Minimizing 3T MRI Geometric Distortions for Stereotactic Radiosurgery via Anterior–Posterior Phase Encoding–A Phantom Study
Abstract
1. Introduction
- Design and construction of a customized geometric phantom to accurately assess geometric distortion. Its filling solution was determined by testing different options on a preliminary phantom.
- Development of distortion quantification software. This tool automated the detection and matching of phantom inserts between MRI and CT scans.
- Optimization of MRI image acquisition by systematically exploring different acquisition parameters. The aim is to identify the most effective sequence to reduce geometric distortion in the MRI scans. This optimization process takes into account crucial factors, such as acquisition time, SAR, SNR, and CNR, while maintaining distortion levels within acceptable clinical thresholds.
2. Materials and Methods
2.1. Phantom Design for Geometric Distortion Measurement
2.2. Phantom Filling Solution Tests
2.3. Development of Distortion Quantification Software
- Image Registration: The registration results obtained using Eclipse planning system (Section 2.2) were used to derive the final outcomes of the developed software.
- Insert Detection: After registration between MRI and CT, the inserts observed in each, as seen on each of the MRI and CT slices, were separately detected after several steps of data pre-processing and filtering.
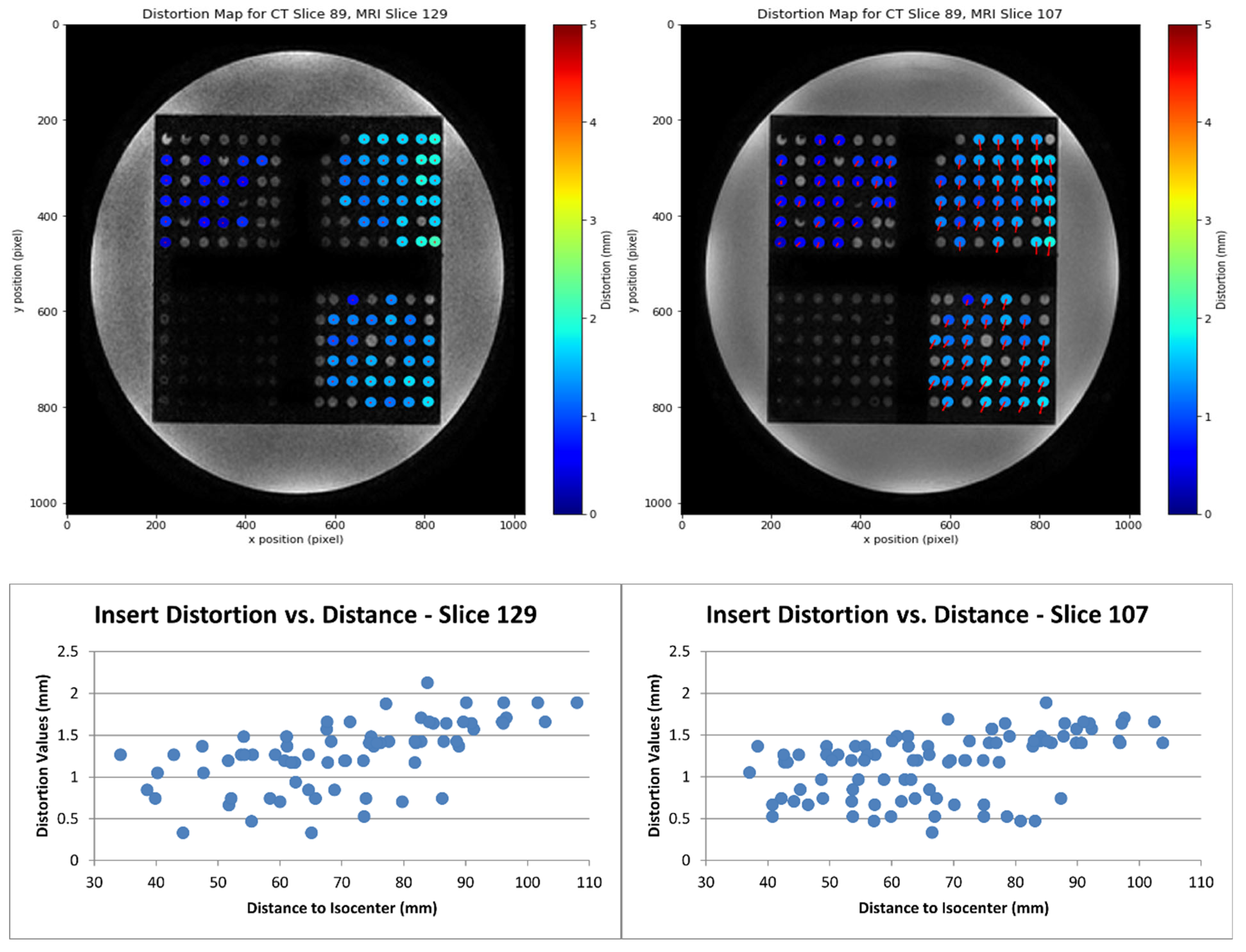
- Distortion Calculation: The geometric distortion was calculated as the distance between each matched insert centroid. The process generated a distortion map, illustrating the magnitude and direction of distortion, with outputs including coordinates, distances to the MR scanner isocenter, and distortion values.
