile of those amino acids that serve as direct precursors of biogenic amine formation, as presented in Table 2. The contents of the rest of the determined free amino acids are presented in Supplementary Table S1. Packaging methods do not significantly change the initial levels of amino acids in beef; however, they influence the shelf life and microbial growth, which can indirectly affect the breakdown of amino acids [34,63].
Arginine is a precursor of agmatine, as well as spermine and spermidine. Its levels changed significantly (p ≤ 0.05) during storage time, but depended on the packaging method. In beef stored in MAP80/20/0, arginine content increased over time, reaching the highest values on day 21. For beef stored in MAP70/20/10 and MAP60/20/20, the highest values were observed on days 21 and 14, respectively, followed by a decline on days 21 and 28. For VP beef, arginine content initially increased, but from day 14, a significant decrease was noted, to a level almost 8-fold lower than observed at the beginning of storage (Table 2). The effect of the packaging method was visible from day 14, and VP was characterized by the lowest arginine content (Table 2). During meat aging, the gradual release of amino acids is typical of proteolysis. This increase in amino acids begins after 14 days of storage and is proportional to the aging time [64,65].
Significant (p ≤ 0.05) differences in histidine content, a precursor of histamine, which is produced by the bacterial decarboxylation of histidine via L-histidine decarboxylase, were observed during beef storage under different packaging conditions. Compared to days 0 and 7, histidine content increased (p ≤ 0.05) on day 14. This was followed by a decreasing trend on day 21. On day 28, the decline continued in VP meat, while in MAP beef, a slight increase in histidine content was noted (Table 2). Significant differences (p ≤ 0.05) in histidine content in beef between packaging variants were found on day 14 of storage, and this trend continued till the end of the experiment, with VP beef characterised by lower or significantly lower levels than MAP beef. These differences could be associated with significantly higher counts of Enterobacteriaceae observed in VP beef starting from day 7 (Table 1) and significantly higher amounts of histamine, the decarboxylation product of histidine, in VP beef observed on days 21 and 28 (Table 3). High decarboxylase activity is attributed to the family Enterobacteriaceae, which is particularly associated with the production of biogenic amines such as histamine, cadaverine, and tyramine [61]. In contrast, Vierck et al. [63] found that beef steaks packaged in MAP with a high oxygen content (80% O2 and 20% CO2) were characterized by higher levels of histidine compared to steaks stored in other packaging systems. The authors suggested that proteins with amino acid residues with high electron density, such as histidine and tyrosine, are particularly susceptible to oxidation by singlet oxygen. One such protein is myoglobin, which uses histidine to key structural functions essential in maintaining its structure and function. In a high oxidative environment like MAP with high oxygen content, this may lead to increased histidine release from beef steaks during storage [63].

Table 3.
Changes in the biogenic amine profile of beef steaks during long-term storage and BAI (mean ± standard deviation).
Lysine, a precursor of cadaverine formed through microbial decarboxylation, showed significant changes during beef storage (Table 2). In meat stored in MAP, lysine content increased with storage time. Compared to day 0, significant increases (approximately 2.5-fold) were recorded on days 14, 21, and 28, with the highest levels observed on day 21. Conversely, in VP beef, lysine content decreased 2.5-fold compared to days 0 and 7 on day 14, and remained at a similar level on days 21 and 28. Triki et al. [33] observed a significant increase in lysine content during a 10-day storage time of beef aerobically packed on expanded polystyrene trays. In contrast, Soladoye et al. [66] noted that the reduction or even complete loss of certain amino acids can result from specific chemical reactions. For instance, lysine may react with lipid peroxidation by-products such as malondialdehyde. In our study, no significant (p > 0.05) differences in lysine content were observed between beef packaged using different methods during the first 7 days of storage. However, on days 14, 21, and 28, VP beef showed a significantly lower lysine content, between 5.5- and 7-fold, compared to MAP beef. The significantly lower lysine concentrations found in VP samples could be linked with microbial production of cadaverine, which reached its highest levels in VP beef (Table 3).
At the beginning of storage (days 0 and 7), the content of ornithine, one of the precursors of putrescine, remained at a low level, regardless of packaging method (Table 2). The significant (p ≤ 0.05) increase in ornithine content compared to days 0 and 7 was observed between days 14 and 28 in beef packed in MAP, with the highest values occurring on day 21. Ornithine content in VP beef remained at a stable level throughout storage time. The effect of the packaging method on this free amino acid content was observed at the end of storage time, i.e., on days 21 and 28, with the lowest values for VP beef, and with no differences between the MAP variants. The increase in ornithine content during storage was reported for horse sausages [67]. In case of beef wet-ageing (vacuum-packed), Hangasaki and Asoto [68] reported no significant changes in ornithine content during 28 days of storage.
The phenylalanine content, precursor of 2-phenylethylamine, increased during beef storage, regardless of the packaging method (Table 2). A significant increase was observed on day 14, compared to days 0 and 7. For VP beef, such an increase was higher than for MAP beef. From day 14, the significant differences in phenylalanine content were observed between meat packed under various methods, with higher amounts noted for VP beef. This trend continued to the end of the storage time (Table 2). An increase in phenylalanine content in beef during storage was also reported by Vierck et al. [63].
The content of tryptophan, an amino acid that is the precursor of tryptamine, changed significantly during storage time in all beef samples. Compared to day 0, a significant (p ≤ 0.05) increase was observed on day 14 in meat stored in all MAP variants, with the highest levels noted on the last day of storage (Table 2). In contrast, in VP meat, the tryptophan content increased up to day 14, and then decreased to the initial level. According to Koutsidis et al. [64], the greatest increases in amino acids during beef storage were observed for threonine, leucine, isoleucine, methionine, valine, and tryptophan, and such increase was observed after 14 days of storage. In our studies, the effect of the packaging method was visible only on days 21 and 28 of storage, and VP meat was characterized by significantly (p ≤ 0.05) lower tryptophan content compared to meat packed in all MAP variants. Similar findings were reported by Vierck et al. [63], who observed comparable tryptophan content in beef steaks stored under different MAP gas mixtures, but lower levels in vacuum-packaged ones.
