Abstract
Dental caries remains one of the most prevalent global diseases, and the early detection of occlusal lesions is critical because demineralization often begins deep within pits and fissures where conventional visual–tactile or radiographic inspection cannot detect it. SmarTooth, a newly introduced fluorescence device that irradiates enamel with a 655 nm laser and records the reflected intensity, may provide more objective, quantitative diagnoses. This study assessed its diagnostic performance against the International Caries Detection and Assessment System (ICDAS). We examined 1421 occlusal surfaces from 153 adults, scored each surface with ICDAS codes 0–4, and recorded SmarTooth peak values. Spearman’s rank correlation was used to test the association between codes and peak values; one-way ANOVA with Tukey’s post hoc was used to compare mean values across codes; and sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC) were calculated at three diagnostic thresholds: D1 (0 vs. 1–4), D2 (0–2 vs. 3–4), and D3 (0–3 vs. 4). The SmarTooth values rose with lesion severity and correlated moderately with ICDAS (r = 0.495, p < 0.001). The AUROC ranged from 0.69 to 0.82, with the best accuracy observed at D2 (cut-off: 7.0; AUC: 0.82; sensitivity: 78.3%; specificity: 77.4%). These findings suggest that SmarTooth can complement ICDAS scoring as an adjunctive tool, potentially enhancing diagnostic accuracy and supporting early intervention for occlusal caries in general dental practice.
Keywords:
dental caries; early diagnosis; laser; fluorescence; ICDAS II; permanent teeth; clinical study 1. Introduction
Dental caries is a multifactorial, biofilm-related disease characterized by the progressive demineralization of dental hard tissues due to acid production from bacterial fermentation of dietary carbohydrates [1]. The development and progression of dental caries are influenced by a multifactorial interplay of host-related factors including saliva composition and tooth morphology, microbiological factors such as the presence of acidogenic and aciduric bacteria, behavioral and lifestyle factors including inadequate oral hygiene and frequent intake of fermentable carbohydrates, and socioenvironmental determinants such as socioeconomic status, limited access to dental care, and low educational attainment [2]. Dental caries remains one of the most prevalent and burdensome chronic diseases globally, affecting over 3.09 billion people according to recent estimates from the Global Burden of Disease Study [3]. The prevalence of occlusal caries has been reported to range from 52.7% to 66.3%, primarily due to the unique anatomical characteristics of pits and fissures that promote plaque retention and hinder natural self-cleansing [4]. Similar patterns have also been observed in primary dentition, where meta-analyses indicate that the occlusal surfaces of mandibular molars are among the most frequently affected sites in preschool children, highlighting the universal vulnerability of these surfaces across age groups [5]. These microenvironments are particularly susceptible to early-stage demineralization, often progressing unnoticed without overt clinical symptoms until cavitation occurs [6]. The clinical implications of undetected occlusal caries are substantial, including pulpitis, pain, and eventual tooth loss, thereby affecting individuals’ oral function and quality of life [1]. The early detection of caries lesions, especially at the non-cavitated stage, is critical to enable minimally invasive treatment and long-term preservation of tooth structure.
Despite their widespread use in dental practice, conventional diagnostic approaches such as visual–tactile inspection and radiographic imaging present several limitations in identifying early occlusal caries. Visual–tactile inspection relies on examiner expertise and can be subjective, leading to poor inter-examiner agreement, particularly for subtle enamel changes that are not readily visible [7]. Although radiographic detection such as bitewing imaging offer some utility for detecting proximal caries, they are generally insensitive to early occlusal demineralization due to overlapping anatomical structures and low radiolucency in incipient caries [8]. Additionally, radiographs do not provide real-time feedback or quantitative measurements, making them less suited for monitoring early caries over time. These challenges underscore the need for adjunctive diagnostic tools that are both objective and capable of detecting caries at an early, reversible stage.
To address these limitations, a variety of optical caries lesion detection technologies have been developed over the past two decades, with fluorescence-based methods emerging as some of the most promising approaches [9,10]. Efforts to mitigate caries progression have primarily focused on the development of antimicrobial and anti-biofilm materials, such as chlorhexidine-releasing or nano-silver-fluoride-incorporated orthodontic elastomerics, which have demonstrated significant efficacy against cariogenic biofilms [11,12,13]. Nonetheless, early detection remains a critical component of preventive strategies, particularly in patients undergoing orthodontic treatment or in populations at higher risk of caries. Fluorescence devices operate by detecting changes in tooth fluorescence properties resulting from the presence of chromophores, bacterial metabolites, or porosity within demineralized enamel and dentin [14]. One of the most widely known devices, DIAGNOdent (KaVo, Biberach, Germany), uses a 655 nm diode laser to quantify fluorescence intensity, offering a numeric scale from 0 to 99 as an indicator of caries presence and severity. Recent studies have incorporated fluorescence- and light-based diagnostic devices such as Diagnodent and Diagnocam (KaVo, Biberach, Germany) in conjunction with ICDAS to evaluate demineralization and remineralization processes. For example, Scribante et al. demonstrated that combining visual ICDAS assessment with these adjunctive tools enhanced the evaluation of enamel lesion changes following the application of remineralizing agents [15]. While such studies primarily focused on therapeutic outcomes, their methodologies underscore the growing utility of optical adjuncts in caries detection and monitoring. However, DIAGNOdent has limitations, including variability across users and tooth surfaces, difficulty in achieving consistent probe angulation, and susceptibility to false-positive signals caused by exogenous chromophores unrelated to caries depth. Furthermore, the absence of a standardized classification chart for occlusal surfaces in the DIAGNOcam system limits its utility for quantitative analysis. In response to these issues, SmarTooth (SmarTooth Inc., Seoul, Republic of Korea) was developed as a next-generation fluorescence device specifically optimized for occlusal surface detection. The device uses the same 655 nm laser principle but incorporates an ergonomically enhanced probe tip designed to improve contact with occlusal pits and fissures and ensure more consistent signal acquisition [16]. These improvements position SmarTooth as a potentially valuable tool for enhancing diagnostic accuracy and supporting evidence-based decision-making in dental practice.
