Exploring Protein Misfolding and Aggregate Pathology in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Interventions
Abstract
1. Background
2. Overview of Protein Misfolding
3. Mechanisms of Protein Misfolding in Neurodegenerative Diseases
3.1. Self-Assembly of Native Monomeric Protein
3.2. Aggregation of Conformationally Modified Monomeric Proteins
3.3. Nucleation and Seeding Process
4. The Role of Unfolded Protein Response (UPR) in Protein Misfolding and Neurodegeneration
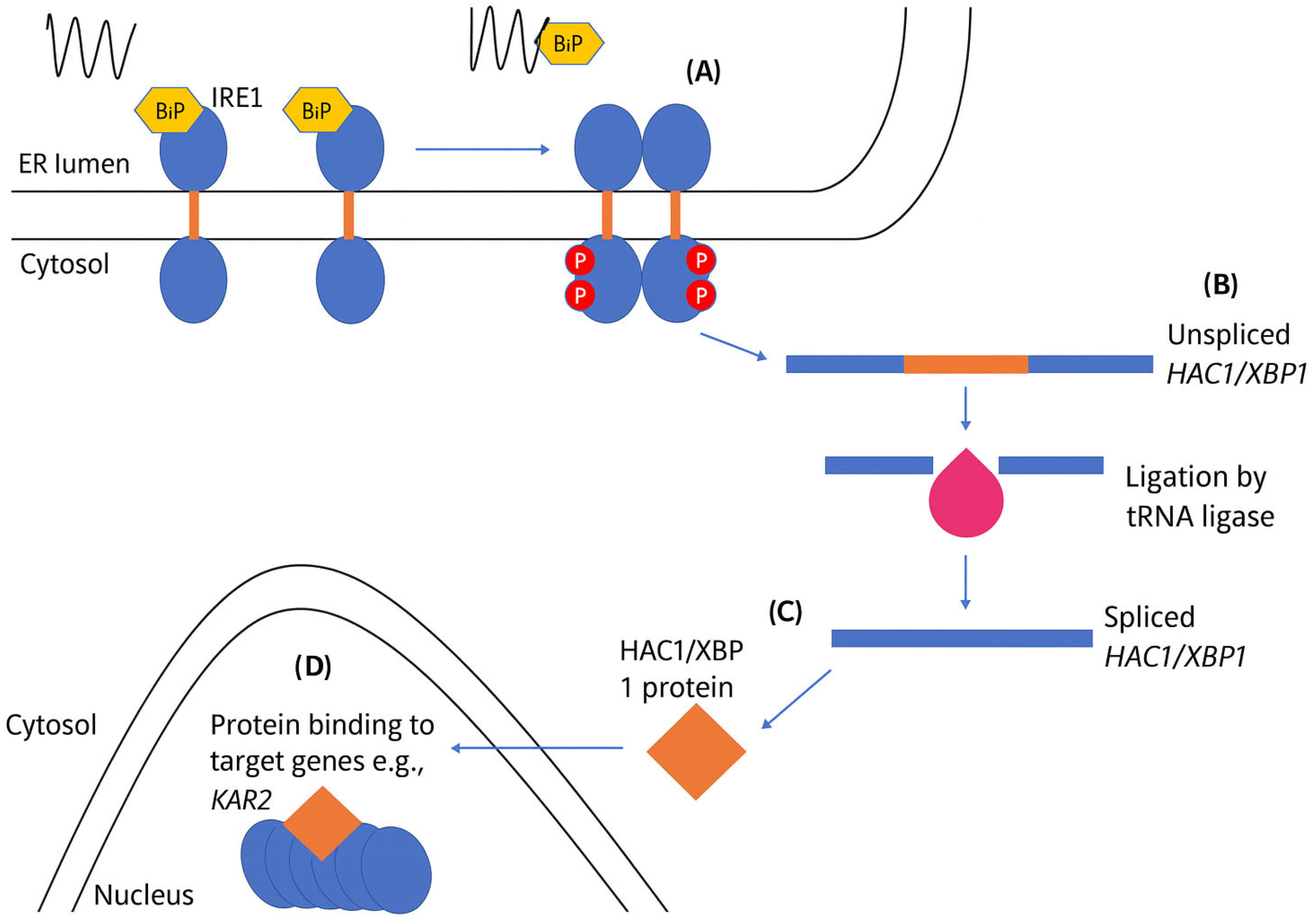
4.1. Activation of the UPR via IRE1
4.2. PERK Activation and Signaling
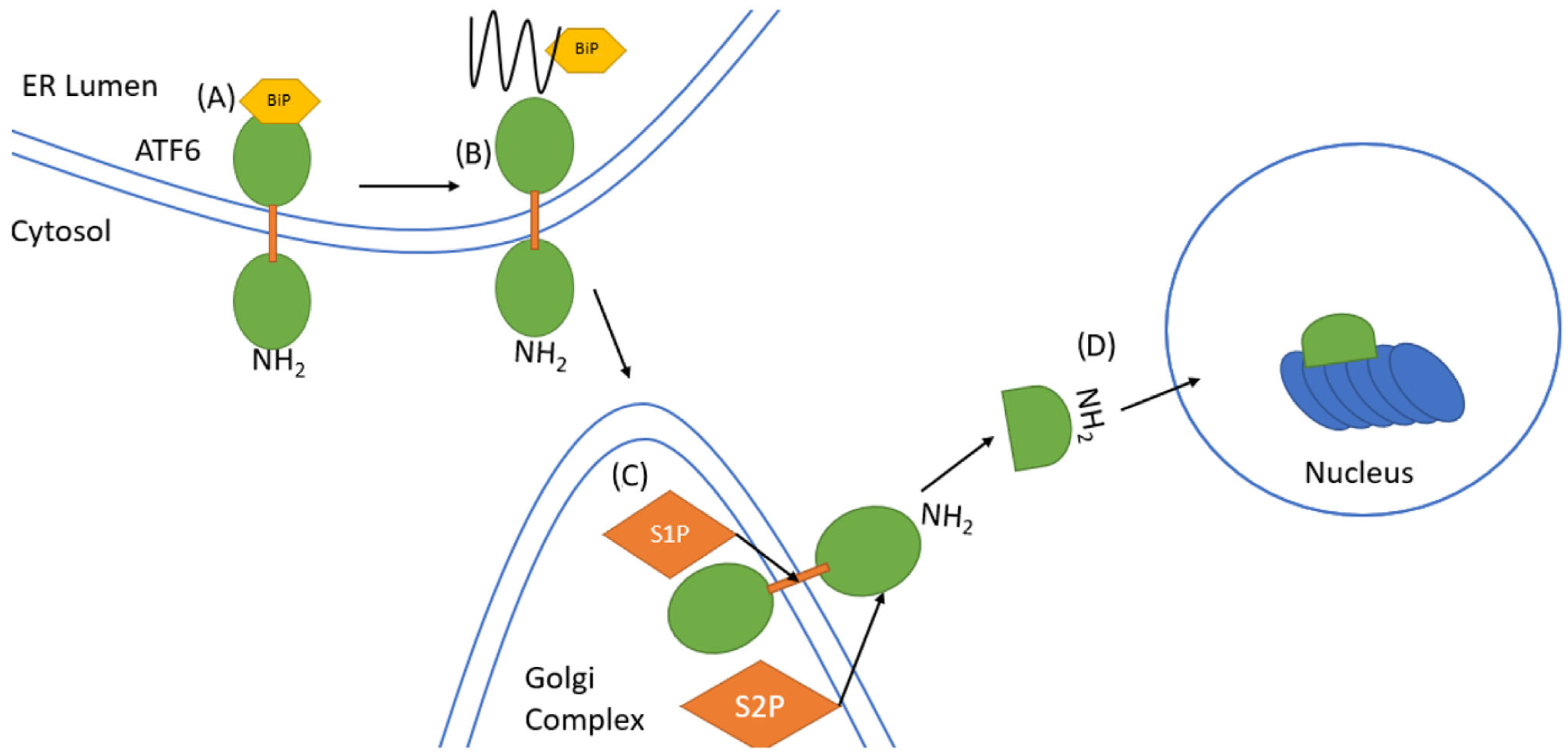
4.3. ATF6 Signaling
5. Protein Aggregation: Mechanism and Consequences
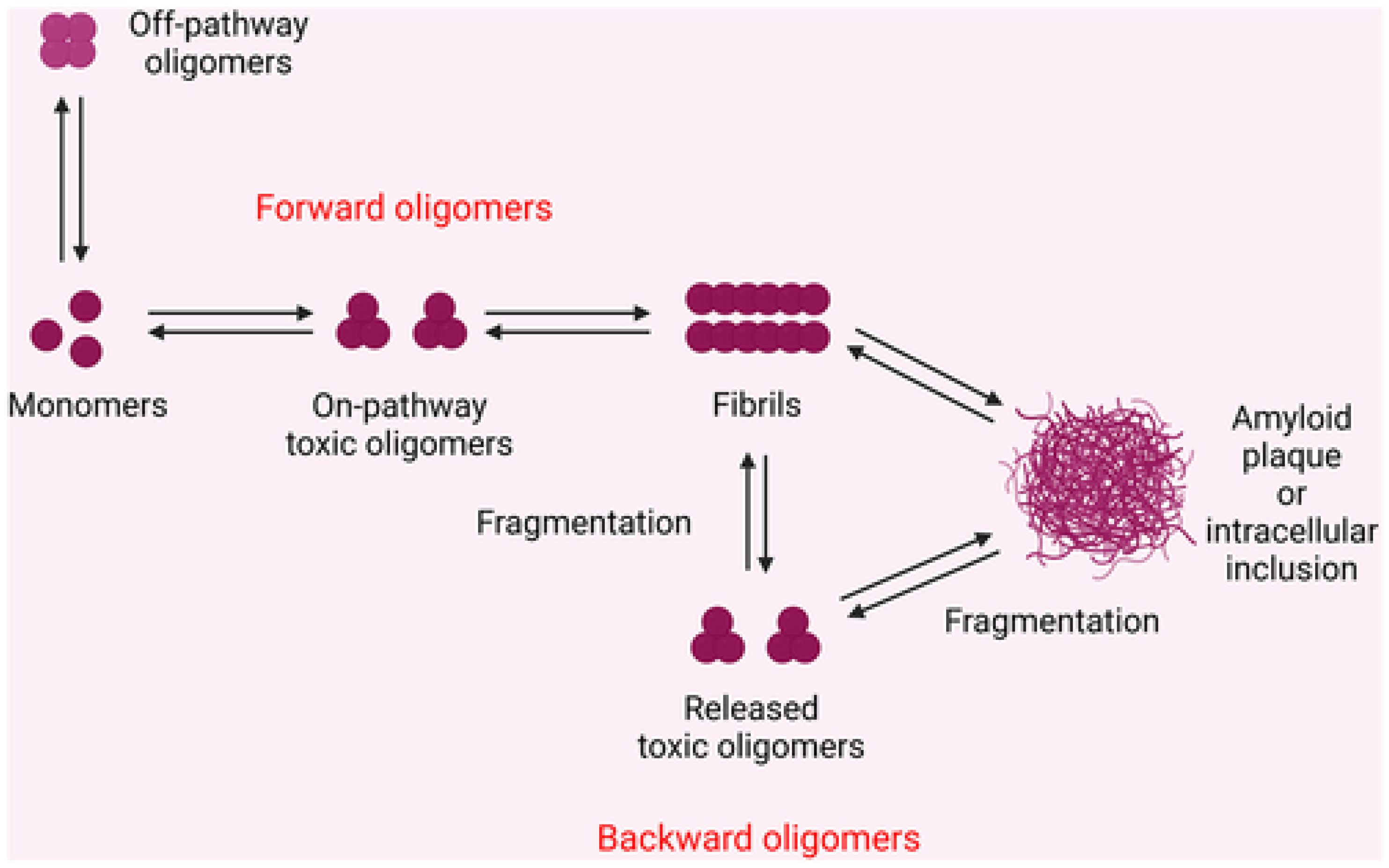
5.1. Definition and Types of Aggregates (Oligomers, Fibrils, Plaques)
5.2. Prion-like Propagation of Misfolded Proteins
5.3. Toxicity of Misfolded Proteins vs. Large Aggregates
6. Cellular Consequences of Protein Aggregation
6.1. Loss of Proteostasis
6.2. TDP-43 Dysfunction
6.3. Synaptic Toxicity
6.4. Escaping Protein Quality Control (PQC)
6.4.1. PQC Failure
6.4.2. Tau-Specific Mechanisms
6.5. Microglial Activation
6.6. Autoantibody Generation
6.7. Oxidative Stress and Mitochondrial Dysfunction
6.8. Disruption of Synaptic Function
6.9. Impaired Autophagy and Proteasome Activity
7. Role of Protein Misfolding and Aggregation in Specific Neurodegenerative Diseases
7.1. Alzheimer’s Disease
7.2. Parkinson’s Disease
7.3. Huntington’s Disease
8. Therapeutic Strategies for the Treatment of Neurodegenerative Diseases
8.1. Antibody-Based Immunotherapy: Targeting Extracellular Aggregates
8.2. Chaperone Therapy: Optimizing Protein Quality Control Mechanisms
8.3. Antioxidant Therapy
9. Diagnostic and Biomarker Potential
9.1. Detection of Misfolded Proteins in Cerebrospinal Fluid (CSF) or Blood
9.2. Imaging Techniques (e.g., PET Scans for Amyloid)
10. Future Directions
10.1. Role of Liquid–Liquid Phase Separation (LLPS)
10.2. Role of RNA-Binding Proteins and Stress Granules
10.3. Advances in Proteomics and Single-Cell Analysis
10.4. Personalized Medicine and Gene Editing Approaches
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Tredici, K.D.; Lee, V.M.-Y.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Breydo, L.; Uversky, V.N. Structural, morphological, and functional diversity of amyloid oligomers. FEBS Lett. 2015, 589, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Knowles, T.P.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef]
- Bigi, A.; Cascella, R.; Chiti, F.; Cecchi, C. Amyloid fibrils act as a reservoir of soluble oligomers, the main culprits in protein deposition diseases. BioEssays 2022, 44, 2200086. [Google Scholar] [CrossRef]
- Goedert, M.; Clavaguera, F.; Tolnay, M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010, 33, 317–325. [Google Scholar] [CrossRef]
- Lu, X.; Lu, J.; Li, S.; Feng, S.; Wang, Y.; Cui, L. The Role of Liquid-Liquid Phase Separation in the Accumulation of Pathological Proteins: New Perspectives on the Mechanism of Neurodegenerative Diseases. Aging Dis. 2024, 16, 769. [Google Scholar] [CrossRef]
- Kitajima, Y.; Tashiro, Y.; Suzuki, N.; Warita, H.; Kato, M.; Tateyama, M.; Ando, R.; Izumi, R.; Yamazaki, M.; Abe, M. Proteasome dysfunction induces muscle growth defects and protein aggregation. J. Cell Sci. 2014, 127, 5204–5217. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, J.; Margittai, M.; Nussinov, R.; Ma, B. How does hyperphopsphorylation promote tau aggregation and modulate filament structure and stability? ACS Chem. Neurosci. 2016, 7, 565–575. [Google Scholar] [CrossRef]
- Read, A.; Schröder, M. The unfolded protein response: An overview. Biology 2021, 10, 384. [Google Scholar] [CrossRef]
- Politis, M. Neuroimaging in Parkinson disease: From research setting to clinical practice. Nat. Rev. Neurol. 2014, 10, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.K.; Siddiqi, M.K.; Alam, P.; Khan, R.H. Protein misfolding and aggregation: Mechanism, factors and detection. Process Biochem. 2016, 51, 1183–1192. [Google Scholar]
- Del Monte, F.; Agnetti, G. Protein post-translational modifications and misfolding: New concepts in heart failure. Proteom. Clin. Appl. 2014, 8, 534–542. [Google Scholar]
- Ramirez-Alvarado, M.; Kelly, J.W.; Dobson, C.M. Protein Misfolding Diseases: Current and Emerging Principles and Therapies; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Agnetti, G.; Halperin, V.L.; Kirk, J.A.; Chakir, K.; Guo, Y.; Lund, L.; Nicolini, F.; Gherli, T.; Guarnieri, C.; Caldarera, C.M. Desmin modifications associate with amyloid-like oligomers deposition in heart failure. Cardiovasc. Res. 2014, 102, 24–34. [Google Scholar] [CrossRef]
- Mannini, B.; Cascella, R.; Zampagni, M.; van Waarde-Verhagen, M.; Meehan, S.; Roodveldt, C.; Campioni, S.; Boninsegna, M.; Penco, A.; Relini, A. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc. Natl. Acad. Sci. USA 2012, 109, 12479–12484. [Google Scholar]
- Clemen, C.S.; Herrmann, H.; Strelkov, S.V.; Schröder, R. Desminopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 47–75. [Google Scholar]
- Chen, W.-T.; Liao, Y.-H.; Yu, H.-M.; Cheng, I.H.; Chen, Y.-R. Distinct effects of Zn2+, Cu2+, Fe3+, and Al3+ on amyloid-β stability, oligomerization, and aggregation: Amyloid-β destabilization promotes annular protofibril formation. J. Biol. Chem. 2011, 286, 9646–9656. [Google Scholar]
- Tay, W.M.; Bryant, J.G.; Martin, P.K.; Nix, A.J.; Cusack, B.M.; Rosenberry, T.L. A mass spectrometric approach for characterization of amyloid-β aggregates and identification of their post-translational modifications. Biochemistry 2012, 51, 3759–3766. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar]
- Krebs, M.R.; Wilkins, D.K.; Chung, E.W.; Pitkeathly, M.C.; Chamberlain, A.K.; Zurdo, J.; Robinson, C.V.; Dobson, C.M. Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the β-domain. J. Mol. Biol. 2000, 300, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Duarte, A.; Ulloa-Aguirre, A. A brief journey through protein misfolding in transthyretin amyloidosis (ATTR amyloidosis). Int. J. Mol. Sci. 2021, 22, 13158. [Google Scholar] [CrossRef] [PubMed]
- Barbiroli, A.; Iametti, S.; Bonomi, F. Beta-lactoglobulin as a model food protein: How to promote, prevent, and exploit its unfolding processes. Molecules 2022, 27, 1131. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.I.; Linse, S.; Luheshi, L.M.; Hellstrand, E.; White, D.A.; Rajah, L.; Otzen, D.E.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 9758–9763. [Google Scholar] [CrossRef]