The content of tyrosine, a precursor of tyramine, significantly (p ≤ 0.05) increased during beef storage in MAP, reaching the highest level on day 14. A significant decrease was then observed on day 21, followed by another increase on day 28. In VP meat, a gradual decrease in tyrosine content was observed from day 7, with significant differences between day 7 and days 21 and 28. This reduction was likely due to the slowing down of oxidation or degradation processes in an anaerobic environment (Table 2). Overall, tyrosine levels tended to increase during beef storage. This increase is often associated with enhanced activity of endogenous proteolytic enzymes, which break down proteins and release amino acids [69,70]. According to Hwang et al. [69], tyrosine (along with arginine) may contribute to the development of spoilage in cold-stored beef. Significant differences in tyrosine content in beef packed using different packaging methods were observed starting from day 14. Up to the end of storage time, VP meat was characterized by significantly (p ≤ 0.05) lower tyrosine levels compared to MAP meat, ranging from 2- to 5-fold lower. This could also be attributed to significantly higher tyramine content (Table 3) and high LAB levels (Table 1), which are found to have the most intensive tyrosine decarboxylase activity [71]. Similar tendencies were observed by Vierck et al. [63]: tyrosine levels were higher for HiOx-MAP steaks and lower in VP steaks. Tyrosine and histidine levels could be higher in beef packed in MAP with high O2 content compared to VP beef due to oxidation-induced protein modifications. High oxygen environments in MAP promote the formation of reactive oxygen species (ROS), which drive oxidative reactions that can alter protein structures and release amino acids such as tyrosine and histidine from peptide chains. Proteins rich in tyrosine residues are particularly prone to oxidation, since the hydroxyl group on the aromatic ring of tyrosine’s side chain makes it especially susceptible to singlet oxygen attack. Consequently, protein oxidation in high-oxygen MAP environments may promote the release of tyrosine, resulting in its higher concentration compared with the anaerobic conditions of VP packaging. Histidine is likewise sensitive to oxidative modification due to its imidazole group, and its accumulation may also reflect its involvement in antioxidant defense. Moreover, ROS can hydroxylate phenylalanine to tyrosine, thereby reducing phenylalanine accumulation. Phenylalanine could be oxidized but is relatively resistant and is most commonly hydroxylated into tyrosine under oxidative stress. In VP beef, the absence of oxygen minimizes oxidative degradation, allowing proteolysis to release and accumulate free phenylalanine without further conversion [18,63,72,73,74].
3.4. Accumulation of Biogenic Amines in Beef During Storage
Table 3 presents the changes in the biogenic amine (BA) profile in beef during storage, depending on the packaging method. Agmatine in meat is mainly formed through microbial decarboxylation of the amino acid arginine. In our study, agmatine levels remained low throughout the storage period, not exceeding 0.3 mg/kg, regardless of the packaging method. The highest concentration was recorded on day 14 of storage. However, no significant effect (p > 0.05) of the packaging method on agmatine content was observed.
Spermine and spermidine are biogenic amines of endogenous origin, potentially derived from arginine and ornithine. These amines occur naturally in fresh meat and are not products of microbial enzymatic decarboxylation [34]. At the beginning of storage, spermine was the predominant amine in beef, followed by spermidine (Table 3). According to Gallas et al. [57] and Triki et al. [33], this may indicate that the development of microflora with decarboxylase activity started later in the storage period, as most other BAs are formed by microbial action. The levels of both amines were typical for this type of meat [33,41]. Storage time and packaging method had no significant effect (p > 0.05) on spermine and spermidine content. Similar results were reported by Rokka et al. [75], who found that spermine and spermidine levels remained stable during the first 12 days of chicken meat storage under MAP without oxygen (80% CO2, 20% N2). In contrast, Li et al. [76] observed a significant decrease in spermine content between days 4 and 7 of beef storage, attributing this decline to bacterial use of spermine as a nitrogen source.
A significant (p ≥ 0.05) increase in histamine content in VP beef was observed during storage time, with the highest values observed on days 21 and 28 (Table 3). On these days, differences between packaging methods were also noted, with histamine levels in VP beef being approximately 5-fold higher than in meat packaged in MAP. This was related to lower histidine content in VP beef compared to MAP beef on those days (Table 2). According to Balamatsia et al. [71], significant histamine formation is typically associated with meat spoilage, particularly when Enterobacteriaceae populations reach approximately 107 cfu/g, levels that were not observed in our study. Nevertheless, VP beef showed higher Enterobacteriaceae counts compared to MAP beef (Table 1). In contrast, Chmiel et al. [21] found no significant effect of packaging technique on histamine content in chicken meat.
Cadaverine levels in beef at day 0 were lower than the Limit of Detection (LOD); however, their content increased during storage, regardless of the packaging method (Table 3). For MAP beef, an increase in cadaverine content was slower than for VP. The highest (p ≤ 0.05) cadaverine contents were observed on the final day of storage for MAP beef and earlier for VP beef, on day 21. Throughout the entire 28-day storage time, VP meat consistently showed significantly higher cadaverine content, ranging from 6- to 18-fold higher, compared to MAP beef. These significant differences became visible starting from day 14 of storage. This was related to significantly lower (or lower) lysine content in VP beef compared to MAP meat (Table 2). For MAP samples, cadaverine levels remained at comparatively low levels even when lysine levels were abundant (Table 1, Table 2). Even if lysine is available, the lack of active decarboxylating bacteria means it is not efficiently converted into cadaverine, resulting in low concentrations of this biogenic amine. This may be attributed to oxygen presence, which promotes oxidative stress, as well as carbon dioxide in the MAP gas mixture, which inhibits the growth of many spoilage microorganisms, including decarboxylating bacteria that produce cadaverine from lysine. As a result, microbial activity and enzyme function are reduced, limiting cadaverine formation despite the presence of lysine [13]. Additionally, factors such as processing methods, the nature of the meat matrix, and other environmental conditions can also influence BAs’ presence in meat [77].
