Abstract
This study presents a comparative analysis of the physicochemical, microbiological, and microstructural characteristics of feed products subjected to electron beam irradiation using the ILU-10 accelerator, as well as non-irradiated samples stored under suboptimal conditions. Feed product samples were obtained from a flour and feed manufacturing facility located in the city of Semey, Abai region. For each type of feed product, 20 samples were irradiated at doses of 3, 6, and 9 kGy, while another 20 samples served as a non-irradiated control group. All samples were stored in conditions that were unfavorable for long-term preservation. After one month, microbiological parameters, mycotoxin content, changes in acidity (pH), and microstructure were assessed using standard analytical methods. This study revealed a significant reduction in the number of microorganisms and preservation of key physicochemical properties of the feed products after one month of storage when irradiated at a dose of 9 kGy. These findings suggest that electron beam treatment can serve as an effective method for feed preservation in cases where storage conditions do not meet regulatory standards. However, further research is required to explore alternative approaches and gain a deeper understanding of the potential applications of electron beam technology in compound feed storage systems.
1. Introduction
Cereal grains are among the most widely consumed staple foods around the world and play a fundamental role in human nutrition and food security. Not only are these grains a primary source of energy in the form of carbohydrates, but they also provide essential proteins, dietary fiber, vitamins (notably thiamin, riboflavin, and niacin), minerals, and bioactive compounds [1]. As such, ensuring the safety, quality, and stability of cereals during their post-harvest handling, storage, and processing is of paramount importance. However, cereals are prone to contamination by a variety of microorganisms, including spoilage organisms, opportunistic pathogens, and toxigenic fungi, all of which pose serious health risks and economic losses. These contaminants can be introduced at various points in the supply chain, during harvesting, threshing, transportation, storage under suboptimal conditions, or even through processing equipment [2].
The microbial flora typically associated with cereal grains include aerobic mesophilic bacteria, coliforms, Enterobacteriaceae, lactic acid bacteria, yeasts, molds, and spore-forming bacteria such as Bacillus cereus. Under favorable environmental conditions, these microorganisms can proliferate, leading to spoilage, deterioration of sensory properties, reduction in shelf life and, in some cases, mycotoxin production. The prevalence of fungi such as Aspergillus, Penicillium, and Alternaria further compounds safety concerns, especially in regions with high humidity and poor storage infrastructure. These issues are particularly pressing in developing countries, where post-harvest losses due to microbial spoilage remain significant [3].
To mitigate microbial risks and extend the shelf life of cereals, a range of decontamination techniques has been explored, including thermal treatments (e.g., autoclaving, roasting), chemical fumigation (e.g., ethylene oxide, methyl bromide), and natural antimicrobials [4]. However, these approaches often face limitations: thermal methods may degrade heat-sensitive nutrients and alter sensory attributes; chemical residues may pose toxicological risks and are subject to regulatory restrictions; and natural antimicrobials are not always effective against the resistance of a spore-forming spoilage bacterium (Bacillus cereus and related genera) [5,6].
In this context, ionizing radiation has emerged as a promising non-thermal preservation method that offers several advantages: it can effectively reduce microbial loads without significantly increasing the temperature, it does not introduce chemical residues, and it can be precisely controlled and standardized. Gamma irradiation using isotopes such as cobalt-60 or cesium-137 has been shown to be particularly effective for the treatment of dry foods, such as cereals. Several studies have reported significant microbial reductions in cereal grains subjected to gamma irradiation. For example, wheat treated with gamma irradiation doses of up to 10 kGy showed complete inactivation of Bacillus cereus and Enterobacteriaceae, with no viable microorganisms detected even after nine months of storage [7]. Similar efficacy has been demonstrated in other cereal grains, where a gamma irradiation dose of 10 kGy typically achieves a 3-log reduction in aerobic bacterial counts, and 15 kGy has been reported to eliminate virtually all viable microorganisms [8].
The efficacy of irradiation is strongly dose-dependent. Lower irradiation doses (e.g., 2–5 kGy) may suffice for reduction in spoilage organisms and fungi in flour and whole grains, while higher doses (10–15 kGy) are required for sterilization-level decontamination or to control spore-forming bacteria and mycotoxigenic fungi. For example, studies have shown that an irradiation dose of 5 kGy significantly reduces total bacterial counts and yeast/mold populations in flours from buckwheat, cowpea, oats, and brown rice [9], while complete inhibition of Alternaria alternata requires a dose of 10 [10]. These findings support the development of customized irradiation protocols based on the intended use of the cereal (e.g., immediate consumption vs. long-term storage), the microbial risks involved, and the desired shelf life.
However, the use of irradiation is not without challenges. Exposure to ionizing radiation can induce physicochemical changes in cereal components, including depolymerization of starch molecules, altered secondary and tertiary structures, and degradation of certain vitamins, particularly those that are heat- and radiation-labile. Although some studies report minimal effects on total amino acid content and functional properties at doses up to 10 kGy, reductions in thiamin and riboflavin levels have been observed [8]. Furthermore, irradiation can affect pasting viscosity, gelatinization behavior, and water absorption capacity, which may have implications for the technological performance of cereals in food processing [11]. Therefore, an optimal balance must be found between achieving microbial safety and preserving nutritional and functional quality.
In response to these concerns, alternative non-thermal technologies such as cold plasma (non-thermal plasma) and far-ultraviolet C (far-UVC) light have gained traction. Non-thermal plasma treatments generate reactive species which are capable of disrupting microbial cell membranes and DNA, offering broad-spectrum antimicrobial effects with minimal thermal or chemical impact on the treated substrate [12]. Unlike conventional UVC (254 nm), far-UVC light (200–230 nm) has a limited penetration depth and is considered safe for surface treatment; moreover, it is especially effective against fungi and bacteria without adversely affecting moisture content or seed viability [13]. These methods have potential as standalone treatments or as part of an integrated hurdle technology approach in cereal processing.
Given the growing emphasis on sustainable, minimally processed feeds that meet stringent safety standards, there is a pressing need to evaluate and compare the effects of both conventional and emerging decontamination technologies on cereals.
Exposure to electron energy has a beneficial effect and can serve as a way to increase the shelf life of grain feed. A special feature of this work is the analysis of the effect of ionizing radiation generated by the ILU-10 electron accelerator during feed storage under suboptimal conditions.
Previous studies lack sufficient data on the effects of electron beam treatment on the storage stability of grain and feed products, particularly under unfavorable conditions.
The research deepens our knowledge about feed processing methods using the ILU-10 accelerator and provides promising approaches to improve grain quality during storage. As part of this study, a detailed analysis of how processing changes the physicochemical characteristics and microstructure of feed products was performed, which allows for the development of new strategies and techniques for their storage, especially when deviating from established storage standards.
The present study was limited to typical feed samples prevalent in a specific region, which were determined by both logistical and methodological factors. The main focus was on studying the initial effects of electron beam treatment during the first month of storage under unfavorable conditions and its short-term stability. At the same time, we acknowledge the necessity of expanding future research to encompass a broader range of feed types, different geographical regions, and extended storage periods of up to six months. Such an approach will enable a more comprehensive evaluation of the effectiveness of electron beam irradiation and its impact on various feed products.
The purpose of this study is to conduct a comprehensive evaluation of ionizing radiation with respect to its efficacy in reducing microbiological activity and its impact on the functional properties and storage stability of various feed products. The findings are expected to inform best practices for industrial applications and support the development of evidence-based protocols for the safe processing of grain-based feed products.
2. Materials and Methods
2.1. Experimental Procedure
In Kazakhstan, ILU-10 electron accelerators are being actively integrated into agricultural technologies, while research continues to determine the optimal parameters for processing agricultural raw materials and animal feed.
The purpose of this study was to study the effects of electron beam irradiation with an ILU-10 electron accelerator using various dosimetric parameters on the physicochemical, microstructural, and microbiological properties of feed products, as well as the presence of mycotoxins during storage in artificially created unfavorable conditions for 30 days.
The scheme of the experimental research is shown in Figure 1.
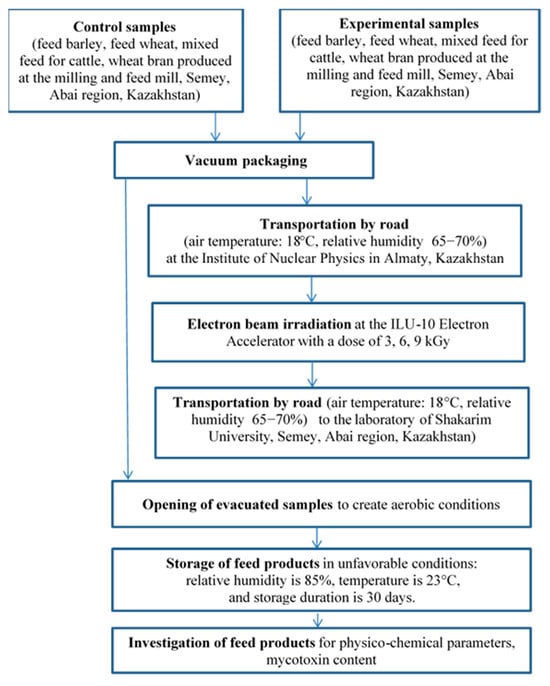
Figure 1.
Experimental research scheme.
This study focused on feed wheat, feed barley, wheat bran, and compound feed for cattle. Feed product samples were collected from a flour and feed mill located in Semey (Abai region, Kazakhstan).
The selection of the product range was carried out in alignment with the objectives of the research project, taking into account the production activities of local enterprises specializing in feed manufacturing.
Both the control and irradiated samples were packed in polypropylene bags and vacuum sealed.
Packaging of the samples was carried out using polypropylene bags measuring 15 cm × 10 cm, with each sample weighing 0.4 kg. Vacuum sealing was performed using a Henkelman Boxer 42 vacuum packaging system. The vacuum-packed samples are illustrated in Figure 2.

Figure 2.
Packaged samples of feed products.
Vacuum-packed samples were transported by road under controlled environmental conditions (air temperature: 18 °C; relative humidity: 65–70%). The experimental samples were irradiated using the ILU-10 electron accelerator at the Institute of Nuclear Physics in Almaty, Kazakhstan. A photograph of the ILU-10 electron accelerator is shown in Figure 3. The control samples were not subjected to electron beam treatment.