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. [Google Scholar] [CrossRef]
- Sirangelo, I.; Iannuzzi, C. The role of metal binding in the amyotrophic lateral sclerosis-related aggregation of copper-zinc superoxide dismutase. Molecules 2017, 22, 1429. [Google Scholar] [CrossRef]
- Harini, K.; Girigoswami, K.; Anand, A.V.; Pallavi, P.; Gowtham, P.; Elboughdiri, N.; Girigoswami, A. Nano-mediated strategies for metal ion–induced neurodegenerative disorders: Focus on Alzheimer’s and Parkinson’s diseases. Curr. Pharmacol. Rep. 2022, 8, 450–463. [Google Scholar] [CrossRef]
- Ashraf, A.; Clark, M.; So, P.-W. The aging of iron man. Front. Aging Neurosci. 2018, 10, 65. [Google Scholar] [CrossRef]
- Chen, B.; Wen, X.; Jiang, H.; Wang, J.; Song, N.; Xie, J. Interactions between iron and α-synuclein pathology in Parkinson’s disease. Free Radic. Biol. Med. 2019, 141, 253–260. [Google Scholar] [CrossRef]
- Ryu, J.; Girigoswami, K.; Ha, C.; Ku, S.H.; Park, C.B. Influence of multiple metal ions on β-amyloid aggregation and dissociation on a solid surface. Biochemistry 2008, 47, 5328–5335. [Google Scholar] [CrossRef]
- Panda, C.; Kumar, S.; Gupta, S.; Pandey, L.M. Structural, kinetic, and thermodynamic aspects of insulin aggregation. Phys. Chem. Chem. Phys. 2023, 25, 24195–24213. [Google Scholar] [CrossRef] [PubMed]
- Candreva, J.; Chau, E.; Rice, M.E.; Kim, J.R. Interactions between soluble species of β-Amyloid and α-Synuclein promote oligomerization while inhibiting fibrillization. Biochemistry 2019, 59, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef] [PubMed]
- Jokar, S.; Khazaei, S.; Behnammanesh, H.; Shamloo, A.; Erfani, M.; Beiki, D.; Bavi, O. Recent advances in the design and applications of amyloid-β peptide aggregation inhibitors for Alzheimer’s disease therapy. Biophys. Rev. 2019, 11, 901–925. [Google Scholar] [CrossRef]
- Barbu, E.; Joly, M. The globular-fibrous protein transformation. Discuss. Faraday Soc. 1953, 13, 77–93. [Google Scholar] [CrossRef]
- Mallucci, G.R.; Klenerman, D.; Rubinsztein, D.C. Developing therapies for neurodegenerative disorders: Insights from protein aggregation and cellular stress responses. Annu. Rev. Cell Dev. Biol. 2020, 36, 165–189. [Google Scholar] [CrossRef]
- Matlahov, I.; van der Wel, P.C. Conformational studies of pathogenic expanded polyglutamine protein deposits from Huntington’s disease. Exp. Biol. Med. 2019, 244, 1584–1595. [Google Scholar] [CrossRef]
- Pujols, J.; Peña-Díaz, S.; Pallarès, I.; Ventura, S. Chemical chaperones as novel drugs for Parkinson’s disease. Trends Mol. Med. 2020, 26, 408–421. [Google Scholar] [CrossRef]
- Dormann, D.; Lemke, E.A. Adding intrinsically disordered proteins to biological ageing clocks. Nat. Cell Biol. 2024, 26, 851–858. [Google Scholar] [CrossRef]
- Chi, E.Y.; Weickmann, J.; Carpenter, J.F.; Manning, M.C.; Randolph, T.W. Heterogeneous nucleation-controlled particulate formation of recombinant human platelet-activating factor acetylhydrolase in pharmaceutical formulation. J. Pharm. Sci. 2005, 94, 256–274. [Google Scholar] [CrossRef]
- Rochet, J.-C.; Lansbury, P.T., Jr. Amyloid fibrillogenesis: Themes and variations. Curr. Opin. Struct. Biol. 2000, 10, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Unfolded protein response. Curr. Biol. 2012, 22, R622–R626. [Google Scholar] [CrossRef] [PubMed]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Bennett, B.S.; Cullinan, S.B.; Diehl, J.A. PERK and GCN2 contribute to eIF2α phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol. Biol. Cell 2005, 16, 5493–5501. [Google Scholar] [CrossRef]
- Siwecka, N.; Rozpędek-Kamińska, W.; Wawrzynkiewicz, A.; Pytel, D.; Diehl, J.A.; Majsterek, I. The structure, activation and signaling of IRE1 and its role in determining cell fate. Biomedicines 2021, 9, 156. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Su, N.; Kilberg, M.S. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J. Biol. Chem. 2008, 283, 35106–35117. [Google Scholar] [CrossRef]
- Shen, X.; Ellis, R.E.; Lee, K.; Liu, C.-Y.; Yang, K.; Solomon, A.; Yoshida, H.; Morimoto, R.; Kurnit, D.M.; Mori, K. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 2001, 107, 893–903. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Credle, J.J.; Finer-Moore, J.S.; Papa, F.R.; Stroud, R.M.; Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2005, 102, 18773–18784. [Google Scholar]
- Gardner, B.M.; Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 2011, 333, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.C.; Šestak, S.; Ali, A.A.; Sagini, H.A.; Brown, M.; Baty, K.; Treumann, A.; Schröder, M. Bypass of activation loop phosphorylation by aspartate 836 in activation of the endoribonuclease activity of Ire1. Mol. Cell. Biol. 2017, 37, e00655–e00616. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Dey, M.; Neculai, D.; Cao, C.; Dever, T.E.; Sicheri, F. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 2008, 132, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Xu, Z.; Kaufman, R.J. Structure and intermolecular interactions of the luminal dimerization domain of human IRE1α. J. Biol. Chem. 2003, 278, 17680–17687. [Google Scholar] [CrossRef]
- Ma, K.; Vattem, K.M.; Wek, R.C. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J. Biol. Chem. 2002, 277, 18728–18735. [Google Scholar] [CrossRef]
- Vattem, K.M.; Wek, R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11269–11274. [Google Scholar]
- Jiang, H.-Y.; Wek, S.A.; McGrath, B.C.; Lu, D.; Hai, T.; Harding, H.P.; Wang, X.; Ron, D.; Cavener, D.R.; Wek, R.C. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol. Cell. Biol. 2004, 24, 1365–1377. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, X.; Zimmermann, H.R.; Ma, T. Brain-specific suppression of AMPKα2 isoform impairs cognition and hippocampal LTP by PERK-mediated eIF2α phosphorylation. Mol. Psychiatry 2021, 26, 1880–1897. [Google Scholar]
- Brown, M.S.; Ye, J.; Rawson, R.B.; Goldstein, J.L. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell 2000, 100, 391–398. [Google Scholar]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Davé, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Alam, P.; Siddiqi, K.; Chturvedi, S.K.; Khan, R.H. Protein aggregation: From background to inhibition strategies. Int. J. Biol. Macromol. 2017, 103, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Tyedmers, J.; Mogk, A.; Bukau, B. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 2010, 11, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Gsponer, J.; Babu, M.M. Cellular strategies for regulating functional and nonfunctional protein aggregation. Cell Rep. 2012, 2, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef]