Putrescine and cadaverine are considered toxic biogenic amines because they enhance the intestinal absorption of histidine and tyramine and reduce their catabolism, thereby increasing their overall toxicity [33]. According to Borges et al. [50], the aminogenic bacteria, such as Enterobacteriaceae, are responsible for cadaverine formation. This is consistent with our findings, as at the beginning of beef storage, cadaverine levels were low, corresponding with low or undetectable levels of Enterobacteriaceae. From day 7, VP meat showed significantly higher Enterobacteriaceae counts compared to MAP meat (Table 1). In addition to Enterobacteriaceae, Pseudomonas spp. are also known as cadaverine producers [15]. In our studies, among others, cadaverine content was statistically correlated with Enterobacteriaceae counts (Table 4). Studies by Motaghifar et al. [78] reported an increase in cadaverine levels over storage time in beef. Alexi et al. [1] observed a sudden increase in cadaverine at day 11 of pork cutlet storage, following a plateau observed between days 3 and 8. This increase was related to noticeable declines in sensory and microbial quality, rendering the pork cutlets unacceptable for consumption by day 13. These findings were also supported by the correlation of the cadaverine concentrations to the odor freshness assessment and B. thermosphacta counts, which have been associated with pork spoilage and off-odor development.

Table 4.
Pearson’s correlation coefficients between biogenic amines and bacterial groups.
Putrescine levels increased during beef storage, regardless of the packaging method; however, for MAP beef, the increase was slower than for VP. The higher (p ≤ 0.05) putrescine contents, compared to day 0, were observed on the final day of storage for MAP beef and for VP beef earlier, on day 14. Starting from day 14, VP meat was characterised by significantly higher putrescine content, ranging from 4- to 25-fold more, compared to MAP beef (Table 3), which was also related to lower ornithine content in meat (Table 2). According to De Filippis et al. [79] and Lázaro et al. [41], putrescine and cadaverine are major biogenic amines produced by Enterobacteriaceae through decarboxylation of ornithine and lysine, respectively. This relationship was observed in our studies, as Enterobacteriaceae counts were significantly higher for VP beef than MAP beef (Table 1). Moreover, putrescine content was statistically correlated with Enterobacteriaceae counts (Table 4). Chmiel et al. [21] proposed that the concentration of cadaverine or putrescine can potentially serve as a chemical quality indicator of chicken meat freshness. Although toxic at high levels, these biogenic amines are primarily responsible for meat spoilage due to the strong putrefactive odor they produce. Their formation during meat storage has been linked not only to microbial activity but also to storage conditions, particularly the type of gas atmosphere used in packaging [18,52,80].
The 2-phenylethylamine content in MAP beef remained low throughout the storage time (Table 3). In contrast, an increase in 2-phenylethylamine was observed in VP beef starting from day 14, reaching the highest levels on day 28. From day 14 onward, VP beef also showed significantly higher 2-phenylethylamine content compared to MAP beef (Table 3). Ivanov et al. [81] showed increased levels of 2-phenylethylamine, tyramine, histamine, cadaverine, and putrescine in poultry meat. In the studies of Gallas et al. [57] 2-phenylethylamine was not found either at the beginning of or during the storage period in any of the chicken meat tested.
Tryptamine content for MAP80/20/0 beef remained lower than the LOD throughout the whole storage time. In contrast, an increase in tryptamine content was observed in MAP70/20/10, MAP60/20/20 and VP beef was observed, with the highest levels noted on the last day of storage. For VP beef, this increase was observed earlier than in MAP beef, on day 14. Also, starting from day 14, VP meat was characterized by significantly (p ≤ 0.05) higher content of tryptamine than MAP beef, which corresponded to lower tryptophan content (Table 2 and Table 3). On day 28, differences between all packaging methods were noted, and the lower oxygen content in the package, the higher tryptamine content was observed (Table 3). In the studies of Chmiel et al. [56], tryptamine level remained very low (or undetectable) throughout the storage time of beef. An increase in tryptamine content in chicken meat during storage was also noted by Wójcik et al. [81]. Galgano et al. [82] associated tryptamine formation with psychrotrophic bacteria such as B. thermosphacta, a predominant spoilage microorganism in refrigerated fresh meat. Furthermore, Ntzimani et al. [83] detected tryptamine at low but increasing levels in both VP and MAP-packed meat during storage.
In MAP beef, tyramine content remained low up to day 7. A significant (p ≤ 0.05) increase was observed on day 14, and within the storage time, the highest values were noted on day 28. For VP beef, an earlier increase was observed, on day 7, then days 14 and 21, with the highest values noted on day 21 (Table 3). Starting from day 7, VP beef was characterized by significantly (p ≤ 0.05) higher levels of tyramine, ranging from 8- to even 50-fold higher, compared to MAP meat, which was related to lower tyrosine content in VP beef (Table 2 and Table 3). On day 28, differences between all packaging methods were noted, and the lower oxygen content in the package, the higher tyramine content was observed (Table 3). According to Schirone et al. [84] tyramine, along with putrescine, cadaverine and histidine is among the predominant BAs found in meat [8,85]. In the studies on chicken fillets, tyramine, along with cadaverine, dominated in quantitative terms, regardless of packaging technique and storage conditions [21]. In the studies of Li et al. [76], tyramine was not detected in beef during the first 7 days of storage. High levels of tyramine, similar to cadaverine, may indicate a loss of meat freshness of meat or temperature abuse, even when the sensory quality still appears acceptable. This phenomenon may be attributed to ongoing decarboxylase activity in the meat substrate during storage [34]. Tyramine production is primarily associated with the growth of lactic acid bacteria. On the other hand, high decarboxylase activity is attributed to the family Enterobacteriaceae, mainly concerning the production of histamine, cadaverine, putrescine, and tyramine. The production of biogenic amines is a multifactorial process dependent on several factors, such as the amount of precursor amino acids (substrate), bacterial growth kinetics, as well as their proteolytic action and decarboxylase activity [86].
