Efficient Yeast Inactivation and Protein Extraction from Wine Lees Using Pulsed Electric Fields and Ultrasound: A Comparative Energy-Based Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Lees Production
2.2. Autolysis
2.3. Microbiological Analysis
2.4. Protein Content Determination in Autolysates
2.5. Specific Energy Consumption Calculation
2.6. Statistical Analysis
3. Results and Discussion
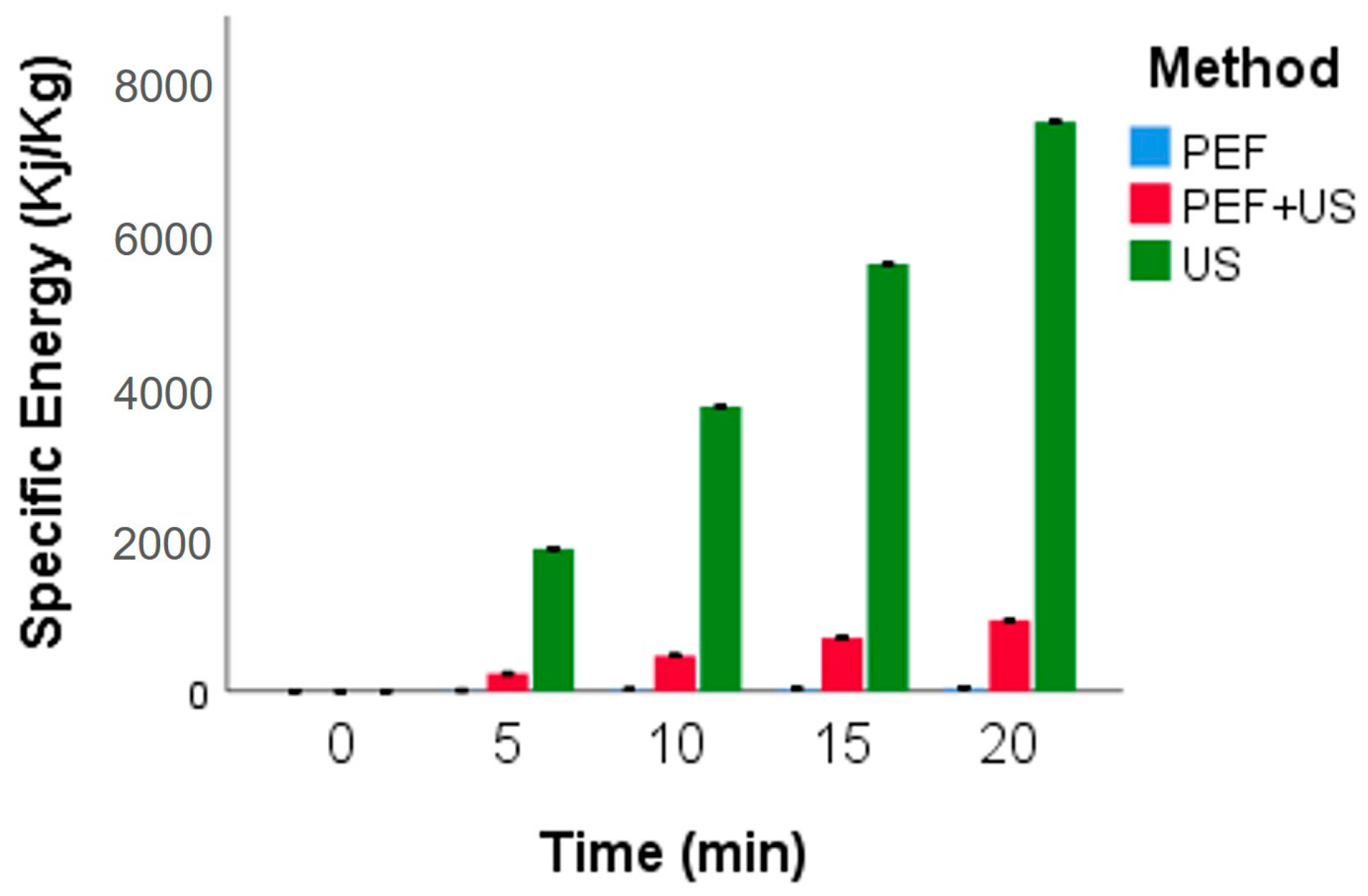
3.1. Specific Energy Consumption
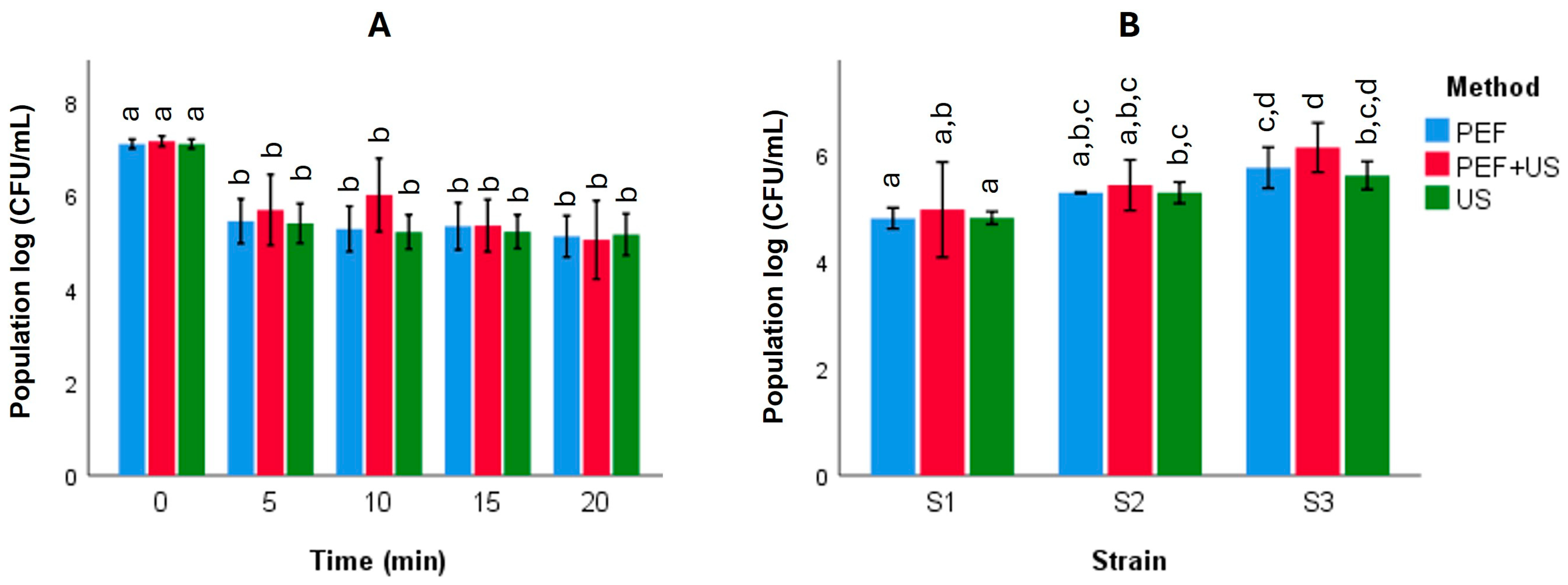
3.2. Yeast Viability and Disruption Dynamics
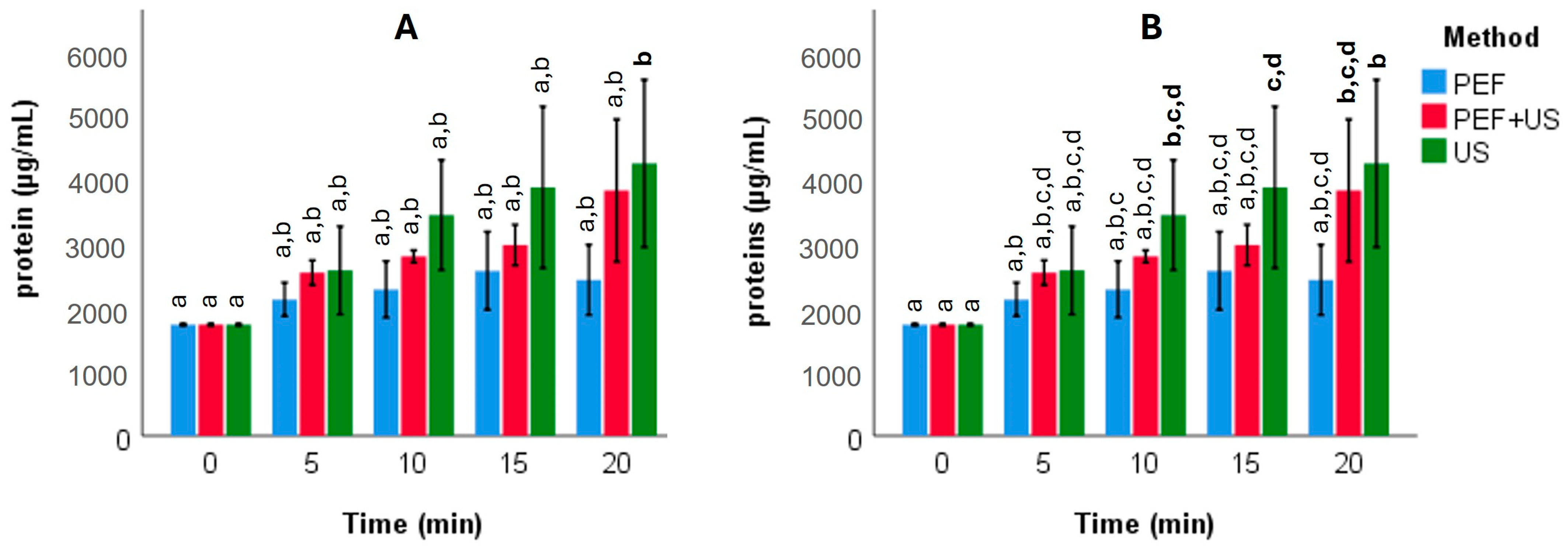
3.3. Protein Recovery Dynamics and Energy Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferrer-Gallego, R.; Silva, P. The Wine Industry By-Products: Applications for Food Industry and Health Benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef]
- De Iseppi, A.; Marangon, M.; Vincenzi, S.; Lomolino, G.; Curioni, A.; Divol, B. A Novel Approach for the Valorization of Wine Lees as a Source of Compounds Able to Modify Wine Properties. LWT 2021, 136, 110274. [Google Scholar] [CrossRef]
- Felix, M.; Martínez, I.; Sayago, A.; Recamales, M.Á.F. Wine Lees: From Waste to O/W Emulsion Stabilizer. Innov. Food Sci. Emerg. Technol. 2021, 74, 102810. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Physicochemical and Nutritional Characterization of Winemaking Lees: A New Food Ingredient. Agronomy 2020, 10, 996. [Google Scholar] [CrossRef]
- Ye, Z.; Qin, Y.; Harrison, R.; Hider, R.; Bekhit, A.E.D.A. Characterization of Bioactive Compounds in Lees from New Zealand Wines with Different Vinification Backgrounds. Antioxidants 2022, 11, 2335. [Google Scholar] [CrossRef]
- Mikuš, J.; Sochor, J.; Ailer, Š.; Baroň, M. The Effect of Yeast Autolysis on the Composition of Wine. Potravin. Slovak. J. Food Sci. 2024, 18, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Pons-Mercadé, P.; Giménez, P.; Vilomara, G.; Conde, M.; Cantos, A.; Rozès, N.; Ferrer, S.; Canals, J.M.; Zamora, F. Monitoring Yeast Autolysis in Sparkling Wines from Nine Consecutive Vintages Produced by the Traditional Method. Aust. J. Grape Wine Res. 2022, 28, 347–357. [Google Scholar] [CrossRef]
