Cymbopogon flexuosus and Eugenol Nanoemulsion: Formulation, Stability, Antimicrobial Efficacy, and In Vitro Safety Assessment
Abstract
1. Introduction
2. Methodology
2.1. Chemicals
2.2. Preparation and Characterization of Nanoemulsion
2.3. NECE Characterization
2.4. Stability Study of Nanoemulsion
2.5. Microbiological Assays
2.5.1. Microorganisms
2.5.2. Minimal Inhibitory Concentration (MIC)
2.5.3. Minimal Bactericide Concentration (MBC)
2.6. Cel Culture
2.6.1. MTT Assay
2.6.2. dsDNA Assay
2.6.3. DCFH-DA Assay
2.7. Statistical Analysis
3. Results and Discussion
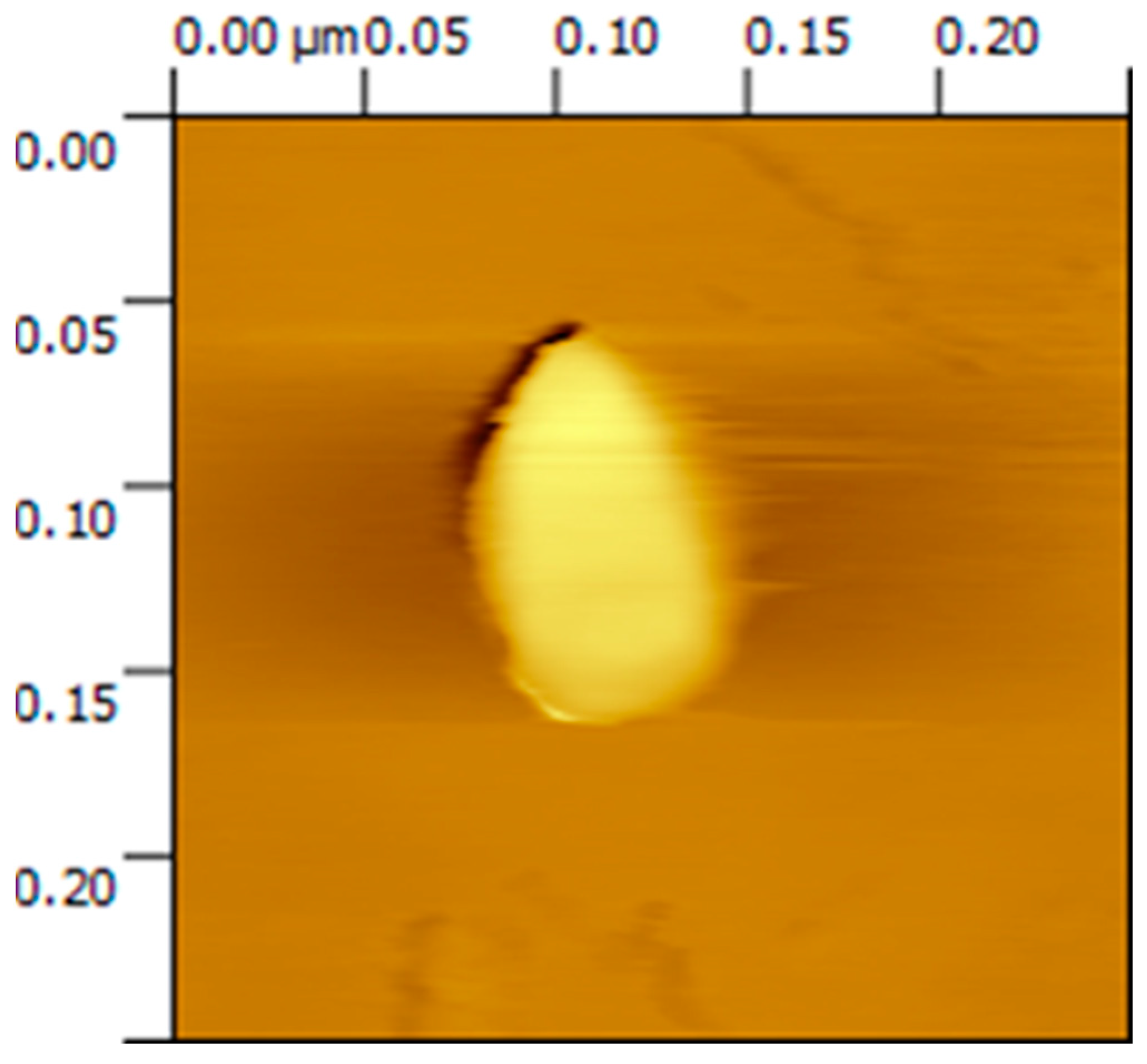
3.1. Atomic Force Microscopy
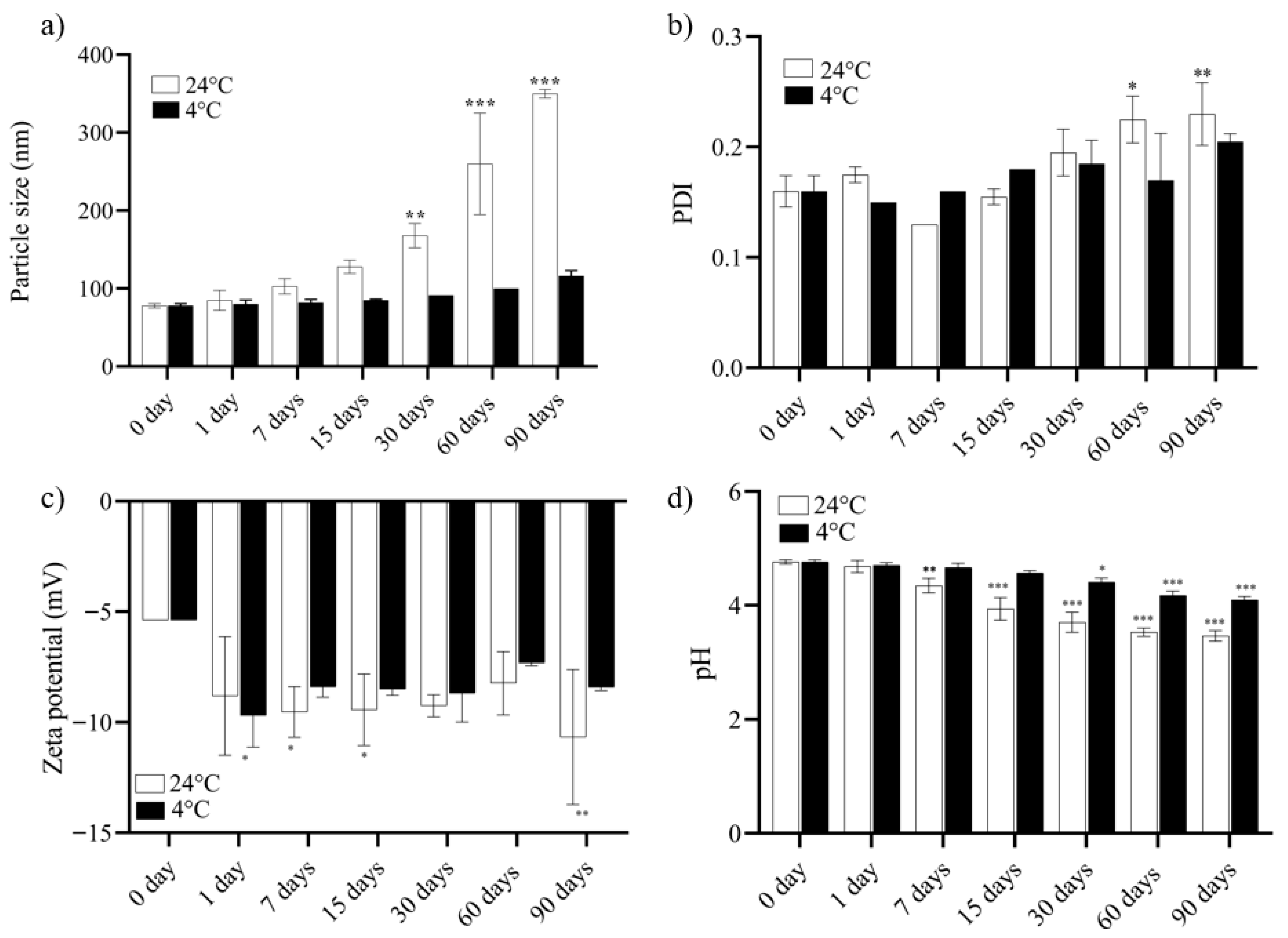
3.2. Determination of Mean Particle Size, Polydispersity Index, Zeta Potential, and pH
3.3. Study of NECE Stability
3.4. Antimicrobial Activity: MIC and MBC
3.5. Biological Assay
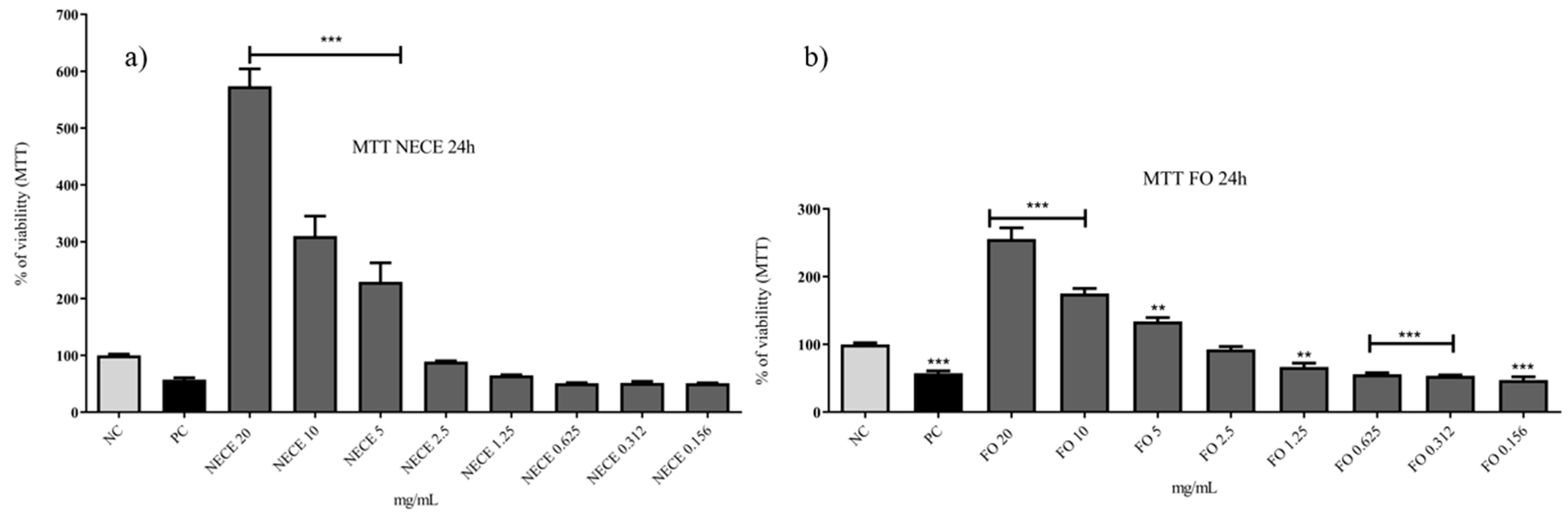
3.5.1. MTT
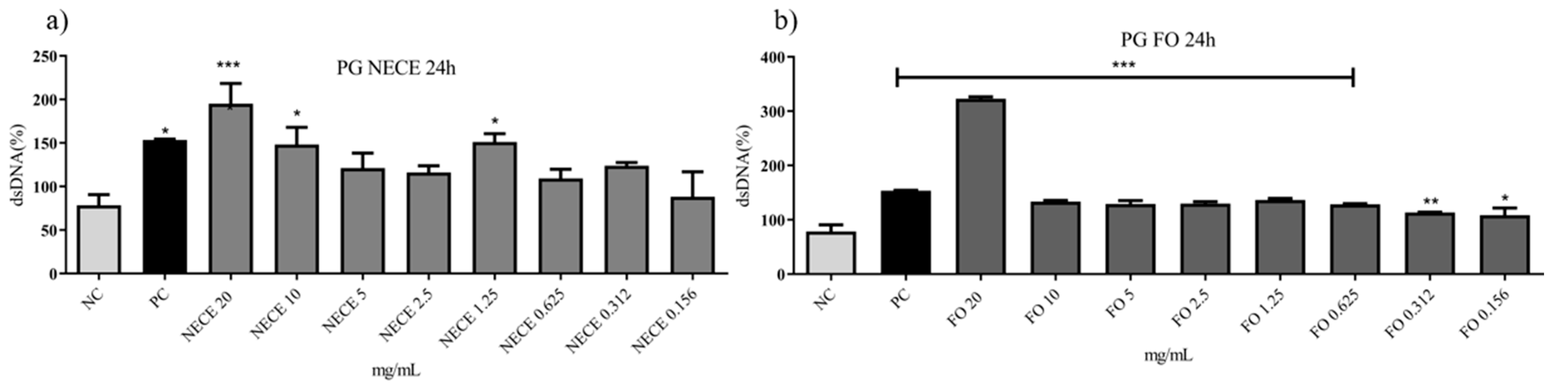
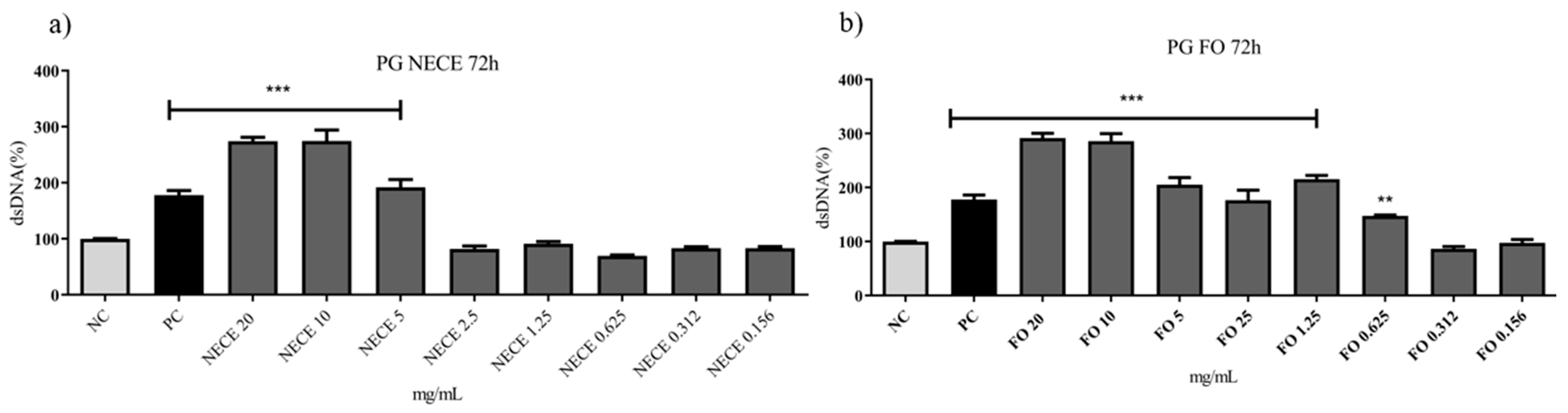
3.5.2. Genotoxicity Assay
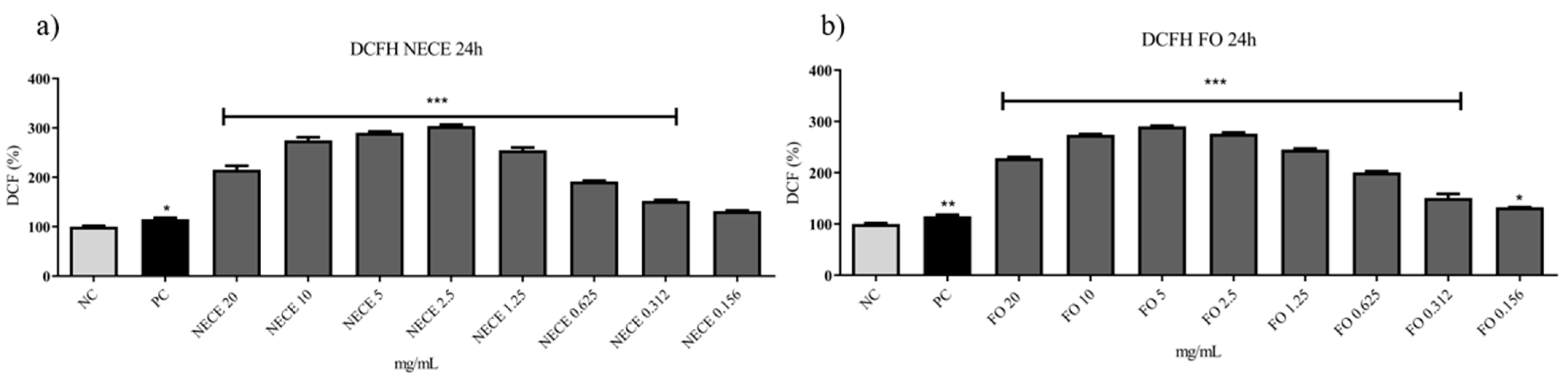
3.5.3. DCFH-DA Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahul, R.; Damodharan, N.; Leela, K.V.; Datchanamoorthy, M.; Gopinathan, A.; Rahul, R.; Damodharan, N.; Leela, K.V.; Datchanamoorthy, M.; Gopinathan, A. Antimicrobial Resistance: A One Health Perspective in India. In Antimicrobial Stewardship—New Insights; Mustafa, G., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/ (accessed on 29 July 2025).[Green Version]
- Sun, J.; Deng, Z.; Yan, A. Bacterial Multidrug Efflux Pumps: Mechanisms, Physiology and Pharmacological Exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef]