2.4. Phantom Positioning and Image Acquisition
2.5. Optimization of MRI Image Acquisition for SRS Treatment Planning
Weighting Factor λ
- First Priority Class: Acquisition time and the mean distortion (squared weighting).
- Second Priority Class: SNR, CNR, and number of detected inserts (linear weighting in Equation (3)). The SNR was calculated in the usual way for MRI images [33], by dividing the mean pixel intensity μsignal in the signal region (phantom volume) by the standard deviation of the background σbackground, while the CNR introduces an additional factor by subtracting the mean pixel intensity μROI of a region of interest (detected inserts for each slice), as follows:
- Third Priority Class: SAR and maximum distortion (fractional linear weighting). SAR is an important parameter, but it only becomes a limiting parameter at approximately 2 W/kg [33], and all SAR values achieved were below 0.5 W/kg (Table 2). Thus, its weight in Equation (3) is defined so that λ = 0 if SAR = 2 W/kg. Regarding the maximum distortion, while it helps to quantify the worst-case scenario, it may also represent an outlier value, so it is considered less important than the mean distortion. Thus, its weighting was implemented such that λ tends to zero as the maximum distortion approaches 10 mm, which is one order of magnitude bigger than the desired threshold.
3. Results
3.1. Phantom Filling Solution Tests
3.2. Distortion Quantification Software
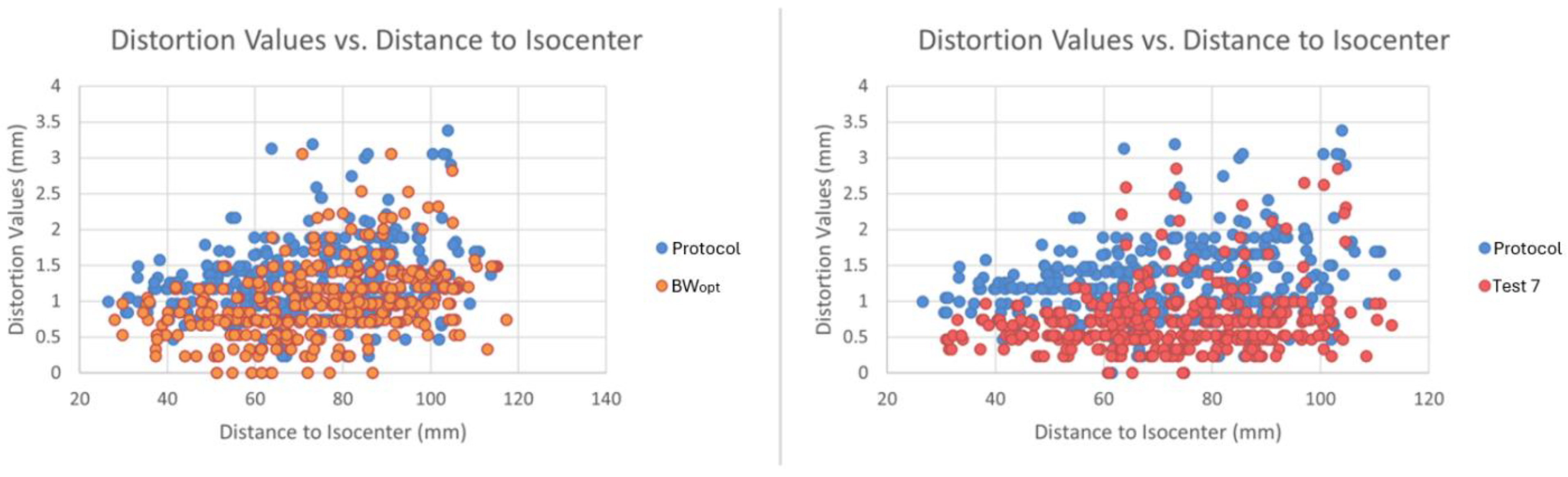
3.3. Optimization of MRI Image Acquisition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoo, V.S.; Joon, D.L. New developments in mri for target volume delineation in radiotherapy. Br. J. Radiol. 2006, 79, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Paulson, E.S.; Crijns, S.P.; Keller, B.M.; Wang, J.; Schmidt, M.A.; Coutts, G.; van der Heide, U.A. Consensus opinion on mri simulation for external beam radiation treatment planning. Radiother. Oncol. 2016, 121, 187–192. [Google Scholar] [CrossRef]
- Baldwin, L.N.; Wachowicz, K.; Thomas, S.D.; Rivest, R.; Fallone, B.G. Characterization, prediction, and correction of geometric distortion in mr images. Med. Phys. 2007, 34, 388–399. [Google Scholar] [CrossRef]
- Zhang, B.; MacFadden, D.; Damyanovich, A.Z.; Rieker, M.; Stainsby, J.; Bernstein, M.; Jaffray, D.A.; Mikulis, D.; Ménard, C. Development of a geometrically accurate imaging protocol at 3 tesla mri for stereotactic radiosurgery treatment planning. Phys. Med. Biol. 2010, 55, 6601–6615. [Google Scholar] [CrossRef]
- Soher, B.J.; Dale, B.M.; Merkle, E.M. A review of mr physics: 3t versus 1.5 t. Magn. Reson. Imaging Clin. N. Am. 2007, 15, 277–290. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, S.-I.; Jin, S.J.; Jin, S.-C.; Kim, J.S.; Jeon, K.D. Reliability of stereotactic coordinates of 1.5-tesla and 3-tesla mri in radiosurgery and functional neurosurgery. J. Korean Neurosurg. Soc. 2014, 55, 136–141. [Google Scholar] [CrossRef]