- Knowles, T.P.; Waudby, C.A.; Devlin, G.L.; Cohen, S.I.; Aguzzi, A.; Vendruscolo, M.; Terentjev, E.M.; Welland, M.E.; Dobson, C.M. An analytical solution to the kinetics of breakable filament assembly. Science 2009, 326, 1533–1537. [Google Scholar] [CrossRef]
- Cascella, R.; Chen, S.W.; Bigi, A.; Camino, J.D.; Xu, C.K.; Dobson, C.M.; Chiti, F.; Cremades, N.; Cecchi, C. The release of toxic oligomers from α-synuclein fibrils induces dysfunction in neuronal cells. Nat. Commun. 2021, 12, 1814. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Cremades, N.; Cohen, S.I.; Deas, E.; Abramov, A.Y.; Chen, A.Y.; Orte, A.; Sandal, M.; Clarke, R.W.; Dunne, P.; Aprile, F.A. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell 2012, 149, 1048–1059. [Google Scholar] [CrossRef]
- Collinge, J.; Clarke, A.R. A general model of prion strains and their pathogenicity. Science 2007, 318, 930–936. [Google Scholar] [CrossRef]
- Wickner, R.B.; Edskes, H.K.; Shewmaker, F.; Nakayashiki, T. Prions of fungi: Inherited structures and biological roles. Nat. Rev. Microbiol. 2007, 5, 611–618. [Google Scholar] [CrossRef]
- Colby, D.W.; Giles, K.; Legname, G.; Wille, H.; Baskakov, I.V.; DeArmond, S.J.; Prusiner, S.B. Design and construction of diverse mammalian prion strains. Proc. Natl. Acad. Sci. USA 2009, 106, 20417–20422. [Google Scholar] [CrossRef]
- Kwiatkowski, T., Jr.; Bosco, D.; Leclerc, A.; Tamrazian, E.; Vanderburg, C.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.; Munsat, T. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Edgeworth, J.A.; Gros, N.; Alden, J.; Joiner, S.; Wadsworth, J.D.; Linehan, J.; Brandner, S.; Jackson, G.S.; Weissmann, C.; Collinge, J. Spontaneous generation of mammalian prions. Proc. Natl. Acad. Sci. USA 2010, 107, 14402–14406. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, G.; Papaleo, E.; Sabate, R.; Ventura, S. Protein aggregation: Mechanisms and functional consequences. Int. J. Biochem. Cell Biol. 2012, 44, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Vats, A.; Taneja, V. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015, 18, 138–145. [Google Scholar] [CrossRef]
- Irvine, G.B.; El-Agnaf, O.M.; Shankar, G.M.; Walsh, D.M. Protein aggregation in the brain: The molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med. 2008, 14, 451–464. [Google Scholar] [CrossRef]
- Guivernau, B.; Bonet, J.; Valls-Comamala, V.; Bosch-Morató, M.; Godoy, J.A.; Inestrosa, N.C.; Perálvarez-Marín, A.; Fernández-Busquets, X.; Andreu, D.; Oliva, B. Amyloid-β peptide nitrotyrosination stabilizes oligomers and enhances NMDAR-mediated toxicity. J. Neurosci. 2016, 36, 11693–11703. [Google Scholar] [CrossRef]
- Kress, G.J.; Mennerick, S. Action potential initiation and propagation: Upstream influences on neurotransmission. Neuroscience 2009, 158, 211–222. [Google Scholar] [CrossRef]
- Stephan, A.; Laroche, S.; Davis, S. Generation of aggregated β-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J. Neurosci. 2001, 21, 5703–5714. [Google Scholar] [CrossRef]
- Morris, G.P.; Clark, I.A.; Vissel, B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol. Commun. 2014, 2, 135. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat. Neurosci. 2015, 18, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Dasari, M.; Espargaro, A.; Sabate, R.; del Amo, J.M.L.; Fink, U.; Grelle, G.; Bieschke, J.; Ventura, S.; Reif, B. Bacterial Inclusion Bodies of Alzheimer’s Disease β-Amyloid Peptides Can Be Employed To Study Native-Like Aggregation Intermediate States. ChemBioChem 2011, 12, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Medeiros, R.; LaFerla, F.M. Transgenic mouse models of Alzheimer disease: Developing a better model as a tool for therapeutic interventions. Curr. Pharm. Des. 2012, 18, 1131–1147. [Google Scholar] [CrossRef]
- Mezler, M.; Barghorn, S.; Schoemaker, H.; Gross, G.; Nimmrich, V. A β-amyloid oligomer directly modulates P/Q-type calcium currents in Xenopus oocytes. Br. J. Pharmacol. 2012, 165, 1572–1583. [Google Scholar]
- Hubin, E.; Van Nuland, N.; Broersen, K.; Pauwels, K. Transient dynamics of Aβ contribute to toxicity in Alzheimer’s disease. Cell. Mol. Life Sci. 2014, 71, 3507–3521. [Google Scholar] [CrossRef]
- Stefani, M. Structural polymorphism of amyloid oligomers and fibrils underlies different fibrillization pathways: Immunogenicity and cytotoxicity. Curr. Protein Pept. Sci. 2010, 11, 343–354. [Google Scholar] [CrossRef]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. eBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef]
- Stefani, M. Structural features and cytotoxicity of amyloid oligomers: Implications in Alzheimer’s disease and other diseases with amyloid deposits. Prog. Neurobiol. 2012, 99, 226–245. [Google Scholar]
- Chen, S.W.; Drakulic, S.; Deas, E.; Ouberai, M.; Aprile, F.A.; Arranz, R.; Ness, S.; Roodveldt, C.; Guilliams, T.; De-Genst, E.J. Structural characterization of toxic oligomers that are kinetically trapped during α-synuclein fibril formation. Proc. Natl. Acad. Sci. USA 2015, 112, E1994–E2003. [Google Scholar] [CrossRef]
- Valastyan, J.S.; Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Models Mech. 2014, 7, 9–14. [Google Scholar]
- Siddiqi, M.K.; Majid, N.; Malik, S.; Alam, P.; Khan, R.H. Amyloid oligomers, protofibrils and fibrils. Macromol. Protein Complexes II Struct. Funct. 2020, 93, 471–503. [Google Scholar]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting proteostasis for disease intervention. Science 2008, 319, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Takahashi, R. Current trends in basic research on Parkinson’s disease: From mitochondria, lysosome to α-synuclein. J. Neural Transm. 2024, 131, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Saxton, A.D.; Kraemer, B.C. Human Ubiquilin 2 and TDP-43 copathology drives neurodegeneration in transgenic Caenorhabditis elegans. G3 2021, 11, jkab158. [Google Scholar]
- Neumann, M.; Kwong, L.K.; Sampathu, D.M.; Trojanowski, J.Q.; Lee, V.M.-Y. TDP-43 proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis: Protein misfolding diseases without amyloidosis. Arch. Neurol. 2007, 64, 1388–1394. [Google Scholar]