- Jibril, A.H.; Bawa, H.; Mohammed, K.; Nuhu, A.; Uhuami, A.O. High Risk of Pseudomonas aeruginosa Infection in Patients Attending Public Hospitals in Sokoto, Nigeria. Microbe 2025, 6, 100271. [Google Scholar] [CrossRef]
- Lapeyre, R.; Rezk, N.; McClean, S.; Moore, A.C. Systematic Mapping Review of Preclinical and Clinical Studies of Staphylococcus aureus Vaccines. Vaccine 2025, 61, 127333. [Google Scholar] [CrossRef] [PubMed]
- Nogrady, B. The Fight against Antimicrobial Resistance. Nature 2023, 624, S30–S32. [Google Scholar] [CrossRef]
- Sosa-Portugal, S.; Dale, L.; Devaney, J.; Sharp, A.; Malalana, F.; Timofte, D. Effectiveness of Steam Cleaning Technology on Reducing the Occurrence of ESKAPE Organisms and Escherichia coli in the Stables of an Equine Referral Hospital. J. Equine Vet. Sci. 2025, 151, 105636. [Google Scholar] [CrossRef]
- Kaur, G.; Panigrahi, C.; Agarwal, S.; Khuntia, A.; Sahoo, M. Recent Trends and Advancements in Nanoemulsions: Production Methods, Functional Properties, Applications in Food Sector, Safety and Toxicological Effects. Food Phys. 2024, 1, 100024. [Google Scholar] [CrossRef]
- Thakur, D.; Rana, P.; Singh, S.K.; Bakshi, M.; Kumar, S.; Singh, S. Nanoemulsion Edible Coating for Shelf-Life Improvement and Quality Control in Perishable Products. Plant Nano Biol. 2024, 10, 100114. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Naebe, M. Lemongrass (Cymbopogon): A Review on Its Structure, Properties, Applications and Recent Developments. Cellulose 2018, 25, 5455–5477. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.A.; Hamid Sadik, N.A.; Shahin, A.M.; Shahin, N.N. Simvastatin and Eugenol Restore Autophagic Flux and Alleviate Oxidative, Inflammatory, and Fibrotic Perturbations in an Arginine-Induced Chronic Pancreatitis Rat Model. Arch. Biochem. Biophys. 2025, 768, 110357. [Google Scholar] [CrossRef]
