First-Principles Investigation of Structural, Electronic, and Optical Transitions in FexZr1−xO2 Solid Solutions
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Properties and Chemical Stability
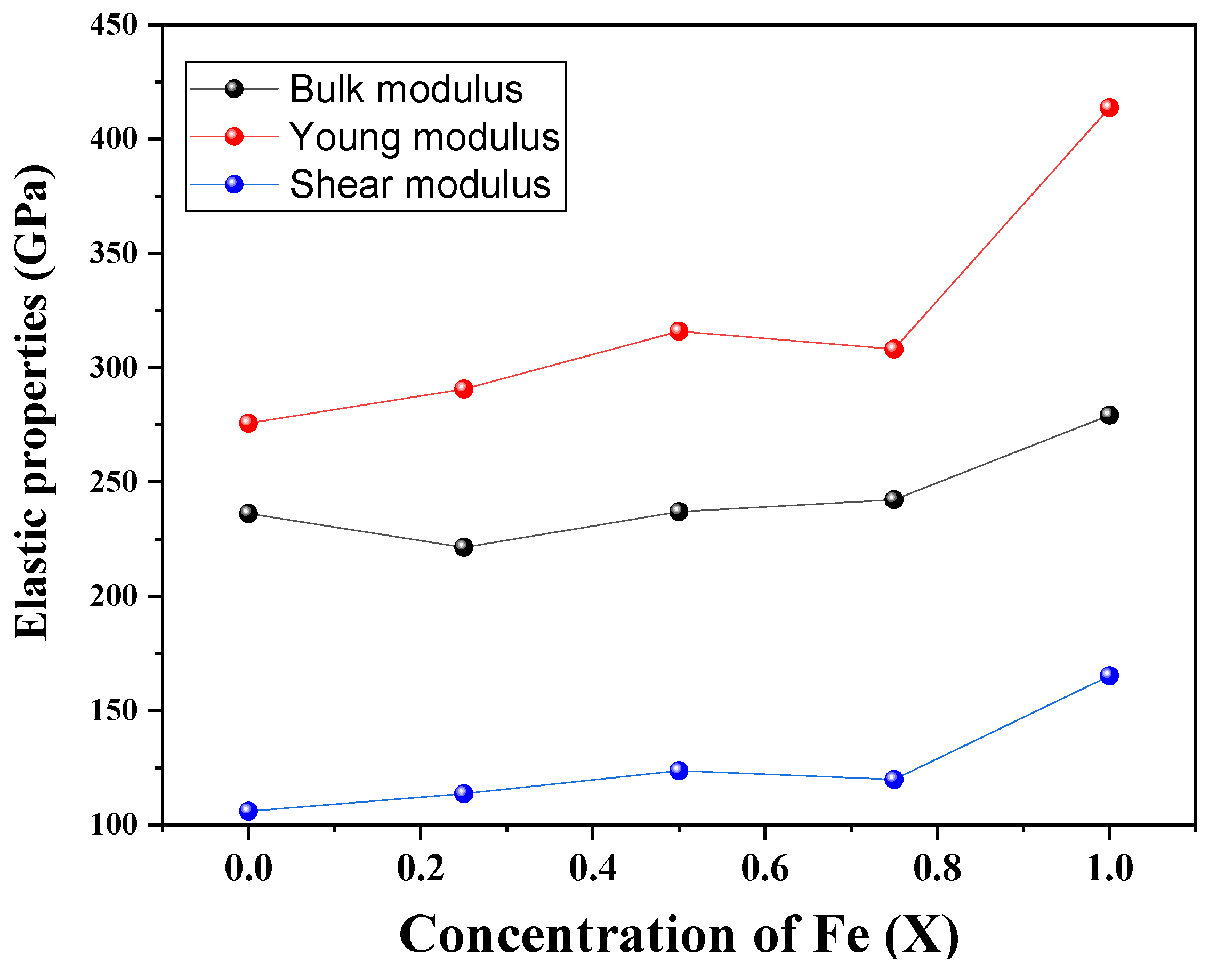
3.2. Mechanical Behavior and Stability
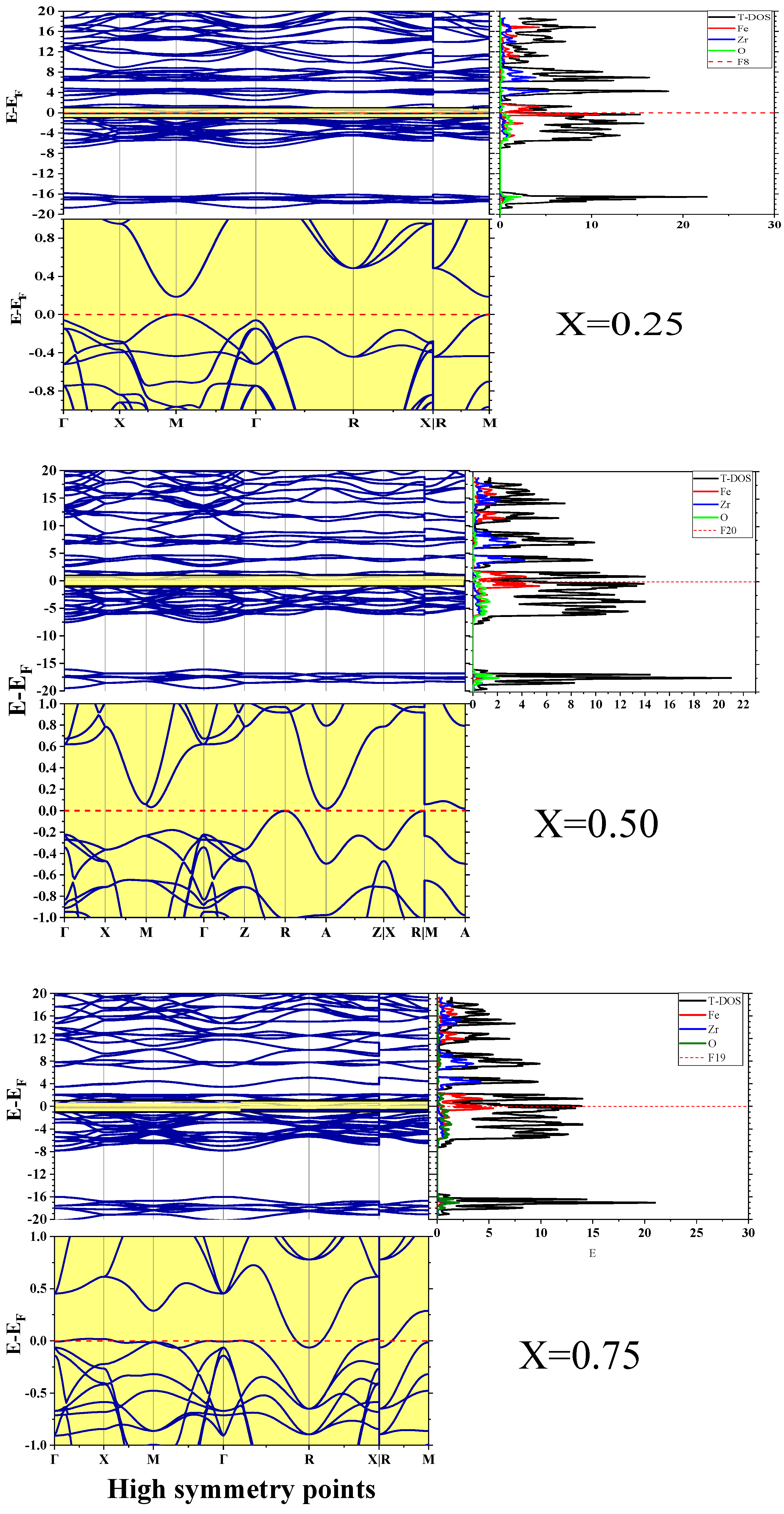
3.3. Electronic Properties
- Transparent Conductive Oxides (TCOs): High electrical conductivity combined with partial optical transparency makes Fe-rich ZrO2 compounds suitable for transparent electrodes in solar cells, LEDs, and display technologies.
- Plasmonic Devices: Semi-metals can support surface plasmon resonances in the near-infrared region, enabling biosensors, metamaterials, and photonic devices.
- Thermoelectric Applications: Coexistence of electron and hole pockets can enhance thermoelectric performance by increasing electrical conductivity while maintaining Seebeck coefficient contributions.
- Electrocatalysis: The metallic nature of Fe0.75Zr0.25O2 may facilitate fast electron transfer reactions, improving performance in oxygen evolution reactions (OER) or hydrogen evolution reactions (HER).
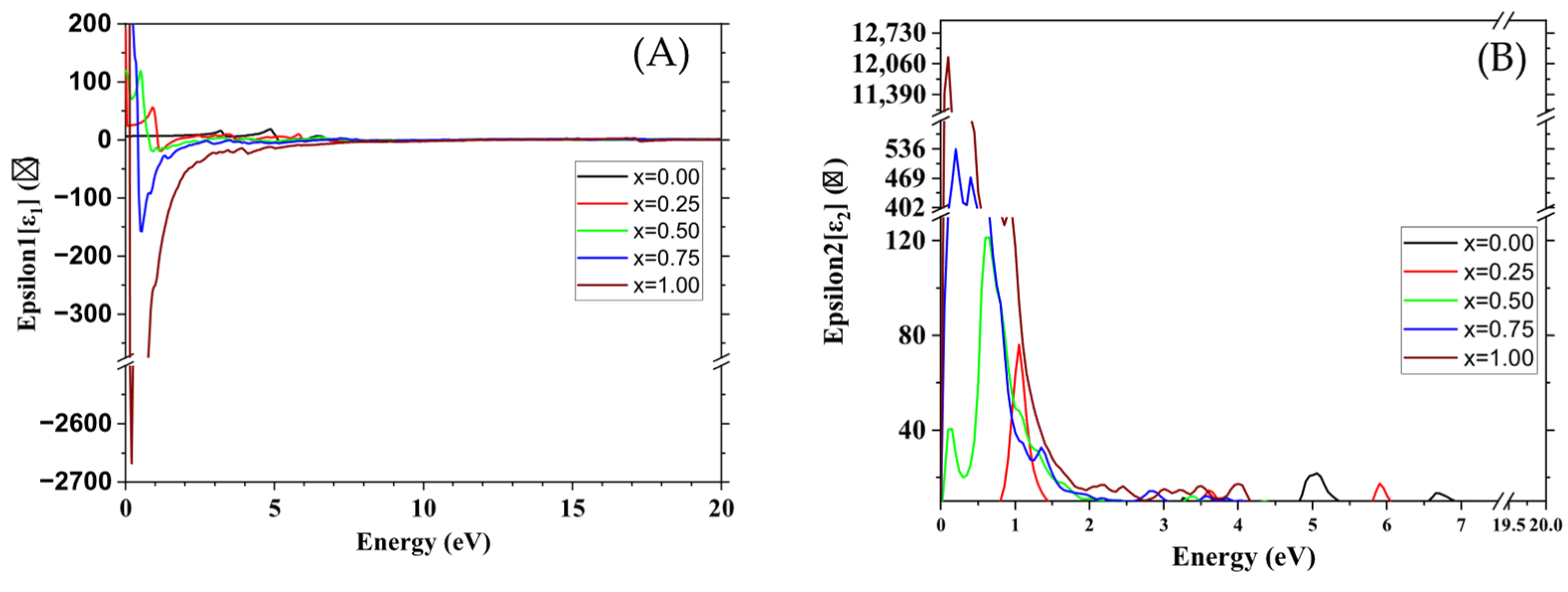
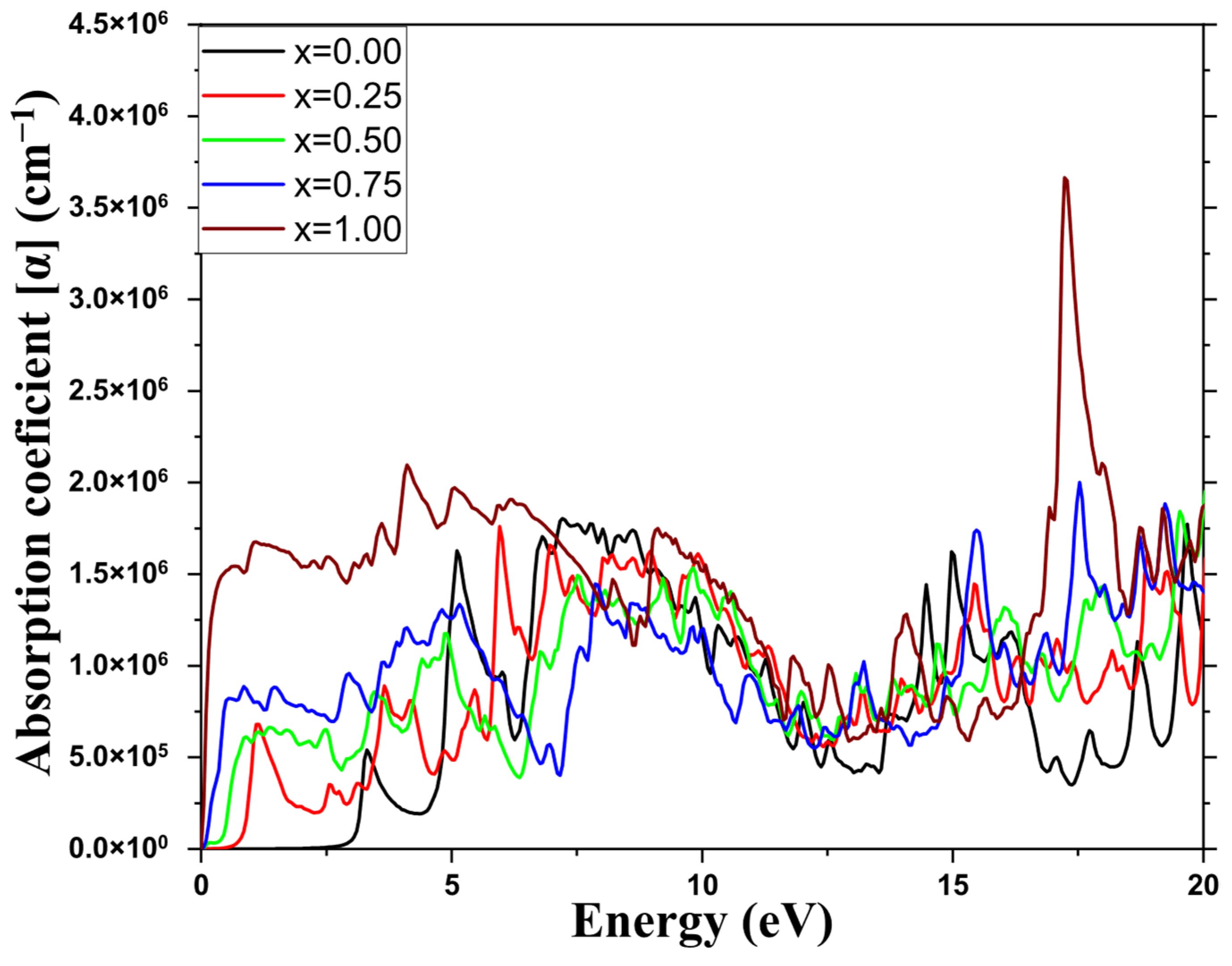
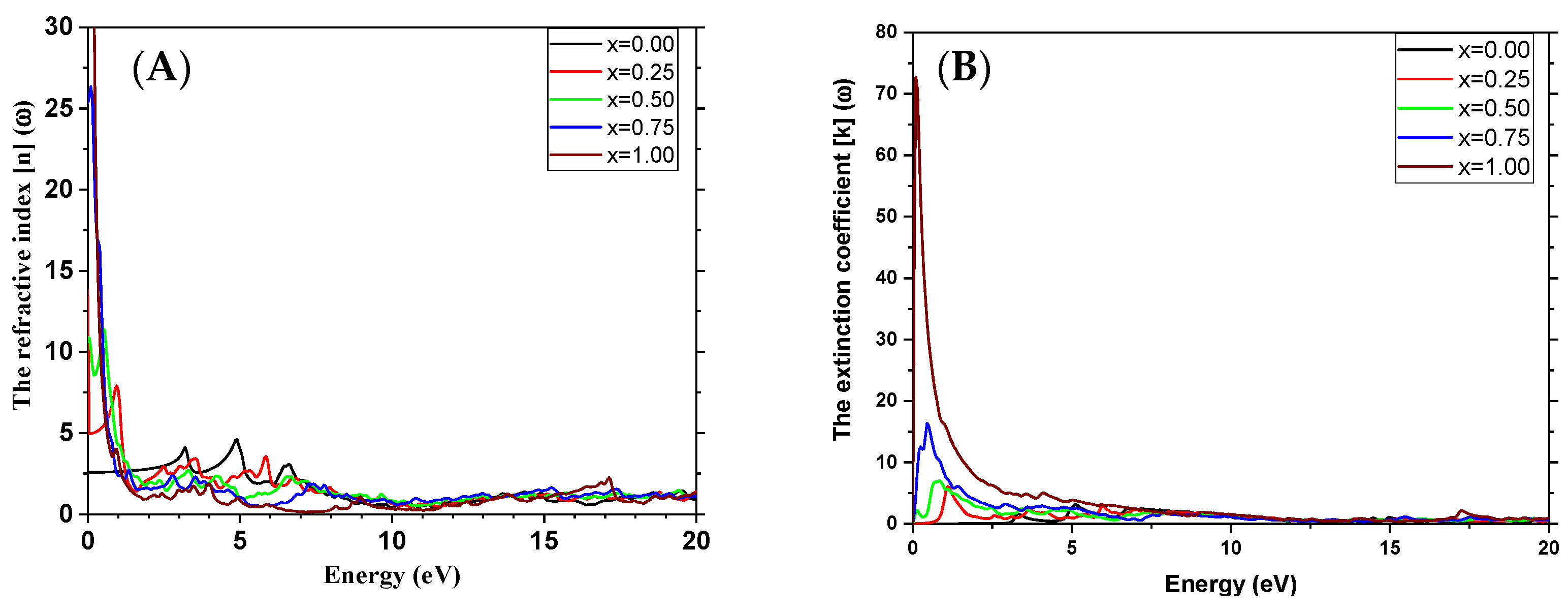
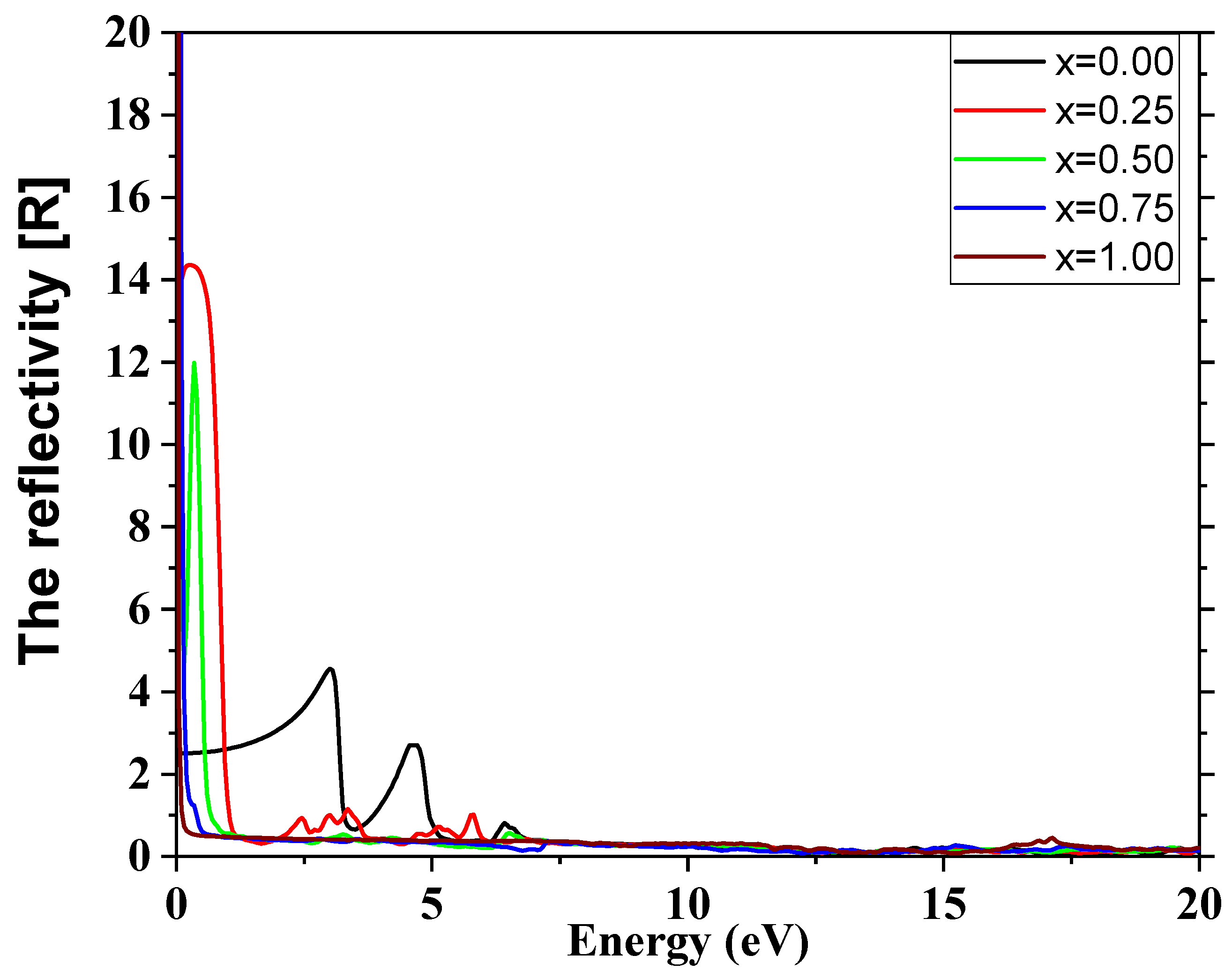
3.4. Optical Properties
| Optical Proprieties | ZrO2 | FeO2 | |
|---|---|---|---|
| Absorption coefficient (Cm−1) | This study | Near-zero | >105 |
| Other studies | Near-zero [49] | About 105 [38] | |
| Dielectric constant | This study | 6.67 | |
| Other studies | 5.85 [44] | Not meaningful for a metal | |
| Refractive index (visible) | This study | 2.6 | / |
| Other studies | 2.15–2.20 [50] | / |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BFGS | Broyden–Fletcher–Goldfarb–Shanno |
| DFT | Density-functional theory |