The sum of histamine, cadaverine, putrescine and tyramine can be used to assess meat freshness, and it is commonly referred to as BAI. Based on the BAI values, meat quality can be classified from good to spoiled [33,35]. In our studies, VP meat was considered spoiled on day 14 of storage according to the BAI classification, while beef stored under all MAP variants was classified as spoiled on the last day of storage. This classification did not clearly correspond with the microbial count observed in our studies. A significant (p ≤ 0.05) effect of both storage time and packaging method on the BAI values of the beef was found. With the extension of storage time, the values of BAI were higher, and for MAP, significant increases in BAI were observed on days 14 and 28. In contrast, for VP beef, such an increase was observed earlier, on day 7, then continuing on days 14 and 28. Moreover, starting from day 7, VP beef was characterized by significantly (p ≤ 0.05) higher BAI values than MAP meat (Table 3).
The permissible maximum level of TPC in refrigerated meat is 107 cfu/g. In our experiment, this threshold was reached on day 28, regardless of packaging method, indicating spoilage according to microbiological standards. However, accordingly to BAI values, VP beef was spoiled already two weeks earlier (on day 14), highlighting a discrepancy between microbial counts (TPC) and BAI-based spoilage assessment. The spoilage of VP beef was primarily attributed to Enterobacteriaceae (Table 2 and Table 3). Triki et al. [33] reported a delay in the formation of biogenic amines in relation to microbial growth. This contrasts with the results obtained in this study, which suggest that BAI can indicate spoilage earlier than traditional microbial counts. This also suggests that the relationship between BAI and the microbiological quality of meat is influenced by both its initial level of contamination and the specific type of microflora present. Currently, there are no universally established legal limits for the BAI, and emerging regulations are typically restricted to individual amines, such as histamine, and specific product groups, particularly fish and certain fermented foods like cheese. For other potentially toxic biogenic amines, such as putrescine, cadaverine, or β-phenylethylamine, regulatory thresholds are difficult to define due to their diverse metabolic pathways and variable toxicological effects. Importantly, consumer sensitivity plays a critical role, as adverse symptoms may occur even at relatively low concentrations of individual biogenic amines, underscoring the need for cautious risk assessment [13,77].
PCA was performed on data containing biochemical and microbiological profiles of beef samples stored under various packaging conditions and over different storage times. The main goals of PCA were to reduce the number of variables while preserving as much information as possible, and to visualise differences between samples based on packaging variant and storage time. PC1 (horizontal axis) and PC2 (vertical axis) are the two principal components that together explain the largest portion of variability in the data, effectively projecting the original variables into a lower-dimensional space. The first two principal components (PC1 and PC2) captured the largest proportion of total variance in the dataset (exact values are shown on the plot axes; Figure 2).
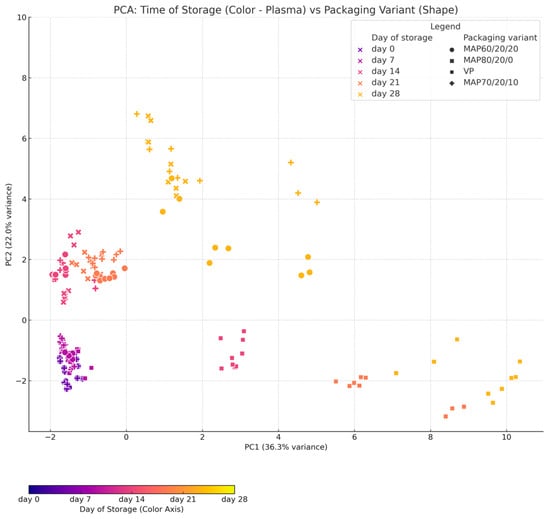
Figure 2.
The principal component analysis of cases between packaging variant (shapes) and storage time (plasma color).
Those explained almost 60% of the total variability. The color of each point represented the Day of storage, allowing time-based trends to be visualized. The shape of each point corresponded to the Packaging variant, facilitating the comparison of technological groups. The PCA plot revealed a clear gradient in sample position over storage time, indicating that the biochemical and microbiological profiles of the samples change over time, likely due to biological and chemical processes such as degradation, microbial growth, and the accumulation of biogenic amines. Furthermore, different packaging variants tended to form distinct clusters in PCA space, suggesting that the packaging method influences the direction and rate of sample evolution. These findings indicate that both packaging strategy and storage duration significantly affect the quality dynamics of the analyzed beef.
4. Conclusions
It was found that beef packaged in modified atmosphere was characterised by longer shelf life than the same meat packaged in vacuum. Modified atmosphere packaging, regardless of the gas mixture used (MAP80/20/0, MAP70/20/10, or MAP60/20/20), ensured better microbiological stability of beef and lower biogenic amine index (BAI) values during storage than vacuum packaging. Vacuum-packed beef was characterised by a significantly higher number of Enterobacteriaceae and showed spoilage symptoms approximately two weeks earlier than MAP beef. This was confirmed by lower levels of free amino acids such as lysine and ornithine and higher concentrations of spoilage-related biogenic amines (cadaverine and putrescine). Among the MAP variants, the 80/20/0 mixture could be recommended for beef packaging, as it slows down the formation of biogenic amines, which is reflected in lower BAI values. Interestingly, this study revealed a discrepancy between the assessment of beef spoilage based on microbial counts and BAI-based spoilage assessment, suggesting that the BA profile may serve as a more sensitive indicator of meat freshness. Developing reliable and rapid tools for assessing the shelf life of packaged culinary beef, beyond traditional, time-consuming, and labor-intensive microbiological methods, could significantly benefit both producers and consumers. Such tools would support efforts to reduce food waste, particularly meat waste, and contribute to more sustainable consumption practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15189882/s1, Table S1: Changes in the free amino acid profile of beef steaks during long-term storage (mean ± standard deviation). Table S1 legend: † the average values presented for day 0 correspond to analyses conducted prior to meat packaging, meaning they are identical for all packaging variants. The symbols identifying significant differences are as follows: A–C—means with different letters differ significantly at p ≤ 0.05 within packaging variants at particular day of storage; a–c—means with different letters differ significantly at p ≤ 0.05 within storage days for particular packaging variant; nd—not detected.