Figure 3.
ILU-10 electron accelerator at the Institute of Nuclear Physics, Almaty, Kazakhstan.
The feed products, contained in their primary packaging, were transported via a pallet conveyor to the irradiation chamber. There, they were exposed to ionizing radiation generated by the linear electron accelerator. After treatment, the products were transported out of the irradiation zone.
The samples of feed products were vacuum-sealed prior to treatment to prevent the presence of oxygen during radiation processing. The packaged samples were irradiated using a pulsed electron beam in the ILU-10 unit, operating at an electron energy of 4 MeV and a constant conveyor speed of 3.6 m/min.
The absorbed doses in the treated products were monitored using B3WinDose chemical dosimeters (Gex Corp., Palm City, FL, USA).
Dosimeter readings were obtained using a standard Genesys 20 spectrophotometer (Thermo Fisher Scientific Corp., Waltham, MA, USA) and subsequently converted to actual dose values. The measurement error for the absorbed doses did not exceed 10–12% at a confidence level.
The processing parameters for the feed products using the ILU-10 electron accelerator are presented in Table 1.

Table 1.
Radiation treatment parameters (ILU-10 electron accelerator).
The radiation treatment of the products was carried out at a temperature of 20 ± 5 °C, and a single treatment mode was used.
For the purpose of conducting an experiment, unfavorable storage conditions were established for the preservation of feed products, with a relative humidity of 85%, a storage temperature of 23 °C, and a storage duration of 30 days. Subsequently, both the irradiated and control samples were stored in a dedicated chamber. In the storage environment, the bags were opened to allow air exchange, creating aerobic conditions.
The selection of a limited storage duration under unfavorable conditions was dictated by the necessity of obtaining preliminary data that capture the most pronounced changes arising in the early storage period, when the risk of recontamination is maximal.
2.2. Microstructure
Observations of the microstructure of feed barley, feed wheat, compound feed for cattle, and wheat bran were carried out using a low-vacuum scanning electron microscope JSM-6390LV JEOL (Tokyo, Japan) [14].
2.3. Determination of Grain Moisture
State standard GOST 13586.5-2015 [15] is a method to determine the moisture content of grain via drying with air heat. The sampling procedure is carried out as follows: batches of crushed grain weighing 300 g are prepared and stored in a refrigerator at a certain temperature from 100 to 150 °C. When the heating capacity of the drying cabinet is such that the set temperature (130 ± 2 °C) is restored in 15 min or less after being filled with samples, the temperature should be lowered to 105 °C for no more than 4 min. The drying time of the grain sample, depending on the humidity previously measured with a moisture meter, is set at a humidity of up to 25% for 7 min, from 25 to 35% for 12 min, and more than 35% for 30 min. The samples are then cooled, and hygrometers are used to measure the humidity level. According to the calculated drying time and humidity, the moisture before and after exposure is compared in the drying unit.
The moisture content of grain and corn kernels X without prior drying, %, is calculated using Equation (1):
where m1 is the mass of the crushed grain or rods before drying, g; m2 is the mass of the suspension of crushed grain or rods after drying, g; and 20 is the coefficient for calculating humidity, %.
X = 20(m1 − m2)
The calculation results are written down to the second decimal place.
The rounding of the humidity measurement results is carried out as follows: if the first of the discarded digits is less than five, then the last saved digit is not changed, but if the first of the discarded digits is more than five, then the last saved digit is increased by one; if the first of the discarded digits is 5, then the last saved digit is increased by one; however, if the digit is odd, it is discarded even if it is an even number [15].
2.4. Cultivation of Toxigenic Fungi
Mycotoxins are known to be produced by the toxigenic fungi Aspergillus spp., Penicillium spp., and Fusarium spp. [16].
To isolate the fungi from the grain, the samples are placed on the surface of the nutrient medium in Petri dishes with Chapek agar or agar wort. A total of 10 pieces of fungi are carefully placed in each Petri dish, ensuring that they do not come into contact with each other in order to prevent contamination from other colonies.
The separation of fungi from concentrated feeds (except grain) of the compound feeds is carried out by sowing a diluted suspension of the samples in a nutrient medium according to regulatory documentation. A sample of granular or briquetted feed is pre-milled in a laboratory mill. An amount of 10 g of compound feed is placed in a 0.1% flask with 100 mL of a sterile solution of a surfactant (Twin-80) in distilled water, and then the sample is disintegrated by shaking on a mechanical shaker for 15–20 min. Subsequent dilutions are prepared from this suspension 1 (1:10) using sterile solutions of the above-mentioned surfactant in the following way: 1 mL of the sample is taken with a graduated sterile pipette (the end of the pipette should be cut off for free passage of feed particles, after which the pipette should be calibrated for 1 mL); a tube with 9 mL of solution is poured to obtain suspension 2 (1:100). From the resulting suspension 2, suspension 3 (1:1000) is prepared in the same way, and if necessary, suspension 4 (1:10,000) is prepared. Before taking the next portion of the suspension, both for further dilution and for sowing, it is necessary to mix the suspension thoroughly with a pipette and also rinse the pipette in the suspension at least 5 times. Feed with normal organoleptic parameters is diluted 1:1000, and spoilage is diluted 1:1000. Sowing is carried out immediately after the preparation of the last suspension (3 or 4), without allowing it to settle, while 1 mL is evenly distributed over the entire surface of the nutrient medium. The number of cups sown depends on the selected degree of dilution: 5 cups at a dilution of 1:1000 and 8 cups at a dilution of 1:10,000.
A Petri dish with crops is placed on a thermostat wrapped in sterile paper and maintained at a temperature of 22–25 °C for 7–10 days. The growth of most fungi becomes noticeable after 3 days. However, the identification of fungi requires long periods of cultivation, approximately 5–7 days. After their cultivation in a thermostat, microscopy of the grown fungi colonies is performed to identify the species [17].
2.5. Determination of Mycotoxins
State standard GOST 28001-88 [18] method consists of the extraction of acetone and the purification of lipids and plant pigments, followed by post-purification on a chromatographic column and double chromatography on a Silufol plate with a standard toxin solution. The sensitivity of the method is 600 micrograms/kg of feed.
The T-2 toxin on the chromatogram in the test sample is detected as a spot with blue fluorescence corresponding to the position and fluorescence of the T-2 toxin spot in the standard sample. When using the toluene–ethyl acetate–formic acid (6:3:1) system in repeated chromatography, the Rf of the T-2 toxin is 0.23–0.24; when using the chloroform–ethyl acetate–acetic acid system (17:3:1), the RF of the T-2 toxin is 0.20–0.23. The result is considered positive for the presence of T-2 toxin if blue fluorescence is detected on the Silufol plate in the test sample corresponding to the fluorescence spot of the standard T-2 toxin solution in at least one of the two parallel samples.
The content of ochratoxin A in the test sample (A) in micrograms per 1 kg of feed is calculated using Equation (2):
where W1 is the minimum volume of the most diverse production, in which a very special glow of the oracle A, cm3 is installed; W2 is the total volume of the product produced, cm3; M is the mass of the sample, g; 0.001 is the minimum amount of ochratoxin A detected on the Silufol plate under the UVL source, mcg; and 2 is a coefficient that takes into account the loss of a substance during the analysis.
The arithmetic mean of the results of two parallel determinations is taken as the final test result. The result is calculated to the third decimal place and rounded to the second decimal place.
The zearalenone content in the sample (X) in milligrams per 1 kg of feed according to the luminescence intensity of the standard and test solution is calculated using Equation (3):
where A is the mass concentration of zearalenone in the standard solution, mcg/cm3; B is the final volume of the extract, taking into account the possible dilution, cm3; C, D are the corresponding global structure and status and the graphic composition of the Sun with the same luminous intensity, cm3; and 12.5 is the mass of the feed sample, corresponding to the volume of the extract subjected to purification, g.
The arithmetic mean of the results of two parallel determinations is taken as the final test result. The result is calculated in the third decimal place and rounded to the second decimal place [18].
State standard GOST R 51116-97 [19] is the method for determining the content of deoxynivalenol (vomitoxin) using a liquid chromatograph “Milichrome.” The essence of the method consists of extracting deoxynivalenol (vomitoxin) from a test sample with a mixture of acetonitrile and water, purifying the extract on two consecutive columns with activated carbon, and quantifying deoxynivalenol (vomitoxin) using a liquid chromatograph “Milichrome” with a spectrophotometric detector for the ultraviolet region (hereinafter used with a UV detector) in a two-wave mode.
The limits of determination in the sample are 0.2–4.0 mg/kg, in solution, 1–20 ng/mm3.
In the case where calibration solutions are chromatographed simultaneously with the test solutions, the mass fraction of deoxynivalenol (vomitoxin) X, ng/g in the test sample is calculated using Equation (4):
where mi is the mass of deoxynivalenol (vomitoxin) introduced into a chromatograph with a calibration solution, the peak height of which is the value that is the least different from the peak height of the test solution, ng; h is the arithmetic mean of three measurements of the height of the peaks of the test solution, mm; hi is the arithmetic mean of the three measurements of the height of the peaks of the calibration solution, mm; 0.2 is the volume of the deoxynivalenol (vomitoxin) solution tested from the sample, cm3; 4 is the volume of the test solution introduced into the chromatograph, mkdm3; 125 is the volume of the extract of the test sample, cm3; 5 is the volume of the extract of the test sample taken for purification, cm3; 25 is the weight of the sample, g; 87 is the degree of extraction of deoxynivalenol (vomitoxin) from the sample, %; and 1000 is the conversion coefficient of cm3 to mcdm3.
The mass fraction of deoxynivalenol (vomitoxin) X, ng/g, in the test sample using a calibration coefficient is calculated using Equation (5):
where K is the calibration coefficient, ng/mm or ng/e.
When analyzing each test sample, two parallel determinations are performed, beginning with taking a sample.
If the discrepancy between the results of parallel definitions does not exceed |x1 − x2| < 0.01 D x, where x1, x2, and X are the results of the first and second parallel definitions and their arithmetic mean, respectively, then the arithmetic mean x is taken as the result of the analysis. Otherwise, the analysis is repeated. The value of norm (d) depends on the range of the mass concentration of deoxygenol (vomixin), ng/g [19].