| DOS | density of states |
| ELF | energy loss function |
| GGA | generalized gradient approximation |
| NCPP | norm-conserving pseudopotentials |
| NIR | near-infrared |
| OER | oxygen evolution reaction |
| PBE | Perdew–Burke–Ernzerhof |
| RPA | random phase approximation |
| SCF | self-consistent field |
| SOFC | solid oxide fuel cell |
| TCO | transparent conductive oxide |
| TDOS | total density of states |
| USPP | Ultrasoft pseudopotentials |
References
- Belkhanchi, H.; Ziat, Y.; Hammi, M.; Laghlimi, C.; Moutcine, A.; Benyounes, A.; Kzaiber, F. Nitrogen doped carbon nanotubes grafted TiO2 rutile nanofilms: Promising material for dye sensitized solar cell application. Optik 2021, 229, 166234. [Google Scholar] [CrossRef]
- Belkhanchi, H.; Ziat, Y.; Hammi, M.; Laghlimi, C.; Moutcine, A.; Benyounes, A.; Kzaiber, F. Synthesis of N-CNT/TiO2 composites thin films: Surface analysis and optoelectronic properties. E3S Web Conf. 2020, 183, 05002. [Google Scholar] [CrossRef]
- Garvie, R.C. The Occurrence of Metastable Tetragonal Zirconia as a Crystallite Size Effect; ACS Publication: Washington, DC, USA, 2002. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S. Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia. Int. Mater. Rev. 2005, 50, 45–64. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic Steel? In Sintering Key Papers; Sōmiya, S., Moriyoshi, Y., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 253–257. [Google Scholar] [CrossRef]
- Mori, M.; Abe, T.; Itoh, H.; Yamamoto, O.; Takeda, Y.; Kawahara, T. Cubic-stabilized zirconia and alumina composites as electrolytes in planar type solid oxide fuel cells. Solid State Ion. 1994, 74, 157–164. [Google Scholar] [CrossRef]
- Ziat, Y.; Belkhanchi, H.; Zarhri, Z. DFT Analysis of Structural, Electrical, and Optical Properties of S, Si, and F-Doped GeO2 Rutile: Implications for UV-Transparent Conductors and Photodetection. Sol. Energy Sustain. Dev. J. 2025, 14, 74–89. [Google Scholar] [CrossRef]