- Henstridge, C.M.; Hyman, B.T.; Spires-Jones, T.L. Beyond the neuron–cellular interactions early in Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2019, 20, 94–108. [Google Scholar] [CrossRef]
- Ciechanover, A.; Kwon, Y.T. Protein quality control by molecular chaperones in neurodegeneration. Front. Neurosci. 2017, 11, 185. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Abdeen, A.; Ashraf, G.M.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Evidence linking protein misfolding to quality control in progressive neurodegenerative diseases. Curr. Top. Med. Chem. 2020, 20, 2025–2043. [Google Scholar] [CrossRef]
- Song, R.; Yin, S.; Wu, J.; Yan, J. Neuronal regulated cell death in aging-related neurodegenerative diseases: Key pathways and therapeutic potentials. Neural Regen. Res. 2025, 20, 2245–2263. [Google Scholar] [CrossRef]
- Koopman, M.B.; Ferrari, L.; Rüdiger, S.G. How do protein aggregates escape quality control in neurodegeneration? Trends Neurosci. 2022, 45, 257–271. [Google Scholar] [CrossRef]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau post-translational modifications: Dynamic transformers of tau function, degradation, and aggregation. Front. Neurol. 2021, 11, 595532. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Opare, S.K.; Xu, X.; Ganesan, A.; Rao, P.P. Post-translational modifications in tau and their roles in Alzheimer’s pathology. Curr. Alzheimer Res. 2024, 21, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Prüss, H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 2021, 21, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C.; Jucker, M. Neurodegenerative diseases: Expanding the prion concept. Annu. Rev. Neurosci. 2015, 38, 87–103. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar]
- Lukens, J.R.; Eyo, U.B. Microglia and neurodevelopmental disorders. Annu. Rev. Neurosci. 2022, 45, 425–445. [Google Scholar] [CrossRef]
- Coomey, R.; Stowell, R.; Majewska, A.; Tropea, D. The role of microglia in neurodevelopmental disorders and their therapeutics. Curr. Top. Med. Chem. 2020, 20, 272–276. [Google Scholar] [CrossRef]
- Yang, X.-P.; Huang, J.-H.; Ye, F.-L.; Yv, Q.-Y.; Chen, S.; Li, W.-W.; Zhu, M. Echinacoside exerts neuroprotection via suppressing microglial α-synuclein/TLR2/NF-κB/NLRP3 axis in parkinsonian models. Phytomedicine 2024, 123, 155230. [Google Scholar] [CrossRef]
- Ayyubova, G. Dysfunctional microglia and tau pathology in Alzheimer’s disease. Rev. Neurosci. 2023, 34, 443–458. [Google Scholar]
- Goldberg, B.S.; Ackerman, M.E. Antibody-mediated complement activation in pathology and protection. Immunol. Cell Biol. 2020, 98, 305–317. [Google Scholar] [CrossRef]
- Shim, S.-M.; Koh, Y.H.; Kim, J.-H.; Jeon, J.-P. A combination of multiple autoantibodies is associated with the risk of Alzheimer’s disease and cognitive impairment. Sci. Rep. 2022, 12, 1312. [Google Scholar] [CrossRef]
- Harackiewicz, O.; Grembecka, B. The role of microglia and astrocytes in the pathomechanism of neuroinflammation in Parkinson’s disease-focus on alpha-synuclein. J. Integr. Neurosci. 2024, 23, 203. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Truscott, T.G. The reactive oxygen species singlet oxygen, hydroxy radicals, and the superoxide radical anion—Examples of their roles in biology and medicine. Oxygen 2021, 1, 77–95. [Google Scholar] [CrossRef]
- Piacenza, L.; Zeida, A.; Trujillo, M.; Radi, R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol. Rev. 2022, 102, 1881–1906. [Google Scholar] [CrossRef]
- Klyubin, I.; Cullen, W.K.; Hu, N.-W.; Rowan, M.J. Alzheimer’s disease Aβ assemblies mediating rapid disruption of synaptic plasticity and memory. Mol. Brain 2012, 5, 25. [Google Scholar] [CrossRef]
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159. [Google Scholar] [CrossRef]
- Chen, S.; Yadav, S.P.; Surewicz, W.K. Interaction between human prion protein and amyloid-β (Aβ) oligomers: Role of N-terminal residues. J. Biol. Chem. 2010, 285, 26377–26383. [Google Scholar] [CrossRef]
- Freir, D.B.; Nicoll, A.J.; Klyubin, I.; Panico, S.; Mc Donald, J.M.; Risse, E.; Asante, E.A.; Farrow, M.A.; Sessions, R.B.; Saibil, H.R. Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nat. Commun. 2011, 2, 336. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.E.; Klyubin, I.; Mc Donald, J.M.; Mably, A.J.; Farrell, M.A.; Scott, M.; Walsh, D.M.; Rowan, M.J. Alzheimer’s disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J. Neurosci. 2011, 31, 7259–7263. [Google Scholar] [CrossRef]
- Solforosi, L.; Criado, J.R.; McGavern, D.B.; Wirz, S.; Sánchez-Alavez, M.; Sugama, S.; DeGiorgio, L.A.; Volpe, B.T.; Wiseman, E.; Abalos, G. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 2004, 303, 1514–1516. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Lacor, P.N.; Velasco, P.T.; Xu, J.; Contractor, A.; Klein, W.L.; Triller, A. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron 2010, 66, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Park, S.-H.; Hartl, F.U. Proteostasis impairment in protein-misfolding and-aggregation diseases. Trends Cell Biol. 2014, 24, 506–514. [Google Scholar] [CrossRef]
- Arndt, V.; Dick, N.; Tawo, R.; Dreiseidler, M.; Wenzel, D.; Hesse, M.; Fürst, D.O.; Saftig, P.; Saint, R.; Fleischmann, B.K. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr. Biol. 2010, 20, 143–148. [Google Scholar] [CrossRef]
- Soto, C.; Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef]
- Amorim, J.A.; Canas, P.M.; Tomé, A.R.; Rolo, A.P.; Agostinho, P.; Palmeira, C.M.; Cunha, R.A. Mitochondria in excitatory and inhibitory synapses have similar susceptibility to amyloid-β peptides modeling Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, 525–536. [Google Scholar] [CrossRef]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; ElIdrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Front. Cell. Neurosci. 2018, 12, 338. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Lin, K.-J.; Lin, K.-L.; Chen, S.-D.; Liou, C.-W.; Chuang, Y.-C.; Lin, H.-Y.; Lin, T.-K. The overcrowded crossroads: Mitochondria, alpha-synuclein, and the endo-lysosomal system interaction in Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 5312. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Ahamad, S.; Dar, N.J.; Siddique, Y.H.; Nazir, A. The emerging landscape of natural small-molecule therapeutics for Huntington’s disease. Curr. Neuropharmacol. 2023, 21, 867–889. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.A.; Kim, S.H.; Tibbetts, R.S. RNA-binding proteins in neurodegenerative disease: TDP-43 and beyond. Wiley Interdiscip. Rev. RNA 2012, 3, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Nik, S.; Bowman, T.V. Splicing and neurodegeneration: Insights and mechanisms. Wiley Interdiscip. Rev. RNA 2019, 10, e1532. [Google Scholar] [CrossRef]