State standard GOST 30711-01 [20] method consists of the extraction of aflatoxins in1 from a product sample, the purification of the extract from interfering substances, and measurement of the mass concentration of aflatoxins in1 using thin-layer chromatography with visual determination of the amount of substance in the stain.
The aflatoxin content in1, x1, mg/kg, for cereals is calculated using Equation (6):
where m1 is the mass of aflatoxin B1 found in the sample stain, ng; V1 is the total volume of the water–acetone extract (the total volume of added solutions of sodium chloride and acetone), cm3; V3 is the volume of the water–acetone extract after adding a solution of lead acetate and water to it, cm3; V5 is the volume to which the extract is concentrated prior to chromatographic separation, cm3; K is the aflatoxin extraction coefficient in1; m is the mass of the sample, g; V2 is the volume of the water–acetone filtrate taken for analysis, cm3; V4 is the volume of the water–acetone filtrate after purification with lead acetate, taken for analysis, cm3; and V6 is the volume of the extract applied to the plate, cm3 [20].
2.6. Determination of the Count of Mesophilic Aerobic and Facultative Anaerobic Microorganisms
The determination of the presence of aerobic and facultative anaerobic microorganisms, CFU/g, is carried out according to State standard GOST 10444.15-94 [21]. The method is based on the sowing of a certain amount of product or diluting it in an agarized nutrient medium, aerobic cultivation of crops at a temperature of 30 ± 1 °C for 72 ± 3 h, counting all visible colonies, and determining the number of aerobic and facultative anaerobic microorganisms in an agarized nutrient medium. After incubation of the crops, the number of colonies grown in Petri dishes is calculated. Petri dishes are selected for counting, on which 15 to 300 colonies have grown. In liquid nutrient media, the presence or absence of visible signs of growth (gas formation and the appearance of turbidity and sediment) is noted [21].
2.7. Determination and Counting of Yeast and Mold Fungi
State standard GOST 10444.12-2013 [22] is applied for the determination and counting of yeast and mold fungi. For ten-fold dilutions, additional Petri dishes are used, which are prepared using the same procedure for sowing the test sample or the initial suspension. The crops in Petri dishes are filled with molten medium and cooled to 45 ± 1 °C. At the same time, 15–20 cm3 of medium is poured into a Petri dish to check its sterility. The cups are incubated under aerobic conditions at a temperature of 25 ± 1 °C for 5 days. After 3 days of incubation, a preliminary count of the number of grown colonies is carried out, and after 5 days, the final count is performed. If flour-bearing (fast-growing) fungi are present in crops in dense media, then the removal of preliminary results should be carried out very carefully, preventing the spores of these fungi from crumbling and giving rise to secondary colonies. If there are no mold fungi and/or yeast in the crops after 5 days of incubation, then the crops can be left at room temperature for 1–2 days to identify slow-growing mold fungi and yeast.
If the number of yeast or mold fungi satisfies 4.1.5 in two consecutive dilutions, then their number per 1 g or 1 cubic centimeter of product (X) is calculated using Equation (7):
where is the sum of all counted colonies in Petri dishes in two consecutive dilutions; n1 is the number of Petri dishes calculated for a lower dilution, i.e., for a more concentrated dilution of the product; n2 is the number of Petri dishes calculated for a greater dilution; and n is the degree of dilution of the product (for a lower dilution).
If the counted number of colonies is less than 15 for yeast or less than 5 for mold fungi—for example, the amount of yeast or mold fungi in 1 g or cm3 of a product is A (less than 15 or 5) and the lower and upper bounds for the 95% confidence interval are indicated in parentheses—then when sowing the initial dilution, the values of A and those indicated in parentheses are multiplied by the dilution coefficient. If the counted number of colonies is 8, the result is expressed as follows:
- -
- the amount of yeast and/or mold fungi in 1 g or 1 cm3 of the product is 8 (from 3 to 13);
- -
- taking into account the tenfold dilution used to prepare the initial dilution, the amount of yeast and/or mold fungi in 1 g or 1 cm3 of the product is 8.0-101 [22].
2.8. Determination of pH
To assess the acidity of the medium (pH), a potentiometric method was used with a 150MI pH meter model (developed by LLC “Measurement Technologies”, Moscow, Russia). The measurement was carried out by immersing two electrodes directly in the test solution, with the pH value displayed on the device screen [23].
2.9. Protein, Fat, Fiber, Calcium, and Phosphorus Research
In feed wheat, fat, protein, and fiber were studied using an Infralume FT-12 device, including the protein content in barley. The principle of operation of the Infralume FT-12 infrared analyzer is rapid analysis of bulk samples based on their absorption (transmission) spectra in the near-infrared (NIR) region using the fractional least squares (PLS) method and other modern chemometric methods. The inverse Fourier transform is used to register the spectra. The crops were analyzed without grinding; for this, the sample was filled into a cuvette, the analysis time was 1 min, and the data was displayed on a computer monitor.
All of the samples were subjected to chemical analysis in accordance with standard methods [24]. The calcium content was measured via titration, and phosphorus was measured using photospectrometric analysis. The Statistical Package for the Social Sciences (SPSS) version 26 software package was used to process the obtained results.
2.10. Statistical Analysis
The Mann–Whitney U test was used to assess the significant differences between the compared samples. To identify the relationships between variables, regardless of their conformity to a normal distribution, the Spearman rank correlation coefficient was calculated. The results were considered statistically significant at a significance level of p < 0.05 [25].
3. Results and Discussion
3.1. Investigation of Microbiological Parameters
Radiation technologies are used to ensure microbiological safety and to extend the shelf life of agricultural raw materials and compound feeds. Various types of irradiation equipment are employed for this purpose, including multipurpose gamma irradiators (Nordion, Ottawa, ON, Canada), electron beam accelerators (ILU-6, ILU-10, and ELV), and Vemata XR-series X-ray systems (Vemata, Novosibirsk, Russia).
Feed safety is influenced by numerous factors, including storage conditions, production processes, environmental hygiene, and the methods used to process raw materials and feed products. The shelf life of livestock feed typically ranges from one to six months, depending on its composition.
The control samples of feed products and experimental samples of feed products irradiated at doses of 3, 6, and 9 kGy were examined for microbiological parameters, including CMAFAM (Count of Mesophilic Aerobic and Facultative Anaerobic Microorganisms) and mold counts. The data are presented in Table 2.

Table 2.
Microbiological parameters in the control samples of feed products and experimental samples of feed products irradiated at doses of 3, 6, and 9 kGy.
The observed changes in the control samples demonstrated that mold was not detected in the feed wheat but was present in the samples of compound feed for cattle, wheat bran, and feed barley. The highest mold content was found in feed barley, 43 CFU/g, followed by compound feed for cattle, 39 CFU/g, while the lowest level was observed in wheat bran, 3 CFU/g.
The maximum CMAFAM value was identified in the sample of compound feed for cattle, where continuous microbial growth was observed. The average values were 6 × 102 CFU/g in feed wheat, 4 × 102 CFU/g in wheat bran, and the lowest value was recorded in feed barley, 2.4 × 102 CFU/g.
It is evident that unfavorable storage conditions contributed to increased moisture levels, which in turn promoted the growth of microorganisms and mold in the control samples of feed products. However, in all samples, the concentrations of mold and CMAFAM did not exceed the maximum permissible limits established by Technical Regulation TR CU 021/2011 [26].
The observed changes demonstrated that the application of ionizing radiation with 3 kGy during the irradiation of feed barley led to a reduction in mold contamination to 10 CFU/g. In compound feed for cattle samples, the contamination level decreased to 6 CFU/g. No mold fungi were detected in the samples of feed wheat and wheat bran, indicating a pronounced antifungal effect of electron beam treatment at the stated dose.
A similar trend was observed in the assessment of CMAFAM. Irradiation at a dose of 3 kGy reduced the CMAFAM count to 2 CFU/g in feed barley, 3 CFU/g in feed wheat, and 4 CFU/g in compound feed for cattle. In wheat bran samples, CMAFAM was not detected, further confirming the high efficacy of electron beam treatment in reducing microbial contamination.
These results suggest that ionizing radiation generates free radicals that are capable of destroying the cellular structures of microorganisms and molds, thereby contributing to the reduction in microbial load.
Previous studies [27] have demonstrated that irradiation of fruit and vegetable products inoculated with microbial cultures (Salmonella spp., Escherichia coli, and Staphylococcus aureus) at doses of 4 to 5 kGy resulted in increased microbial growth under various treatment conditions. However, at higher doses (6, 8, and 10 kGy), a clear inhibitory effect on microbial growth was observed.
The results obtained in the present study confirm the effectiveness of a dose of 3 kGy of electron beam irradiation in reducing microbial contamination in feed wheat and wheat bran.
Irradiation of feed samples at a dose of 6 kGy resulted in complete inhibition of mold growth in feed barley, feed wheat, and wheat bran. In compound feed for cattle, a significant reduction in mold levels was observed, reaching 3 CFU/g, compared with the levels recorded at a dose of 3 kGy. These findings indicate an enhanced fungicidal effect associated with the increased irradiation dose.
The CMAFAM analysis also demonstrated a substantial decrease in microbial contamination. In samples of feed barley and wheat, microbial counts were reduced to 1 × 102 CFU/g, while in compound feed samples, the count reached 2 × 102 CFU/g. Complete suppression of microorganisms was observed in wheat bran, with no CMAFAM detected.
These results confirm the high efficacy of electron beam irradiation at a 6 kGy dose in reducing fungal and bacterial microflora in feed products. Similar findings have been reported in previous research conducted by Asha B. Mohamed et al. [28], who assessed the impact of electron beam irradiation at doses of 5 kGy and higher on maize samples. The results showed a significant decrease in fungal contamination, with levels falling below the detection limit as determined by plating methods (<1.9 log CFU/g).
Additionally, Yakhkeshi examined the effects of electron beam irradiation at doses of 3, 5, and 7 kGy on broiler diets, and demonstrated that irradiation at 5 and 7 kGy completely eliminated microbial contamination in the feed [29].
Observations revealed that electron beam irradiation of feed products with 9 kGy resulted in the complete elimination of mold fungi in experimental samples of feed barley, feed wheat, and wheat bran. In compound feed for cattle, mold content was significantly reduced compared with the previously tested irradiation doses (3 and 6 kGy), reaching 1 CFU/g, indicating a pronounced fungicidal effect of high-dose irradiation.