- Shabalin, I.L.; Roach, D.L.; Shabalin, L.I. Oxidation of titanium carbide–graphite hetero-modulus ceramics with low carbon content: II. Physico-chemical interpretation of the ridge effect. J. Eur. Ceram. Soc. 2008, 28, 3177–3188. [Google Scholar] [CrossRef]
- Safari, E.; Rahemi, N.; Kahforoushan, D.; Allahyari, S. Copper adsorptive removal from aqueous solution by orange peel residue carbon nanoparticles synthesized by combustion method using response surface methodology. J. Environ. Chem. Eng. 2019, 7, 102847. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, J.; Chen, H.; Qu, Y.; Deng, Q.; Lin, Z. One-pot synthesis of echinus-like Fe-doped SnO2 with enhanced photocatalytic activity under simulated sunlight. J. Alloys Compd. 2017, 695, 3318–3323. [Google Scholar] [CrossRef]
- Velardi, L.; Valentini, A.; Cicala, G. Highly efficient and stable ultraviolet photocathode based on nanodiamond particles. Appl. Phys. Lett. 2016, 108, 083503. [Google Scholar] [CrossRef]
- Ahmed, W.; Iqbal, J.; Aisida, S.O.; Badshah, A.; Ahmad, I.; Alamgir, K.; Gul, I.H. Structural, magnetic and dielectric characteristics of optically tuned Fe doped ZrO2 nanoparticles with visible light driven photocatalytic activity. Mater. Chem. Phys. 2020, 251, 122999. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, W.; Chen, Q.; Zhu, J.; Li, D.; Fu, L.; Zhou, L. Fe-doped zirconia nanoparticles with highly negative conduction band potential for enhancing visible light photocatalytic performance. Appl. Surf. Sci. 2020, 530, 147291. [Google Scholar] [CrossRef]
- Cui, H.; Heath, G.; Remo, T.; Ravikumar, D.; Silverman, T.; Deceglie, M.; Kempe, M.; Engel-Cox, J. Technoeconomic analysis of high-value, crystalline silicon photovoltaic module recycling processes. Sol. Energy Mater. Sol. Cells 2022, 238, 111592. [Google Scholar] [CrossRef]