- Blanco-Huerta, C.; Rodríguez-Nogales, J.M.; Vila-Crespo, J.; Ruipérez, V.; Fernández-Fernández, E. Impact of High Hydrostatic Pressure and Ultrasound Technologies in the Autolytic Process of Saccharomyces cerevisiae in a Model Wine System. Food Biosci. 2024, 57, 103614. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Stefanou, N.; Andreou, V.; Taoukis, P. Effect of Pulsed Electric Fields on the Production of Yeast Extract by Autolysis. Innov. Food Sci. Emerg. Technol. 2018, 48, 287–295. [Google Scholar] [CrossRef]
- García Martín, J.F.; Guillemet, L.; Feng, C.; Sun, D.W. Cell Viability and Protein Release during Ultrasound-Assisted Yeast Lysis of Light Lees in Model Wine. Food Chem. 2013, 141, 934–939. [Google Scholar] [CrossRef]
- Marín-Sánchez, J.; Berzosa, A.; Álvarez, I.; Raso, J.; Sánchez-Gimeno, C. Yeast Protein Extraction Assisted by Pulsed Electric Fields: Balancing Electroporation and Recovery. Food Hydrocoll. 2025, 168, 111527. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Altunakar, B. Pulsed Electric Fields Processing of Foods: An Overview. In Food Engineering Series; Springer: New York, NY, USA, 2006; pp. 3–26. [Google Scholar] [CrossRef]
- Aldrich, J.E. Basic Physics of Ultrasound Imaging. Crit. Care Med. 2007, 35 (Suppl. S5), S131–S137. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in Application of Ultrasound in Food Processing: A Review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhang, M.; Adhikari, B. The Inactivation of Enzymes by Ultrasound—A Review of Potential Mechanisms. Crit. Rev. Food Sci. Nutr. 2013, 30, 1–21. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, R.; Rahaman, A.; Zeng, X.A.; Brennan, C.S. Combined Effects of Pulsed Electric Field and Ultrasound Pretreatments on Mass Transfer and Quality of Mushrooms. LWT 2021, 147, 112008. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Dourtoglou, V.G.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Lalas, S.I. Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment. AgriEngineering 2022, 4, 847–854. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Dourtoglou, V.G.; Elhakem, A.; Sami, R.; Ashour, A.A.; Shafie, A.; et al. Combination of Pulsed Electric Field and Ultrasound in the Extraction of Polyphenols and Volatile Compounds from Grape Stems. Appl. Sci. 2022, 12, 6219. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Liu, S. Power Ultrasound and Its Applications: A State-of-the-Art Review. Ultrason. Sonochem. 2020, 62, 104722. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Tsapou, E.A.; Drosou, F.; Bozinou, E.; Lalas, S.L.; Tataridis, P.; Dourtoglou, V. Pulsed Electric Field Extraction of α- and β-Acids from Pellets of Humulus lupulus (Hop). Front. Bioeng. Biotechnol. 2020, 8, 297. [Google Scholar] [CrossRef]
- Tzamourani, A.; Lytra, G.; Goulioti, E.; Gammacurta, M.; Marchal, A.; Evangelou, A.; Arapitsas, P.; Kotseridis, Y.; Par-askevopoulos, I.; Dimopoulou, M. Profound Analysis of the Impact of Indigenous Saccharomyces cerevisiae Strains on the Chemical and Sensorial Profile of Assyrtiko Wine. ACS Food Sci. Technol. 2025, 6, 2286–2295. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In Protein Protocols Handbook; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar] [CrossRef]
- Sebastià, A.; Calleja-Gómez, M.; Pallarés, N.; Barba, F.J.; Berrada, H.; Ferrer, E. Impact of Combined Processes Involving Ultrasound and Pulsed Electric Fields on ENNs, and OTA Mitigation of an Orange Juice–Milk Based Beverage. Foods 2023, 12, 1582. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current Applications and New Opportunities for the Use of Pulsed Electric Fields in Food Science and Industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M.; Pecyna, A.; Buczaj, A. Process Efficiency and Energy Consumption during the Ultrasound-Assisted Extraction of Bioactive Substances from Hawthorn Berries. Energies 2021, 14, 7638. [Google Scholar] [CrossRef]
- Pobiega, K.; Matys, A.; Trusinska, M.; Rybak, K.; Witrowa-Rajchert, D.; Nowacka, M. The Effect of Ultrasound and Pulsed Electric Field on the Osmotic Dehydration Process of Strawberries. Appl. Sci. 2023, 13, 12335. [Google Scholar] [CrossRef]
- Raso, J.; Heinz, V.; Alvarez, I.; Toepfl, S. (Eds.) Pulsed Electric Fields Technology for the Food Industry; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Berzosa, A.; Marín-Sánchez, J.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Extraction of Yeast Cell Compounds: Comparing Pulsed Electric Fields with Traditional Thermal Autolysis. Food Res. Int. 2025, 217, 116852. [Google Scholar] [CrossRef]