CMAFAM analysis showed no detectable microbial contamination in samples of feed barley, compound feed for cattle, and wheat bran. In feed wheat samples, CMAFAM levels remained at 1 CFU/g, consistent with the values observed after irradiation at a dose of 6 kGy.
Electron beam irradiation using the ILU-10 accelerator significantly reduced microbiological contamination, even under unfavorable storage conditions. Increasing the irradiation dose from 3 to 9 kGy led to a more pronounced and stable reduction in bacterial load in feed products, confirming the effectiveness of this method in improving the microbiological safety of animal feeds.
3.2. Investigation of Moisture Characteristics in Feed Products
Moisture content is one of the key quality parameters during feed storage. An increase in moisture level beyond the standardized threshold can trigger self-heating processes, which subsequently promote mold development. The dynamics of the moisture content in the untreated control samples are illustrated in Figure 4.
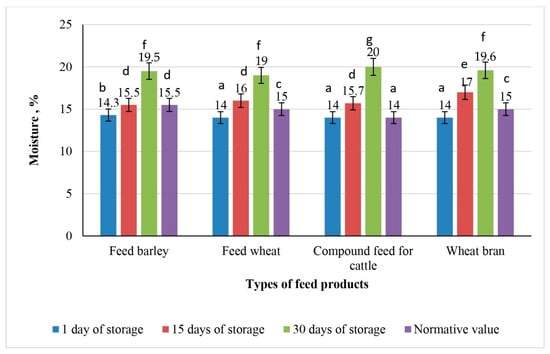
Figure 4.
Dynamics of moisture changes in the control samples of feed products during 30 days of storage (the values for bars sharing different letters (a–g) are significantly different (p < 0.05)).
Photos of the control samples after 30 days of storage at 23 °C are presented in Figure 5.

Figure 5.
Control samples of feed products after 30 days of storage at 23 °C.
Observations indicated that storage of the control samples of feed products under unfavorable conditions with oxygen access led to an increase in moisture content.
On the 15th day of storage, the moisture levels increased in all feed product samples. By the 30th day of storage, the moisture levels in all samples exceeded the permissible regulatory limits.
The highest moisture level was recorded in the sample of compound feed for cattle, reaching 20%. This value can be explained by the sample composition, as this feed contains a higher proportion of particles capable of absorbing moisture and may possess a more porous structure. The moisture content was 19.6% in wheat bran, 19.5% in feed barley, and the lowest value of 19% was observed in feed wheat (p > 0.05). When storage conditions are compromised, dry feed products are capable of accumulating moisture.
Mold growth typically occurs at moisture levels above 16–17%, particularly in the presence of oxygen. This confirms that oxygen exposure and elevated humidity are key factors contributing to microbial development.
Mold fungi not only degrade the organoleptic properties of feed but can also produce mycotoxins—substances that are hazardous to animal health.
All samples exhibited pronounced visual changes characteristic of microbiological contamination processes. Visible mold growth was observed on the surface of the feed products.
Figure 6, Figure 7 and Figure 8 illustrate dynamic fluctuations in the moisture content between samples of the experimental feed products irradiated at doses of 3, 6, and 9 kGy.
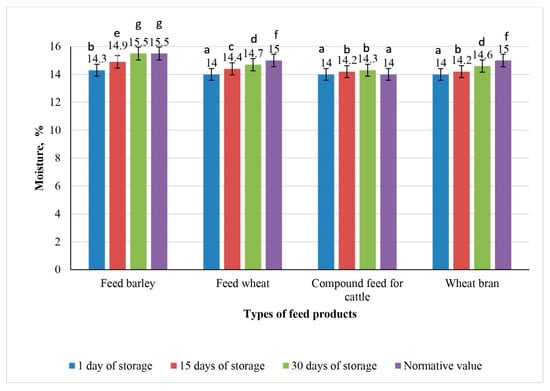
Figure 6.
Dynamics of moisture changes in experimental samples of feed products irradiated with 3 kGy during 30 days of storage (the values for bars sharing different letters (a–g) are significantly different (p < 0.05)).
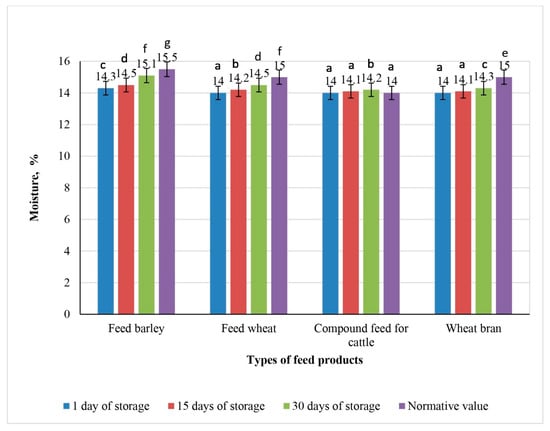
Figure 7.
Dynamics of moisture changes in experimental samples of feed products irradiated with 6 kGy during 30 days of storage (the values for bars sharing different letters (a–g) are significantly different (p < 0.05)).
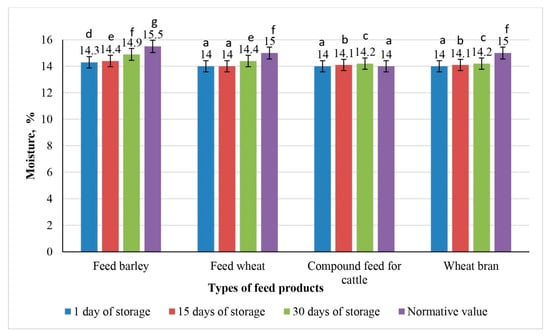
Figure 8.
Dynamics of moisture changes in experimental samples of feed products irradiated with 9 kGy during 30 days of storage (the values for bars sharing different letters (a–g) are significantly different (p < 0.05)).
The initial moisture content in all feed samples, including feed barley, feed wheat, compound feed for cattle, and wheat bran, remained stable on the first day of storage: 14.3% for feed barley and 14.0% for the other samples.
Graph analysis revealed that moisture accumulation in feed products irradiated at doses of 3, 6, and 9 kGy progressed less intensively by day 15 of storage compared with the non-irradiated control samples. All irradiated samples showed lower moisture content than the control samples on day 15.
At an irradiation dose of 3 kGy, the moisture content in feed barley increased to the upper permissible limit of 15.5% by day 30 (p < 0.05).
In feed wheat and wheat bran, the moisture levels remained within acceptable limits (14.7 and 14.6%, respectively), whereas in the compound feed for cattle, the moisture levels slightly exceeded the regulatory threshold of 14.2% (p < 0.05).
Exposure to 6 kGy of irradiation led to a moderate increase in moisture content across all feed types. By day 30, the moisture content in feed barley again reached the maximum permissible value of 15.5%. In feed wheat and wheat bran, the moisture levels were 14.5 and 14.3% (p < 0.05), respectively, remaining below the regulatory threshold of 15%. However, in the compound feed for cattle, the moisture content exceeded the permissible limit, reaching 14.2% (p < 0.05).
Following irradiation at a dose of 9 kGy, the moisture content in all feed samples remained within regulatory limits throughout the storage period. Notably, no changes in moisture content were observed in feed wheat or the compound feed for cattle, indicating increased moisture stability.
These findings suggest that irradiation at a dose of 9 kGy is optimal for minimizing moisture accumulation and ensuring microbiological stability during storage. Among all samples, the compound feed for cattle exhibited the highest sensitivity to moisture increase, likely due to its complex, multicomponent composition and greater hygroscopicity.
Similar observations were reported by Lund, who studied barley stored at various humidity levels under controlled conditions at 20 °C. Barley with a moisture content of 12–14% was effectively preserved for up to 30 days without visible mold growth [30]. As moisture levels increased, a strong correlation was observed between fungal growth (particularly Aspergillus spp.), elevated CO2 production, and an increase in reducing sugars.
In general, storage under high humidity significantly elevates the risk of feed spoilage and mycotoxin formation, highlighting the critical importance of both irradiation treatment and controlled storage conditions to ensure feed quality and safety.
3.3. Investigation of pH in Feed Products
One of the key indicators of feed quality is the active acidity of the medium. The pH level plays a crucial role in ensuring microbiological stability and, consequently, the overall quality of the feed products. For most dry mixtures, a pH range of 5.5 to 6.5 is considered optimal. Deviations from this range increase the risk of mold, yeast, and opportunistic microorganism growth, especially under conditions of high humidity and improper storage. A pH level below 5.0 may indicate the onset of fermentation, while a pH above 7.0 may suggest bacterial contamination or protein degradation. Maintaining the pH within the optimal range not only extends the shelf life of feed and minimizes nutrient losses, but also improves the bioavailability of macro and microelements such as calcium, phosphorus, and zinc. Scientific evidence indicates that deviations from the recommended pH range can negatively affect animal productivity, disrupt digestion, and contribute to disease development.
Therefore, monitoring of pH at all stages of feed production and storage—along with control of moisture and control of water activity—is considered one of the most important parameters to ensure feed quality and microbiological stability.
Figure 9 presents the changes in the pH of the control feed product samples during a 30-day storage period.
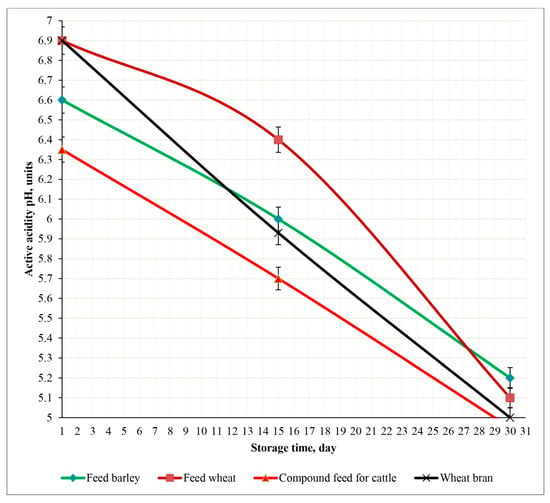
Figure 9.
Changes in the pH of the control feed samples during a 30-day storage period at 23 °C.
Observations revealed a gradual decrease in the pH of various feed types over 30 days of storage, indicating increased acidity as a result of microbial activity.