Author Contributions
Conceptualization, M.C.; methodology, M.C., O.Ś., D.P. (Daria Padewska), M.Ł.R., M.B., L.A. and E.H.-S.; validation, O.Ś., M.Ł.R. and D.P. (Daria Padewska); formal analysis, D.P. (Dorota Pietrzak) and A.C.; investigation, E.H.-S., T.F., L.A., D.P. (Dorota Pietrzak), O.Ś., D.P. (Daria Padewska) and I.S.; data curation, M.C.; writing—original draft preparation, M.C., O.Ś. and M.Ł.R.; writing—review and editing, A.C. and I.S.; visualization, M.Ł.R. and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
The research for this publication was carried out using research equipment purchased as part of the “Food and Nutrition Centre-modernization of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Alexi, N.; Thamsborg, K.; Hvam, J.; Lund, B.W.; Nsubuga, L.; De Oliveira Hansen, R.M.; Byrne, D.V.; Leisner, J.J. Novel cadaverine non-invasive biosensor technology for the prediction of shelf life of modified atmosphere packed pork cutlets. Meat Sci. 2022, 192, 108876. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- FAO. The state of food and agriculture 2019. In Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Guidance on date marking and related food information: Part 1 (Date Marking). EFS2 2020, 18, e06306. [Google Scholar] [CrossRef]
- Tarlak, F. The use of predictive microbiology for the prediction of the shelf life of food products. Foods 2023, 12, 4461. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Makia, R.S.; Joshua, O.A.; Akpoghelie, P.O.; Gaaz, T.S.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; et al. A review on food spoilage mechanisms, food borne diseases and commercial aspects of food preservation and processing. Food Chem. Adv. 2024, 5, 100852. [Google Scholar] [CrossRef]
- Luong, N.-D.M.; Coroller, L.; Zagorec, M.; Membré, J.-M.; Guillou, S. Spoilage of chilled fresh meat products during storage: A quantitative analysis of literature data. Microorganisms 2020, 8, 1198. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, W.; Łukasiewicz-Mierzejewska, M.; Damaziak, K.; Bień, D. Biogenic amines in poultry meat and poultry products: Formation, appearance, and methods of reduction. Animals 2022, 12, 1577. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Prats-Moya, M.S. Free amino acids and biogenic amines in Alicante Monastrell wines. Food Chem. 2012, 135, 1511–1519. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Xu, J.; Sun, F.; Liu, H.; Kong, B. Effects of modified atmosphere packaging with various CO2 concentrations on the bacterial community and shelf-life of smoked chicken legs. Foods 2022, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Abril, B.; Bou, R.; García-Pérez, J.V.; Benedito, J. Role of enzymatic reactions in meat processing and use of emerging technologies for process intensification. Foods 2023, 12, 1940. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Herrero, A. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Li, H.; Jia, D.; Fu, L.; Chen, J.; Zhang, D.; Wang, Y. Biogenic amines detection in meat and meat products: The mechanisms, applications, and future trends. J. Future Foods 2024, 4, 21–36. [Google Scholar] [CrossRef]
- Thamsborg, K.K.M.; Lund, B.W.; Byrne, D.V.; Leisner, J.J.; Alexi, N. Cadaverine as a potential spoilage indicator in skin-packed beef and modified-atmosphere-packed beef. Foods 2023, 12, 4489. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Trindade, M.A.; Palu, A.F.; Baldin, J.C.; De Lima, C.G.; De Alvarenga Freire, M.T. Modified atmosphere packaging for lamb meat: Evaluation of gas composition in the extension of shelf life and consumer acceptance. J. Food Sci. Technol. 2018, 55, 3547–3555. [Google Scholar] [CrossRef]
- Lu, X.; Cornforth, D.P.; Carpenter, C.E.; Zhu, L.; Luo, X. Effect of oxygen concentration in modified atmosphere packaging on color changes of the M. longissimus thoraces et lumborum from dark cutting beef carcasses. Meat Sci. 2020, 161, 107999. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein oxidation in muscle foods: A comprehensive review. Antioxidants 2021, 11, 60. [Google Scholar] [CrossRef]
- Bonny, D.; Li, X.; Li, Z.; Li, M.; Du, M.; Gao, L.; Zhang, D. Colour stability and lipid oxidation of beef longissimus lumborum under different packaging conditions. Pol. J. Food Nutr. Sci. 2017, 67, 275–281. [Google Scholar] [CrossRef][Green Version]
- Liang, R.; Zhang, W.; Mao, Y.; Zhang, Y.; Li, K.; Luo, X.; Yang, X. Effects of CO2 on the physicochemical, microbial, and sensory properties of pork patties packaged under optimized O2 levels. Meat Sci. 2024, 209, 109422. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Roszko, M.; Hać-Szymańczuk, E.; Cegiełka, A.; Adamczak, L.; Florowski, T.; Pietrzak, D.; Bryła, M.; Świder, O. Changes in the microbiological quality and content of biogenic amines in chicken fillets packed using various techniques and stored under different conditions. Food Microbiol. 2022, 102, 103920. [Google Scholar] [CrossRef]
- Chmiel, M.; Hać-Szymańczuk, E.; Dasiewicz, K.; Szymańska, I.; Adamczak, L.; Cegiełka, A.; Pietrzak, D.; Florowski, T.; Reder, K.; Bryła, M. Microbiological and physicochemical quality changes of pork ham slices packed using various methods during long-term refrigerated storage. LWT-Food Sci. Technol. 2025, 225, 117933. [Google Scholar] [CrossRef]
- PN-EN ISO 6887-2:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension, and Decimal Dilutions for Microbiological Examination—Part 2: Specific Rules for the Preparation of Meat and Meat Products. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN ISO 4833-2:2013-12; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 Degrees C by the Surface Plating Technique. Polish Committee for Standardization: Warsaw, Poland, 2013.