- Zhao, J.; Fleet, G.H. Degradation of DNA during the Autolysis of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2003, 30, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Jouany, J.; Yiannikouris, A.; Bertin, G. The Chemical Bonds between Mycotoxins and Cell Wall Components of Saccharomyces cerevisiae Have Been Identified. Mycotoxin Res. 2009, 25, 43–55. [Google Scholar]
- Wang, J.; Li, M.; Zheng, F.; Niu, C.; Liu, C.; Li, Q.; Sun, J. Cell Wall Polysaccharides: Before and after Autolysis of Brewer’s Yeast. World J. Microbiol. Biotechnol. 2018, 34, 139. [Google Scholar] [CrossRef]
- Teparić, R.; Lozančić, M.; Mrša, V. Evolutionary Overview of Molecular Interactions and Enzymatic Activities in the Yeast Cell Walls. Int. J. Mol. Sci. 2020, 21, 8996. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, Plasmolysis and Enzymatic Hydrolysis of Baker’s Yeast (Saccharomyces cerevisiae): A Comparative Study. World J. Microbiol. Biotechnol. 2020, 36, 65. [Google Scholar] [CrossRef]
- Seredyński, R.; Wolna, D.; Kędzior, M.; Gutowicz, J. Different Patterns of Extracellular Proteolytic Activity in W303a and BY4742 Saccharomyces cerevisiae Strains. J. Basic. Microbiol. 2017, 57, 34–40. [Google Scholar] [CrossRef]
- Schiavone, M.; Sieczkowski, N.; Castex, M.; Dague, E.; François, J.M. Effects of the Strain Background and Autolysis Process on the Composition and Biophysical Properties of the Cell Wall from Two Different Industrial Yeasts. FEMS Yeast Res. 2015, 15, fov012. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter Cultures for Sparkling Wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications: A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Toepfl, S.; Heinz, V.; Knorr, D. High-Intensity Pulsed Electric Fields Applied for Food Preservation. Chem. Eng. Process. 2007, 46, 537–546. [Google Scholar] [CrossRef]
- Martín-Belloso, O.; Sobrino-López, A. Combination of Pulsed Electric Fields with Other Preservation Techniques. Food Bioprocess. Technol. 2011, 4, 954–968. [Google Scholar] [CrossRef]