Although fluorescence-based caries detection technologies have demonstrated promising diagnostic capabilities in laboratory and controlled settings, their performance in clinical environments remains insufficiently characterized [17]. Variability in diagnostic accuracy has been observed and depends on multiple factors, including lesion severity, tooth surface, and examiner technique [18]. While previous studies have reported moderate correlations between fluorescence intensity values and caries progression, many of these studies were limited by methodological constraints. In particular, a substantial proportion relied on in vitro models or only examined a narrow spectrum of lesion types, thereby limiting their clinical applicability [19]. Moreover, heterogeneity in measurement protocols, such as surface preparation, drying time, probe angulation, and calibration procedures, further complicates the comparability of findings across studies and devices [20]. Despite the increasing clinical interest in such technologies, there is currently a paucity of well-designed in vivo studies assessing the diagnostic validity of SmarTooth across different degrees of caries severity as classified by the International Caries Detection and Assessment System (ICDAS). To ensure reliable integration of SmarTooth into everyday dental practice, clinical validation under standardized conditions using recognized diagnostic frameworks such as ICDAS is essential. Such efforts are critical not only for confirming its diagnostic accuracy but also for supporting evidence-based implementation of fluorescence devices in preventive and operative dentistry.
The integration of objective, device-based diagnostics such as SmarTooth into routine caries assessment protocols holds significant promise for improving early detection, reducing clinician’s bias, and enhancing treatment planning [21]. In particular, in general dental practice where variability in clinical experience and training may influence diagnostic decisions, tools that provide quantifiable, reproducible data can play a valuable role in supporting evidence-based care. Furthermore, the use of SmarTooth may facilitate patient education and motivation by transforming abstract clinical observations into visible, trackable data points [22]. This not only strengthens communication between the clinician and patient, but may also encourage better oral hygiene compliance and preventive behaviors. From a public health perspective, improved early detection of occlusal caries could contribute to reducing treatment costs and preserving natural dentition, especially in populations at high risk of dental disease.
To objectively evaluate the diagnostic accuracy of such emerging devices, a validated reference standard is essential. ICDAS provides a comprehensive visual scoring method that classifies caries lesions, ranging from sound enamel (code 0) to extensive cavitation (code 6), based on observable changes in enamel color and breakdown [23]. Its hierarchical coding system has been widely adopted in both clinical research and epidemiological studies due to its demonstrated correlation with histological depth and strong inter-examiner reliability when appropriately calibrated [24]. However, certain ICDAS codes, particularly code 1, which identifies early enamel opacity visible only after air-drying, are susceptible to examiner variability and may require adjunctive methods to increase diagnostic confidence [25]. In this context, fluorescence-based devices such as SmarTooth have been introduced to offer objective and quantifiable caries detection and may support earlier intervention and monitoring consistency in comparison to conventional methods.
Therefore, this study aimed to evaluate the diagnostic performance of the SmarTooth fluorescence device for detecting occlusal caries in permanent teeth using ICDAS codes as the reference standard. Specifically, we examined SmarTooth’s ability to discriminate between different ICDAS-defined caries stages using three thresholds: D1 (ICDAS 0 vs. 1–4), D2 (0–2 vs. 3–4), and D3 (0–3 vs. 4). Diagnostic accuracy was assessed through sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC). By comparing SmarTooth peak values to ICDAS codes on a per-surface basis, this study sought to provide clinically relevant evidence for the use of SmarTooth as an adjunctive diagnostic tool in general dental practice.
2. Materials and Methods
2.1. Study Subjects
This prospective clinical study strictly adhered to the ethical principles of the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of Catholic Kwandong University (IRB No. 2502-0404). Based on an AUROC-based sample size estimation (θ = 0.85, P = 0.40, W = 0.05, CL = 0.95), a minimum of 1072 occlusal surfaces was required to ensure precise estimation of diagnostic accuracy. To accommodate potential exclusions, including the assumption that up to half of occlusal surfaces may be unsuitable for evaluation, as well as the skewed distribution of ICDAS codes and the need to maintain robust statistical power for all planned analyses, we ultimately included 157 subjects [26]. Prior to participation, all eligible individuals received a detailed explanation of the study objectives, procedures, potential risks, and benefits, and provided written informed consent. The participants were healthy adults aged 18 years or older, with no history of systemic diseases or medications that could affect oral conditions. The unit of analysis was the individual tooth; therefore, teeth with enamel hypoplasia or a history of restorative treatment on the occlusal surface were excluded. Additionally, teeth with extensive cavitation (ICDAS codes 5 and 6), severe discoloration, or structural anomalies were excluded to ensure accurate assessment of non-cavitated caries. Before examination, all the participants underwent professional prophylaxis to remove plaque, debris, and extrinsic stains that could interfere with optical measurements. A total of 157 subjects were initially recruited, but 4 were excluded due to incomplete visualization of the occlusal surface. The final sample included 1421 occlusal surfaces from 153 individuals (Figure 1).
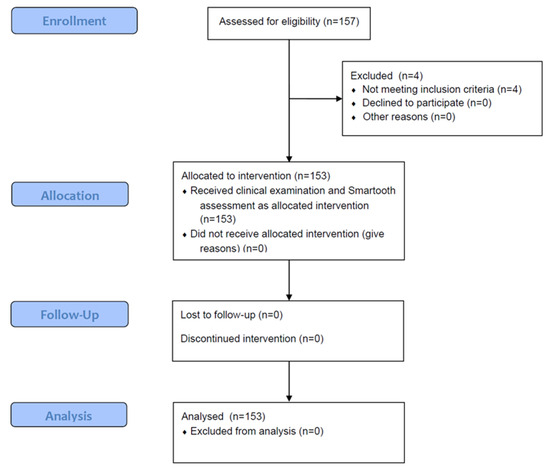
Figure 1.
Study design and participant selection in alignment with CONSORT 2010.