- PN-ISO 17410:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Psychrotrophic Microorganisms. Polish Committee for Standardization: Warsaw, Poland, 2004.
- PN-ISO 15214:2002; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Plate Method at 30 °C. Polish Committee for Standardization: Warsaw, Poland, 2002.
- PN-EN ISO 21528-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 1: Detection of Enterobacteriaceae. Polish Committee for Standardization: Warsaw, Poland, 2017.
- Nowak, A.; Rygala, A.; Oltuszak-Walczak, E.; Walczak, P. The prevalence and some metabolic traits of Brochothrix thermosphacta in meat and meat products packaged in different ways. J. Sci. Food Agric. 2012, 92, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 13720:2010; Meat and Meat Products-Enumeration of Presumptive Pseudomonas sp. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Chmiel, M.; Roszko, M.; Hać-Szymańczuk, E.; Adamczak, L.; Florowski, T.; Pietrzak, D.; Cegiełka, A.; Bryła, M. Time evolution of microbiological quality and content of volatile compounds in chicken fillets packed using various techniques and stored under different conditions. Poult. Sci. 2020, 99, 1107–1116. [Google Scholar] [CrossRef]
- Cegiełka, A.; Piątkowska, J.; Chmiel, M.; Hać-Szymańczuk, E.; Kalisz, S.; Adamczak, L. Changes in quality features of pork burgers prepared with chokeberry pomace during storage. Appl. Sci. 2025, 15, 2337. [Google Scholar] [CrossRef]
- Świder, O.; Roszko, M.Ł.; Wójcicki, M.; Szymczyk, K. Biogenic amines and free amino acids in traditional fermented vegetables—Dietary risk evaluation. J. Agric. Food Chem. 2020, 68, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Quality assessment of fresh meat from several species based on free amino acid and biogenic amine contents during chilled storage. Foods 2018, 7, 132. [Google Scholar] [CrossRef]
- Feddern, V.; Mazzuco, H.; Fonseca, F.N.; De Lima, G.J.M.M. A review on biogenic amines in food and feed: Toxicological aspects, impact on health and control measures. Anim. Prod. Sci. 2019, 59, 608. [Google Scholar] [CrossRef]
- Algahtani, F.D.; Morshdy, A.E.; Hussein, M.A.; Abouelkheir, E.S.; Adeboye, A.; Valentine, A.; Elabbasy, M.T. Biogenic amines and aflatoxins in some imported meat products: Incidence, occurrence, and public health impacts. J. Food Qual. 2020, 2020, 718179. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Liang, R.; Zhu, L.; Mao, Y.; Dong, P.; Hopkins, D.L.; Luo, X.; Zhang, Y. The response of bacterial communities to carbon dioxide in high-oxygen modified atmosphere packaged beef steaks during chilled storage. Food Res. Int. 2022, 151, 110872. [Google Scholar] [CrossRef]
- Guillard, V.; Couvert, O.; Stahl, V.; Hanin, A.; Denis, C.; Huchet, V.; Chaix, E.; Loriot, C.; Vincelot, T.; Thuault, D. Validation of a predictive model coupling gas transfer and microbial growth in fresh food packed under modified atmosphere. Food Microbiol. 2016, 58, 43–55. [Google Scholar] [CrossRef]
- Conte-Junior, C.A.; Monteiro, M.L.G.; Patrícia, R.; Mársico, E.T.; Lopes, M.M.; Alvares, T.S.; Mano, S.B. The effect of different packaging systems on the shelf life of refrigerated ground beef. Foods 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; Walsh, H.; Allen, P.; Kerry, J.P. Sensory comparison of commercial low and high oxygen modified atmosphere packed sirloin beef steaks. Meat Sci. 2011, 88, 198–202. [Google Scholar] [CrossRef]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT-Food Sci. Technol. 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte-Júnior, C.A.; Canto, A.C.; Monteiro, M.L.G.; Costa-Lima, B.; Cruz, A.G.D.; Mársico, E.T.; Franco, R.M. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT-Food Sci. Technol. 2015, 60, 15–21. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.; Kim, H.J.; Song, S.O.; Song, Y.H.; Jang, A. Evaluation of the microbiological status of raw beef in Korea: Considering the suitability of aerobic plate count guidelines. Food Sci. Anim. Resour. 2018, 38, 43–51. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Duan, X.; Li, K.; Cheng, H.; Sun, G.; Luo, X.; Hopkins, D.L.; Holman, B.W.B.; Zhang, Y.; et al. Investigation of oxygen packaging to maintain beef color stability and microbiology safety after periods of long-term superchilled storage. Meat Sci. 2024, 215, 109548. [Google Scholar] [CrossRef] [PubMed]
- Sai-Ut, S.; Indriani, S.; Srisakultiew, N.; Kingwascharapong, P.; Suriyarak, S.; Issara, U.; Phongthai, S.; Rawdkuen, S.; Pongsetkul, J. The Role of CO2 levels in high-oxygen modified atmosphere packaging on microbial communities of chilled goat meat during storage and their relationship with quality attributes. Foods 2025, 14, 1837. [Google Scholar] [CrossRef]
- Al-Mazrouei, M.A.; Al-Kharousi, Z.S.; Al-Kharousi, J.M.; Al-Barashdi, H.M. Microbiological evaluation of local and imported raw beef meat at retail sites in oman with emphasis on spoilage and pathogenic psychrotrophic bacteria. Microorganisms 2024, 12, 2545. [Google Scholar] [CrossRef] [PubMed]
