Orally Delivered Hyaluronic Acid Tetrasaccharide Improves Skin Barrier Function in UVB-Irradiated Mice: A Bioactive Approach for Cosmetic and Nutritional Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Permeation Test
2.4. Quantitative Determination of HA and HA4
2.5. Animals
2.6. Oral Administration to Mice and UVB Irradiation
2.7. Histological Procedures
2.8. Statistical Analysis
3. Results and Discussion
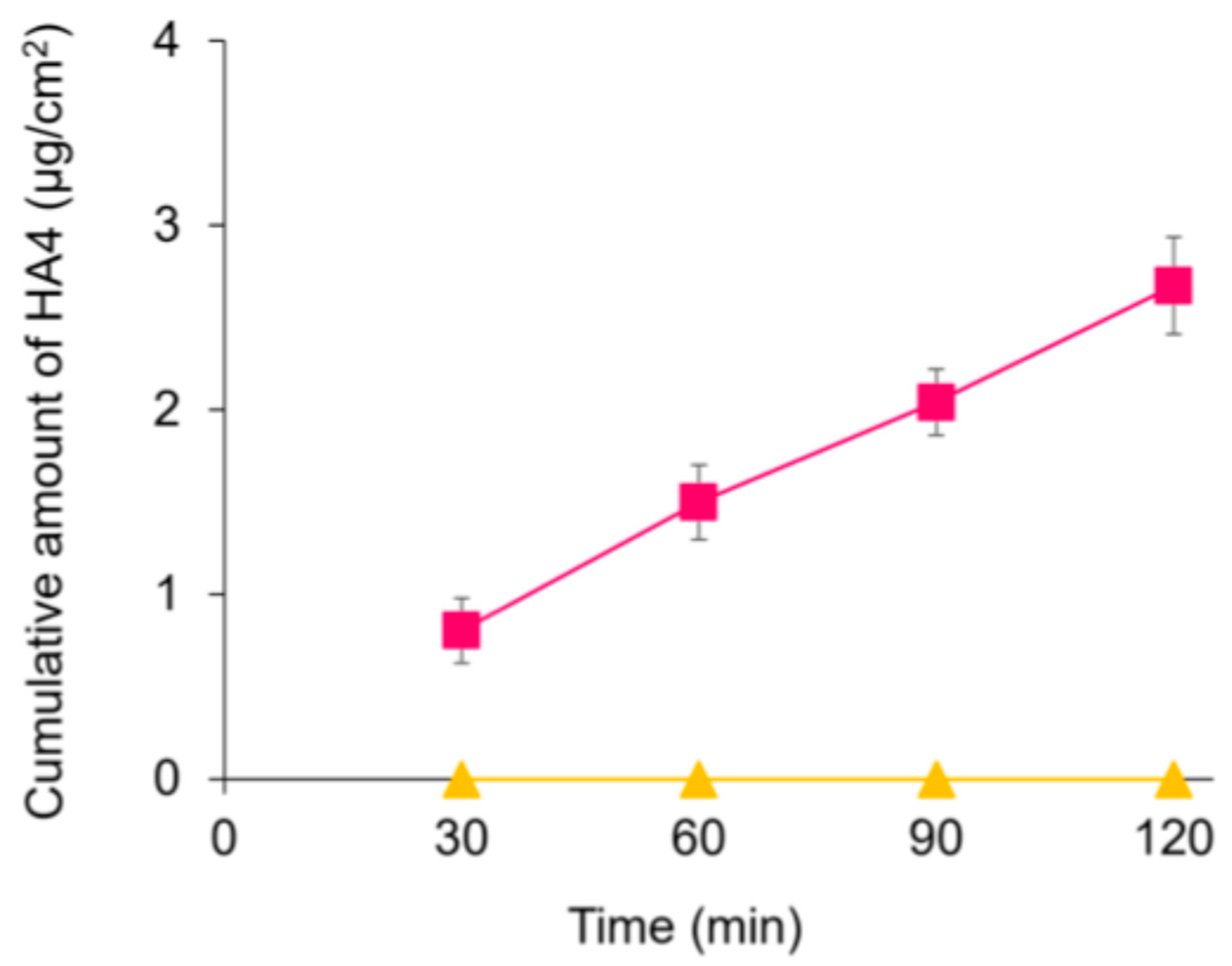
3.1. HA4 Was Absorbed Intact in the Intestine
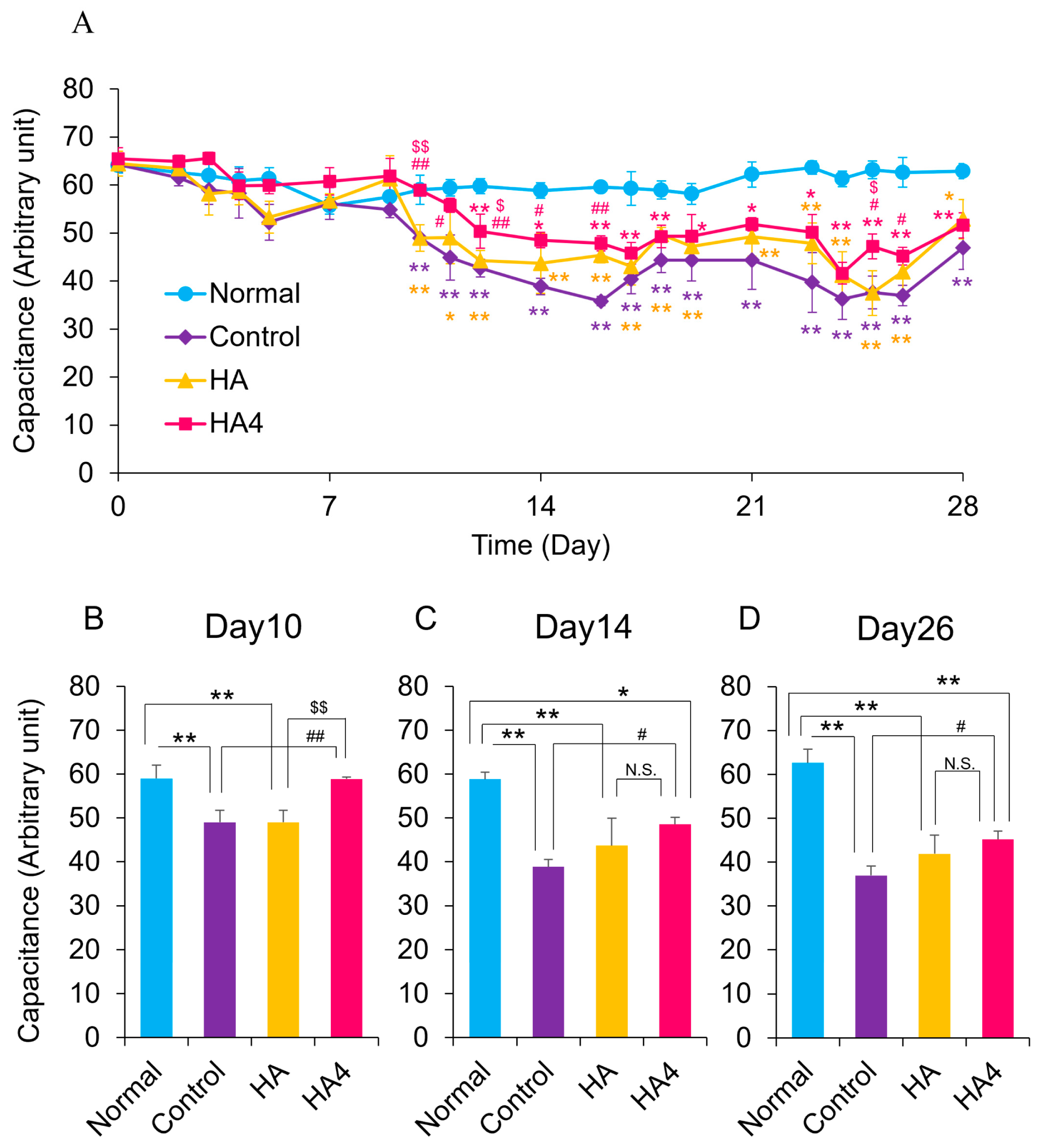
3.2. Oral Administration of HA4 Suppresses the Deterioration of Water Content of SC and Improves Barrier Function Caused by UVB Irradiation
3.3. Oral Administration of HA4 Suppresses UVB-Induced Epidermal Thickening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, T.; Mayuzumi, J. The change and recovery of human skin barrier functions after ultraviolet light irradiation. Chem. Pharm. Bull. 1979, 27, 458–462. [Google Scholar] [CrossRef]
- Haratake, A.; Uchida, Y.; Schmuth, M.; Tanno, O.; Yasuda, R.; Epstein, J.H.; Elias, P.M.; Holleran, W.M. UVB-induced alterations in permeability barrier function: Roles for epidermal hyperproliferation and thymocyte-mediated response. J. Investig. Dermatol. 1997, 108, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Kawada, C.; Yoshida, T.; Yoshida, H.; Sakamoto, W.; Odanaka, W.; Sato, T.; Yamasaki, T.; Kanemitsu, T.; Masuda, Y.; Urushibata, O. Ingestion of hyaluronans (molecular weights 800 k and 300 k) improves dry skin conditions: A randomized, double blind, controlled study. J. Clin. Biochem. Nutr. 2015, 56, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.F.; Su, Z.R.; Hsieh, Y.H.; Wang, M.F.; Oe, M.; Matsuoka, R.; Masuda, Y. Oral hyaluronan relieves wrinkles and improves dry akin: A 12-week double-blinded, placebo-controlled study. Nutrients 2021, 13, 2220. [Google Scholar] [CrossRef]
- Meyer, K.; Palmer, J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- da Silva Xavier, A.A.; da Rosa, P.P.; de Brum Mackmill, L.; Roll, V.F.B. An assessment of the effectiveness of hyaluronic acid and polyacrylamide hydrogel in horses with osteoarthritis: Systematic review and network meta-analysis. Res. Vet. Sci 2021, 134, 42–50. [Google Scholar] [CrossRef]
