Abstract
This study addresses the development of advanced anode materials for lithium-ion batteries by investigating the high-entropy perovskite La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3. The material was synthesized via spray drying of aqueous metal nitrate solutions, followed by calcination at various temperatures (800 °C/1 h, 1000 °C/1 h, 1000 °C/2 h, 1100 °C/1 h) to optimize structural properties. Structural analysis using X-ray diffraction confirmed the formation of a single-phase perovskite in the sample calcined at 1100 °C for 1 h, while SEM/EDS revealed homogeneous elemental distribution. Electrochemical testing of the powders as anode materials in coin-type lithium-ion cells revealed a trend of slightly increasing capacity over 150 cycles, with capacity ultimately reaching 617 mAh g−1, indicating progressive electrochemical activation. Although the samples share the same composition, variations in calcination conditions resulted in differences in capacity and cycling behavior. These results demonstrate that synthesis parameters critically influence the electrochemical performance of high-entropy perovskites. The findings suggest that such materials have potential as stable anodes for next-generation lithium-ion batteries, contributing to improved durability and efficiency in energy-storage technologies.
1. Introduction
Lithium-ion batteries (LIBs) are among the most promising energy-storage technologies due to their high energy density and competitive cost. They dominate applications ranging from portable electronics to electric vehicles and grid storage [1]. Currently, graphite is the standard anode material, but its low theoretical capacity (372 mAh g−1), slow lithium-ion diffusion, and classification as a critical raw material in the EU limit its long-term use [1,2]. Challenges such as poor fast-charging performance, low initial coulombic efficiency, and lithium trapping further reduce battery lifespan, motivating the search for advanced anode materials with higher capacity and improved stability [1,2,3].
A promising strategy is the use of high-configurational-entropy materials (HEMs), in which compositional complexity and structural disorder enhance mechanical and functional properties [4,5]. Initially developed for metallic alloys, the concept has expanded to oxides, carbides, fluorides, and perovskites [6]. HEMs exhibit unique features such as entropy-stabilized phases, lattice distortion, sluggish diffusion, and the cocktail effect [5], often forming single-phase structures despite their chemical complexity [4,5,6,7].
Perovskites, which have the general formula ABX3, allow versatile substitution at the A and B sites, enabling tunable properties for applications in batteries, catalysts, fuel cells, and solar cells [7,8]. High-entropy perovskite oxides (HEPOs) can be synthesized by diverse methods, including solid-state reaction, sol–gel, solution combustion, co-precipitation, and hydrothermal approaches. For example, LaMn0.2Fe0.2Co0.2Ni0.2Cu0.2O3–δ has been prepared by EDTA–citrate complexation [9], enthalpy-change-driven synthesis yielding nanoparticles [10], and carbon-sphere templating to obtain hollow orthorhombic structures with high surface area and oxygen-vacancy content [11]. While materials with this composition have not yet been used in lithium-ion batteries, the most extensively studied HEPO is La(FeCoNiCrMn)O3, synthesized by sol–gel [12], solution combustion [13,14], solid-state reaction [15], deep eutectic solvent-assisted processes [16,17], and co-precipitation [18].
Its electrochemical performance is highly dependent on structure and morphology. The cubic phase exhibits the lowest capacity (~93 mAh g−1 at 1C after 200 cycles) [12], whereas hollow spheres deliver ~349 mAh g−1 [11]. Orthorhombic mesoporous structures achieve 569 mAh g−1 after 500 cycles [14], and the highest capacity (879 mAh g−1 after 250 cycles) was reported for a mesoporous cubic perovskite prepared by solution combustion [13], with this performance attributed to SEI evolution and pseudocapacitive effects.