- Aamlid, S.S.; Kim, M.; González-Rivas, M.U.; Oudah, M.; Takagi, H.; Hallas, A.M. Effect of high pressure synthesis conditions on the formation of high entropy oxides. Appl. Phys. Lett. 2024, 125, 021901. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Pinto da Silva, L.; Zegaoui, O.; Esteves da Silva, J.C.G. Synthesis of Fe- and Co-Doped TiO2 with Improved Photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef]
- Feng, C.; Faheem, M.B.; Fu, J.; Xiao, Y.; Li, C.; Li, Y. Fe-Based Electrocatalysts for Oxygen Evolution Reaction: Progress and Perspectives. ACS Catal. 2020, 10, 4019–4047. [Google Scholar] [CrossRef]
- Lai, C.; Shi, X.; Li, L.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; Yi, H.; Qin, L.; Zhang, M.; et al. Enhancing iron redox cycling for promoting heterogeneous Fenton performance: A review. Sci. Total Environ. 2021, 775, 145850. [Google Scholar] [CrossRef] [PubMed]
- Sarhani, M.E.; Belkhir, M.L.; Begagra, A. Study of Photo-Conversion Performance of Carbon-Based Perovskite Solar Cells. Phys. Status Solidi B 2025, 2500069. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Choudhary, K.; Tavazza, F. Convergence and machine learning predictions of Monkhorst-Pack k-points and plane-wave cut-off in high-throughput DFT calculations. Comput. Mater. Sci. 2019, 161, 300–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, V.; Xu, K.-W. The detailed orbital-decomposed electronic structures of tetragonal ZrO2. Phys. B Condens. Matter 2013, 411, 126–130. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, J. Deep mantle hydrogen in the pyrite-type FeO2–FeO2H system. Geosci. Front. 2021, 12, 975–981. [Google Scholar] [CrossRef]