- Serra Aguado, C.I.; Ramos-Plá, J.J.; Soler, C.; Segarra, S.; Moratalla, V.; Redondo, J.I. Effects of Oral Hyaluronic Acid Administration in Dogs Following Tibial Tuberosity Advancement Surgery for Cranial Cruciate Ligament Injury. Animals 2021, 11, 1264. [Google Scholar] [CrossRef]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuroczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. Absorption, uptake and tissue affinity of high-molecular-weight hyaluronan after oral administration in rats and dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Oe, M.; Mitsugi, K.; Odanaka, W.; Yoshida, H.; Matsuoka, R.; Seino, S.; Kanemitsu, T.; Masuda, Y. Dietary hyaluronic acid migrates into the skin of rats. Sci. World J. 2014, 2014, 378024. [Google Scholar] [CrossRef]
- Kimura, M.; Maeshima, T.; Kubota, T.; Kurihara, H.; Masuda, Y.; Nomura, Y. Absorption of Orally Administered Hyaluronan. J. Med. Food 2016, 19, 1172–1179. [Google Scholar] [CrossRef]
- Nakamura, K.; Yokohama, S.; Yoneda, M.; Okamoto, S.; Tamaki, Y.; Ito, T.; Okada, M.; Aso, K.; Makino, I. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J. Gastroenterol. 2004, 39, 346–354. [Google Scholar] [CrossRef]
- Spessotto, P.; Rossi, F.M.; Degan, M.; Francia, R.D.; Perris, R.; Colombatti, A.; Gattei, V. Hyaluronan-CD44 interaction hampers migration of osteoclast-like cells by down-regulating MMP-9. J. Cell Biol. 2002, 158, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Goomer, R.S.; Harwood, F.; Kubo, T.; Hirasawa, Y.; Amiel, D. The effects of hyaluronan on matrix metal-loproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthr. Cartil. 1999, 7, 182–190. [Google Scholar] [CrossRef]
- Neumann, A.; Schinzel, R.; Palm, D.; Riederer, P.; Munch, G. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-kappaB activation and cytokine expression. FEBS Lett. 1999, 453, 283–287. [Google Scholar] [CrossRef]
- Hodge-Dufour, J.; Noble, P.W.; Horton, M.R.; Bao, C.; Wysoka, M.; Burdick, M.D.; Strieter, R.M.; Trinchieri, G.; Pure, E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 1997, 159, 2492–2500. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef]