Spray drying followed by calcination offers key advantages over other methods for perovskite synthesis. It ensures better cation mixing, enabling phase formation at lower temperatures and shorter calcination times, while producing fine, homogeneous powders. Compared to sol–gel or freeze-drying, it is more scalable and reproducible, yielding spherical, flowable particles ideal for further processing. This versatile method suits various perovskite compositions, including doped systems, making it practical for both laboratory research and industrial-scale production [19]. For a detailed comparison of preparation methods, see Supplementary Data.
In this work, we applied spray drying for the preparation of the high-entropy perovskite LaMn0.2Fe0.2Co0.2Ni0.2Cu0.2O3, a material neither previously synthesized by this method nor previously studied as an anode material in lithium-ion batteries. This approach supports the scalable fabrication of high-entropy materials with tailored microstructures and electrochemical functionalities. We further investigated the effect of calcination temperature on structural and electrochemical properties, hypothesizing that higher temperatures improve crystallinity and morphological stability, thereby enhancing capacity and cycle life.
2. Materials and Methods
2.1. Material
The high-entropy perovskite La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3 was synthesized via spray drying of an aqueous solution of mixed metal salts followed by calcination. A schematic representation of the synthesis process is shown in Figure 1. To prepare the mixed-salt solution, metal nitrates with a concentration of 1 mol dm−3 were used. Details on materials and their sources are given in Supplementary Data. The concentrations of the prepared solutions were verified using inductively coupled plasma optical emission spectrometry (ICP-OES, Thermo Scientific iCAP 6200 DUO, Waltham, MA, USA). The precursor solutions were mixed in volume ratios corresponding to the stoichiometric molar ratios required for the target perovskite composition.
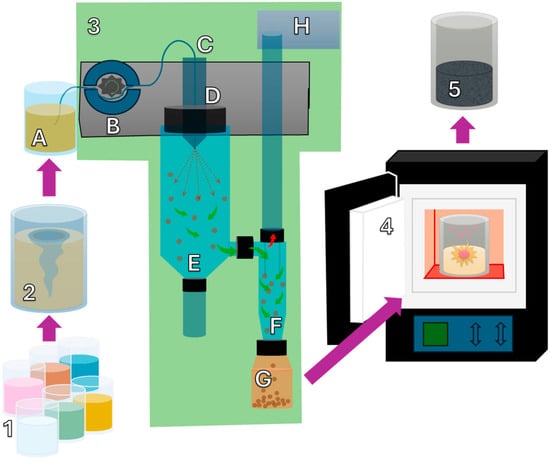
Figure 1.
Schematic of perovskite synthesis via spray drying and calcination. Precursor solutions (1) are mixed (2) and spray-dried (3), and the resulting powder is calcined (4) to obtain the final perovskite product (5).
The mixed aqueous nitrate solution was processed using a spray dryer (TEFIC Biotech, TFS-2L, Xi’an, China) at an inlet air temperature of 300 °C. The solution was delivered by a peristaltic pump at 0.2 L h−1 to an air atomizer equipped with a 1 mm nozzle and operated at an air pressure of 80 kPa and an exit velocity of ~300 m s−1. The resulting aerosol was introduced into a hot-air stream (300 °C) generated by a 4 kW flow heater at 4 m3 min−1. The outlet air temperature was 125 °C, and the average drying time was 1–1.5 s. The solution feed rate was adjusted to 30% of the pump capacity (corresponding to an air flow of 0.83 mL min−1), and the cyclone fan speed was set to 70% to minimize particle entrainment and ensure efficient powder collection. These conditions were optimized in preliminary trials to achieve a low residual water content in the spray-dried precursor.
The obtained fine powder was subsequently calcined in air at 800, 1000, and 1100 °C for dwell times of 1 and 2 h, with a heating rate of 5 °C min−1. The choice of calcination temperatures was guided by differential thermal analysis (DTA). This treatment yielded the crystalline perovskite phase La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3. Additional information regarding synthesis yields can be found in Table S3 of the Supplementary Material.