- Boulard, E.; Guyot, F.; Menguy, N.; Corgne, A.; Auzende, A.-L.; Perrillat, J.-P.; Fiquet, G. CO2-induced destabilization of pyrite-structured FeO2Hx in the lower mantle. Natl. Sci. Rev. 2018, 5, 870–877. [Google Scholar] [CrossRef]
- Lu, C.; Amsler, M.; Chen, C. Unraveling the structure and bonding evolution of the newly discovered iron oxide FeO2. Phys. Rev. B 2018, 98, 054102. [Google Scholar] [CrossRef]
- Howard, C.J.; Hill, R.J.; Reichert, B.E. Structures of ZrO2 polymorphs at room temperature by high-resolution neutron powder diffraction. Acta Crystallogr. B 1988, 44, 116–120. [Google Scholar] [CrossRef]
- Zhao, X.; Vanderbilt, D. Phonons and lattice dielectric properties of zirconia. Phys. Rev. B 2002, 65, 075105. [Google Scholar] [CrossRef]
- Salikhodzha, Z.M.; Bairbayeva, G.B.; Kassymkhanova, R.N.; Konuhova, M.; Zhangylyssov, K.B.; Popova, E.; Popov, A.I. Density Functional Theory Study of Pressure-Dependent Structural and Electronic Properties of Cubic Zirconium Dioxide. Ceramics 2025, 8, 41. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Fan, X.-L.; Liu, C.; Ran, R.-X. First principles study of structural and electronic properties of cubic phase of ZrO2 and HfO2. Phys. B Condens. Matter 2014, 434, 7–13. [Google Scholar] [CrossRef]
- Zhang, S.; Li, K.; Ma, Y.; Guo, F.; Jiang, C.; Liang, Z.; Bu, Y.; Zhang, J. Density Functional Studies on the Atomistic Structure and Properties of Iron Oxides: A Parametric Study. Materials 2022, 15, 8316. [Google Scholar] [CrossRef]
- Hadbi, M.; Demmouche, K.; Eddine Mellah, D.; Coutinho, J. Theoretical insights into off-stoichiometric Zr(x)Ti(1−x)IrSb half-Heusler alloys: A first principle calculations. J. Phys. Condens. Matter Inst. Phys. J. 2024, 37, 045702. [Google Scholar] [CrossRef]