- Noble, P.W.; McKee, C.M.; Cowman, M.; Shin, H.S. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J. Exp. Med. 1996, 183, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- West, D.C.; Hampson, I.N.; Arnold, F.; Kumar, S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Kawada, C.; Kimura, M.; Masuda, Y.; Nomura, Y. Oral administration of hyaluronan prevents skin dryness and epidermal thickening in ultraviolet irradiated hairless mice. J. Photochem. Photobiol. B 2015, 153, 215–221. [Google Scholar] [CrossRef]
- Kawada, C.; Yoshida, T.; Yoshida, H.; Matsuoka, R.; Sakamoto, W.; Odanaka, W.; Sato, T.; Yamasaki, T.; Kanemitsu, T.; Masuda, Y.; et al. Ingested hyaluronan moisturizes dry skin. Nutr. J. 2014, 13, 70. [Google Scholar] [CrossRef]
- Yun, M.K.; Lee, S.J.; Song, H.J.; Yu, H.J.; Rha, C.S.; Kim, D.O.; Choe, S.Y.; Sohn, J. Protein expression level of skin wrinkle-related factors in hairless mice fed hyaluronic acid. J. Med. Food 2017, 20, 420–424. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.W.; Hyunc, J.W.; Yoon, S.-P. Biological effects of chitosan oligosaccharides on the skin and their clinical applicability. Carbohydr. Res. 2025, 555, 109588. [Google Scholar] [CrossRef]
- Gruber, J.V.; Holtz, R.; Riemer, J. Hyaluronic acid (HA) stimulates the in vitro expression of CD44 proteins but not HAS1 proteins in normal human epidermal keratinocytes (NHEKs) and is HA molecular weight dependent. J. Cosmet. Dermatol. 2022, 21, 1193–1198. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.; Sugahara, K. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Prestipino, V.; Calatroni, A.; Campo, S. Hyaluronan in part mediates IL-1beta-induced inflammation in mouse chondrocytes by up-regulating CD44 receptors. Gene 2012, 494, 24–35. [Google Scholar] [CrossRef]
- Kage, M.; Tokudome, Y.; Matsunaga, Y.; Hariya, T.; Hashimoto, F. Effect of hyaluronan tetrasaccharides on epidermal differentiation in normal human epidermal keratinocytes. Int. J. Cosmet. Sci. 2014, 36, 109–115. [Google Scholar] [CrossRef]
- Kage, M.; Tokudome, Y. Hyaluronan tetrasaccharides stimulate ceramide production through upregulated mRNA expression of ceramide synthesis-associated enzymes. Arch. Dermatol. Res. 2016, 308, 95–101. [Google Scholar] [CrossRef]
- Kage, M.; Tokudome, Y.; Hashimoto, F. Permeation of hyaluronan tetrasaccharides through hairless mouse skin: An in vitro and in vivo study. Arch. Dermatol. Res. 2013, 305, 69–77. [Google Scholar] [CrossRef]