Feed barley demonstrated the greatest stability, with pH declining steadily from approximately 6.6 to 5.2 without significant fluctuations. Feed wheat had the highest initial pH of 6.9 but experienced the largest drop, reaching 5.1 by day 30, particularly after day 15, indicating a higher susceptibility to acidification (p < 0.05).
The pH of compound feed for cattle decreased from 6.35 to around 5.0, following a trend of wheat bran, which dropped from 6.9 to 5.0 (p < 0.05). The pH decline in wheat bran was more consistent, though relatively rapid.
Overall, feed barley exhibited the highest pH stability, while feed wheat was the least stable. By day 30, all feed types showed increased acidity.
Figure 10, Figure 11 and Figure 12 illustrate the changes in the pH values of experimental feed samples subjected to irradiation at doses of 3, 6, and 9 kGy, respectively.
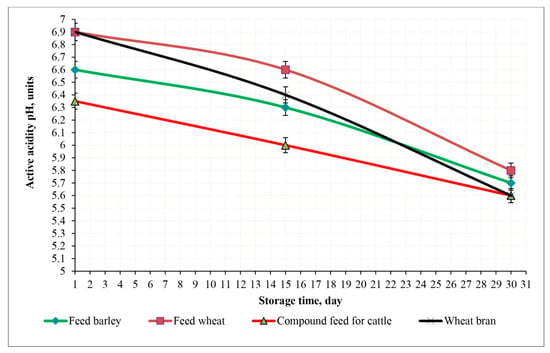
Figure 10.
Changes in the pH of experimental feed samples irradiated with a dose of 3 kGy during a 30-day storage period at 23 °C.
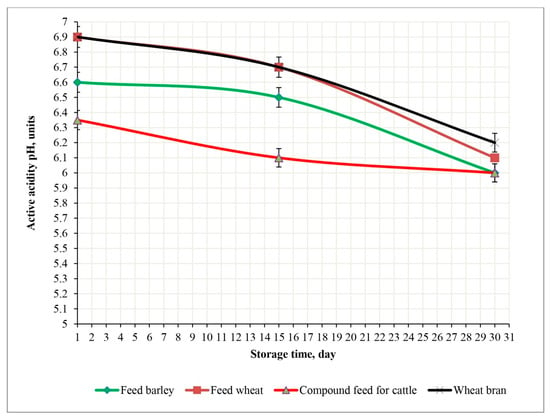
Figure 11.
Changes in the pH of experimental feed samples irradiated with a dose of 6 kGy during a 30-day storage period at 23 °C.
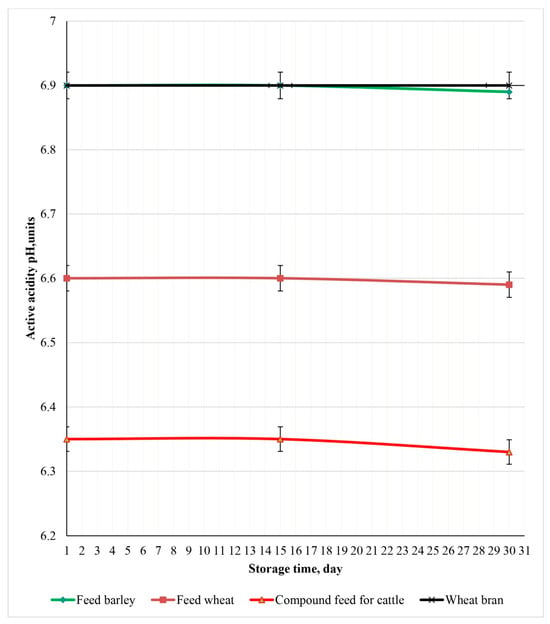
Figure 12.
Changes in the pH of experimental feed samples irradiated with a dose of 9 kGy during a 30-day storage period at 23 °C.
The observations showed that feed wheat was the most resistant to pH reduction (from 6.9 to 5.8) at an irradiation dose of 3 kGy, demonstrating the highest resistance to acidification. In feed barley and wheat bran, the pH decreased from 6.6 and 6.9 to 5.7 and 5.6 (p < 0.05), respectively, indicating moderate stability. Compound feed for cattle lowered the pH from 6.35 to 5.6.
Irradiation at a dose of 3 kGy slowed the pH decrease and suppressed the growth of acid-forming microorganisms, especially in feed wheat. This effect is probably due to the lower formation of carbon dioxide through respiration during storage which, in combination with free water, leads to a decrease in the formation of hydrogen ions.
At an irradiation dose of 6 kGy, the compound feed for cattle maintained greater pH stability (from 6.35 to 6.0), although its initial pH was lower than that of other feeds. In wheat and wheat bran, the pH decreased from 6.9 to 6.1 and from 6.9 to 6.2 (p < 0.05), which is probably due to the activation of acid-forming microflora. Feed barley decreased the pH from 6.6 to 6.0, respectively, while wheat bran showed the most pronounced stabilizing effect, which indicates a high efficiency of irradiation in suppressing microbial activity.
Irradiation at a dose of 9 kGy resulted in minimal pH changes in all samples. In feed wheat and feed barley, the pH remained virtually unchanged (from 6.9 to 6.59 and from 6.9 to 6.89, respectively); in wheat bran, it remained at the same level of 6.9; and in cattle feed, it changed minimally from 6.35 to 6.33 (p < 0.05). These results confirm the high efficiency of the 9 kGy dose in maintaining the pH stability of the feed, even under suboptimal storage conditions.
Electron beam treatment reduced acidification in feed products, likely as a result of both the inactivation of acid-producing bacteria by ionizing radiation and the stabilization of moisture content during storage, which together help maintain the initial pH level.
Figure 13 presents experimental samples of feed products irradiated with a dose of 9 kGy and stored for 30 days at 23 °C.
Figure 13.
Experimental samples of feed products irradiated with a dose of 9 kGy and stored for 30 days at 23 °C.
Irradiation at a dose of 9 kGy improved feed product safety under unfavorable storage conditions. The pH remained more stable, with only a slight decrease observed. Visually, the samples maintained a satisfactory appearance. Observations indicate that irradiated feed products exhibited reduced microbiological spoilage over time, suggesting an increase in shelf life and safety. These results suggest that electron beam treatment at this dose can be an effective method for preserving the quality and extending the shelf life of feed products.
Electron beam irradiation helps to slow down physicochemical and microbiological processes during storage.
3.4. Investigation of Feed Products for Mycotoxins
Samples of feed products irradiated at doses of 3, 6, and 9 kGy were analyzed for the qualitative content of mycotoxins. This study was carried out in the accredited laboratory of the Joint Stock Company National Center for Expertise and Certification (Semey, Kazakhstan).
The following mycotoxins were identified: aflatoxin B1, T-2 toxin, zearalenone (F-2), ochratoxin A, and deoxynivalenol.
The results of the analysis of the control samples and experimental samples of feed products irradiated at doses of 3, 6, and 9 kGy are presented in Table 3.

Table 3.
Qualitative detection of mycotoxins in the control samples and experimental samples of feed products irradiated at doses of 3, 6, and 9 kGy.
The results of this study revealed the presence of aflatoxin B1 in the control feed barley sample. Mycotoxins were not detected in the other control samples (feed wheat, wheat bran, and compound feed) or in the irradiated experimental samples. The increased moisture content in the feed barley contributed to the deterioration of its quality, promoted mold growth, and led to the formation of aflatoxin B1.
Wheat, corn, barley, and other major agricultural crops are the primary raw materials for feed production, and are particularly susceptible to mycotoxin contamination [31].
Aflatoxin B1 (AFB1), fumonisin B1 (FB1), zearalenone (ZEA), and deoxynivalenol (DON) are recognized as the most common and highly toxic mycotoxin pollutants in China [32].
Feed contaminated with mycotoxins, even at concentrations below regulatory thresholds, can lead to chronic intoxication in animals [33].
Some mycotoxins present in commercial feed originate from contaminated raw materials, while others form during processing or storage. In 2016, the detection rates of ZEA, AFB1, and DON reached 99.5%, 100%, and 120%, respectively. Furthermore, it was found that more than 96.4% of feed samples collected in various regions of China contained all three toxins simultaneously [34].
The content of mycotoxins in feed products derived from grain crops grown in Kazakhstan depends on various factors, including the grain variety and herbicide treatment, as confirmed by numerous studies.
In studies conducted by Kazakhstani researchers [35], the contamination of cereal crops in Kazakhstan with mycotoxins was shown to depend on the type of mycotoxin, the crop variety, and the extent of seedling infection by root rot. Mycotoxins were not detected in 60 out of 72 triticale samples analyzed.
The consumption of cereals contaminated with mycotoxins poses a serious health risk to both humans and animals. Mycotoxins were analyzed in cereal crops used for the preparation of the traditional grain product Talkan. The presence of mycotoxins was investigated in 50 cereal samples cultivated in Kazakhstan. Mycotoxins were confirmed and quantitatively determined using HPLC-MS (High-Performance Liquid Chromatography–Mass Spectrometry). More than 28% of the samples tested positive for at least one mycotoxin [36].
Field trials were conducted in Kazakhstan to assess the impact of soybean cultivars and the severity of Fusarium root rot on crop physiology, chlorophyll content, and amino acid composition. Despite the presence of Fusarium root rot, no mycotoxins were detected in the seeds, indicating that the toxins do not spread beyond the site of their biosynthesis to other plant organs. Twelve soybean cultivars—including Tanais and Isidor, which showed susceptibility to Fusarium root rot—demonstrated potential for agricultural use under the agroclimatic conditions of Kazakhstan, even under high precipitation levels observed in 2020 [37].
Pesticide treatment of cereal crops affects mycotoxin levels. A three-year field study was conducted in two regions of Kazakhstan using wheat cultivars from both Kazakhstan and Poland. The treatments included herbicides (aryloxyalkanoic and benzoic acids), fungicides (benzimidazole, strobilurin, triazole, and morpholine), and their combinations. The greatest reduction in mycotoxin levels (trichothecenes, fumonisins, and zearalenone) was observed following applications of MCPA/dicamba (BBCH 19–28), thiophanate-methyl/azoxystrobin (BBCH 44–58), and a five-component fungicide mixture (BBCH 68–77). The highest concentrations of zearalenone (571.0 µg/kg) and deoxynivalenol (151.0 µg/kg) were found in untreated plots [38].