- Lavrador, R.F.D. Correction of Image Distortions in Magnetic Resonance Imaging: Intensity Inhomogeneities Correction and Evaluation. PhD Thesis, Universidade de Coimbra, Coimbra, Portugal, 2010. [Google Scholar]
- Irarrazabal, P.; Meyer, C.H.; Nishimura, D.G.; Macovski, A. Inhomogeneity correction using an estimated linear field map. Magn. Reson. Med. 1996, 35, 278–282. [Google Scholar] [CrossRef]
- Fransson, A.; Andreo, P.; Pötter, R. Aspects of mr image distortions in radiotherapy treatment planning. Strahlenther. Onkol. 2001, 177, 59–73. [Google Scholar] [CrossRef]
- Jeffrey, H. Simpson. Chapter 2-instrumental considerations. In Organic Structure Determination Using 2-D NMR Spectroscopy, 2nd ed.; Simpson, J.H., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 21–57. [Google Scholar]
- Juchem, C.; de Graaf, R.A. B0 magnetic field homogeneity and shimming for in vivo magnetic resonance spectroscopy. Anal. Biochem. 2017, 529, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. Sense: Sensitivity encoding for fast mri. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 1999, 42, 952–962. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Rong, Y.; Cao, M.; Li, H.; Jia, C.; Shi, L.; Lu, W.; Gong, G.; Yin, Y.; et al. A comparison of the distortion in the same field mri and mr-linac system with a 3d printed phantom. Front. Oncol. 2021, 11, 579451. [Google Scholar] [CrossRef]
- Walker, A.; Liney, G.; Metcalfe, P.; Holloway, L. Mri distortion: Considerations for mri based radiotherapy treatment planning. Australas. Phys. Eng. Sci. Med. 2014, 37, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.; Broadbent, D.A.; Chuter, R.; Al-Qaisieh, B.; Jackson, S.; Michael, H.; Johnstone, R.I.; Shah, S.; Wetscherek, A.; Chick, H.J.; et al. Audit feasibility for geometric distortion in magnetic resonance imaging for radiotherapy. Phys. Imaging Radiat. Oncol. 2020, 15, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, F.; Zhou, Z.; Cao, M.; Kaprealian, T.; Kamrava, M.; Wang, C.; Neylon, J.; Low, D.A.; Yang, Y.; et al. Distortion-free diffusion mri using an mri-guided tri-cobalt 60 radiotherapy system: Sequence verification and preliminary clinical experience. Med. Phys. 2017, 44, 5357–5366. [Google Scholar] [CrossRef]
- Karaiskos, P.; Moutsatsos, A.; Pappas, E.; Georgiou, E.; Roussakis, A.; Torrens, M.; Seimenis, I. A simple and efficient methodology to improve geometric accuracy in gamma knife radiation surgery: Implementation in multiple brain metastases. Int. J. Radiat. Oncol. 2014, 90, 1234–1241. [Google Scholar] [CrossRef]
- Glide-Hurst, C.K.; Paulson, E.S.; McGee, K.; Tyagi, N.; Hu, Y.; Balter, J.; Bayouth, J. Task group 284 report: Magnetic resonance imaging simulation in radiotherapy: Considerations for clinical implementation, optimization, and quality assurance. Med. Phys. 2021, 48, E636–E670. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Doddrell, D.M. Geometric distortion in structural magnetic resonance imaging. Curr. Med. Imaging Rev. 2005, 1, 49–60. [Google Scholar] [CrossRef]