2.2. Clinical Examination Protocol
All clinical examinations were performed in a standard dental unit under consistent ambient lighting conditions. Occlusal surfaces were initially inspected visually to ensure the absence of biofilm, debris, or calculus. Any residual contaminants were gently removed using gauze or an air–water syringe. Each surface was then air-dried for at least 5 s to standardize moisture conditions prior to both ICDAS scoring and fluorescence-based assessment. All procedures were conducted by a trained examiner using a consistent, standardized protocol across all subjects.
2.3. Visual Examination Using ICDAS
Visual caries assessments were performed by a single calibrated examiner who had completed the official ICDAS e-learning calibration program and had prior clinical experience with ICDAS-based scoring. The ICDAS criteria were applied to each occlusal surface, with scores ranging from code 0 (sound enamel) to code 4 (underlying dark shadow with or without localized enamel breakdown). Teeth with ICDAS codes 5 and 6, indicating extensive cavitated lesions, were excluded from the analysis. Prior to inspection, each occlusal surface was dried with air for 5 s to enhance the visibility of early enamel opacities, especially those corresponding to codes 1 and 2. All ICDAS scores were recorded immediately following inspection and documented electronically (Figure 2).
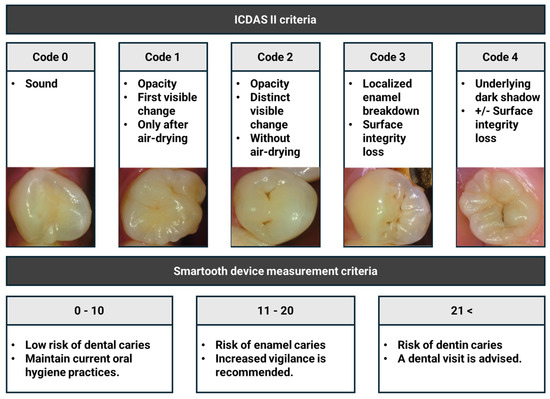
Figure 2.
ICDAS and SmarTooth caries diagnosis criteria used in this study.
2.4. Fluorescence Assessment Using SmarTooth
Fluorescence-based caries detection was conducted using the SmarTooth device, which emits a 655 nm diode laser and features an ergonomically designed occlusal probe tip. The device was calibrated prior to each examination session according to the manufacturer’s instructions. For each occlusal surface, the probe was held as perpendicular as possible to the surface, maintaining direct enamel contact. The examiner gently rotated the probe within the fissure area to detect fluorescence signals from multiple angles, thereby minimizing measurement variability due to angulation artifacts. Multiple scans were performed when necessary, and the highest fluorescence peak value was automatically recorded by the device for analysis. Based on the manufacturer’s guidelines, fluorescence values were categorized as follows: 0–10 indicates a low risk, 11–20 indicates a moderate risk, and ≥21 indicates a high risk. These peak values were then used for correlation with ICDAS codes and for subgroup analysis (Figure 2).
2.5. Statistical Analysis
All statistical analyses were performed using SPSS software version 26.0 (SPSS Inc., Chicago, IL, USA) Cross-tabulations were constructed to explore categorical associations between SmarTooth caries diagnosis criteria and ICDAS codes. The relationship between SmarTooth caries diagnosis criteria and ICDAS codes was assessed using Spearman’s rank correlation coefficient, which was chosen due to the ordinal nature of the ICDAS scale and the non-parametric distribution of fluorescence data. Differences in mean SmarTooth peak values across ICDAS code groups were assessed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for pairwise comparisons. Diagnostic validity was evaluated at three thresholds frequently used in caries detection research: D1 (ICDAS code 0 vs. codes 1–4), D2 (codes 0–2 vs. 3–4), and D3 (codes 0–3 vs. 4). For each threshold, sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC) were calculated.
3. Results
3.1. Correlation Between ICDAS and SmarTooth Caries Diagnostic Criteria
To assess the association between caries severity identified by visual examination and fluorescence intensity, we conducted a cross-tabulation analysis comparing ICDAS codes with the SmarTooth diagnostic categories (low: 0–10; moderate: 11–20; high: ≥21), as defined by the manufacturer. The results showed a clear tendency for higher fluorescence categories to align with higher ICDAS codes. Additionally, Spearman’s rank correlation analysis was performed using the full dataset of 1421 occlusal surfaces, revealing a moderate but statistically significant positive correlation (ρ = 0.495, p < 0.001) between SmarTooth peak values and ICDAS codes (Table 1).

Table 1.
Correlation between ICDAS codes and SmarTooth caries risk categories.
3.2. Comparative Analysis of SmarTooth Peak Values Across ICDAS Code Groups
SmarTooth peak values exhibited a consistent upward trend corresponding to increasing ICDAS codes, reflecting a statistically significant association with lesion severity. The mean peak fluorescence value for sound enamel surfaces (ICDAS code 0) was 3.9, which rose markedly to 20.8 for ICDAS code 4, which is indicative of dentin involvement, representing over a 5.3-fold increase across the caries progression spectrum. Post hoc comparisons using Tukey’s test further validated that the differences in mean peak values between most adjacent ICDAS codes (0 vs. 1, 1 vs. 2, and 2 vs. 3) were statistically significant (p < 0.05), although no significant difference was observed between codes 3 and 4 (p > 0.05), suggesting potential overlap in fluorescence values at more advanced stages (Table 2).

Table 2.
SmarTooth peak value in each ICDAS code.
3.3. Sensitivity, Specificity, and AUROC According to ICDAS Thresholds
To assess the diagnostic utility of SmarTooth in clinical settings, performance metrics including sensitivity, specificity, and the area under the receiver operating characteristic curve (AUROC) were calculated across three ICDAS-defined thresholds. For the D1 threshold (code 0 vs. codes 1–4), SmarTooth demonstrated a sensitivity of 71.1%, specificity of 67.4%, and AUROC of 0.76, indicating a good level of diagnostic accuracy (Figure 3A). At the D2 threshold (codes 0–2 vs. codes 3–4), the diagnostic performance improved, with the sensitivity reaching 78.3%, the specificity reaching 77.4%, and the AUROC reaching 0.82, suggesting very good discriminative ability (Figure 3A). In contrast, the D3 threshold (codes 0–3 vs. code 4) yielded a lower sensitivity (60.9%) and specificity (63.5%), with an AUROC of 0.69, reflecting moderate but clinically acceptable accuracy (Figure 4).