- Hilgarth, M.; Behr, J.; Vogel, R.F. Monitoring of spoilage-associated microbiota on modified atmosphere packaged beef and differentiation of psychrophilic and psychrotrophic strains. J. Appl. Microbiol. 2018, 124, 740–753. [Google Scholar] [CrossRef]
- Patsias, A.; Chouliara, I.; Badeka, A.; Savvaidis, I.N.; Kontominas, M.G. Shelf-life of a chilled precooked chicken product stored in air and under modified atmospheres: Microbiological, chemical, sensory attributes. Food Microbiol. 2006, 23, 423–429. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, H.-J.; Lee, J.-W.; Park, H.-J.; Ryu, G.-H.; Kang, I.-J.; Byun, M.-W. Effects of gamma irradiation on the biogenic amines in pepperoni with different packaging conditions. Food Chem. 2005, 89, 199–205. [Google Scholar] [CrossRef]
- Bassey, A.P.; Chen, Y.; Zhu, Z.; Odeyemi, O.A.; Gao, T.; Olusola, O.O.; Ye, K.; Li, C.; Zhou, G. Evaluation of spoilage indexes and bacterial community dynamics of modified atmosphere packaged super-chilled pork loins. Food Control 2021, 130, 108383. [Google Scholar] [CrossRef]
- Borges, A.F.; Cózar, A.; Patarata, L.; Gama, L.T.; Alfaia, C.M.; Fernandes, M.J.; Fernandes, M.H.; Pérez, H.V.; Fraqueza, M.J. Effect of high hydrostatic pressure challenge on biogenic amines, microbiota, and sensory profile in traditional poultry- and pork-based semidried fermented sausage. J. Food Sci. 2020, 85, 1256–1264. [Google Scholar] [CrossRef]
- Stella, S.; Ripamonti, B.; Vandoni, S.; Bernardi, C.; Sgoifo Rossi, C.A. Microbiological and physicochemical quality evaluation of vacuum-packed Argentine beef imported into Italy. J. Food Qual. 2013, 36, 253–262. [Google Scholar] [CrossRef]
- Li, M.; Tian, L.; Zhao, G.; Zhang, Q.; Gao, X.; Huang, X.; Sun, L. Formation of biogenic amines and growth of spoilage-related microorganisms in pork stored under different packaging conditions applying PCA. Meat Sci. 2014, 96, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Kaur, M.; Pillidge, C.J.; Torley, P.J. Evaluation of the potential of protective cultures to extend the microbial shelf-life of chilled lamb meat. Meat Sci. 2021, 181, 108613. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhao, H.; Yan, L.; Li, S.; Chen, X.; Fan, J. Effect of CO2 on the preservation effectiveness of chilled fresh boneless beef knuckle in modified atmosphere packaging and microbial diversity analysis. LWT-Food Sci. Technol. 2023, 187, 115262. [Google Scholar] [CrossRef]
- Nauman, K.; Jaspal, M.H.; Asghar, B.; Manzoor, A.; Akhtar, K.H.; Ali, U.; Ali, S.; Nasir, J.; Sohaib, M.; Badar, I.H. Effect of different packaging atmosphere on microbiological shelf life, physicochemical attributes, and sensory characteristics of chilled poultry fillets. Food Sci. Anim. Resour. 2022, 42, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Cegiełka, A.; Świder, O.; Roszko, M.; Hać-Szymańczuk, E.; Adamczak, L.; Pietrzak, D.; Florowski, T.; Bryła, M.; Florowska, A. Effect of high pressure processing on biogenic amines content in skin-packed beef during storage. LWT-Food Sci. Technol. 2023, 175, 114483. [Google Scholar] [CrossRef]
- Gallas, L.; Standarová, E.; Steinhauserová, I.; Steinhauser, L.; Vorlová, L. Formation of biogenic amines in chicken meat stored under modified atmosphere. Acta Vet. Brno 2010, 79, 107–116. [Google Scholar] [CrossRef]
- Bassey, A.P.; Chen, Y.; Zhu, Z.; Odeyemi, O.A.; Frimpong, E.B.; Ye, K.; Li, C.; Zhou, G. Assessment of quality characteristics and bacterial community of modified atmosphere packaged chilled pork loins using 16S rRNA amplicon sequencing analysis. Food Res. Inter. 2021, 145, 110412. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang, R.; Mao, Y.; Dong, P.; Zhu, L.; Luo, X.; Zhang, Y.; Yang, X. Potential inhibitory effect of carbon dioxide on the spoilage behaviors of Pseudomonas Fragi in high-oxygen packaged beef during refrigerated storage. Food Microbiol. 2023, 112, 104229. [Google Scholar] [CrossRef]
- Gálvez, F.; Domínguez, R.; Maggiolino, A.; Pateiro, M.; Carballo, J.; De Palo, P.; Barba, F.J.; Lorenzo, J.M. Meat quality of commercial chickens reared in different production systems: Industrial, range and organic. Ann. Anim. Sci. 2020, 20, 263–285. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Cañedo, A.; Martínez-Onandi, N.; Gaya, P.; Nuñez, M.; Picon, A. Effect of high-pressure processing and chemical composition on lipid oxidation, aminopeptidase activity and free amino acids of serrano dry-cured ham. Meat Sci. 2021, 172, 108349. [Google Scholar] [CrossRef]
- Vierck, K.R.; Legako, J.F.; Kim, J.; Johnson, B.; Brooks, J.C. Determination of package and muscle-type influence on proteolysis, beef-flavor-contributing free amino acids, final beef flavor, and tenderness. Meat Muscle Biol. 2020, 4, 10933. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Oruna-Concha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-soluble precursors of beef flavour. Part II: Effect of post-mortem conditioning. Meat Sci. 2008, 79, 270–277. [Google Scholar] [CrossRef]
