1. Introduction
The emergence of sustainable chemistry and the urge for the development of strategies for tackling climate change-related issues have been the driving forces of new innovative chemistry thinking and modern technologies [
1,
2,
3,
4,
5]. The exploitation of renewable resources and materials appears to be one of the most promising strategies to overcome the challenges faced with conventional limited resources such as petroleum [
3,
4,
5,
6,
7,
8].
Considering feedstock as a valuable and renewable natural resource opens up opportunities for developing innovative approaches or revisiting traditional strategies, offering a potential economic model for the future [
8,
9,
10,
11]. Thus, many synthetic processes nowadays are switching to more ecofriendly chemical processes, starting with adopting new green solvents as they represent most of the carbon footprint in different industrial areas [
3,
5]. Green solvents, particularly those derived from biomass, are emerging, such as limonene, glycerol, γ-valerolactone, cyrene, pinene, and 2-methyl tetrahydrofuran (2-MeTHF), which are gaining traction within the chemical community for various reactions. For example, research has been done into the exploration of 2-MeTHF in Suzuki–Miyaura reactions [
12], γ-valerolactone for cross-coupling reactions [
13], and glycerol for Sonogashira coupling [
14]. On the other hand, some of these biorenewable terpene feedstocks could be transformed into more valuable terpenes via sustainable approaches. For example, the aromatization of limonene leads to
p-cymene, a less abundant terpene in essential oil that is commonly obtained by a fastidious process involving hazardous Friedel–Crafts alkylation of benzene derivatives with alkyl halides and AlCl
3 as a catalyst [
15].
p-Cymene has been explored and demonstrated to hold significant value across various industries, including in fragrances, perfumes, flavorings, and pharmaceuticals [
15]. On the other hand,
p-cymene can be obtained from waste tires by catalytic processes in order to produce this highly valued terpene [
16]. From the same perspective,
p-menthane, a hydrogenated version of
p-cymene, is an even more highly valued terpene. It has been used as substrate for the synthesis of
p-menthane hydroperoxide, which is an excellent initiator for polymerization reactions [
17]. On top of that,
p-menthane was utilized as a green solvent for cleaning applications [
18] and natural product extraction [
19].
p-Menthane has a great combustion ability and can play the role of an additive, with
p-cymene, in green blend fuel [
20,
21,
22].
p-Menthane is typically produced through the hydrogenation of feedstock using a metal-based catalytic reaction. For example, the reduction of limonene over a Cu–Ni-supported catalytic system produced a mixture of
p-menthene and
p-menthane (58:42) under the best conditions [
23]. On the other hand, menthane was obtained as a mixture with the aromatized
p-cymene by hydrogenation of
p-menthadienes over palladium [
24]. Linalool can also be transformed into a mixture of
p-menthane and 2,6-dimethyloctane using commercial catalysts [
25]. To the best of our knowledge, only a few works on the synthesis of
p-menthane have been reported involving
p-cymene hydrogenation over palladium, providing a
trans/
cis mixture (69/31) [
26]. Consequently, developing an eco-friendly method for the synthesis of
p-menthane would greatly facilitate deeper research into its potential applications. Such advancements could offer valuable insights into its role in sustainable chemistry and energy solutions. Previously, we investigated the hydrogenation of limonene over various catalysts, and
p-cymene was detected at various stages of the reaction kinetics [
27]. This
p-cymene results from the dehydrogenation of limonene and/or menthene, yet it was never detected at the end of the reaction. Following our preliminary tests of
p-cymene hydrogenation as part of our investigation of limonene hydrogenation, we have undertaken a complete investigation of the solvent-free
p-cymene hydrogenation to
cis- and
trans-menthane isomers in this study (
Scheme 1).