- Mwithiga, G.; Maina, S.; Muturi, P.; Gitari, J. Lemongrass (Cymbopogon flexuosus) Growth Rate, Essential Oil Yield and Composition as Influenced by Different Soil Conditioners under Two Watering Regimes. Heliyon 2024, 10, e25540. [Google Scholar] [CrossRef] [PubMed]
- Tavvabi-Kashani, N.; Hasanpour, M.; Baradaran Rahimi, V.; Vahdati-Mashhadian, N.; Askari, V.R. Pharmacodynamic, Pharmacokinetic, Toxicity, and Recent Advances in Eugenol’s Potential Benefits against Natural and Chemical Noxious Agents: A Mechanistic Review. Toxicon 2024, 238, 107607. [Google Scholar] [CrossRef]
- Kusuma, I.Y.; Perdana, M.I.; Vágvölgyi, C.; Csupor, D.; Takó, M. Exploring the Clinical Applications of Lemongrass Essential Oil: A Scoping Review. Pharmaceuticals 2024, 17, 159. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Saad, A.M.; Salem, H.M.; Ashry, N.M.; Abo Ghanima, M.M.; Shukry, M.; Swelum, A.A.; Taha, A.E.; El-Tahan, A.M.; et al. Essential Oils and Their Nanoemulsions as Green Alternatives to Antibiotics in Poultry Nutrition: A Comprehensive Review. Poult. Sci. 2022, 101, 101584. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhong, Q. Physical and Antimicrobial Properties of Self-Emulsified Nanoemulsions Containing Three Synergistic Essential Oils. Int. J. Food Microbiol. 2022, 365, 109557. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, N.K.; Srivastava, A.; Kataria, A.; Dubey, S.; Sharma, S.; Kundu, B. Clove and Lemongrass Oil Based Non-Ionic Nanoemulsion for Suppressing the Growth of Plant Pathogenic Fusarium oxysporum f. sp. lycopersici. Ind. Crops Prod. 2018, 123, 353–362. [Google Scholar] [CrossRef]