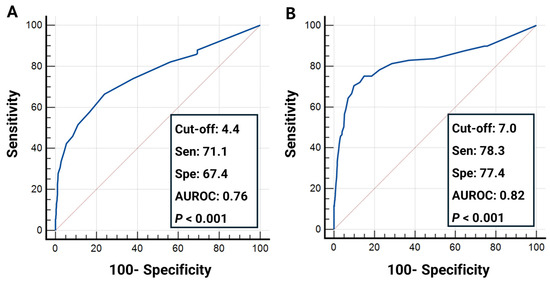
Figure 3.
Receiver operating characteristic (ROC) curves depicting the diagnostic performance of SmarTooth across three ICDAS classification thresholds: (A) code 0 vs. codes 1–4 (D1 threshold); (B) codes 0–2 vs. codes 3–4 (D2 threshold).
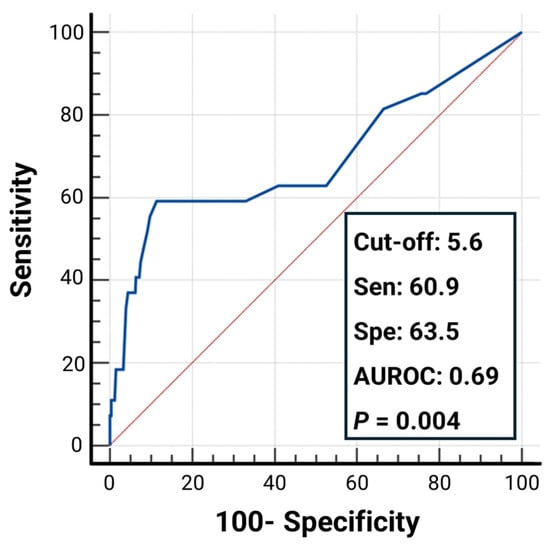
Figure 4.
Receiver operating characteristic (ROC) curve illustrating the diagnostic performance of the SmarTooth device for distinguishing between ICDAS codes 0–3 vs. code 4 (D3 threshold).
4. Discussion
This study aimed to evaluate the diagnostic accuracy of the SmarTooth fluorescence-based device in detecting occlusal caries in vivo using the ICDAS as the clinical reference standard. The findings revealed a statistically significant moderate positive correlation (ρ = 0.495, p < 0.001) between ICDAS codes and SmarTooth caries diagnosis criteria, indicating partial but meaningful agreement that supports SmarTooth’s role in assisting the differentiation of occlusal caries severity across a clinically relevant spectrum. A distinct pattern was observed wherein higher ICDAS codes were consistently associated with increased SmarTooth fluorescence peak values, thereby reinforcing the convergent validity between visual examination and fluorescence-based assessment.
The discrepancies observed between SmarTooth and ICDAS stem fundamentally from the differences in underlying principles and mechanisms of each diagnostic method. ICDAS is based on the visual inspection of the surface texture, translucency, and discoloration, and requires air-drying and trained clinical judgment. In addition to its subjectivity, the ICDAS system presents several structural limitations that may affect diagnostic consistency and efficiency [27]. Accurate application of ICDAS requires thorough air-drying of the tooth surface for at least 5 s to reveal subtle changes in enamel translucency or opacity, a step that may be inconsistently performed in busy clinical settings. Moreover, precise differentiation between codes, especially for early caries lesions such as codes 1 and 2, depends on examiner training and calibration, which can vary across institutions and among clinicians [28]. These procedural demands introduce variability and limit the scalability of ICDAS-based screening in large-scale or resource-constrained environments. Consequently, a sole reliance on ICDAS may compromise diagnostic reliability in real-world contexts unless supported by adjunctive tools or standardized training protocols. While widely adopted and validated, ICDAS scoring is inherently subjective and may vary between examiners depending on their training and experience. Conversely, SmarTooth utilizes 655 nm laser-induced fluorescence to detect metabolic by-products of cariogenic bacteria or changes in enamel porosity, generating a numerical output that is designed to support objective and reproducible measurement. This quantitative nature may help reduce inter-examiner variability and facilitate more consistent monitoring over time, particularly when used with standardized protocols. However, SmarTooth peak values may be affected by confounding factors such as residual biofilms, extrinsic stains, or restorative materials present on the tooth surface [29]. Although professional prophylaxis was performed prior to examination in this study to minimize such interference, these factors can still lead to false-positive readings or the attenuation of fluorescence signals, potentially reducing the diagnostic accuracy. For example, extrinsic stains and resin-based restorations may generate fluorescence unrelated to caries activity, thereby complicating the interpretation [30]. These considerations highlight the need for careful surface preparation, standardized protocols, and the complementary use of visual scoring systems such as ICDAS to enhance the diagnostic reliability. Future research should further investigate the extent to which these clinical variables influence fluorescence-based detection in everyday dental practice.
Moreover, SmarTooth’s diagnostic performance varied according to the ICDAS thresholds applied. Using ICDAS as the clinical reference standard, SmarTooth demonstrated very good diagnostic performance (AUROC = 0.82) in distinguishing non-cavitated enamel caries (ICDAS codes 0–2) from cavitated caries with substantive tissue loss (ICDAS codes 3–4), according to established AUROC interpretive criteria. This superior performance likely reflects the pronounced changes in enamel microstructure and the increased accumulation of organic matter and metabolic byproducts at active cavitated sites, which produce a more distinct fluorescence differential [29]. Conversely, dentin caries (ICDAS code 4) often lie deep beneath an intact or partially intact enamel surface; in these cases, the laser light must penetrate the enamel, scatter within subsurface tissues, and then transmit the reflected fluorescence back to the probe, a process susceptible to signal attenuation and scattering [31]. Additionally, dentin caries may exhibit overt discoloration or surface collapse detectable by visual inspection [32], a feature which SmarTooth may be less sensitive to. These inherent differences in sensitivity to superficial versus subsurface changes explain the variability in SmarTooth’s caries detection performance across different ICDAS thresholds.