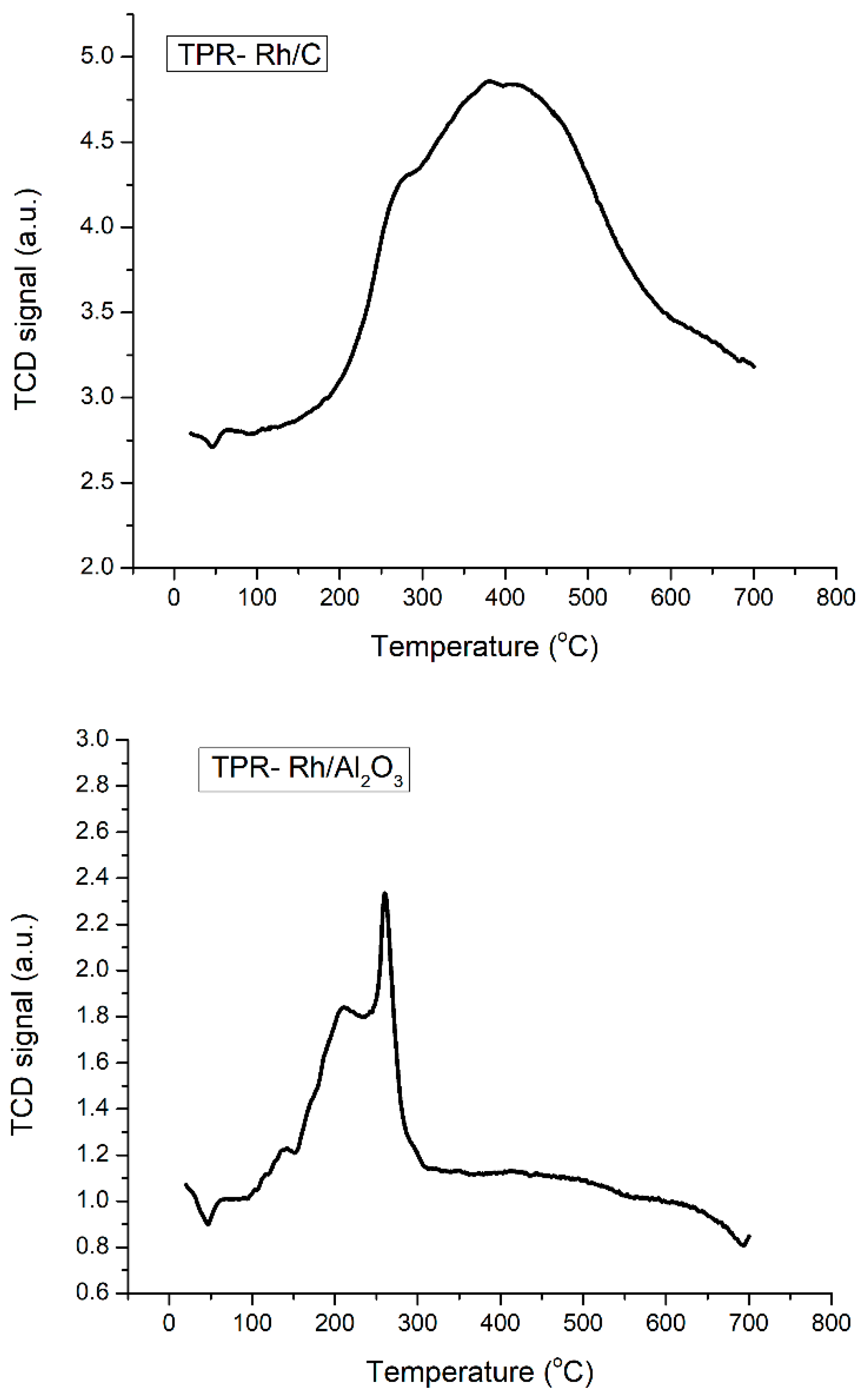
Four platinum-group metals, palladium (Pd), platinum (Pt), ruthenium (Ru), and rhodium (Rh), were used for their potential in hydrogenation reactions due to their high strong ability to dissociate hydrogen. Additionally, charcoal (C) and alumina (Al2O3) were selected as catalyst supports among a range of other options (e.g., silica, zeolite). This choice was taken in consideration of the morphological characteristics offered by these two supports for enhancing the efficiency of the selected metals in heterogeneous hydrogenation reactions. Fortunately, these catalysts offer the benefit of commercial availability, with comparable metal loading. The metals cited earlier, supported on charcoal (C) or alumina (Al2O3), and were tested for the solvent-free hydrogenation of p-cymene. In this study, the hydrogenation of p-cymene was systematically investigated under different catalytic and process conditions. The effects of metal, support, pressure, and temperature were each examined in detail: the present investigation allows us to provide a comprehensive analysis of the key parameters and their interplay, supported by plausible mechanistic considerations. Thus, the outcome of this study will provide more insights into sustainable hydrogenation for feedstocks, especially the aromatic derivative, looking toward full hydrogenation.