- da Silva Gündel, S.; de Souza, M.E.; Quatrin, P.M.; Klein, B.; Wagner, R.; Gündel, A.; de Almeida Vaucher, R.; Santos, R.C.V.; Ourique, A.F. Nanoemulsions Containing Cymbopogon Flexuosus Essential Oil: Development, Characterization, Stability Study and Evaluation of Antimicrobial and Antibiofilm Activities. Microb. Pathog. 2018, 118, 268–276. [Google Scholar] [CrossRef] [PubMed]
- De Godoi, S.N.; Quatrin, P.M.I.; Sagrillo, M.R.; Nascimento, K.; Wagner, R.; Klein, B.; Santos, R.C.V.; Ourique, A.F. Evaluation of Stability and In Vitro Security of Nanoemulsions Containing Eucalyptus Globulus Oil. Biomed. Res. Int. 2017, 2017, 2723418. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI supplement M100; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ho, T.M.; Abik, F.; Mikkonen, K.S. An Overview of Nanoemulsion Characterization via Atomic Force Microscopy. Crit. Rev. Food Sci. Nutr. 2022, 62, 4908–4928. [Google Scholar] [CrossRef]
- Prasad, J.; Das, S.; Maurya, A.; Soni, M.; Yadav, A.; Singh, B.; Dwivedy, A.K. Encapsulation of Cymbopogon khasiana × Cymbopogon pendulus Essential Oil (CKP-25) in Chitosan Nanoemulsion as a Green and Novel Strategy for Mitigation of Fungal Association and Aflatoxin B1 Contamination in Food System. Foods 2023, 12, 722. [Google Scholar] [CrossRef]
- Ramadan, M.M.; El Haggar, E.F.; Mohamed, R.S.; Mahmoud, K.F.; Mabrouk, A.M.; Hussien, A.G.; Mahmoud, A.E.; Mohawed, O.A.M.; El-Messery, T.M. Development of a Functional Cake with Probiotics and Micro-Encapsulated Essential Oils: Evaluation of Nutritional Properties, Liver Protection, and Immune Boosting. Heliyon 2024, 10, e22918. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Jam, S.; Greenwood, R.W.; Waters, K.E. An Overview of the Temperature Dependence of the Zeta Potential of Aqueous Suspensions. Results Eng. 2025, 27, 105698. [Google Scholar] [CrossRef]