Previous research evaluating the diagnostic performance of DIAGNOdent, which uses the same fluorescence-based detection principle as SmarTooth, reported an AUROC of 0.372, sensitivity of 61%, and specificity of 51% at the D1 threshold, indicating a relatively lower accuracy for early caries detection compared to SmarTooth [33]. These discrepancies likely arise from the different evaluation criteria employed in the two studies. For example, the DIAGNOdent study included 44 participants with 230 occlusal surfaces and focused exclusively on incipient lesions within ICDAS codes 0–3. Although two examiners were trained and calibrated to ensure high inter-examiner reliability, the relatively small sample size and restricted lesion range may have limited the generalizability of the findings. Because sound surfaces (ICDAS code 0) were excluded, that study could not evaluate how effectively the device distinguishes healthy enamel from carious tissue. In contrast, our study aimed to more closely replicate clinical conditions by including a wider spectrum of caries stages (sound surfaces (ICDAS code 0) through ICDAS code 4) and analyzing the performance across three thresholds (0/1–4, 0–2/3–4, and 0–3/4) [34]. Additionally, our study incorporated a much larger and more diverse sample (153 participants and 1421 occlusal surfaces) with strict exclusion criteria to minimize confounding factors such as restorations, hypoplasia, or extensive cavitation, thereby enhancing the robustness of the results. While our design relied on a single calibrated examiner—ensuring internal consistency but precluding inter-examiner assessment—this methodological choice distinguishes it from prior DIAGNOdent research and should be considered when interpreting the diagnostic performance. The results demonstrate the device’s clinical utility, especially in differentiating early from advanced lesions, which is critical for preventive intervention. Significant mean differences were observed between ICDAS codes 0 and 1, 1 and 2, and 2 and 3 (p < 0.05 for all), indicating that SmarTooth is capable of distinguishing early-stage non-cavitated caries based on fluorescence intensity. However, the difference between ICDAS codes 3 and 4 was not statistically significant (p > 0.05), possibly reflecting the fluorescence signal saturation effect at later stages of caries progression or overlapping optical characteristics in more advanced caries. Furthermore, examiner techniques may also influence diagnostic outcomes: both SmarTooth and DIAGNOdent require rotation of the probe to record the maximum peak value [35], and examiner proficiency in maintaining consistent probe angulation and contact pressure can introduce variability in measured peak values, a consideration that is important for the clinical use of both devices. To date, only one in vitro study has directly compared SmarTooth with other fluorescence-based devices using micro-computed tomography (micro-CT) as the gold standard [21]. That study reported that SmarTooth achieved the highest overall diagnostic accuracy at both enamel and dentin thresholds, showing a stronger correlation with micro-CT than either DIAGNOdent or ICDAS II. While these results reinforce the potential advantages of SmarTooth under controlled laboratory conditions, further well-designed in vivo studies remain essential to validate its diagnostic performance across diverse populations and clinical contexts.
In addition to fluorescence-based devices, other non-invasive diagnostic modalities have emerged, offering alternative approaches for caries detection. Several such modalities, namely Quantitative Light-induced Fluorescence (QLF), Optical Coherence Tomography (OCT), and Near-Infrared Imaging (NIRI), have shown potential for enhancing caries diagnostics through alternative mechanisms. QLF allows for the visualization and quantification of demineralized enamel by capturing fluorescence loss under blue light excitation. While QLF offers high sensitivity for early enamel caries and is valuable for longitudinal monitoring, it typically requires specialized imaging systems and software analysis [36]. OCT, on the other hand, generates high-resolution cross-sectional images of the tooth structure, enabling visualization of subsurface caries. However, its high cost, technical complexity, and limited accessibility restrict its widespread adoption in general dental clinics [37]. OCT studies frequently involve small sample sizes and are often limited to experimental environments, which contrasts with our large-scale in vivo study in general adult populations [38,39]. NIRI provides images based on differences in light scattering and absorption between sound and demineralized enamel, allowing for caries detection without ionizing radiation. Although promising, NIRI systems are still in relatively early stages of clinical validation and often require controlled environments for optimal image acquisition [40]. Furthermore, NIRI has primarily been investigated in in vitro studies and proximal lesions, making its diagnostic performance less generalizable at this stage [41]. In contrast, the present study incorporated a broad adult cohort with stringent exclusion criteria, thereby offering clinically relevant evidence under conditions that closely replicate everyday practice.
According to the manufacturer, SmarTooth incorporates ergonomic enhancements intended to improve probe stability and optimize contact with occlusal surfaces, thereby facilitating more consistent fluorescence measurements. In addition, the device is programmed to automatically record the highest fluorescence peak value obtained across multiple angulations, which is intended to capture the most representative signal while reducing potential underestimation due to transient probe misalignment or inconsistent surface contact. These design features may offer practical benefits in routine clinical settings, particularly where standardization across users is difficult to achieve. From a clinical standpoint, the incorporation of such device-based diagnostics into caries detection protocols may contribute to improving the consistency and objectivity of diagnostic outcomes [42]. In practices where clinician experience varies widely, such as public dental clinics, school-based programs, or rural healthcare centers, SmarTooth may function as a diagnostic aid to assist practitioners in making evidence-informed diagnostic decisions. Furthermore, its numerical output enables better documentation and facilitates longitudinal monitoring of caries progression or arrest, providing an advantage over conventional methods that rely solely on visual impressions.