- Scheckel, C.; Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018, 19, 405–418. [Google Scholar] [CrossRef]
- Sarnataro, D. Attempt to untangle the prion-like misfolding mechanism for neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3081. [Google Scholar] [CrossRef]
- Meraz-Ríos, M.A.; Franco-Bocanegra, D.; Rios, D.T.; Campos-Peña, V. Early onset Alzheimer’s disease and oxidative stress. Oxidative Med. Cell. Longev. 2014, 2014, 375968. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer′ s disease. Oxidative Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium ions in neuronal degeneration. IUBMB Life 2008, 60, 575–590. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Sochocka, M.; Koutsouraki, E.S.; Gasiorowski, K.; Leszek, J. Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: A new approach to therapy. CNS Neurol. Disord. Drug Targets 2013, 12, 870–881. [Google Scholar] [CrossRef]
- Feeney, C.J.; Frantseva, M.V.; Carlen, P.L.; Pennefather, P.S.; Shulyakova, N.; Shniffer, C.; Mills, L.R. Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res. 2008, 1198, 1–15. [Google Scholar] [CrossRef]
- Tahavvori, A.; Gargari, M.K.; Yazdani, Y.; Mamalo, A.S.; Beilankouhi, E.A.V.; Valilo, M. Involvement of antioxidant enzymes in Parkinson’s disease. Pathol. Res. Pr. 2023, 249, 154757. [Google Scholar] [CrossRef] [PubMed]
- Filosto, M.; Scarpelli, M.; Cotelli, M.S.; Vielmi, V.; Todeschini, A.; Gregorelli, V.; Tonin, P.; Tomelleri, G.; Padovani, A. The role of mitochondria in neurodegenerative diseases. J. Neurol. 2011, 258, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Irrcher, I.; Aleyasin, H.; Seifert, E.; Hewitt, S.; Chhabra, S.; Phillips, M.; Lutz, A.; Rousseaux, M.; Bevilacqua, L.; Jahani-Asl, A. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010, 19, 3734–3746. [Google Scholar] [CrossRef] [PubMed]
- Goehler, H.; Lalowski, M.; Stelzl, U.; Waelter, S.; Stroedicke, M.; Worm, U.; Droege, A.; Lindenberg, K.S.; Knoblich, M.; Haenig, C. A protein interaction network links GIT1, an enhancer of huntingtin aggregation, to Huntington’s disease. Mol. Cell 2004, 15, 853–865. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Carmichael, J. Huntington’s disease: Molecular basis of neurodegeneration. Expert Rev. Mol. Med. 2003, 5, 1–21. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Fenech, M. Mutations that affect mitochondrial functions and their association with neurodegenerative diseases. Mutat. Res. Rev. Mutat. Res. 2014, 759, 1–13. [Google Scholar] [CrossRef]
- Sadri-Vakili, G.; Cha, J.-H.J. Mechanisms of disease: Histone modifications in Huntington’s disease. Nat. Clin. Pr. Neurol. 2006, 2, 330–338. [Google Scholar] [CrossRef]
- Choi, M.L.; Gandhi, S. Crucial role of protein oligomerization in the pathogenesis of Alzheimer’s and Parkinson’s diseases. FEBS J. 2018, 285, 3631–3644. [Google Scholar] [CrossRef]
- Danzer, K.M.; Kranich, L.R.; Ruf, W.P.; Cagsal-Getkin, O.; Winslow, A.R.; Zhu, L.; Vanderburg, C.R.; McLean, P.J. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 2012, 7, 42. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Iljina, M.; Hong, L.; Horrocks, M.H.; Ludtmann, M.H.; Choi, M.L.; Hughes, C.D.; Ruggeri, F.S.; Guilliams, T.; Buell, A.K.; Lee, J.-E. Nanobodies raised against monomeric α-synuclein inhibit fibril formation and destabilize toxic oligomeric species. BMC Biol. 2017, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, H.O.; Murray, E.D.; Price, B.H.; Tarazi, F.I. Bapineuzumab and solanezumab for Alzheimer’s disease: Is the ‘amyloid cascade hypothesis’ still alive? Expert Opin. Biol. Ther. 2013, 13, 1075–1084. [Google Scholar] [CrossRef]
- Neațu, M.; Covaliu, A.; Ioniță, I.; Jugurt, A.; Davidescu, E.I.; Popescu, B.O. Monoclonal antibody therapy in Alzheimer’s disease. Pharmaceutics 2023, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.; Biocca, S. The potential of intracellular antibodies for therapeutic targeting of protein-misfolding diseases. Trends Mol. Med. 2008, 14, 373–380. [Google Scholar] [CrossRef]
- Finka, A.; Goloubinoff, P. Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones 2013, 18, 591–605. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Wahlster, L.; McLean, P.J. Protein degradation pathways in Parkinson’s disease: Curse or blessing. Acta Neuropathol. 2012, 124, 153–172. [Google Scholar] [CrossRef]
- Danzer, K.M.; Ruf, W.P.; Putcha, P.; Joyner, D.; Hashimoto, T.; Glabe, C.; Hyman, B.T.; McLean, P.J. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011, 25, 326. [Google Scholar] [CrossRef] [PubMed]
- Daturpalli, S.; Waudby, C.A.; Meehan, S.; Jackson, S.E. Hsp90 inhibits α-synuclein aggregation by interacting with soluble oligomers. J. Mol. Biol. 2013, 425, 4614–4628. [Google Scholar] [CrossRef]
- Ojha, J.; Masilamoni, G.; Dunlap, D.; Udoff, R.A.; Cashikar, A.G. Sequestration of toxic oligomers by HspB1 as a cytoprotective mechanism. Mol. Cell. Biol. 2011, 31, 3146–3157. [Google Scholar] [CrossRef] [PubMed]
- Sörgjerd, K.M.; Zako, T.; Sakono, M.; Stirling, P.C.; Leroux, M.R.; Saito, T.; Nilsson, P.; Sekimoto, M.; Saido, T.C.; Maeda, M. Human prefoldin inhibits amyloid-β (Aβ) fibrillation and contributes to formation of nontoxic Aβ aggregates. Biochemistry 2013, 52, 3532–3542. [Google Scholar] [CrossRef]
- Wyatt, A.R.; Yerbury, J.J.; Berghofer, P.; Greguric, I.; Katsifis, A.; Dobson, C.M.; Wilson, M.R. Clusterin facilitates in vivo clearance of extracellular misfolded proteins. Cell. Mol. Life Sci. 2011, 68, 3919–3931. [Google Scholar] [CrossRef]
- Laslo, A.; Laslo, L.; Arbănași, E.-M.; Ujlaki-Nagi, A.-A.; Chinezu, L.; Ivănescu, A.D.; Arbănași, E.-M.; Cărare, R.O.; Cordoș, B.A.; Popa, I.A. Pathways to Alzheimer’s disease: The intersecting roles of clusterin and apolipoprotein E in Amyloid-β regulation and neuronal health. Pathophysiology 2024, 31, 545–558. [Google Scholar] [CrossRef]
- Narita, M.; Holtzman, D.M.; Schwartz, A.L.; Bu, G. α2-macroglobulin complexes with and mediates the endocytosis of β-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J. Neurochem. 1997, 69, 1904–1911. [Google Scholar] [CrossRef]