- da Silva Gündel, S.; Velho, M.C.; Diefenthaler, M.K.; Favarin, F.R.; Copetti, P.M.; de Oliveira Fogaça, A.; Klein, B.; Wagner, R.; Gündel, A.; Sagrillo, M.R.; et al. Basil Oil-Nanoemulsions: Development, Cytotoxicity and Evaluation of Antioxidant and Antimicrobial Potential. J. Drug Deliv. Sci. Technol. 2018, 46, 378–383. [Google Scholar] [CrossRef]
- Tinh, N.Q.; Van Thanh, D.; Van Thu, N.; Quynh Nhung, B.T.; Ngoc Huyen, P.; Phu Hung, N.; Thi Thuy, N.; Dieu Thuy, P.; Hoa Mi, N.; Thi Tam, K. Preparation of Nanoemulsions from Elsholtzia kachinensis and Elsholtzia ciliata Essential Oils via Ultrasonic Homogenization and Their Antibacterial and Anticancer Activities. RSC Adv. 2025, 15, 11243–11256. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Kumar, S. Effect of Surfactant Concentration on Physicochemical and Antibacterial Properties of Eugenol Nanoemulsions. Curr. Indian. Sci. 2023, 2, e2210299X262244. [Google Scholar] [CrossRef]
- Devi, L.S.; Mukherjee, A.; Das, M.R.; Kumar, S. Antimicrobial Efficacy of Eugenol and Neem Oil Nanoemulsions Against Foodborne Pathogens and Food Spoilage Fungi. Food Bioeng. 2025, 4, 101–112. [Google Scholar] [CrossRef]
- Putra, P.S.U.; Adhika, D.R.; Genecya, G.; Al Madanie, M.S.; Asri, L.A.T.W. Evaluation of Chitosan-Encapsulated Lemongrass (Cymbopogon citratus) Essential Oil Nanoemulsion for Fruit Edible Coating. OpenNano 2025, 24, 100246. [Google Scholar] [CrossRef]