- Kim, H.C.; Baek, K.H.; Ko, Y.-J.; Lee, H.J.; Yim, D.-G.; Jo, C. Characteristic metabolic changes of the crust from dry-aged beef using 2D NMR spectroscopy. Molecules 2020, 25, 3087. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Comp. Rev. Food Sci. Food Safe 2015, 14, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Rabie, M.A.; Peres, C.; Malcata, F.X. Evolution of amino acids and biogenic amines throughout storage in sausages made of horse, beef and turkey meats. Meat Sci. 2014, 96, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hanagasaki, T.; Asato, N. Changes in free amino acids and hardness in round of Okinawan delivered cow beef during dry- and wet-aging processes. J. Anim. Sci. Technol. 2018, 60, 23. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, J.; Nam, T.G.; Koo, M.; Cho, Y.S. Changes in physicochemical properties and bacterial communities in aged korean native cattle beef during cold storage. Food Sci. Nutr. 2022, 10, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Kanokruangrong, S.; Kebede, B.; Carne, A.; Stewart, I.; Bekhit, A.E.-D.A. Metabolomic investigation of fresh beef, lamb and venison using nuclear magnetic resonance spectroscopy in relation to colour stability. Food Chem. 2025, 463, 141447. [Google Scholar] [CrossRef]
- Balamatsia, C.C.; Paleologos, E.K.; Kontominas, M.G.; Savvaidis, I.N. Correlation between microbial flora, sensory changes and biogenic amines formation in fresh chicken meat stored aerobically or under modified atmosphere packaging at 4 °C: Possible role of biogenic amines as spoilage indicators. J. Microbiol. 2006, 89, 9–17. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Comp. Rev. Food Sci. Food Safe 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Matthews, D.E. An overview of phenylalanine and tyrosine kinetics in humans. J. Nutr. 2007, 137, 1549–1555. [Google Scholar] [CrossRef]
- Hellwig, M. Analysis of protein oxidation in food and feed products. J Agric Food Chem. 2020, 68, 12870–12885. [Google Scholar] [CrossRef]
- Rokka, M.; Eerola, S.; Smolander, M.; Alakomi, H.-L.; Ahvenainen, R. Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions. Food Control 2004, 15, 601–607. [Google Scholar] [CrossRef]
- Li, S.; Johansson, M.; Vidanarachchi, J.K.; Pickova, J.; Zamaratskaia, G. Determination of biogenic amines in aerobically stored beef using high-performance thin-layer chromatography densitometry. Acta Agric. Scand. A Anim. Sci. 2016, 66, 199–205. [Google Scholar] [CrossRef]
- Banicod, R.J.S.; Ntege, W.; Njiru, M.N.; Abubakar, W.H.; Kanthenga, H.T.; Javaid, A.; Khan, F. Production and transformation of biogenic amines in different food products by the metabolic activity of the lactic acid bacteria. Int. J. Food Microbiol. 2025, 428, 110996. [Google Scholar] [CrossRef] [PubMed]
- Motaghifar, A.; Akbari-Adergani, B.; Rokney, N.; Mottalebi, A. Evaluating red meat putrefaction in long term storage in freezing condition based on co-variation of major biogenic amines and total volatile nitrogen. Food Sci. Technol. 2021, 41, 123–128. [Google Scholar] [CrossRef]
- De Filippis, F.; Pennacchia, C.; Di Pasqua, R.; Fiore, A.; Fogliano, V.; Villani, F.; Ercolini, D. Decarboxylase gene expression and cadaverine and putrescine production by Serratia proteamaculans in vitro and in beef. Int. J. Food Microbiol. 2013, 165, 332–338. [Google Scholar] [CrossRef]
- Ivanov, G.; Ivanova, I.; Slavchev, A.; Vassilev, K. Biogenic amines and their role as index of freshness in chicken meat. J. Appl. Life Sci. Int. 2015, 3, 55–62. [Google Scholar] [CrossRef]
- Wójcik, W.; Damaziak, K.; Łukasiewicz-Mierzejewska, M.; Świder, O.; Niemiec, J.; Wójcicki, M.; Roszko, M.; Gozdowski, D.; Riedel, J.; Marzec, A. Correlation between biogenic amines and their precursors in stored chicken meat. Appl. Sci. 2023, 13, 12230. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Bonadio, M.; Lorusso, V.; Romano, P. Role of biogenic amines as index of freshness in beef meat packed with different biopolymeric materials. Food Res. Int. 2009, 42, 1147–1152. [Google Scholar] [CrossRef]
- Ntzimani, A.G.; Paleologos, E.K.; Savvaidis, I.N.; Kontominas, M.G. Formation of biogenic amines and relation to microbial flora and sensory changes in smoked turkey breast fillets stored under various packaging conditions at 4 °C. Food Microbiol. 2008, 25, 509–517. [Google Scholar] [CrossRef]
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef]
- Pang, Z.; Lee, J.-W.; Lee, Y.; Moon, B. Changes in quality characteristics and biogenic amine contents in beef by cooking methods. Food Sci. Biotechnol. 2024, 33, 2313–2321. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Monteiro, M.L.G.; Conte-Junior, C.A. Combined effect of modified atmosphere packaging and UV-C radiation on pathogens reduction, biogenic amines, and shelf life of refrigerated tilapia (Oreochromis niloticus) fillets. Molecules 2020, 25, 3222. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).