- Hirtl, A.; Bergmann, H.; Knäusl, B.; Beyer, T.; Figl, M.; Hummel, J. Fully-automated analysis of jaszczak phantom measurements as part of routine spect quality control. Med. Phys. 2017, 44, 1638–1645. [Google Scholar] [CrossRef]
- Jaszczak, R.J.; Coleman, R.E.; Lim, C.B. Spect: Single photon emission computed tomography. IEEE Trans. Nucl. Sci. 1980, 27, 1137–1153. [Google Scholar] [CrossRef]
- MODUS. Measuring Geometric Distortion with Sub-Millimeter Accuracy in Mrgrt qa. White Paper, 2021. Available online: https://modusqa.com/wp-content/uploads/2020/02/MRID3D_WhitePaper-v3_PRINT.pdf (accessed on 29 August 2025).
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef]
- Kanal, E. Gadolinium-based magnetic resonance contrast agents for neuroradiology: An overview. Magn. Reson. Imaging Clin. N. Am. 2012, 20, 625–631. [Google Scholar] [CrossRef]
- Laurent, S.; Elst, L.V.; Muller, R.N. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol. Imaging 2006, 1, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, M.; Aragno, D.; Bencivenghi, A. RM phantom preparation with CuSO4 and NiCl2 water solutions. In Proceedings of the Congresso Nazionale AIFM, Torino, Italy, 16–19 November 2013. [Google Scholar]
- Thangavel, K.; Saritaş, E.Ü. Aqueous paramagnetic solutions for mri phantoms at 3 t:A detailed study on relaxivities. Turk. J. Electr. Eng. Comput. Sci. 2017, 25, 2108–2121. [Google Scholar] [CrossRef]
- Monzari, S.F.; Geraily, G.; Nia, T.H.; Salmanian, S.; Toolee, H.; Farzin, M. Fabrication of anthropomorphic phantoms for use in total body irradiations studies. J. Radiother. Pract. 2020, 19, 242–247. [Google Scholar] [CrossRef]
- Kleck, J.H.; Smathers, J.B.; Holly, F.E.; Myers, L.T. Anthropomorphic radiation therapy phantoms: A quantitative assessment of tissue substitutes. Med. Phys. 1990, 17, 800–806. [Google Scholar] [CrossRef]
- DeWerd, L.A.; Kissick, M. The Phantoms of Medical and Health Physics; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Silva, S.; Pinto, S.; Conde, P.; Lencart, J.; Machado, E.; Santos, J.A.M. Optimization of the 3t mri acquisition for radiosurgery patients using a customized head phantom. In Proceedings of the FÍSICA 2022–23ª Conferência Nacional de Física e 32º Encontro Ibérico para o Ensino da Física, Porto, Portugal, 7–10 September 2022. [Google Scholar]
- Dietrich, O.; Raya, J.G.; Reeder, S.B.; Reiser, M.F.; Schoenberg, S.O. Measurement of signal-to-noise ratios in mr images: Influence of multichannel coils, parallel imaging, and reconstruction filters. J. Magn. Reson. Imaging 2007, 26, 375–385. [Google Scholar] [CrossRef]
- Seo, Y.; Wang, Z.J. Measurement and evaluation of specific absorption rate and temperature elevation caused by an artificial hip joint during mri scanning. Sci. Rep. 2021, 11, 1134. [Google Scholar] [CrossRef]