- Chang, C.; Tang, X.; Woodley, D.T.; Chen, M.; Li, W. Previously unrecognized and potentially consequential challenges facing Hsp90 inhibitors in cancer clinical trials. Cell Stress Chaperones 2024, 29, 642–653. [Google Scholar] [CrossRef]
- Tai, L.M.; Mehra, S.; Shete, V.; Estus, S.; Rebeck, G.W.; Bu, G.; LaDu, M.J. Soluble apoE/Aβ complex: Mechanism and therapeutic target for APOE4-induced AD risk. Mol. Neurodegener. 2014, 9, 2. [Google Scholar] [CrossRef]
- Peng, K.Y.; Pérez-González, R.; Alldred, M.J.; Goulbourne, C.N.; Morales-Corraliza, J.; Saito, M.; Saito, M.; Ginsberg, S.D.; Mathews, P.M.; Levy, E. Apolipoprotein E4 genotype compromises brain exosome production. Brain 2019, 142, 163–175. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Gambardella, S.; Familiari, P.; Frati, A.; Fornai, F. Promiscuous roles of autophagy and proteasome in neurodegenerative proteinopathies. Int. J. Mol. Sci. 2020, 21, 3028. [Google Scholar] [CrossRef]
- Montine, T.; Beal, M.; Cudkowicz, M.; O’donnell, H.; Margolin, R.; McFarland, L.; Bachrach, A.; Zackert, W.; Roberts, L.; Morrow, J. Increased CSF F2-isoprostane concentration in probable AD. Neurology 1999, 52, 562. [Google Scholar] [CrossRef] [PubMed]
- Pratico, D.; Lee, V.M.-Y.; Trojanowski, J.Q.; Rokach, J.; Fitzgerald, G.A. Increased F2-isoprostanes in Alzheimer’s disease: Evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998, 12, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Markesbery, W.R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 2001, 22, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, S.; Xie, C.; Markesbery, W.; Lovell, M. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J. Neurochem. 2005, 93, 953–962. [Google Scholar] [CrossRef]
- Chi, H.; Chang, H.-Y.; Sang, T.-K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Ozougwu, J.C. The role of reactive oxygen species and antioxidants in oxidative stress. Int. J. Res. 2016, 1, 1–8. [Google Scholar]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- di Biase, L.; Pecoraro, P.M.; Di Lazzaro, V. Validating the accuracy of Parkinson’s disease clinical diagnosis: A UK Brain bank case–control study. Ann. Neurol. 2025, 97, 1110–1121. [Google Scholar] [CrossRef]
- Reichmann, H. Clinical criteria for the diagnosis of Parkinson’s disease. Neurodegener. Dis. 2010, 7, 284–290. [Google Scholar] [CrossRef]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.L.; Brown, D.R. Seeking a mechanism for the toxicity of oligomeric α-synuclein. Biomolecules 2015, 5, 282–305. [Google Scholar] [CrossRef] [PubMed]
- Rockenstein, E.; Nuber, S.; Overk, C.R.; Ubhi, K.; Mante, M.; Patrick, C.; Adame, A.; Trejo-Morales, M.; Gerez, J.; Picotti, P. Accumulation of oligomer-prone α-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain 2014, 137, 1496–1513. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, F.; Betti, L.; Palego, L.; Giannaccini, G. Parkinson’s disease and alpha-synucleinopathies: From arising pathways to therapeutic challenge. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 109–116. [Google Scholar] [CrossRef]
- Compta, Y.; Valente, T.; Saura, J.; Segura, B.; Iranzo, A.; Serradell, M.; Junqué, C.; Tolosa, E.; Valldeoriola, F.; Munoz, E. Correlates of cerebrospinal fluid levels of oligomeric-and total-α-synuclein in premotor, motor and dementia stages of Parkinson’s disease. J. Neurol. 2015, 262, 294–306. [Google Scholar] [CrossRef]
- Tokuda, T.; Qureshi, M.; Ardah, M.; Varghese, S.; Shehab, S.; Kasai, T.; Ishigami, N.; Tamaoka, A.; Nakagawa, M.; El-Agnaf, O. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 2010, 75, 1766–1770. [Google Scholar] [CrossRef]
- Hansson, O.; Hall, S.; Öhrfelt, A.; Zetterberg, H.; Blennow, K.; Minthon, L.; Nägga, K.; Londos, E.; Varghese, S.; Majbour, N.K. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimer’s Res. Ther. 2014, 6, 25. [Google Scholar] [CrossRef]
- Lei, Y.; Han, H.; Yuan, F.; Javeed, A.; Zhao, Y. The brain interstitial system: Anatomy, modeling, in vivo measurement, and applications. Prog. Neurobiol. 2017, 157, 230–246. [Google Scholar] [CrossRef]
- Shetty, A.K.; Zanirati, G. The interstitial system of the brain in health and disease. Aging Dis. 2020, 11, 200. [Google Scholar] [CrossRef]
- Cirrito, J.R. Extracellular Amyloid-β Protein Dynamics in Alzheimer’s Disease. In Microdialysis in Drug Development; Springer: Berlin/Heidelberg, Germany, 2012; pp. 163–178. [Google Scholar]
- Espourteille, J.; Rouaud, O.; Njamnshi, A.K.; Allali, G.; Richetin, K. Circulating Biomarkers for Alzheimer’s Disease: Unlocking the Diagnostic Potential in Low-and Middle-Income Countries, Focusing on Africa. Neurodegener. Dis. 2024, 24, 26–40. [Google Scholar]
- Campese, N.; Beatino, M.F.; Del Gamba, C.; Belli, E.; Giampietri, L.; Del Prete, E.; Galgani, A.; Vergallo, A.; Siciliano, G.; Ceravolo, R. Ultrasensitive techniques and protein misfolding amplification assays for biomarker-guided reconceptualization of Alzheimer’s and other neurodegenerative diseases. Expert Rev. Neurother. 2021, 21, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, M.; Tokuda, T.; Waragai, M.; Mendez, N.; Ishii, R.; Trenkwalder, C.; Mollenhauer, B.; Soto, C. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 2017, 74, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bidesi, N.S.; Andersen, I.V.; Windhorst, A.D.; Shalgunov, V.; Herth, M.M. The role of neuroimaging in Parkinson’s disease. J. Neurochem. 2021, 159, 660–689. [Google Scholar] [CrossRef]
- Loftus, J.R.; Puri, S.; Meyers, S.P. Multimodality imaging of neurodegenerative disorders with a focus on multiparametric magnetic resonance and molecular imaging. Insights Into Imaging 2023, 14, 8. [Google Scholar] [CrossRef]
- Dupont, A.-C.; Largeau, B.; Guilloteau, D.; Ribeiro, M.J.S.; Arlicot, N. The place of PET to assess new therapeutic effectiveness in neurodegenerative diseases. Contrast Media Mol. Imaging 2018, 2018, 7043578. [Google Scholar] [CrossRef]
- Nordberg, A.; Rinne, J.O.; Kadir, A.; Långström, B. The use of PET in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 78–87. [Google Scholar] [CrossRef]
- Mosconi, L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin. Transl. Imaging 2013, 1, 217–233. [Google Scholar] [CrossRef]
- Palermo, G.; Giannoni, S.; Bellini, G.; Siciliano, G.; Ceravolo, R. Dopamine transporter imaging, current status of a potential biomarker: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 11234. [Google Scholar] [CrossRef]