2.2. Material Characterization
Differential thermal analysis and thermogravimetric analysis (DTA and TG) were performed using a simultaneous DTA–TG analyzer (NETZSCH STA 449 F3 Jupiter, Selb, Germany) in a synthetic air atmosphere (20 mL min−1), with an additional protective Ar flow (20 mL min−1). Measurements were carried out from room temperature (20 °C) up to 1200 °C at a heating rate of 10 °C min−1.
Phase analysis of the calcined material was conducted at room temperature using a Philips X’Pert Pro laboratory X-ray diffractometer (Philips, Amsterdam, The Netherlands) in Bragg–Brentano geometry with Co Kα radiation, an iron filter, and an X’celerator high-speed detector, operating in reflection mode at 40 kV and 40 mA. Data were collected with a step size of 0.03° and a counting time of 60 s per step over a 2θ range of 10–110°. The diffraction patterns were refined using GSAS-II software (Version 5799) [20]. Phase identification was performed using the free software QualX2.0 (version 2.24) [21] together with the Crystallographic Open Database (COD). Instrumental broadening was experimentally determined using a LaB6 powder standard (SRM 660c).
Powder morphology was examined by scanning electron microscopy (SEM) using a Tescan Vega 3 LMU microscope (Tescan, Brno, Czech Republic) operated at an accelerating voltage of 15 kV and a working distance of 15.4 mm. Qualitative and semi-quantitative elemental analysis, including elemental mapping of the samples, was carried out using an energy-dispersive X-ray spectroscopy (EDS) detector (Bruker, Billerica, MA, USA) integrated into the SEM system.
2.3. Coin Cell Preparation
Electrochemical properties were investigated using lithium-ion coin cells. The preparation of electrodes and assembly of the cells were carried out following standard protocols [22]. Detailed experimental conditions and procedures are provided in the Supplementary Information.
2.4. Electrochemical Characterization
The electrochemical properties of the prepared samples were evaluated by galvanostatic charge–discharge cycling within a voltage window of 0–3 V vs. Li/Li+, performed using a battery tester (Landt Instruments, New York, NY, USA). Complementary cyclic voltammetry (CV) measurements were carried out at a scan rate of 0.1 mV s−1 over a voltage range of 0.01–3 V vs. Li/Li+ to provide a more detailed analysis of the electrochemical behavior of the electrode material. These measurements were conducted using a BioLogic SP-150 potentiostat/galvanostat (BioLogic, Seyssinet-Pariset, France).
3. Results
3.1. Material Characterization
The appropriate calcination temperature for synthesizing La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3 via thermal decomposition of the spray-dried high-entropy perovskite (HEPO) precursor was determined using differential thermal analysis (DTA) and thermogravimetric analysis (TG). The corresponding TG–DTA curves are presented in Figure 2.
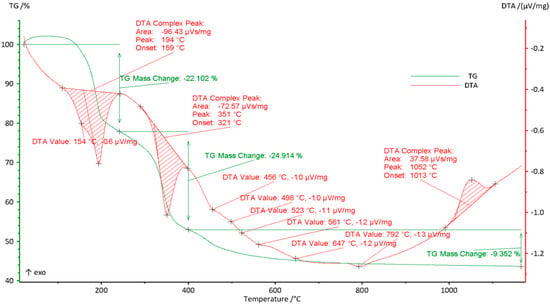
Figure 2.
TG–DTA curves of the HEPO precursor. The TG profile exhibits three major weight-loss steps: (i) below 220 °C, corresponding to the removal of adsorbed and bound water (~22% mass loss); (ii) between 220–400 °C, associated with the decomposition of hydrated nitrate species (~25% mass loss); and (iii) between 400–800 °C, reflecting the gradual decomposition of anhydrous nitrate salts (~10% mass loss). The DTA curve shows a broad exothermic peak at ~1050 °C, which can be attributed to oxide recrystallization and the crystallization of the perovskite/spinel phase.