Electron irradiation can significantly reduce mycotoxin contamination. In a study by Yakhkeshi, a significant decrease in mycotoxins (p = 0.03) was observed in corn samples irradiated with an electron beam, with an average reduction of 0.3 logarithmic units (ng/g) at a dose of 20 kGy (range of −0.9 to 1.4 logarithmic units (ng/g)). These results confirm the sensitivity of fungal contaminants in naturally contaminated corn and demonstrate that a 20 kGy dose is effective for the degradation of aflatoxins [29]. However, radiation was not effective against all types of toxins.
Electron beam irradiation can effectively suppress the normal growth of microorganisms and the production of toxins. Radiation increases the permeability of cell membranes and disrupts the integrity of cell walls [39].
3.5. Investigation of the Microstructure of Feed Products
The primary objective of this study was to assess structural changes resulting from 30-day storage under adverse environmental conditions. Therefore, the microstructural characteristics of the control and experimental feed samples were analyzed. Electron microscopy was performed using a JEOL JSM-63690LV electron microscope (JEOL Ltd., Japan).
Figure 14 presents a representative micrograph illustrating the surface microstructure of the control and experimental samples.
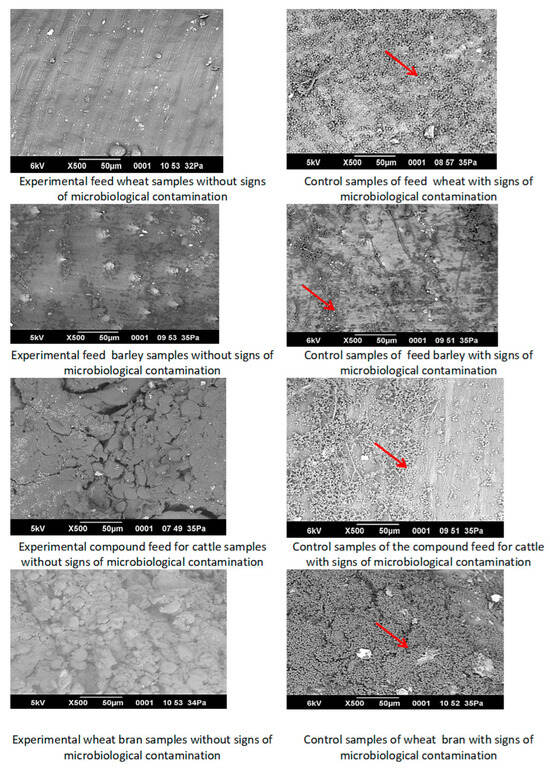
Figure 14.
Microstructural analysis of the experimental and control samples of feed products for contamination with microscopic fungi (The arrow indicates the presence of contamination).
Distinct zones of microbiological degradation, associated with mold contamination (indicated by arrows), were observed on the surface of the control samples of feed wheat, feed barley, compound cattle feed, and wheat bran.
Similar studies have been conducted on four barley varieties (Esma, AC Metcalfe, Tradition, and AB Cattlelac) stored at 17% humidity for eight weeks, revealing a decline in quality by the end of the storage period. Progressive microstructural degradation due to spoilage was noted [40].
Examination of the surface microstructure of irradiated experimental samples (feed barley, feed wheat, wheat bran, and animal feed treated with a dose of 9 kGy) revealed a homogeneous structure with no signs of microbiological contamination. No mold growth was detected.
Feed barley and feed wheat exhibited increased structural density. The compound feed for cattle had a homogeneous, slightly porous texture with no signs of structural damage or decomposition. Wheat bran showed slight particle separation and moderate loosening, likely due to the morphological characteristics of the raw material.
These morphological features—particularly the compact arrangement and structural integrity—are typically associated with favorable sensory and functional properties, including increased resistance to external stressors.
Post-irradiation analysis revealed that the structure of the feed products remained intact and free from mold contamination compared with the untreated control samples.
A clearly distinguishable structural density and compactness after irradiation demonstrate advantages over the control samples.
The described structural morphology may serve as a basis for future studies evaluating changes in the nutritional value of feeds caused by irradiation, facilitating a more accurate assessment of structural modifications induced using electron beam treatment.
This study highlights the critical role of initial microstructural integrity in determining the shelf life of feeds, particularly under suboptimal storage conditions.
3.6. Investigation of the Nutritional Value of Feed Products
The quality of feed is determined primarily by the content of nutrients such as crude protein, fat, fiber, as well as essential macronutrients—calcium and phosphorus. During storage, feed products undergo a range of biochemical and oxidative changes, which can significantly reduce their nutritional value.
To evaluate the stability of nutritional content under storage conditions and the effectiveness of radiation treatment in preserving feed quality, we analyzed the chemical composition of both the control and irradiated samples over a 30-day period.
The results are presented in Table 4, which summarizes the variations in chemical composition of the four feed types—wheat, barley, wheat bran, and compound cattle feed—on the 1st and 30th days of storage, including irradiated samples treated at doses of 3, 6, and 9 kGy.

Table 4.
Changes in the chemical composition of feed products (the control and irradiated samples) on days 1 and 30 of storage.
On the first day of storage, feed wheat exposed to ionizing radiation at doses ranging from 3 to 9 kGy demonstrated a slight reduction in crude protein content. Protein levels decreased to 11.80 g at 3 kGy, to 11.74 g at 6 kGy, and to 11.90 g at 9 kGy; the protein level in the control sample was 12.0 g. While these variations fall within acceptable analytical margins, they may indicate partial degradation of protein molecules under the influence of radiation.
The crude fat content in feed wheat also decreased compared with the control (1.70 g), dropping to 1.67 g at 3 kGy, to 1.68 g at 6 kGy, and to 1.69 g at 9 kGy. This reduction is likely associated with oxidative processes induced via irradiation. A slight decrease in crude fiber was observed at all irradiation doses—from 2.70 g (control) to 2.68 g at 3 kGy, to 2.65 g at 6 kGy, and to 2.67 g at 9 kGy—suggesting partial degradation of structural polysaccharides.
It has previously been confirmed that ionizing radiation induces the depolymerization of polysaccharides through the radiolysis of starch [41].
Interestingly, calcium content showed a non-linear response: it initially dropped to 1.18 g at a dose of 3 kGy, rose slightly to 1.19 g at 6 kGy, and returned to 1.20 g (control value) at 9 kGy. This may be explained by the redistribution or release of calcium salts within the grain matrix. Phosphorus content decreased slightly, most notably with 6 kGy (4.69 g), but returned to 4.78 g at 9 kGy, approaching the control level (4.84 g).
It has been established that storage for 30 days without irradiation leads to a significant reduction in the levels of all components. Specifically, the crude protein content decreases from 12.00 to 10.3 g, fat from 1.7 to 1.2 g, fiber from 2.7 to 1.7 g, calcium from 1.2 to 0.6 g, and phosphorus from 4.8 to 3.4 g.
Irradiation at doses of 3, 6, and 9 kGy contributes to a partial compensation of these losses. The doses of 6 and 9 kGy proved to be particularly effective: by day 30, the protein content reached 11.75 and 11.86 g, respectively, while fat and fiber levels were restored almost to their initial values. The calcium content (up to 1.18 g at 9 kGy) and phosphorus (up to 4.7 g) were also recorded, indicating the potential of electron beam irradiation to stabilize the mineral composition.
On the first day of storage in feed barley, moderate changes were observed after irradiation. The protein content decreased slightly to 9.48 g at 3 kGy, to 9.45 g at 6 kGy, and increased slightly to 9.49 g at 9 kGy; the protein content in the control sample was 9.50 g.
Similarly, the crude fat content decreased to 2.38 g at 3 kGy and 2.37 g at 6 kGy, with a slight rise to 2.39 g at 9 kGy; the crude fat content in the control samples was 2.44 g. However, these fluctuations are statistically insignificant.
The crude fiber content gradually decreased to 4.88 g at 3 kGy, to 4.95 g at 6 kGy, and 4.99 g at 9 kGy; the crude fiber content in the control was 5.04. A notable change was observed in phosphorus, which declined from 3.84 g (control) to 3.27 g at a dose of 6 kGy. This could indicate disruption of phosphorus-binding complexes or partial leaching. Calcium content also showed a minor decrease, from 1.24 g (control samples) to 1.17–1.19 g, suggesting slight mineral losses due to irradiation.
Barley also exhibited a deterioration in chemical composition during storage. By day 30 without irradiation, the protein content decreased from 9.5 to 8.2 g, fiber content decreased from 5.0 to 4.1 g, and calcium content decreased from 1.2 to 0.7 g.
In the study [42], the chemical composition of both raw and electron beam-irradiated barley grains was examined at doses of 10, 20, 30, and 40 kGy under room-temperature conditions. The findings indicated that electron irradiation can induce protein denaturation, decrease starch crystallinity, and enhance the digestibility of barley flour. Furthermore, electron beam treatment was found to increase the ammonia content in barley grains.
Irradiation at doses of 3, 6, and 9 kGy had a positive effect. At a dose of 3 kGy, the protein content increased to 9.32 g, and at 9 kGy it reached 9.46 g, nearly fully compensating for storage-related losses. Furthermore, irradiation doses of 6 and 9 kGy led to increases in fiber content (up to 4.96 g), fat (up to 2.38 g), and phosphorus (up to 3.28 g), confirming the effectiveness of the method.
On the first day of storage in the case of compound feed for cattle, the protein content remained relatively stable. The control sample had 14.0 g of protein, which was maintained at 9 kGy, while it slightly decreased to 13.84 g at 3 kGy and 13.76 g at 6 kGy.
Research conducted by Shawrang et al. [43] revealed that irradiation induces protein denaturation, which in turn enhances its digestibility.
The crude fat content remained unchanged at 2.54 g at a dose of 3 kGy, but dropped slightly to 2.48 g at 6 kGy and 2.49 g at 9 kGy. The crude fiber content also showed minimal changes: it remained at 12.04 g in both the control and with a dose of 9 kGy, with slight decreases to 11.95 g and 11.86 g at doses of 3 and 6 kGy, respectively.