- Doufar, N.; Benamira, M.; Lahmar, H.; Trari, M.; Avramova, I.; Caldes, M.T. Structural and photochemical properties of Fe-doped ZrO2 and their application as photocatalysts with TiO2 for chromate reduction. J. Photochem. Photobiol. Chem. 2020, 386, 112105. [Google Scholar] [CrossRef]
- Sarhani, M.E.; Dahame, T.; Belkhir, M.L.; Bentria, B.; Begagra, A. AB-INITIO study of electronic, mechanical, optical and thermoelectric properties of KGeCl3 for photovoltaic application. Heliyon 2023, 9, e19808. [Google Scholar] [CrossRef] [PubMed]
- Mayr-Schmölzer, W.; Planer, J.; Redinger, J.; Grüneis, A.; Mittendorfer, F. Many-electron calculations of the phase stability of ZrO2 polymorphs. Phys. Rev. Res. 2020, 2, 043361. [Google Scholar] [CrossRef]
- Choudhary, K.; Zhang, Q.; Reid, A.C.E.; Chowdhury, S.; Van Nguyen, N.; Trautt, Z.; Newrock, M.W.; Congo, F.Y.; Tavazza, F. Computational screening of high-performance optoelectronic materials using OptB88vdW and TB-mBJ formalisms. Sci. Data 2018, 5, 180082. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.G.; Kim, D.Y.; Shim, J.H. Metal-insulator transition and the role of electron correlation in FeO2. Phys. Rev. B 2017, 95, 075144. [Google Scholar] [CrossRef]
- Yashima, M.; Kakihana, M.; Yoshimura, M. Metastable-stable phase diagrams in the zirconia-containing systems utilized in solid-oxide fuel cell application. Solid State Ion. 1996, 86–88, 1131–1149. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-Type Doped TiO2: Nature of Ti3+ Species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Sathupun, K.; Kotmool, K.; Tsuppayakorn-aek, P.; Pluengphon, P.; Majumdar, A.; Bovornratanaraks, T. Fe-doped effects on phase transition and electronic structure of CeO2 under compressed conditions from ab initio calculations. Appl. Phys. A 2021, 127, 784. [Google Scholar] [CrossRef]
- Szade, J.; Kajewski, D.; Kubacki, J.; Szot, K.; Koehl, A.; Lenser, C.; Dittmann, R. Impact of Fe doping on the electronic structure of SrTiO3 thin films determined by resonant photoemission. arXiv 2015, arXiv:1509.08006. [Google Scholar] [CrossRef]