4. Conclusions
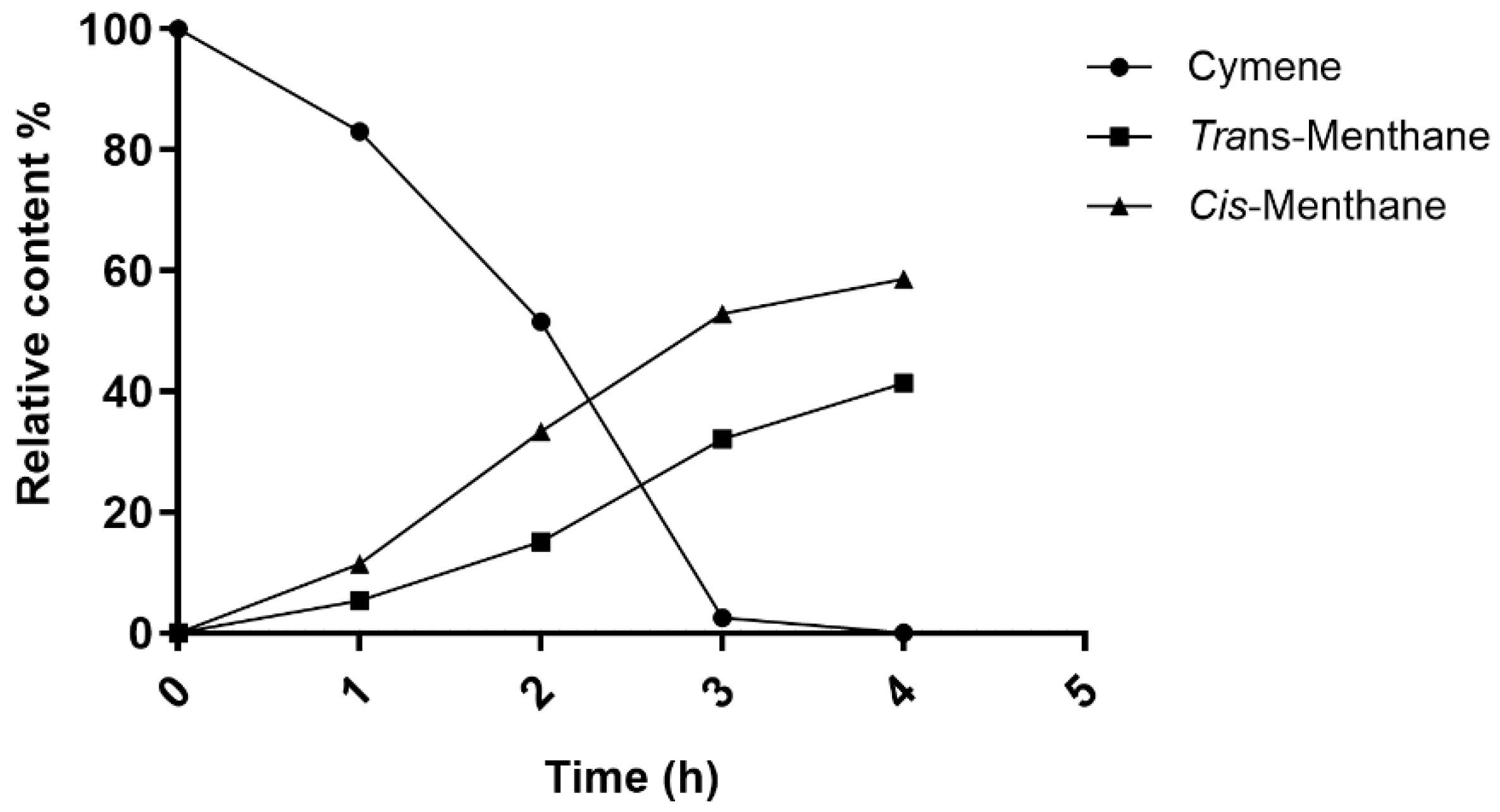
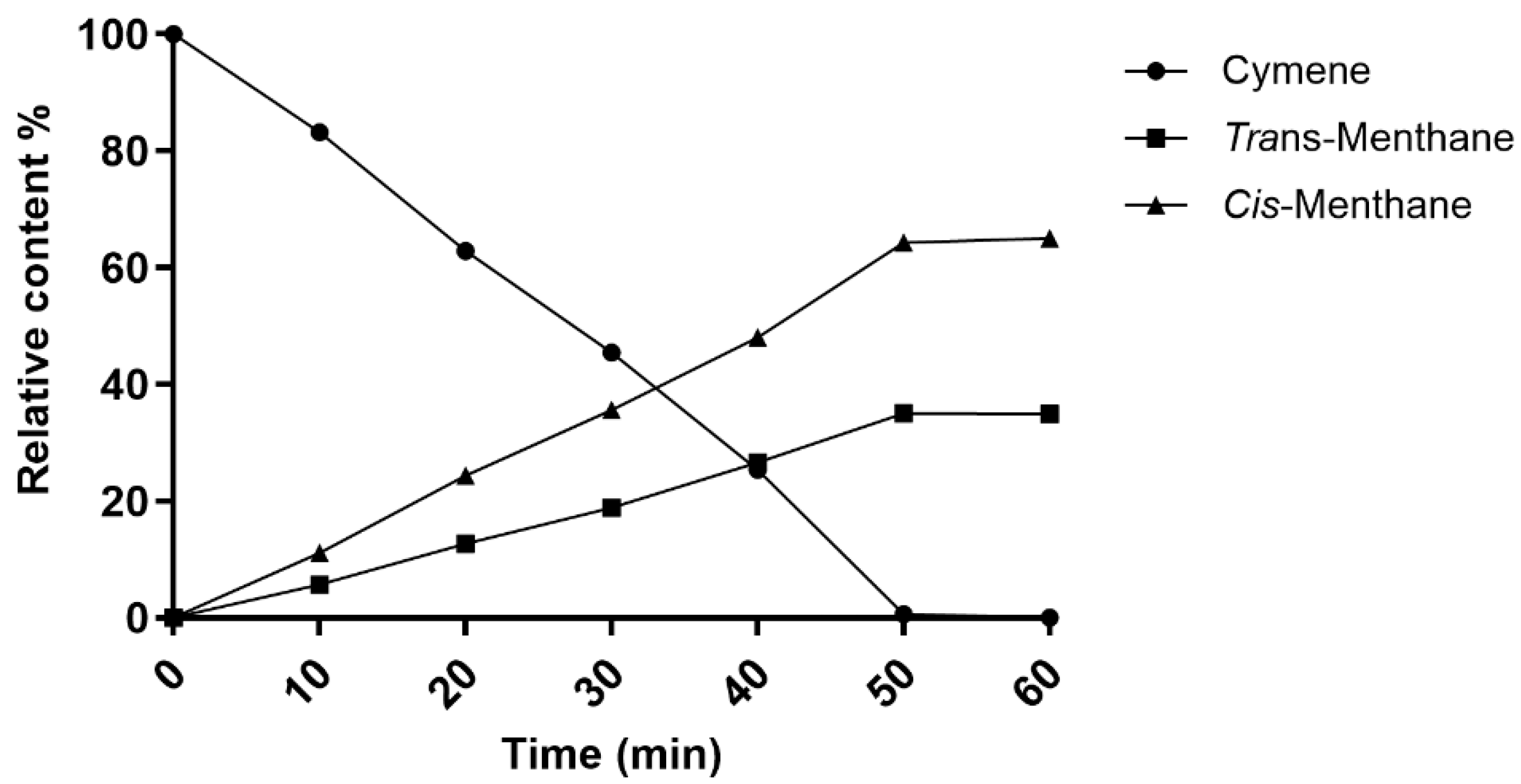
This study evaluated the performance of four catalysts, each on two different supports, for the hydrogenation reaction of p-cymene. Among the four investigated platinum-group catalysts, Rh/C was the most efficient. This catalyst fully converted p-cymene to p-menthane with two stereoisomers at both low (4 °C) and high (150 °C) temperatures, thereby offering excellent reactivity. The effect of the temperature on the selectivity was also observed: at 4 °C, the kinetically favored cis-p-menthane isomer dominated (72%), while at 150 °C, the thermodynamically favored trans-p-menthane isomer dominated (91%). These results highlight the importance of the temperature not only for complete conversion, but also for controlling stereoselectivity, allowing the reaction to be directed towards the desired product depending on the chosen experimental conditions.
Whether the reaction was carried out at medium or high pressure, conversion remained complete, accompanied by a marked preference for the cis-p-menthane isomer, with selectivity up to 65%. This highlights not only the robustness of the catalyst, but also its effectiveness in maintaining pure, well-defined products, which is crucial in industrial applications.
A particularly remarkable aspect of this study was the stability of Rh/C, which demonstrated excellent recyclability, with over 60 cycles of reuse without any significant loss of performance. This durability confirms that Rh/C is not only efficient but also cost-effective for large-scale processes. To the best of our knowledge, the use of Rh/C for the hydrogenation of p-cymene under solvent-free conditions has not been previously reported. Our study highlights the high efficiency and recyclability of Rh/C under green conditions, which makes it particularly attractive for the sustainable valorization of this natural aromatic compound.
In conclusion, this research establishes Rh/C as a catalyst of choice for the hydrogenation of p-cymene to cis- and trans-p-menthane, combining efficiency, selectivity, and recyclability, essential evaluation criteria for potential industrial applications.