- Mosconi, L.; Berti, V.; Glodzik, L.; Pupi, A.; De Santi, S.; De Leon, M.J. Pre-clinical detection of Alzheimer’s disease using FDG-PET, with or without amyloid imaging. J. Alzheimer’s Dis. 2010, 20, 843–854. [Google Scholar] [CrossRef]
- Roy, R.; Niccolini, F.; Pagano, G.; Politis, M. Cholinergic imaging in dementia spectrum disorders. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-M.; Ren, W.-M.; Tang, X.-C.; Hu, Y.-H.; Zhang, H.-Y. Advances in development of fluorescent probes for detecting amyloid-β aggregates. Acta Pharmacol. Sin. 2016, 37, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Lendel, C.; Bolognesi, B.; Wahlstrom, A.; Dobson, C.M.; Graslund, A. Detergent-like interaction of Congo red with the amyloid β peptide. Biochemistry 2010, 49, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.P.; Chatterjee, U.; Xie, L.; Johansson, J.; Gothelid, E.; Arvidsson, P.I. Effects of Congo red on Aβ1–40 fibril formation process and morphology. ACS Chem. Neurosci. 2010, 1, 315–324. [Google Scholar] [CrossRef]
- Pedersen, M.Ø.; Mikkelsen, K.; Behrens, M.A.; Pedersen, J.S.; Enghild, J.J.; Skrydstrup, T.; Malmendal, A.; Nielsen, N.C. NMR reveals two-step association of Congo Red to amyloid β in low-molecular-weight aggregates. J. Phys. Chem. B 2010, 114, 16003–16010. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.M.; Kim, H.Y.; Kim, Y. Fluorescence chemicals to detect insoluble and soluble amyloid-β aggregates. ACS Chem. Neurosci. 2019, 10, 2647–2657. [Google Scholar] [CrossRef]
- Pontecorvo, M.J.; Siderowf, A.; Dubois, B.; Doraiswamy, P.M.; Frisoni, G.B.; Grundman, M.; Nobili, F.; Sadowsky, C.H.; Salloway, S.; Arora, A.K. Effectiveness of florbetapir PET imaging in changing patient management. Dement. Geriatr. Cogn. Disord. 2017, 44, 129–143. [Google Scholar] [CrossRef]
- Chapleau, M.; Iaccarino, L.; Soleimani-Meigooni, D.; Rabinovici, G.D. The role of amyloid PET in imaging neurodegenerative disorders: A review. J. Nucl. Med. 2022, 63, 13S–19S. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Carrillo, M.C.; Apgar, C.; Gareen, I.F.; Gutman, R.; Hanna, L.; Hillner, B.E.; March, A.; Romanoff, J.; Siegel, B.A. Amyloid positron emission tomography and subsequent health care use among medicare beneficiaries with mild cognitive impairment or dementia. JAMA Neurol. 2023, 80, 1166–1173. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Ding, M.; Xu, W.; Pei, G.; Li, P. Long way up: Rethink diseases in light of phase separation and phase transition. Protein Cell 2024, 15, 475–492. [Google Scholar] [CrossRef]
- Vanderweyde, T.; Youmans, K.; Liu-Yesucevitz, L.; Wolozin, B. Role of stress granules and RNA-binding proteins in neurodegeneration: A mini-review. Gerontology 2013, 59, 524–533. [Google Scholar] [CrossRef]
- Vanderweyde, T.E.; Wolozin, B. RNA Binding Proteins in Health and Disease. In Neuroimmune Pharmacology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 299–312. [Google Scholar]
- Yang, H.; Hu, H.Y. Sequestration of cellular interacting partners by protein aggregates: Implication in a loss-of-function pathology. FEBS J. 2016, 283, 3705–3717. [Google Scholar] [CrossRef]
- Argueti-Ostrovsky, S.; Alfahel, L.; Kahn, J.; Israelson, A. All roads lead to Rome: Different molecular players converge to common toxic pathways in neurodegeneration. Cells 2021, 10, 2438. [Google Scholar] [CrossRef]
- Yu, Q.-Y.; Ye, L.-Q.; Li, H.-L. Molecular interaction of stress granules with Tau and autophagy in Alzheimer’s disease. Neurochem. Int. 2022, 157, 105342. [Google Scholar] [CrossRef]
- Liu-Yesucevitz, L.; Bilgutay, A.; Zhang, Y.-J.; Vanderwyde, T.; Citro, A.; Mehta, T.; Zaarur, N.; McKee, A.; Bowser, R.; Sherman, M. Tar DNA binding protein-43 (TDP-43) associates with stress granules: Analysis of cultured cells and pathological brain tissue. PLoS ONE 2010, 5, e13250. [Google Scholar] [CrossRef]
- Richter, J.D.; Zhao, X. The molecular biology of FMRP: New insights into fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Liu-Yesucevitz, L.; Bassell, G.J.; Gitler, A.D.; Hart, A.C.; Klann, E.; Richter, J.D.; Warren, S.T.; Wolozin, B. Local RNA translation at the synapse and in disease. J. Neurosci. 2011, 31, 16086–16093. [Google Scholar] [CrossRef]
- Wolozin, B. Regulated protein aggregation: Stress granules and neurodegeneration. Mol. Neurodegener. 2012, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.Y.-Y.; Ho, P.W.-L.; Liu, H.-F.; Leung, C.-T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.-L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef]
- Pozojevic, J.; Spielmann, M. Single-cell sequencing in neurodegenerative disorders. Mol. Diagn. Ther. 2023, 27, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 2023, 24, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Plum, S.; Steinbach, S.; Abel, L.; Marcus, K.; Helling, S.; May, C. Proteomics in neurodegenerative diseases: Methods for obtaining a closer look at the neuronal proteome. Proteom. Clin. Appl. 2015, 9, 848–871. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Oh, Y.J. A novel 2-DE-based proteomic analysis to identify multiple substrates for specific protease in neuronal cells. In Neuroproteomics: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 229–245. [Google Scholar]
- Collins, M.A.; An, J.; Hood, B.L.; Conrads, T.P.; Bowser, R.P. Label-free LC–MS/MS proteomic analysis of cerebrospinal fluid identifies protein/pathway alterations and candidate biomarkers for amyotrophic lateral sclerosis. J. Proteome Res. 2015, 14, 4486–4501. [Google Scholar] [CrossRef]
- Li, K.W.; Gonzalez-Lozano, M.A.; Koopmans, F.; Smit, A.B. Recent developments in data independent acquisition (DIA) mass spectrometry: Application of quantitative analysis of the brain proteome. Front. Mol. Neurosci. 2020, 13, 564446. [Google Scholar] [CrossRef]
- Aslebagh, R.; Wormwood, K.L.; Channaveerappa, D.; Wetie, A.G.N.; Woods, A.G.; Darie, C.C. Identification of posttranslational modifications (PTMs) of proteins by mass spectrometry. Adv. Mass Spectrom. Biomed. Res. 2019, 1140, 199–224. [Google Scholar]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Mnatsakanyan, R.; Shema, G.; Basik, M.; Batist, G.; Borchers, C.H.; Sickmann, A.; Zahedi, R.P. Detecting post-translational modification signatures as potential biomarkers in clinical mass spectrometry. Expert Rev. Proteom. 2018, 15, 515–535. [Google Scholar] [CrossRef]