- Mohd Daud, I.S.; Mahmud Ab Rashid, N.K.; Palmer, J.; Flint, S. Characterization, Antibacterial Activity, and Stability of Supercritical Fluid Extracted Lemongrass Nanoemulsion on Bacillus cereus. Food Biosci. 2025, 68, 106526. [Google Scholar] [CrossRef]
- Shabani, P.; Davati, N.; Emamifar, A. Effect of Zataria multiflora Boiss L. Essential Oil and Its Nanoemulsion on Microbial Growth, Physicochemical and Organoleptic Properties of Doogh during Refrigerated Storage. Appl. Food Res. 2025, 5, 101003. [Google Scholar] [CrossRef]
- Showkat, S.; Anjum, N.; Ayaz, Q.; Mustafa, S.; Malik, A.R.; Beigh, M.A.; Banday, N.; Gulzar, B.; Wani, S.M. Enhancing Shelflife of Plum Fruit by Chitosan-Based Nanoemulsion Coating Incorporated with Ginger Essential Oil. Appl. Food Res. 2025, 5, 100768. [Google Scholar] [CrossRef]
- Sobhy, M.; Abdelkarim, E.A.; Hussein, M.A.; Aziz, T.; Al-Asmari, F.; Alabbosh, K.F.; Cui, H.; Lin, L. Essential Oils as Antibacterials against Multidrug-Resistant Foodborne Pathogens: Mechanisms, Recent Advances, and Legal Considerations. Food Biosci. 2025, 64, 105937. [Google Scholar] [CrossRef]
- Hassan, M.; Alijaniha, M.; Jafari, S.; Ghafari, A. Physicochemical Properties and Antimicrobial Efficacy of Eugenol Nanoemulsion Formed by Spontaneous Emulsification. Chem. Pap. 2025, 79, 1155–1163. [Google Scholar] [CrossRef]
- Nunes, F.B.; da Silva Bruckmann, F.; Viana, A.R.; da Rosa Salles, T.; Zancanaro, L.V.; Rhoden, D.S.B.; Franco, C.; Schuch, A.P.; Dotto, G.L.; Silva, L.F.O.; et al. Removal of Selective Serotonin Reuptake Inhibitor Using Magnetic Graphene Oxide Derivatives: Adsorption Study in Low Drug Concentration Using HPLC Quantification, in Vitro Safety, and Phytotoxicity. J. Environ. Chem. Eng. 2024, 12, 112336. [Google Scholar] [CrossRef]