The mineral composition varied slightly across radiation doses. The calcium content increased from 0.64 g (control) to 0.66 g at a dose of 9 kGy, while the phosphorus content was 0.81 g at 3 kGy, 0.82 g at 6 kGy, and 0.83 g at 9 kGy. These results may reflect radiation-induced disruption of organic complexes, such as phytates or protein–mineral structures, leading to the release of previously bound Ca2+ and PO43− ions, which then become detectable during analysis.
It has been previously established that the treatment of soybeans with electron or gamma irradiation at doses of 20 kGy and above resulted in a significant decrease in phytic acid content and a simultaneous increase in the level of inorganic phosphorus. These changes indicated the degradation of phytic acid and, consequently, an increase in the bioavailability of mineral elements [44].
The results of this study show that storage for 30 days without treatment resulted in a decrease in protein to 13.1 g, fat to 1.3 g, fiber to 11.3 g, and calcium and phosphorus to 0.45 g and 0.63 g, respectively.
Irradiation at a dose of 3 kGy shows a positive effect: protein increases to 13.78 g and fiber to 11.56 g. The best results were observed at doses of 6 and 9 kGy, with protein levels restored to 13.86 g and 13.94 g, respectively, fat to 2.48 g, and fiber to 11.94 g. The mineral composition also returned to near-initial levels.
Irradiation induces changes in the morphology and molecular mass of polysaccharides, altering their solubility and facilitating the release of associated components, including proteins and lipids [45]. Thus, irradiation at doses of 6–9 kGy ensures an almost complete restoration of the original parameters.
On the first day of storage for wheat bran, the protein content exhibited a minor decline from 16.06 g (control) to 15.94 g at a dose of 3 kGy, 15.74 g at 6 kGy, and 15.96 g at 9 kGy. The crude fat content slightly decreased to 4.00 g at a dose of 3 kGy but remained unchanged at 4.14 g at doses of 6 and 9 kGy. Radiation may generate free radicals, which can interact with proteins (e.g., oxidizing side chains) but do not fully degrade them. These changes remain within analytical error margins.
At a dose of 3 kGy, oxidation of lipids—particularly free fatty acids and phospholipids—may initiate fat reduction. However, at higher doses (6 and 9 kGy), more complex reactions such as polymerization or binding of oxidized lipids to proteins may occur, stabilizing fat content at the control levels.
The crude fiber content showed a minor decline from 10 g to 9.99 g (3 and 9 kGy) and 9.97 g (6 kGy). Ionizing radiation likely causes partial breakdown of cell wall components (cellulose, hemicellulose, and lignin), leading to depolymerization of long polysaccharide chains into smaller fragments. This microscopic decomposition of fiber explains the slight reduction observed.
Minor changes were also seen in the mineral composition of wheat bran. Calcium increased slightly from 1.84 g (control) to 1.81 g at doses of 3 and 9 kGy, remaining constant at 1.84 g at a dose of 6 kGy. Phosphorus showed a minimal decrease to 9.98 g at 3 kGy and 10.00 g at 6 kGy, before returning to 10.10 g at 9 kGy (control level). This may be due to the breakdown of phytic complexes, making phosphorus more available for detection. A partial decomposition of organic phosphorus compounds or formation of volatile phosphate products during initial irradiation (3 kGy) could explain the slight reduction.
These fluctuations (in the range of 0.01–0.12 g) fall within standard analytical error but may still indicate the onset of chemical transformations under ionizing radiation.
When stored for 30 days without treatment, a decrease in all parameters was observed: protein dropped to 14.8 g, fat to 3.6 g, fiber to 9.1 g, calcium to 0.7 g, and phosphorus to 8.9 g.
Irradiation at doses of 3, 6, and 9 kGy resulted in a consistent recovery of these parameters. At a dose of 3 kGy, the protein content increased to 15.76 g, and at 6 and 9 kGy, the protein content increased to 15.89 g and 15.92 g, respectively. The fat content reached 3.96 g at 9 kGy, and fiber increased to 9.96 g. The mineral levels were also higher compared with the non-irradiated samples.
On the first day of storage, experimental observations showed that irradiation at doses of 3 kGy and 6 kGy resulted in a slight decrease in nutrient content across all tested feed samples. In contrast, irradiation at 9 kGy led to an increase in nutrient content, approaching the values of the control sample, which is likely due to internal chemical transformations occurring within the product matrix.
Storage of feed for 30 days without treatment led to a regular decrease in the content of protein, fat, fiber, calcium, and phosphorus in all studied samples. Losses were most pronounced in wheat and barley.
Ionizing radiation at doses of 3, 6, and 9 kGy had a positive effect on the chemical composition of feed, effectively compensating for storage losses. The optimal doses are 6 and 9 kGy, which provide the nutritional value of the feed. Future research may aim to study the effects of radiation on the microbiological safety and digestibility of the components.
Although quantitative changes in protein, fat, and fiber content were identified, it is worth noting that the molecular mechanisms underlying these changes are beyond the scope of this study. Specifically, lipid oxidation, protein denaturation, and structural modifications of polysaccharides caused by ionizing radiation warrant further in-depth investigation in future work.
In general, all the results obtained should be considered in the context of the study’s limitations: only typical feed samples from a particular region were used. This is due to both logistical and methodological reasons. Our study mainly focused on the initial effects of electron beam treatment during storage under unfavorable conditions and on its short-term stability. At the same time, there is a clear need for further research, including an expansion of the range of feed under study, wider sampling geography, and an extension of the shelf life up to 6 months, which would enable a more objective assessment of the effectiveness of electron beam irradiation technology.
4. Conclusions
In this study, samples of barley, feed, wheat, and compound feed for cattle and wheat bran—both irradiated with electron accelerators and untreated—were analyzed to perform a comparative assessment of their physicochemical properties and microbiological parameters. Moreover, the microstructure of the samples under unfavorable storage conditions was examined over a period of one month.
The experimental results demonstrated that feed products stored at high humidity (85%) and a temperature of 20 °C without irradiation treatment using the ILU-10 electron accelerator exhibited significant quality degradation. The effects included increased moisture content and elevated pH levels. Moreover, substantial microstructural damage to the grains was observed. In particular, the untreated samples showed progressive mold growth, with visible fungal colonization and the presence of aflatoxin B1.
In contrast, irradiation treatment at doses of 3 and 6 kGy effectively reduced microbial contamination and slowed the rate of increase in both acidity and moisture content during storage. However, the physicochemical parameters of these treated samples were not fully in line with standard quality requirements.
The most favorable results were obtained with a radiation dose of 9 kGy, which maintained the feed quality parameters within acceptable limits throughout the storage period. Therefore, this study highlights the effectiveness of electron accelerator-based irradiation at a dose of 9 kGy in preserving the safety and quality of feed products, even under suboptimal storage conditions.
A limitation of this study is the limited availability and accessibility of electron accelerator irradiation equipment. The use of such technology is not yet widespread in the feed industry, which may restrict the practical application of the findings on a larger industrial scale.
To conduct a more in-depth investigation of the effects of electron beam treatment, storage periods will be extended in future research.
The study showed that focusing on the initial effects of electron beam treatment and their short-term stability (when stored under unfavorable conditions) was justified when using typical feed samples from a certain region. However, the limited sample size in terms of feed type, geography, and shelf life (no more than one month) highlights the need for further research. Testing a wider range of feeds and extending the storage period to 6 months would provide more reliable confirmation of the effectiveness of electron beam irradiation and help clarify its practical significance. In addition, future studies will also assess its effects on nutritional value and animal performance. Finally, industrial-scale applications in the feed industry will also be considered.
Author Contributions
D.O.: Conceptualization, Investigation, Methodology, Visualization, Writing—Original Draft, Software, Validation. Z.K.: Writing—Review and Editing, Conceptualization, Supervision. A.N.: Conceptualization, Writing—Review and Editing, Investigation. G.N.: Writing—Review and Editing, Methodology. F.S.: Writing—Review and Editing, Writing—Original Draft, Supervision. M.K.: Writing—Review and Editing, Methodology. B.R.: Investigation, Writing—Review and Editing. G.B.: Methodology, Writing—Review and Editing. A.B.: Conceptualization, Validation, Writing—Original Draft. E.A.: Investigation, Visualization, Writing—Original Draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR21882447).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Arendt, E.K.; Zannini, E. Cereal Grains for the Food and Beverage Industries; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Khapre, A.P.; Deshpande, H.W.; Katke, S.D. A review on microbial contamination of cereal grains. Int. J. Chem. Stud. 2020, 8, 1829–1832. [Google Scholar] [CrossRef]
- Cook, F.K.; Johnson, B.L. Microbiological spoilage of cereal products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Iztayev, A.; Akkozha, I.; Yakiyayeva, M.; Iztayev, B.; Mamyrayev, M.; Uykassova, Z. The role of particle size in improving the quality of wheat, pumpkin, melon, and carrot powders. J. Food Sci. Technol. 2024, 21, 16–25. [Google Scholar] [CrossRef]
- Kiskó, G.; Hladicekova, N.; Taczmann-Brückner, A.; Mohácsi-Farkas, C. Studies on the heat and disinfectant resistance of a spore-forming spoilage bacterium. Acta Univ. Sapientiae. Aliment. 2019, 12, 94–103. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Boshevska, M.; Sandeva, I.; Cabo Verde, S.; Spasevska, H.; Jankuloski, Z. Effects of different irradiation doses and storage period on microbiological characteristics of wheat (Triticum aestivum L.). Food Control 2024, 158, 110201. [Google Scholar] [CrossRef]
- Aziz, N.H.; Souzan, R.M.; Shahin Azza, A. Effect of γ-irradiation on the occurrence of pathogenic microorganisms and nutritive value of four principal cereal grains. Appl. Radiat. Isot. 2006, 64, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Singla, M.; Goraya, R.K.; Kumar, Y.; Jan, K.; Bashir, K. Dose-Dependent Effects of Gamma Irradiation on Microbiological, Antioxidant, and Functional Properties of Buckwheat, Cowpea, Oat, and Brown Rice Flour. J. Food Process. Preserv. 2024, 2024, 1196594. [Google Scholar] [CrossRef]
- Braghini, R.; Pozzi, C.R.; Aquino, S.; Rocha, L.O.; Corrêa, B. Effects of γ-radiation on the fungus Alternaria alternata in artificially inoculated cereal samples. Appl. Radiat. Isot. 2009, 67, 1622–1628. [Google Scholar] [CrossRef]
- Nasab, S.S.; Zare, L.; Tahmouzi, S.; Nematollahi, A.; Mollakhalili-Meybodi, N.; Abedi, A.-S.; Delshadian, Z. Effect of irradiation treatment on microbial, nutritional and technological characteristics of cereals: A comprehensive review. Radiat. Phys. Chem. 2023, 212, 111124. [Google Scholar] [CrossRef]
- Scholtz, V.; Jirešová, J.; Šerá, B.; Julák, J. A review of microbial decontamination of cereals by non-thermal plasma. Foods 2021, 10, 2927. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, Y.C. ‘Mitigating fungal contamination of cereals: The efficacy of microplasma-based far-UVC lamps against Aspergillus flavus and Fusarium graminearum. Food Res. Int. 2024, 190, 114550. [Google Scholar] [CrossRef]
- Yessimbekov, Z.; Kakimov, A.; Kabdylzhar, B.; Suychinov, A.; Baikadamova, A.; Akimova, D.; Abdilova, G. Chemical, physical properties, microstructure and granulometric composition of ultra-finely ground chicken bone paste. Appl. Food Res. 2023, 3, 100318. [Google Scholar] [CrossRef]
- GOST 13586.5-2015; Grain. Method of Moisture Content Determination. Moscow: Standartinform. Rosstandart: Moscow, Russia, 2015. Available online: https://files.stroyinf.ru/Data2/1/4293759/4293759564.pdf (accessed on 15 March 2025).