Patient communication also stands to benefit from SmarTooth integration. By translating complex diagnostic observations into tangible, quantifiable data, clinicians can more effectively educate patients about their oral health status [43]. For instance, showing an increasing fluorescence score over time may motivate behavioral change and reinforce oral hygiene compliance. This visual feedback may improve communication with pediatric, adolescent, or elderly patients who may have difficulty understanding abstract diagnostic information. Additionally, the tool has potential to support teledentistry applications by providing standardized digital data that can be shared for remote consultation or follow-up.
From a public health standpoint, early detection and non-invasive intervention are key strategies in reducing the burden of untreated dental caries, which remains a major global health issue. Fluorescence-based diagnostic tools may be considered for community-level screening strategies aimed at improving risk-based referral decisions [44]. By enabling objective documentation of subclinical caries, SmarTooth may also contribute to epidemiologic surveillance and program evaluation efforts in school dental programs or regional oral health campaigns.
As a future direction, the applicability of the SmarTooth device should be explored in populations with systemic conditions that increase caries susceptibility. For example, pediatric asthma patients undergoing long-term corticosteroid therapy are known to have an elevated risk of dental caries due to reduced salivary flow and altered oral microbiota [2]. The non-invasive and quantitative nature of SmarTooth may offer valuable benefits for caries monitoring in such medically compromised patients.
From a practical perspective, the integration of fluorescence-based devices such as SmarTooth into busy clinical workflows requires consideration of preparation time, training, and cost. Although plaque, stains, and calculus may interfere with fluorescence readings, these influences can be minimized with brief prophylactic cleaning and air-drying, which can be incorporated into the routine examination sequence without imposing substantial time burdens. Compared with image-based technologies such as QLF or OCT, SmarTooth requires a relatively modest learning curve, as it provides immediate numerical outputs rather than relying on complex image acquisition and software-based analysis. Nonetheless, proper training in probe angulation and rotation remains essential to ensure reproducibility. Cost considerations are also relevant: while the device requires initial investment and the use of disposable probe tips increases recurring expenses, these can be offset by reduced diagnostic uncertainty and earlier, minimally invasive interventions. Taken together, SmarTooth may be realistically suited for general dental practice as a chairside adjunct to ICDAS while also offering value in specialist or academic contexts where more detailed monitoring and validation of preventive protocols are required.
Nonetheless, this study has several limitations. First, all examinations in this study were conducted by a single calibrated examiner. While this approach ensured internal consistency in both ICDAS scoring and SmarTooth measurements, it did not allow for the assessment of inter-examiner reliability. This constraint may have particularly influenced the evaluation of early enamel lesions (ICDAS codes 1 and 2) where the diagnostic outcomes are highly dependent on examiner calibration and visual interpretation. Furthermore, the reproducibility of fluorescence peak values may also vary with differences in probe angulation and handling technique among clinicians. To overcome these limitations, future studies should include multiple calibrated examiners and assess the inter-examiner agreement, thereby enhancing the generalizability and robustness of fluorescence-based diagnostic devices in diverse clinical settings. Second, although ICDAS is widely adopted as a reference framework, it is a subjective visual system and does not allow for direct histological validation of lesion depth. Recent in vitro research comparing SmarTooth, DIAGNOdent, and ICDAS II against micro-computed tomography (micro-CT) as the gold standard demonstrated that SmarTooth achieved the highest overall diagnostic accuracy, particularly at the enamel and dentin thresholds [21]. While such findings reinforce the validity of fluorescence-based devices under controlled conditions, further in vivo studies incorporating histological or advanced imaging validation are warranted to substantiate diagnostic accuracy across diverse clinical contexts. Third, the present study was restricted to healthy adults, which limits the applicability of our findings to other populations such as children, older adults, and medically compromised patients. Tooth morphology, enamel thickness, and caries dynamics differ substantially across age groups and clinical conditions. For instance, pediatric teeth have thinner enamel and deeper fissures, which may increase both the risk of early caries and the sensitivity of fluorescence detection [45]. In geriatric patients, age-related changes such as enamel wear, dentin exposure, and a higher prevalence of root caries may alter fluorescence signals and complicate interpretation. Similarly, in medically compromised individuals—such as those with xerostomia or systemic conditions affecting salivary flow—fluorescence-based devices may play an important role in monitoring early lesions but require additional validation. Future research should therefore extend the evaluation of SmarTooth to these diverse patient groups to determine its diagnostic reliability and optimize its clinical implementation across different populations. To address these limitations, future research should consider multi-center study designs that include examiners with varying levels of clinical expertise. In addition, implementing standardized examiner calibration protocols would facilitate robust evaluation of inter-examiner reliability and enhance the generalizability of fluorescence-based diagnostic devices such as SmarTooth in dental practice.
5. Conclusions
This study demonstrated that SmarTooth performs well in detecting early occlusal caries and can effectively differentiate between non-cavitated and cavitated lesions. Given its non-invasive, objective, and user-friendly nature, clinicians are encouraged to integrate such fluorescence-based devices into routine caries screening, especially in general practice settings where early intervention is critical. Further implementation studies could help establish clinical guidelines for optimized use across diverse patient populations. Nevertheless, owing to the potential for false positives and operator-dependent variability, SmarTooth is best interpreted as an adjunctive tool used alongside ICDAS scoring and clinical judgment rather than as a standalone diagnostic modality.