- Cometta, S.; Hutmacher, D.W. Assessing the Biocompatibility of Tannic Acid-Based Biomaterials: Addressing Challenges in Standard Cytotoxic Assays. Bioengineering 2025, 12, 660. [Google Scholar] [CrossRef]
- Koba, M.; Szostek, A.; Konopa, J. Limitation of Usage of PicoGreen Dye in Quantitative Assays of Double-Stranded DNA in the Presence of Intercalating Compounds. Acta Biochem. Polim. 2007, 54, 883–886. [Google Scholar] [CrossRef]
- Ban, E.; Kim, A. PicoGreen Assay for Nucleic Acid Quantification—Applications, Challenges, and Solutions. Anal. Biochem. 2024, 692, 115577. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive Oxygen Species as Intracellular Messengers During Cell Growth and Differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Diebold, L.; Chandel, N.S. Mitochondrial ROS Regulation of Proliferating Cells. Free Radic. Biol. Med. 2016, 100, 86–93. [Google Scholar] [CrossRef]
- Meng, D.; Shi, X.; Jiang, B.H.; Fang, J. Insulin-like Growth Factor-I (IGF-I) Induces Epidermal Growth Factor Receptor Transactivation and Cell Proliferation through Reactive Oxygen Species. Free Radic. Biol. Med. 2007, 42, 1651–1660. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The Initiation of Oxidative Stress and Therapeutic Strategies in Wound Healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]



| NECE Characterization | |||
|---|---|---|---|
| Zeta Potential (Mv) | PDI | Mean Particle Size (nm) | pH |
| −5.38 ± 0 | 0.16 ± 0.014 | 76.33 ± 0.014 | 4.76 ± 0.035 |
| Antimicrobial Activity of NECE Against P. aeruginosa | ||||
|---|---|---|---|---|
| MIC NECE mg/mL | MBC NECE mg/mL | MIC FO mg/mL | MBC FO mg/mL | |