- Maza, M.A.; Martínez, J.M.; Delso, C.; Camargo, A.; Raso, J.; Álvarez, I. PEF-Dependency on Polyphenol Extraction during Maceration/Fermentation of Grenache Grapes. Innov. Food Sci. Emerg. Technol. 2020, 60, 102303. [Google Scholar] [CrossRef]
- García Martín, J.F.; Sun, D.W. Ultrasound and Electric Fields as Novel Techniques for Assisting the Wine Ageing Process: The State-of-the-Art Research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of Protein-Rich Extracts from Spent Brewer’s Yeast (Saccharomyces cerevisiae): An Overview. Biomass Convers. Biorefin. 2022, 15, 1771–1793. [Google Scholar] [CrossRef]
- Bystryak, S.; Santockyte, R.; Peshkovsky, A.S. Cell Disruption of S. cerevisiae by Scalable High-Intensity Ultrasound. Biochem. Eng. J. 2015, 99, 99–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntourtoglou, G.; Tzamourani, A.; Kasioura, A.; Tsioka, A.; Gimenez-Gil, P.; Gkizi, D.; Dimopoulou, M.; Arapitsas, P.; Evangelou, A. Efficient Yeast Inactivation and Protein Extraction from Wine Lees Using Pulsed Electric Fields and Ultrasound: A Comparative Energy-Based Approach. Appl. Sci. 2025, 15, 9860. https://doi.org/10.3390/app15189860
Ntourtoglou G, Tzamourani A, Kasioura A, Tsioka A, Gimenez-Gil P, Gkizi D, Dimopoulou M, Arapitsas P, Evangelou A. Efficient Yeast Inactivation and Protein Extraction from Wine Lees Using Pulsed Electric Fields and Ultrasound: A Comparative Energy-Based Approach. Applied Sciences. 2025; 15(18):9860. https://doi.org/10.3390/app15189860
Chicago/Turabian StyleNtourtoglou, George, Aikaterini Tzamourani, Angeliki Kasioura, Artemis Tsioka, Pol Gimenez-Gil, Danai Gkizi, Maria Dimopoulou, Panagiotis Arapitsas, and Alexandra Evangelou. 2025. "Efficient Yeast Inactivation and Protein Extraction from Wine Lees Using Pulsed Electric Fields and Ultrasound: A Comparative Energy-Based Approach" Applied Sciences 15, no. 18: 9860. https://doi.org/10.3390/app15189860
APA StyleNtourtoglou, G., Tzamourani, A., Kasioura, A., Tsioka, A., Gimenez-Gil, P., Gkizi, D., Dimopoulou, M., Arapitsas, P., & Evangelou, A. (2025). Efficient Yeast Inactivation and Protein Extraction from Wine Lees Using Pulsed Electric Fields and Ultrasound: A Comparative Energy-Based Approach. Applied Sciences, 15(18), 9860. https://doi.org/10.3390/app15189860