- Sacco, C.; Donato, R.; Zanella, B.; Pini, G.; Pettini, L.; Marino, M.F.; Rookmin, A.D.; Marvasi, M. Mycotoxins and flours: Effect of type of crop, organic production, packaging type on the recovery of fungal genus and mycotoxins. Int. J. Food Microbiol. 2020, 334, 108808. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Sanitary Mycological Assessment and Improvement of Feed Quality. 1986. Available online: https://gostrf.com/normadata/1/4293849/4293849318.pdf (accessed on 15 March 2025).
- GOST 28001-88; Feed Grains. Products of Its Processing. Compound Feed. Methods for the Determination of Mycotoxins: T-2 Toxin. Zearalenone (F-2) and Ochratoxin A. Rosstandart: Moscow, Russia, 1990. Available online: https://meganorm.ru/Data2/1/4294826/4294826766.pdf (accessed on 15 March 2025).
- GOST R 51116-97; Compound Feed. Grain and Grain By-Products. Method for Determining the Content of Deoxynivalenol (Vomitoxin). Rosstandart: Moscow, Russia, 1997. Available online: https://meganorm.ru/Data2/1/4294819/4294819031.pdf (accessed on 15 March 2025).
- GOST 30711-2001; Food-Stuff’s. Methods for Detection and Determination of Aflatoxins B1 and M1 Content. Rosstandart: Moscow, Russia, 2001. Available online: https://meganorm.ru/Data2/1/4294813/4294813777.pdf (accessed on 15 March 2025).
- GOST 10444.15-94; Food Products. Methods for Determining the Number of Mesophilic Aerobes and Facultative Anaerobes. Rosstandart: Moscow, Russia, 1994. Available online: https://meganorm.ru/Data2/1/4294840/4294840290.pdf (accessed on 15 March 2025).
- GOST 10444.12-2013; Microbiology of Food and Animal Feeding Stuffs. Methods for the Detection and Colony Count of Yeasts and Moulds. Rosstandart: Moscow, Russia, 2013. Available online: https://meganorm.ru/Data2/1/4293773/4293773820.pdf (accessed on 15 March 2025).
- GOST 26180-84; Methods for the Determination of Ammonia Nitrogen and Active Acidity (pH). Rosstandart: Moscow, Russia, 1984. Available online: https://files.stroyinf.ru/Data2/1/4294828/4294828294.pdf (accessed on 15 March 2025).
- AOAC. Official Methods of Analytical Chemist, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Perme, M.P.; Manevski, D. Confidence intervals for the Mann-Whitney test. Stat. Methods Med. Res. 2019, 28, 64–71. [Google Scholar] [CrossRef]
- About the Draft Decision of the Council of the Eurasian Economic Commission “On Amendments to the Technical Regulations of the Customs Union “On Food Safety” (TR CU 021/2011)”; Eurasian Economic Union: Moscow, Russia, 2011; Available online: https://docs.eaeunion.org/documents/303/2318/ (accessed on 15 March 2025).
- Zavyalov, M.A.; Kukhto, V.A.; Ilyukhina, N.V.; Kolokolova, A.Y. Irradiation of biological objects using an ionizing beam to inhibit conditionally pathogenic and pathogenic microflora in agricultural raw materials. Bull. Voronezh State Univ. Eng. Technol. 2018, 80, 278–282. [Google Scholar] [CrossRef]
- Mohamed, A.B.; Chavez, R.A.; Wagacha, M.J.; Mutegi, C.K.; Muthomi, J.W.; Pillai, S.D.; Stasiewicz, M.J. Efficacy of electron beam irradiation in reduction of mycotoxin-producing fungi, aflatoxin, and fumonisin, in naturally contaminated maize slurry. Toxicon X 2022, 16, 100141. [Google Scholar] [CrossRef]
- Yakhkeshi, S.; Rahimi, S.; Shawrang, P. Effects of electron-beam irradiation of the diet on microbial population, intestinal morphology, ileal digestibility and performance of broilers. Iran. J. Appl. Anim. Sci. 2013, 3, 747–752. Available online: https://api.semanticscholar.org/CorpusID:54194772 (accessed on 15 March 2025).
- Lund, A.; Pedersen, H.; Sigsgaard, P. Storage experiments with barley at different moisture contents. J. Sci. Food Agric. 1971, 22, 458–463. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Hao, S.; Hu, J.; Song, S.; Huang, D.; Xu, H.; Qian, G.; Gan, F.; Huang, K. Selenium alleviates aflatoxin B1-induced immune toxicity through improving glutathione peroxidase 1 and selenoprotein S expression in primary porcine splenocytes. J. Agric. Food Chem. 2016, 64, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A. Toxicity of Three Biological Derivatives of Deoxynivalenol: Deepoxy-Deoxynivalenol, 3-Epi-Deoxynivalenol and Deoxynivalenol-3-Glucoside on Pigs. Doctoral Dissertation, Paul Sabatier University, Toulouse, France, 2016. [Google Scholar]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.-T.; Xie, W.-M.; Zhang, N.-Y.; Dai, J.-F.; Wang, Y.; Rajput, S.A.; Qi, D.-S.; et al. Individual and Combined Occurrence of Mycotoxins in Feed Ingredients and Complete Feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Dutbayev, Y.; Kharipzhanova, A.; Yessimbekova, M.; Toishimanov, M.; Lozowicka, B.; Iwaniuk, P.; Bastaubaeva, S.; Kokhmetova, A. Ochratoxin A and Deoxynivalenol Mycotoxin Profile in Triticale Seedlings with Different Susceptibility to the Root Rot. OnLine J. Biol. Sci. 2023, 23, 87–93. [Google Scholar] [CrossRef]
- Tattibayeva, D.; Nebot, C.; Miranda, J.M.; Abuova, A.B.; Kizatova, M.Z.; Vazquez, B.; Cepeda, A.; Franco, C.M. Occurrence of mycotoxins in talkan: A cereal-based food traditional for Turkic population. Pol. J. Vet. Sci. 2018, 21, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kuldybayev, N.; Dutbayev, Y.; Lozowicka, B.; Iwaniuk, P.; Slyamova, A.; Tsygankov, V. Effects of Root Rot in Soybean Cultivars with Diverse Susceptibility to the Disease on Plant Physiology, Yield, Amino Acids and Mycotoxins Profile in Climatic Conditions of Kazakhstan. OnLine J. Biol. Sci. 2021, 21, 312–321. [Google Scholar] [CrossRef]
- Nugmanov, A.; Beishova, I.; Kokanov, S.; Lozowicka, B.; Kaczynski, P.; Konecki, R.; Snarska, K.; Wołejko, E.; Sarsembayeva, N.; Abdigaliyeva, T. Systems to reduce mycotoxin contamination of cereals in the agricultural region of Poland and Kazakhstan. Crop Prot. 2018, 106, 64–71. [Google Scholar] [CrossRef]
- Fan, L.; Li, L.; Shang, F.; Xie, Y.; Duan, Z.; Cheng, Q.; Zhang, Y. Study on antibacterial mechanism of electron beam radiation on Aspergillus flavus. Food Biosci. 2023, 51, 102197. [Google Scholar] [CrossRef]
- Indore, N.S.; Jayas, D.S.; Karunakaran, C.; Stobbs, J.; Bondici, V.F.; Vu, M.; Tu, K.; Marinos, O. Study of microstructural, nutritional, and biochemical changes in hulled and hulless barley during storage using X-ray and infrared techniques. Foods 2023, 12, 3935. [Google Scholar] [CrossRef]
- Wu, D.; Shu, Q.; Wang, Z.; Xia, Y. Effect of gamma irradiation on starch viscosity and physicochemical properties of different rice. Radiat. Phys. Chem. 2002, 65, 79–86. [Google Scholar] [CrossRef]
- Bornaei, L.; Salari, S.; Erfanimajd, N. Determination of chemical composition and metabolisable energy irradiated barley grain in broiler chickens and cockerels. Iran. J. Anim. Sci. Res. 2020, 11, 451–462. [Google Scholar] [CrossRef]
- Shawrang, P.; Nikkhah, A.; Zare-Shahneh, A.; Sadeghi, A.A.; Raisali, G.; Moradi-Shahrebabak, M. Effects of gamma irradiation on chemical composition and ruminal protein degradation of canola meal. Radiat. Phys. Chem. 2008, 77, 918–922. [Google Scholar] [CrossRef]
- Nielsen, A.; Meyer, A. Phytase-mediated mineral solubilization from cereals under in vitro gastric conditions. J. Sci. Food Agric. 2016, 96, 3755–3761. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Jae, K.K.; Periasamy, S.; Jae, H.K.; Hyun, J.P.; Myung, W.B.; Ju, W.L. Comparison of gamma ray and electron beam irradiation on extraction yield, morphological and antioxidant properties of polysaccharides from tamarind seed. Radiat. Phys. Chem. 2009, 78, 605–609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).