- Nazir, M.A.; Mahmood, T.; Zafar, A.A.; Akhtar, N.; Hussain, T.; Saeed, M.A.; Aleem, F.; Saeed, A.; Raza, J.; Cao, C. Electronic, optical and elastic properties of cubic zirconia (c-ZrO2) under pressure: A DFT study. Phys. B Condens. Matter 2021, 604, 412462. [Google Scholar] [CrossRef]
- Kilic, U.; Traouli, Y.; Hilfiker, M.; Bryant, K.; Schoeche, S.; Feder, R.; Argyropoulos, C.; Schubert, E.; Schubert, M. Nanocolumnar Metamaterial Platforms: Scaling Rules for Structural Parameters Revealed from Optical Anisotropy. Adv. Opt. Mater. 2024, 12, 2302767. [Google Scholar] [CrossRef]
- Harrop, P.J.; Wanklyn, J.N. The dielectric constant of zirconia. Br. J. Appl. Phys. 1967, 18, 739. [Google Scholar] [CrossRef]
- Sanesi, M.; Cremante, G.; Pizzini, S.; Wagner, V. On the Dielectric Constant of Zirconia and Lime-zirconia. Z. Für Naturforschung A 1971, 26, 159–164. [Google Scholar] [CrossRef]
- Elouafi, A.; Moubah, R.; Tizliouine, A.; Derkaoui, S.; Omari, L.H.; Lassri, H. Effects of Ru doping and of oxygen vacancies on the optical properties in α-Fe2O3 powders. Appl. Phys. A 2020, 126, 228. [Google Scholar] [CrossRef]
- Polyanskiy, M.N. Refractiveindex.info database of optical constants. Sci. Data 2024, 11, 94. [Google Scholar] [CrossRef]
- Wood, D.L.; Nassau, K. Refractive index of cubic zirconia stabilized with yttria. Appl. Opt. 1982, 21, 2978–2981. [Google Scholar] [CrossRef]
- YU, P.; Cardona, M. Fundamentals of Semiconductors: Physics and Materials Properties; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
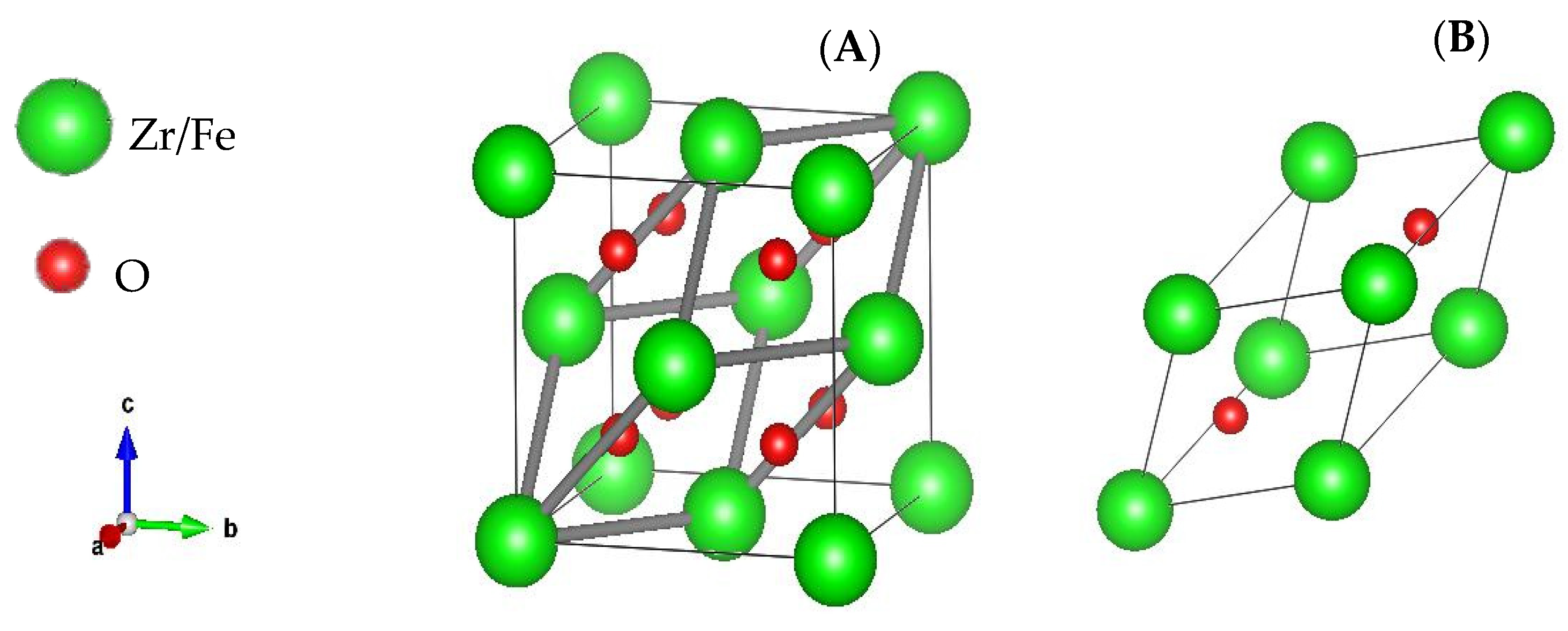
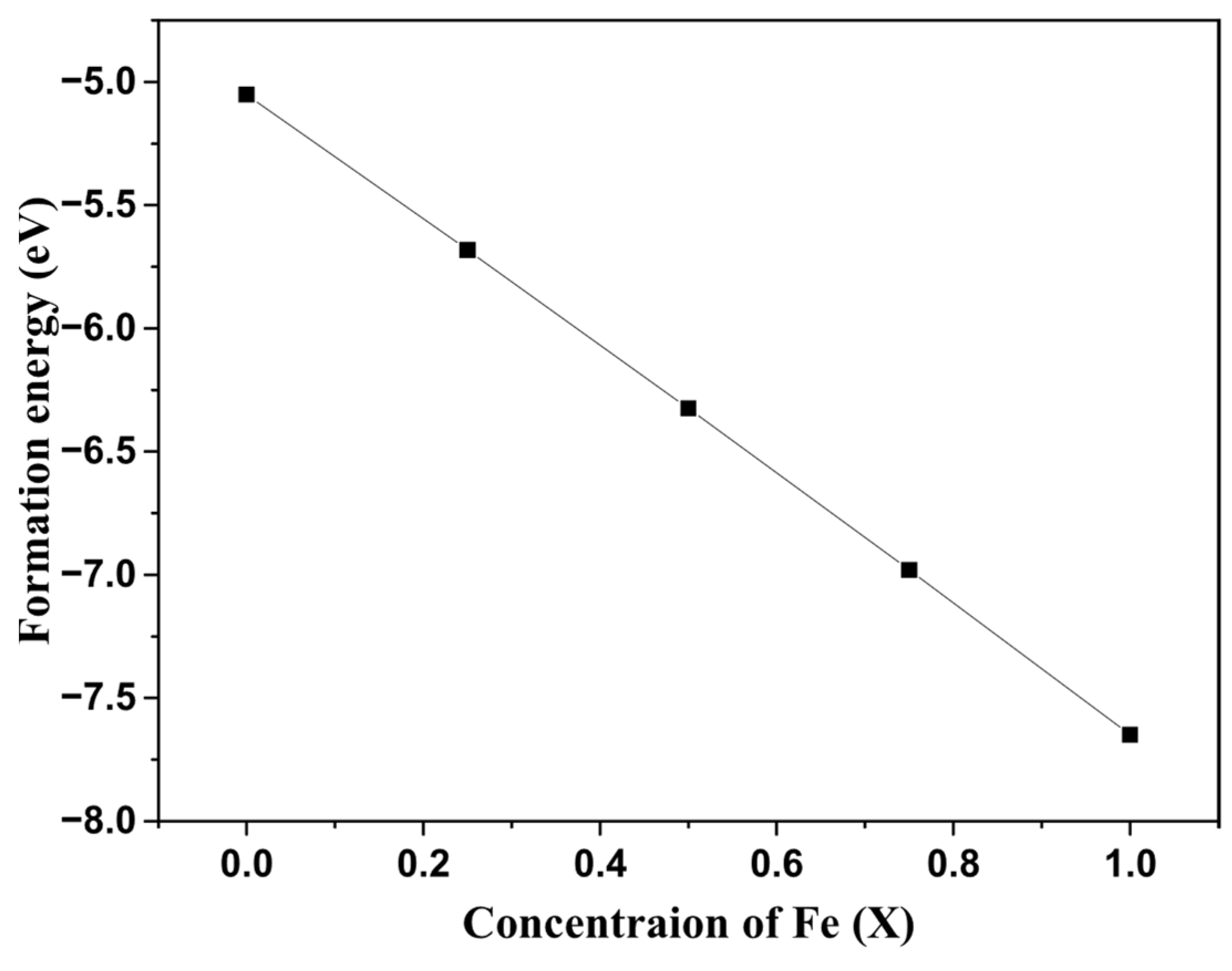



| Lattice Parameter (Å) | Formation Energy (eV) | |
|---|---|---|
| FeO2 | 4.6 | −7.65 |