Author Contributions
Investigation, data curation, writing—original draft preparation, and funding acquisition, Y.J. Conceptualization, methodology, supervision, visualization, and writing—review and editing, J.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Catholic Kwandong University (IRB No. 2502-0404).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding authors.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of children caries risk factors: A narrative review of nutritional aspects, oral hygiene habits, and bacterial alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Qin, X.; Zi, H.; Zeng, X. Changes in the global burden of untreated dental caries from 1990 to 2019: A systematic analysis for the Global Burden of Disease study. Heliyon 2022, 8, e10714. [Google Scholar] [CrossRef]
- Demirci, M.; Tuncer, S.; Yuceokur, A.A. Prevalence of caries on individual tooth surfaces and its distribution by age and gender in university clinic patients. Eur. J. Dent. 2010, 4, 270–279. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yon, M.J.Y.; Liu, F.; Lo, E.C.M.; Yiu, C.K.Y.; Chu, C.H.; Lam, P.P.Y. Prevalence of caries patterns in the 21st century preschool children: A systematic review and meta-analysis. J. Evid.-Based Dent. Pract. 2024, 24, 101992. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Ekstrand, K.R.; Foundation, I. International Caries Detection and Assessment System (ICDAS) and its International Caries Classification and Management System (ICCMS)–methods for staging of the caries process and enabling dentists to manage caries. Community Dent. Oral Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Rechmann, P.; Charland, D.; Rechmann, B.M.; Featherstone, J.D. Performance of laser fluorescence devices and visual examination for the detection of occlusal caries in permanent molars. J. Biomed. Opt. 2012, 17, 036006. [Google Scholar] [CrossRef]
- Schwendicke, F.; Tzschoppe, M.; Paris, S. Radiographic caries detection: A systematic review and meta-analysis. J. Dent. 2015, 43, 924–933. [Google Scholar] [CrossRef]
- Gomez, J.; Tellez, M.; Pretty, I.; Ellwood, R.; Ismail, A. Non-cavitated carious lesions detection methods: A systematic review. Community Dent. Oral Epidemiol. 2013, 41, 55–66. [Google Scholar] [CrossRef]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Rohban, M.H.; Krois, J.; Uribe, S.E.; Mahmoudinia, E.; Rokhshad, R.; Nadimi, M.; Schwendicke, F. Deep learning for caries detection: A systematic review. J. Dent. 2022, 122, 104115. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jung, E.-H.; Lee, E.-S.; Jung, H.-I.; Kim, B.-I. Anti-biofilm activity of chlorhexidine-releasing elastomerics against dental microcosm biofilms. J. Dent. 2022, 122, 104153. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, E.-S.; Jung, H.-I.; Kim, B.-I. Drug delivery and antibiofilm efficacy of nano silver fluoride sustained release orthodontic elastomerics against Streptococcus mutans. Sci. Rep. 2024, 14, 19912. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, E.-S.; Jung, H.-I.; Kim, B.-I. Caries prevention effects of nano silver fluoride sustained release orthodontic elastomerics in dental microcosm biofilms. J. Dent. 2025, 156, 105649. [Google Scholar] [CrossRef] [PubMed]
- Warkhankar, A.; Tanpure, V.R.; Wajekar, N.A. Using light fluorescence technique as an emerging approach in treating dental caries. Int. J. Prev. Clin. Dent. Res. 2023, 10, 69–72. [Google Scholar] [CrossRef]
- Scribante, A.; Cosola, S.; Pascadopoli, M.; Genovesi, A.; Battisti, R.A.; Butera, A. Clinical and Technological Evaluation of the Remineralising Effect of Biomimetic Hydroxyapatite in a Population Aged 6 to 18 Years: A Randomized Clinical Trial. Bioengineering 2025, 12, 152. [Google Scholar] [CrossRef]
- Neuhaus, K.W.; Lussi, A. DIAGNOdent. In Detection and Assessment of Dental Caries: A Clinical Guide; Springer: Berlin/Heidelberg, Germany, 2019; pp. 171–175. [Google Scholar]
- Walsh, T.; Macey, R.; Ricketts, D.; Carrasco Labra, A.; Worthington, H.; Sutton, A.; Freeman, S.; Glenny, A.; Riley, P.; Clarkson, J. Enamel caries detection and diagnosis: An analysis of systematic reviews. J. Dent. Res. 2022, 101, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, K.W.; Eggmann, F.; Kühnisch, J.; Kapor, S.; Janjic Rankovic, M.; Schüler, I.; Krause, F.; Lussi, A.; Michou, S.; Ekstrand, K. Standard reporting of caries detection and diagnostic studies (STARCARDDS). Clin. Oral Investig. 2022, 26, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Kapor, S.; Rankovic, M.J.; Khazaei, Y.; Crispin, A.; Schüler, I.; Krause, F.; Lussi, A.; Neuhaus, K.; Eggmann, F.; Michou, S. Systematic review and meta-analysis of diagnostic methods for occlusal surface caries. Clin. Oral Investig. 2021, 25, 4801–4815. [Google Scholar] [CrossRef]
- Lenzi, T.L.; Piovesan, C.; Mendes, F.M.; Braga, M.M.; Raggio, D.P. In vitro performance of QLF system and conventional methods for detection of occlusal caries around tooth-colored restorations in primary molars. Int. J. Paediatr. Dent. 2016, 26, 26–34. [Google Scholar] [CrossRef]
- Kim, K.; Jung, H.-I.; Park, W.; Lee, K.E.; Kang, C.-M.; Song, J.S. Optimizing Fluorescence-Based Caries Detection: A Diagnostic Performance Analysis of SmarTooth and DIAGNOdent Pen. Photodiagnosis Photodyn. Ther. 2025, 55, 104731. [Google Scholar] [CrossRef]
- Michou, S.; Benetti, A.R.; Vannahme, C.; Hermannsson, P.G.; Bakhshandeh, A.; Ekstrand, K.R. Development of a fluorescence-based caries scoring system for an intraoral scanner: An in vitro study. Caries Res. 2020, 54, 324–335. [Google Scholar] [CrossRef]