- Delie, F.; Rubas, W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: Advantages and limitations of the Caco-2 model. Crit. Rev. Ther. Drug Carr. Syst. 1997, 14, 221–286. [Google Scholar] [CrossRef]
- Meyer, K.; Rapport, M.M. Hyaluronidases. Adv. Enzymol. Relat. Subj. Biochem. 1952, 13, 199–236. [Google Scholar]
- Chun, L.E.; Koob, T.J.; Eyre, D.R. Quantitation of hyaluronic acid in tissues by ion-pair reverse-phase high-performance liquid chromatography of oligosaccharide cleavage products. Anal. Biochem. 1988, 171, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, M.H.; Lee, J.W.; No, K.O.; Park, S.K.; Lee, H.S.; Kang, S.; Cho, W.G.; Park, H.J.; Oh, K.W.; et al. Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation. Photodermatol. Photoimmunol. Photomed. 2007, 23, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Koyama, Y.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Bock, U.; Flötotto, T.; Haltner, E. Validation of cell culture models for the intestine and the blood-brain barrier and comparison of drug permeation. ALTEX—Altern. Anim. Exp. 2004, 3, 57–64. [Google Scholar]
- Yamashita, S.; Tanaka, Y.; Endoh, Y.; Taki, Y.; Sakane, T.; Nadai, T.; Sezaki, H. Analysis of drug permeation across Caco-2 monolayer: Implication for predicting in vivo drug absorption. Pharm. Res. 1997, 14, 486–491. [Google Scholar] [CrossRef]
- Hisada, N.; Satsu, H.; Mori, A.; Totsuka, M.; Kamei, J.; Nozawa, T.; Shimizu, M. Low-molecular-weight hyaluronan permeates through human intestinal Caco-2 cell monolayers via the paracellular pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1111–1114. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.K.; Yang, H.J.; Park, K.M. Effects of egg shell membrane hydrolysates on UVB-radiation-induced wrinkle formation in SKH-1 hairless mice. Korean J. Food Sci. Anim. Resour. 2015, 35, 58–70. [Google Scholar] [CrossRef]
- Takagi, Y.; Nakagawa, H.; Kondo, H.; Takema, Y.; Imokawa, G. Decreased levels of covalently bound ceramide are associated with ultraviolet B-induced perturbation of the skin barrier. J. Investig. Dermatol. 2004, 123, 1102–1109. [Google Scholar] [CrossRef]