| Py-FeO2 | 4.55exp [26] 4.36exp [27] 4.36DFT+U [28] | - |
| ZrO2 | 5.12 | −5.06 |
| ZrO2 | 5.07exp [29] 5.09exp [30] 5.11hf [31] 5.15PBE [32] | - |
| x | C11 | C12 | C13 | C22 | C33 | C44 | C66 |
|---|---|---|---|---|---|---|---|
| 0.00 | 520.77 | 93.90 | 93.90 | 520.77 | 520.77 | 63.59 | 63.59 |
| 0.25 | 482.36 | 90.90 | 90.90 | 482.36 | 482.36 | 77.61 | 77.61 |
| 0.50 | 475.86 | 113.55 | 107.22 | 475.86 | 525.97 | 86.89 | 102.08 |
| 0.75 | 519.96 | 103.26 | 103.26 | 519.96 | 519.96 | 81.25 | 81.25 |
| 1.00 | 623.45 | 107.07 | 107.07 | 623.45 | 623.45 | 121.58 | 121.58 |
| X Content | PBE | PBE+U | Other Studies |
|---|---|---|---|
| 0.00 | 3.41 | - | 3.31 1, 3.37 *2 3.86 **2 |
| 0.25 | 0.184 | 0.275 | |
| 0.50 | 0.018 | 0.275 | |
| 0.75 | −0.062 | 0.025 | |
| 1.00 | 0.252 | 0.30 | Metal 3 Metal 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouar, D.; Hamdi, A.; Benghia, A.; Sarhani, M.E. First-Principles Investigation of Structural, Electronic, and Optical Transitions in FexZr1−xO2 Solid Solutions. Appl. Sci. 2025, 15, 10224. https://doi.org/10.3390/app151810224
Nouar D, Hamdi A, Benghia A, Sarhani ME. First-Principles Investigation of Structural, Electronic, and Optical Transitions in FexZr1−xO2 Solid Solutions. Applied Sciences. 2025; 15(18):10224. https://doi.org/10.3390/app151810224
Chicago/Turabian StyleNouar, Djelloul, Ahmed Hamdi, Ali Benghia, and Mohammed ElSaid Sarhani. 2025. "First-Principles Investigation of Structural, Electronic, and Optical Transitions in FexZr1−xO2 Solid Solutions" Applied Sciences 15, no. 18: 10224. https://doi.org/10.3390/app151810224
APA StyleNouar, D., Hamdi, A., Benghia, A., & Sarhani, M. E. (2025). First-Principles Investigation of Structural, Electronic, and Optical Transitions in FexZr1−xO2 Solid Solutions. Applied Sciences, 15(18), 10224. https://doi.org/10.3390/app151810224