- Angnes, V.; Angnes, G.; Batisttella, M.; Grande, R.; Loguercio, A.; Reis, A. Clinical effectiveness of laser fluorescence, visual inspection and radiography in the detection of occlusal caries. Caries Res. 2005, 39, 490–495. [Google Scholar] [CrossRef]
- Ismail, A.I.; Sohn, W.; Tellez, M.; Amaya, A.; Sen, A.; Hasson, H.; Pitts, N.B. The International Caries Detection and Assessment System (ICDAS): An integrated system for measuring dental caries. Community Dent. Oral Epidemiol. 2007, 35, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, K.R.; Gimenez, T.; Ferreira, F.R.; Mendes, F.M.; Braga, M.M. The international caries detection and assessment system–ICDAS: A systematic review. Caries Res. 2018, 52, 406–419. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 2014, 48, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Honkala, E.; Runnel, R.; Honkala, S.; Olak, J.; Vahlberg, T.; Saag, M.; Mäkinen, K.K. Measuring dental caries in the mixed dentition by ICDAS. Int. J. Dent. 2011, 2011, 150424. [Google Scholar] [CrossRef] [PubMed]
- Dhanavel, C.; Sai, C.K.; Neelamurthy, P.S.; Raja, S.V.; Vigneshwari, S.; Gokulapriyan, K.; Usha, C. Evaluation of reliability and validity of occlusal caries detection by direct visual, indirect visual and fluorescence camera using ICDAS II (codes 0, 1, and 2): An in vivo study. Int. J. Clin. Pediatr. Dent. 2023, 16, 74. [Google Scholar] [CrossRef]
- Lussi, A.; Hibst, R.; Paulus, R. DIAGNOdent: An optical method for caries detection. J. Dent. Res. 2004, 83, 80–83. [Google Scholar] [CrossRef]
- Cochrane Oral Health Group; Macey, R.; Walsh, T.; Riley, P.; Glenny, A.-M.; Worthington, H.V.; Fee, P.A.; Clarkson, J.E.; Ricketts, D. Fluorescence devices for the detection of dental caries. Cochrane Database Syst. Rev. 1996, 2021, CD013811. [Google Scholar]
- Lussi, A.; Imwinkelried, S.; Pitts, N.; Longbottom, C.; Reich, E. Performance and reproducibility of a laser fluorescence system for detection of occlusal caries in vitro. Caries Res. 1999, 33, 261–266. [Google Scholar] [CrossRef]
- Bertella, N.; Moura, d.S.; Alves, L.; Damé-Teixeira, N.; Fontanella, V.; Maltz, M. Clinical and radiographic diagnosis of underlying dark shadow from dentin (ICDAS 4) in permanent molars. Caries Res. 2013, 47, 429–432. [Google Scholar] [CrossRef]
- Achilleos, E.; Rahiotis, C.; Kavvadia, K.; Vougiouklakis, G. In Vivo validation of Diagnodent and Vista proof devices vs ICDAS clinical criteria on incipient carious lesions in adults. Photodiagnosis Photodyn. Ther. 2021, 34, 102252. [Google Scholar] [CrossRef]
- Jung, E.-H.; Lee, E.-S.; Jung, H.-I.; Kang, S.-M.; de Jong, E.d.J.; Kim, B.-I. Development of a fluorescence-image scoring system for assessing noncavitated occlusal caries. Photodiagnosis Photodyn. Ther. 2018, 21, 36–42. [Google Scholar] [CrossRef]
- Khalife, M.A.; Boynton, J.R.; Dennison, J.B.; Yaman, P.; Hamilton, J.C. In Vivo evaluation of DIAGNOdent for the quantification of occlusal dental caries. Oper. Dent. 2009, 34, 136–141. [Google Scholar] [CrossRef]
- Ko, H.-Y.; Kang, S.-M.; Kim, H.E.; Kwon, H.-K.; Kim, B.-I. Validation of quantitative light-induced fluorescence-digital (QLF-D) for the detection of approximal caries in vitro. J. Dent. 2015, 43, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Yoshiyama, M.; Tagami, J.; Sumi, Y. Evaluation of dental caries, tooth crack, and age-related changes in tooth structure using optical coherence tomography. Jpn. Dent. Sci. Rev. 2020, 56, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Sadr, A.; Burrow, M.F.; Tagami, J.; Ozawa, N.; Sumi, Y. Validation of swept-source optical coherence tomography (SS-OCT) for the diagnosis of occlusal caries. J. Dent. 2010, 38, 655–665. [Google Scholar] [CrossRef]
- Nakajima, Y.; Shimada, Y.; Sadr, A.; Wada, I.; Miyashin, M.; Takagi, Y.; Tagami, J.; Sumi, Y. Detection of occlusal caries in primary teeth using swept source optical coherence tomography. J. Biomed. Opt. 2014, 19, 016020. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, G.; Pitchika, V.; Litzenburger, F.; Hickel, R.; Kühnisch, J. Evaluation of occlusal caries detection and assessment by visual inspection, digital bitewing radiography and near-infrared light transillumination. Clin. Oral Investig. 2018, 22, 2431–2438. [Google Scholar] [CrossRef]
- Rodrigues, N.; Martinez-Rus, F.; Miguel-Calvo, A.; Pradíes, G.; Salido, M.P. Accuracy Assessment of Human and AI-Assisted Bitewing Radiography and NIRI-Based Methods for Interproximal Caries Detection: A Histological Validation. Caries Res. 2025, 30, 1–12. [Google Scholar] [CrossRef]
- Galuscan, A.; Jumanca, D.; Fratila, A.D. Caries Management Aided by Fluorescence-Based Devices. In Dental Caries-The Selection of Restoration Methods and Restorative Materials; IntechOpen: London, UK, 2022. [Google Scholar]
- Inquimbert, C.; Hirata-Tsuchiya, S.; Yoshii, S.; Molinari, N.; Nogue, E.; Roy, C.; Morotomi, T.; Washio, A.; Cuisinier, F.; Tassery, H. Concordance study between regular face-to-face dental diagnosis and dental telediagnosis using fluorescence. J. Telemed. Telecare 2021, 27, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Tetuan, T.M.; McGlasson, D.; Meyer, I. Oral health screening using a caries detection device. J. Sch. Nurs. 2005, 21, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; van der Veen, M.H.; Schemehorn, B.R.; Stookey, G.K. Comparative study to quantify demineralized enamel in deciduous and permanent teeth using laser–and light–induced fluorescence techniques. Caries Res. 2001, 35, 464–470. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).