- Kawai, K.; Kamochi, R.; Oiki, S.; Murata, K.; Hashimoto, W. Probiotics in human gut microbiota can degrade host glycosaminoglycans. Sci. Rep. 2018, 8, 10674–10687. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, J.H.; Nam, K.T.; Lee, S.H. Molecular events associated with apoptosis and proliferation induced by ultraviolet-B radiation in the skin of hairless mice. J. Dermatol. Sci. 2003, 32, 171–179. [Google Scholar] [CrossRef]
- Kambayashi, H.; Yamashita, M.; Odake, Y.; Takada, K.; Funasaka, Y.; Ichihashi, M. Epidermal changes caused by chronic low-dose UV irradiation induce wrinkle formation in hairless mouse. J. Dermatol. Sci. 2001, 27 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef] [PubMed]
- El-Abaseri, T.B.; Putta, S.; Hansen, L.A. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 2006, 27, 225–231. [Google Scholar] [CrossRef]
- Pauloin, T.; Dutot, M.; Joly, F.; Warnet, J.M.; Rat, P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol. Vis. 2009, 15, 577–583. [Google Scholar]
- Haneke, E. Adverse effects of fillers and their histopathology. Facial Plast. Surg. 2014, 30, 599–614. [Google Scholar] [CrossRef]
- Mashiko, T.; Kinoshita, K.; Kanayama, K.; Feng, J.; Yoshimura, K. Perpendicular strut injection of hyaluronic acid filler for deep wrinkles. Plast. Reconstr. Surg. Glob. Open 2015, 3, e567. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, O.; Odanaka, W.; Sakamoto, W.; Yoshida, K.; Takahashi, T. Clinical effects of hyaluronic acid for dry skin. J. New Rem. Clin. 2001, 50, 548–560. [Google Scholar]
- Sato, T.; Sakamoto, W.; Odanaka, W.; Yoshida, K.; Urushibata, O. Clinical effects of hyaluronic acid diet for dry and rough skin. Aesthetic Dermatol. 2002, 12, 109–120. [Google Scholar]
- Sato, T.; Yoshida, T.; Kanemitsu, T.; Yoshida, K.; Hasegawa, M.; Urushibata, O. Clinical effects of hyaluronic acid diet for moisture content of dry skin. Aesthetic Dermatol. 2007, 17, 33–39. [Google Scholar]
- Yoshida, T.; Kanemitsu, T.; Narabe, O.; Tobita, M. Improvement of dry skin by a food containing hyaluronic acids derived from microbial fermentation. J. New Rem. Clin. 2009, 58, 143–155. [Google Scholar]
- Kim, H.K.; Moon, T.K.; Kim, N.S. Effect of hyaluronan on wrinkle. Food Style 2007, 11, 42–46. [Google Scholar]
- Watanabe, M.; Matsui, K.; Kondo, S. Effects of low molecular weight hyaluronic acid by oral intake to beautify skin -placebo-controlled double-blind comparative study. Jpn. Pharmacol. Ther. 2015, 43, 57–64. [Google Scholar]