| P. aeruginosa Pa01 | 2.5 | 2.5 | 1.25 | 2.5 |
| S. aureus ATCC 29213 | 0.625 | 0.625 | 0.625 | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, F.B.; Barin, R.; Silveira, L.d.S.; Sagrillo, M.R.; Zancanaro, L.V.; Novais, V.F.B.; Ourique, A.F.; Gündel, A.; Bohn Rhoden, C.R.; Santos, R.C.V. Cymbopogon flexuosus and Eugenol Nanoemulsion: Formulation, Stability, Antimicrobial Efficacy, and In Vitro Safety Assessment. Appl. Sci. 2025, 15, 10214. https://doi.org/10.3390/app151810214
Nunes FB, Barin R, Silveira LdS, Sagrillo MR, Zancanaro LV, Novais VFB, Ourique AF, Gündel A, Bohn Rhoden CR, Santos RCV. Cymbopogon flexuosus and Eugenol Nanoemulsion: Formulation, Stability, Antimicrobial Efficacy, and In Vitro Safety Assessment. Applied Sciences. 2025; 15(18):10214. https://doi.org/10.3390/app151810214
Chicago/Turabian StyleNunes, Franciane Batista, Ruth Barin, Larissa da Silva Silveira, Michele Rorato Sagrillo, Leonardo Vidal Zancanaro, Vitória Fernanda Belmonte Novais, Aline Ferreira Ourique, André Gündel, Cristiano Rodrigo Bohn Rhoden, and Roberto Christ Vianna Santos. 2025. "Cymbopogon flexuosus and Eugenol Nanoemulsion: Formulation, Stability, Antimicrobial Efficacy, and In Vitro Safety Assessment" Applied Sciences 15, no. 18: 10214. https://doi.org/10.3390/app151810214
APA StyleNunes, F. B., Barin, R., Silveira, L. d. S., Sagrillo, M. R., Zancanaro, L. V., Novais, V. F. B., Ourique, A. F., Gündel, A., Bohn Rhoden, C. R., & Santos, R. C. V. (2025). Cymbopogon flexuosus and Eugenol Nanoemulsion: Formulation, Stability, Antimicrobial Efficacy, and In Vitro Safety Assessment. Applied Sciences, 15(18), 10214. https://doi.org/10.3390/app151810214