- Runge, V.M. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the ema’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Investig. Radiol. 2017, 52, 317–323. [Google Scholar] [CrossRef]
- Leyba, K.; Wagner, B. Gadolinium-based contrast agents: Why nephrologists need to be concerned. Curr. Opin. Nephrol. Hypertens. 2019, 28, 154–162. [Google Scholar] [CrossRef]
- Guo, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Zhao, L.; Chen, K.; Deng, J. Nickel chloride (nicl2) in hepatic toxicity: Apoptosis, g2/m cell cycle arrest and inflammatory response. Aging 2016, 8, 3009–3027. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Mondello, M.R.; Cimino, F.; Cristani, M.; Pergolizzi, S.; Saija, A. Toxic effect of nickel in an in vitro model of human oral epithelium. Toxicol. Lett. 2005, 159, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gamage, G.C.V.; Goh, J.K.; Choo, W.S. Natural blue colourant preparations from blue pea flower and spirulina: A comparison stability study. Food Chem. Adv. 2023, 3, 100457. [Google Scholar] [CrossRef]
- FDA. Dotarem® (Gadoterate Meglumine) Injection for Intravenous Use; FDA: Silver Spring, MD, USA, 2013.
- Schilling, K.G.; Blaber, J.; Hansen, C.; Cai, L.; Rogers, B.; Anderson, A.W.; Smith, S.; Kanakaraj, P.; Rex, T.; Resnick, S.M.; et al. Distortion correction of diffusion weighted MRI without reverse phase-encoding scans or field-maps. PLoS ONE 2020, 15, e0236418. [Google Scholar] [CrossRef] [PubMed]
- Kennis, M.; van Rooij, S.; Kahn, R.; Geuze, E.; Leemans, A. Choosing the polarity of the phase-encoding direction in diffusion MRI: Does it matter for group analysis? NeuroImage Clin. 2016, 11, 539–547. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Jiang, L.; Fu, K.; Xie, H.; Ran, Q. Effect of the phase encoding direction on the image quality of breast diffusion-weighted magnetic resonance images. Preprint 2023. [Google Scholar] [CrossRef]
- Lüdeke, K.; Röschmann, P.; Tischler, R. Susceptibility artefacts in NMR imaging. Magn. Reson. Imaging 1985, 3, 329–343. [Google Scholar] [CrossRef]
- Lide, D.R. Magnetic susceptibility of the elements and inorganic compounds. In Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; Volume 81, pp. 130–135. [Google Scholar]
- Hargreaves, B.A.; Worters, P.W.; Pauly, K.B.; Pauly, J.M.; Koch, K.M.; Gold, G.E. Metal-Induced Artifacts in MRI. Am. J. Roentgenol. 2011, 197, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Tudor, G.S.J.; Bernstein, D.; Riley, S.; Rimmer, Y.; Thomas, S.J.; van Herk, M.; Webster, A. Geometric uncertainties in daily online IGRT: Refining the CTV-PTV margin for contemporary photon radiotherapy. Br. Inst. Radiol. 2020, 93, 20200346. [Google Scholar]