- Elkjaer, M.L.; Röttger, R.; Baumbach, J.; Illes, Z. A systematic review of tissue and single cell transcriptome/proteome studies of the brain in multiple sclerosis. Front. Immunol. 2022, 13, 761225. [Google Scholar] [CrossRef]
- Zhu, B.; Park, J.-M.; Coffey, S.R.; Russo, A.; Hsu, I.-U.; Wang, J.; Su, C.; Chang, R.; Lam, T.T.; Gopal, P.P. Single-cell transcriptomic and proteomic analysis of Parkinson’s disease Brains. Sci. Transl. Med. 2024, 16, eabo1997. [Google Scholar] [CrossRef]
- Voicu, V.; Toader, C.; Șerban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Systemic Neurodegeneration and Brain Aging: Multi-Omics Disintegration, Proteostatic Collapse, and Network Failure Across the CNS. Biomedicines 2025, 13, 2025. [Google Scholar] [CrossRef]
- Ruffini, N.; Klingenberg, S.; Schweiger, S.; Gerber, S. Common factors in neurodegeneration: A meta-study revealing shared patterns on a multi-omics scale. Cells 2020, 9, 2642. [Google Scholar] [CrossRef] [PubMed]
- Smajić, S.; Prada-Medina, C.A.; Landoulsi, Z.; Ghelfi, J.; Delcambre, S.; Dietrich, C.; Jarazo, J.; Henck, J.; Balachandran, S.; Pachchek, S. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain 2022, 145, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Tărlungeanu, D.C.; Novarino, G. Genomics in neurodevelopmental disorders: An avenue to personalized medicine. Exp. Mol. Med. 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Tsai, P.T.; Hull, C.; Chu, Y.; Greene-Colozzi, E.; Sadowski, A.R.; Leech, J.M.; Steinberg, J.; Crawley, J.N.; Regehr, W.G.; Sahin, M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 2012, 488, 647–651. [Google Scholar] [CrossRef]
- Zhou, J.; Blundell, J.; Ogawa, S.; Kwon, C.-H.; Zhang, W.; Sinton, C.; Powell, C.M.; Parada, L.F. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 2009, 29, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Oguro-Ando, A.; Rosensweig, C.; Herman, E.; Nishimura, Y.; Werling, D.; Bill, B.; Berg, J.; Gao, F.; Coppola, G.; Abrahams, B. Increased CYFIP1 dosage alters cellular and dendritic morphology and dysregulates mTOR. Mol. Psychiatry 2015, 20, 1069–1078. [Google Scholar] [CrossRef]
- Budde, K.; Zonnenberg, B.A.; Frost, M.; Cheung, W.; Urva, S.; Brechenmacher, T.; Stein, K.; Chen, D.; Kingswood, J.C.; Bissler, J.J. Pharmacokinetics and pharmacodynamics of everolimus in patients with renal angiomyolipoma and tuberous sclerosis complex or lymphangioleiomyomatosis. Br. J. Clin. Pharmacol. 2016, 81, 958–970. [Google Scholar] [CrossRef]
- Kohrman, M.H. Emerging treatments in the management of tuberous sclerosis complex. Pediatr. Neurol. 2012, 46, 267–275. [Google Scholar] [CrossRef]
- Kline, D.D.; Ogier, M.; Kunze, D.L.; Katz, D.M. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J. Neurosci. 2010, 30, 5303–5310. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Garcia, R.I.; Kwok, S.; Banerjee, A.; Petravicz, J.; Woodson, J.; Mellios, N.; Tropea, D.; Sur, M. Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett Syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 9941–9946. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.C.; Klann, E. The translation of translational control by FMRP: Therapeutic targets for FXS. Nat. Neurosci. 2013, 16, 1530–1536. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Des Portes, V.; Hagerman, R.; Jacquemont, S.; Charles, P.; Visootsak, J.; Brinkman, M.; Rerat, K.; Koumaras, B.; Zhu, L. Mavoglurant in fragile X syndrome: Results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med. 2016, 8, ra325–ra321. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Ward, A.J.; Chun, S.; Bennett, C.F.; Beaudet, A.L.; Rigo, F. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature 2015, 518, 409–412. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Chen, H.-M.; Swann, J.W.; Hao, S.; Tang, B.; Wu, Z.; Tang, J.; Wan, Y.-W.; Liu, Z.; Rigo, F. Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides. Nature 2015, 528, 123–126. [Google Scholar] [CrossRef]


| Proteins That Aggregate | Main Associated Disease(s) | Mechanism of Toxicity | References |
|---|---|---|---|
| Amyloid-β (Aβ) | Alzheimer’s disease | Synaptic dysfunction, mitochondrial impairment, excitotoxicity, ion channel formation disrupting Ca2+ homeostasis. | [130] |
| Tau | Alzheimer’s, frontotemporal dementia | Hyperphosphorylation which leads to the formation of neurofibrillary tangles, thereby causing microtubule destabilization, synaptic failure, and trans-neuronal spread. | [131,132] |
| α-Synuclein | Parkinson’s, Lewy body dementia | Oligomerization into Lewy bodies, mitochondrial and lysosomal dysfunction, calcium dysregulation, neuroinflammation. | [133] |
| Huntingtin (mHTT) | Huntington’s disease | Expansion of polyglutamine which causes protein misfolding and aggregation, transcriptional dysregulation, impaired axonal transport, excitotoxicity. | [134] |
| TDP-43 | ALS, frontotemporal lobar degeneration (FTLD) | Abnormal aggregation, loss of RNA-binding function, impaired splicing, cytoplasmic inclusions are toxic to neurons. | [135,136] |
| Prion protein (PrPSc) | Prion diseases (Creutzfeldt–Jakob disease, Kuru, Bovine Spongiform Encephalopathy) | Misfolded prion proteins (PrPSc) act as pathological templates that induce the misfolding of normal cellular prion protein (PrPC), leading to the accumulation of insoluble aggregates in the brain, causing spongiform changes, synaptic loss, and progressive neurodegeneration. | [137,138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.T.; Awosiminiala, F.W.; Anumudu, C.K. Exploring Protein Misfolding and Aggregate Pathology in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Interventions. Appl. Sci. 2025, 15, 10285. https://doi.org/10.3390/app151810285
Johnson JT, Awosiminiala FW, Anumudu CK. Exploring Protein Misfolding and Aggregate Pathology in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Interventions. Applied Sciences. 2025; 15(18):10285. https://doi.org/10.3390/app151810285
Chicago/Turabian StyleJohnson, Joel Theophilus, Fila Winifred Awosiminiala, and Christian Kosisochukwu Anumudu. 2025. "Exploring Protein Misfolding and Aggregate Pathology in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Interventions" Applied Sciences 15, no. 18: 10285. https://doi.org/10.3390/app151810285
APA StyleJohnson, J. T., Awosiminiala, F. W., & Anumudu, C. K. (2025). Exploring Protein Misfolding and Aggregate Pathology in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Interventions. Applied Sciences, 15(18), 10285. https://doi.org/10.3390/app151810285