- Oe, M.; Sakai, S.; Yoshida, H.; Okado, N.; Kaneda, H.; Masuda, Y.; Urushibata, O. Oral hyaluronan relieves wrinkles: A double-blinded, placebo-controlled study over a 12-week period. Clin. Cosmet. Investig. Dermatol. 2017, 10, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am. J. Pathol. 2014, 184, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Kage, M.; Tokudome, Y.; Matsunaga, Y.; Hariya, T.; Hashimoto, F. Expression of hyaluronan synthase and collagen type I mRNA by hyaluronan tetrasaccharides in normal human dermal fibroblasts. J. Jpn. Cosmet. Sci. Soc. 2013, 37, 1–5. [Google Scholar]
- Greco, R.M.; Iocono, J.A.; Ehrlich, H.P. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J. Cell. Physiol. 1998, 177, 465–473. [Google Scholar] [CrossRef]
- Lüke, H.J.; Prehm, P. Synthesis and shedding of hyaluronan from plasma membranes of human fibroblasts and metastatic and non-metastatic melanoma cells. Biochem. J. 1999, 343, 71–75. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kage, M.; Okawara, M.; Asami, T.; Tokudome, Y. Orally Delivered Hyaluronic Acid Tetrasaccharide Improves Skin Barrier Function in UVB-Irradiated Mice: A Bioactive Approach for Cosmetic and Nutritional Applications. Appl. Sci. 2025, 15, 10182. https://doi.org/10.3390/app151810182
Kage M, Okawara M, Asami T, Tokudome Y. Orally Delivered Hyaluronic Acid Tetrasaccharide Improves Skin Barrier Function in UVB-Irradiated Mice: A Bioactive Approach for Cosmetic and Nutritional Applications. Applied Sciences. 2025; 15(18):10182. https://doi.org/10.3390/app151810182
Chicago/Turabian StyleKage, Madoka, Masaki Okawara, Takehiro Asami, and Yoshihiro Tokudome. 2025. "Orally Delivered Hyaluronic Acid Tetrasaccharide Improves Skin Barrier Function in UVB-Irradiated Mice: A Bioactive Approach for Cosmetic and Nutritional Applications" Applied Sciences 15, no. 18: 10182. https://doi.org/10.3390/app151810182
APA StyleKage, M., Okawara, M., Asami, T., & Tokudome, Y. (2025). Orally Delivered Hyaluronic Acid Tetrasaccharide Improves Skin Barrier Function in UVB-Irradiated Mice: A Bioactive Approach for Cosmetic and Nutritional Applications. Applied Sciences, 15(18), 10182. https://doi.org/10.3390/app151810182