| Sequence Name | Acquisition Type | Slice Thickness (mm) | TR (ms) | TE (ms) | Number of Excitations | BW (kHz) | Matrix Size (Pixels2) | Flip Angle (°) | FOV (cm) | Resolution (Pixels/mm) | Additional Changes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Protocol | 3D | 1 | 5.8 | 2.1 | 1 | 62.5 | 256 × 256 | 12 | 24 × 24 | 1.1 | |
| BWₒₚₜ | 3D | 0.8 | 6.5 | 1.8 | 1 | 200 | 256 × 256 | 12 | 24 × 24 | 1.1 | |
| Test 1 | 3D | 0.6 | 6.9 | 2.6 | 1 | 41.7 | 256 × 256 | 12 | 24 × 24 | 1.1 | |
| Test 2 | 3D | 1 | 5.8 | 2.1 | 1 | 62.5 | 256 × 256 | 18 | 24 × 24 | 1.1 | |
| Test 3 | 3D | 1 | 5.8 | 2.1 | 1 | 62.5 | 256 × 256 | 12 | 24 × 24 | 1.1 | Manual shimming |
| Test 4 | 3D | 1 | 5.6 | 1.7 | 2 | 200 | 256 × 256 | 12 | 24 × 24 | 1.1 | |
| Test 5 | 3D | 1 | 5.8 | 2.1 | 1 | 62.5 | 256 × 256 | 10 | 24 × 24 | 1.1 | |
| Test 6 | 2D | 1.9 | 12 | 5.8 | 1 | 83.3 | 256 × 256 | 12 | 24 × 24 | 1.1 | Multi-echo GE seq. |
| Test 7 | 3D | 1 | 5.8 | 2.1 | 1 | 62.5 | 256 × 256 | 12 | 24 × 24 | 1.1 | AP Phase |
| Sequence Name | Acquisition Time (min) | SAR (W/kg) | SNR | CNR | No. of Detected Inserts | Mean Distortion (mm) | Max Distortion (mm) | λ Factor |
|---|---|---|---|---|---|---|---|---|
| Protocol | 4.80 | 0.29 | 19.21 | 5.30 | 376 | 1.30 | 3.38 | 0.28 |
| BWopt | 5.72 | 0.27 | 14.79 | 4.43 | 404 | 0.97 | 3.06 | 0.26 |
| Test 1 | 5.90 | 0.26 | 19.93 | 5.65 | 368 | 1.22 | 3.36 | 0.23 |
| Test 2 | 4.78 | 0.47 | 20.12 | 7.29 | 412 | 1.18 | 3.32 | 0.48 |
| Test 3 | 4.77 | 0.29 | 17.79 | 4.78 | 360 | 1.21 | 3.33 | 0.26 |
| Test 4 | 18.30 | 0.30 | 10.96 | 2.74 | 439 | 0.98 | 3.13 | 0.01 |
| Test 5 | 4.78 | 0.25 | 15.60 | 4.74 | 263 | 1.19 | 3.29 | 0.18 |
| Test 6 | 3.15 | 0.04 | 10.79 | 5.19 | 88 | 1.22 | 2.36 | 0.13 |
| Test 7 | 4.78 | 0.29 | 19.55 | 5.24 | 384 | 0.73 | 2.85 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campilho, B.; Silva, S.; Pinto, S.; Conde, P.; Lencart, J.; Mendes, B.; Santos, J. Minimizing 3T MRI Geometric Distortions for Stereotactic Radiosurgery via Anterior–Posterior Phase Encoding–A Phantom Study. Appl. Sci. 2025, 15, 9864. https://doi.org/10.3390/app15189864
Campilho B, Silva S, Pinto S, Conde P, Lencart J, Mendes B, Santos J. Minimizing 3T MRI Geometric Distortions for Stereotactic Radiosurgery via Anterior–Posterior Phase Encoding–A Phantom Study. Applied Sciences. 2025; 15(18):9864. https://doi.org/10.3390/app15189864
Chicago/Turabian StyleCampilho, Bernardo, Sofia Silva, Sara Pinto, Pedro Conde, Joana Lencart, Bruno Mendes, and João Santos. 2025. "Minimizing 3T MRI Geometric Distortions for Stereotactic Radiosurgery via Anterior–Posterior Phase Encoding–A Phantom Study" Applied Sciences 15, no. 18: 9864. https://doi.org/10.3390/app15189864
APA StyleCampilho, B., Silva, S., Pinto, S., Conde, P., Lencart, J., Mendes, B., & Santos, J. (2025). Minimizing 3T MRI Geometric Distortions for Stereotactic Radiosurgery via Anterior–Posterior Phase Encoding–A Phantom Study. Applied Sciences, 15(18), 9864. https://doi.org/10.3390/app15189864