The TG curve reveals a multi-step weight-loss pattern corresponding to dehydration processes and the decomposition of nitrate salts. An initial sharp mass loss of approximately 22% occurs up to 220 °C and is attributable to the desorption of physically adsorbed water and the initial dehydration of hydrated species. Above 220 °C, a gradual mass loss of about 35% is observed in association with the continued removal of residual water and the thermal decomposition of anhydrous nitrate salts, accompanied by the evolution of NO2 and O2 gases [23].
The DTA curve supports these observations, displaying two distinct endothermic peaks at 154 °C and 194 °C, which correspond to removal of residual moisture and partial dehydration of salts. A broader endothermic peak around 351 °C is linked to the elimination of remaining water. Several smaller, less distinct endothermic peaks at higher temperatures (456 °C, 498 °C, 523 °C, 561 °C, 647 °C, and 792 °C) are attributed to the stepwise thermal decomposition of individual metal nitrates into their respective oxides. Finally, a broad exothermic peak with a maximum at 1052 °C is ascribed to oxide recrystallization and the progressive formation of the La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3 perovskite crystalline structure.
To investigate the influence of crystallinity on the electrochemical performance of the synthesized La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3, an initial calcination temperature of 800 °C was selected to promote the formation of the perovskite phase. Samples were subsequently calcined at temperatures ranging from 800 °C to 1100 °C, with dwell times of 1 or 2 h.
To evaluate the phase composition of the synthesized materials at different calcination temperatures, X-ray diffraction (XRD) measurements were carried out (Figure 3). Phase analysis of the diffraction data revealed that a single-phase perovskite structure, comparable to LaMnO3 (COD #1542144), was obtained only for the sample calcined at 1100 °C for 1 h. Rietveld refinement of this sample confirmed a trigonal structure with space group Rc and refined lattice parameters a = 5.508 Å and c = 13.265 Å (in the conventional hexagonal setting). The corresponding refinement profiles are provided in the Supplementary Information (Figure S1), and the refined structural parameters are summarized in Table S4. In contrast, samples calcined at lower temperatures exhibited multiphase compositions, mainly containing phases with orthorhombic symmetry. Due to the structural complexity and peak overlap, these secondary phases could not be unambiguously indexed.
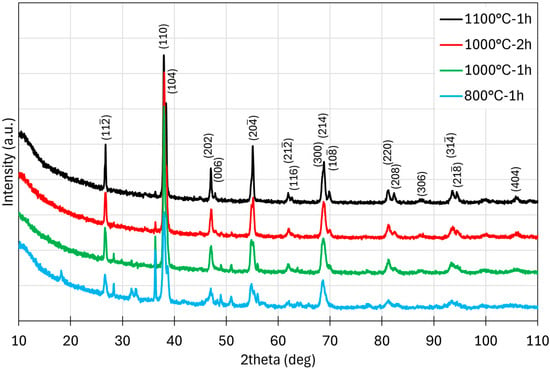
Figure 3.
XRD patterns of HEPO samples calcined under different conditions (1100 °C for 1 h, 1000 °C for 2 h, 1000 °C for 1 h, and 800 °C for 1 h), recorded using Co Kα radiation in the 2θ range of 10–110°.
Comparison of the main perovskite phase across all samples revealed slight differences in lattice parameters, reflected in the shifts of the main diffraction peaks (30–40°). Figure 4a,b shows the evolution of lattice parameters (a and c) and unit cell volume as a function of calcination temperature and dwell time. With increasing calcination temperature, both the lattice parameters and the unit cell volume decreased. The most pronounced variation occurred in the c parameter (average change ~0.9 Å), while changes in the a parameter were on the order of thousandths of an angstrom. This trend can be attributed to the incorporation of different cations into the perovskite structure. At higher calcination temperatures, enhanced cation diffusion promotes the transformation of secondary phases present at lower temperatures into the single-phase perovskite. This process is governed by both temperature and dwell time, leading to a clear correlation between calcination conditions and the fraction of the perovskite phase.
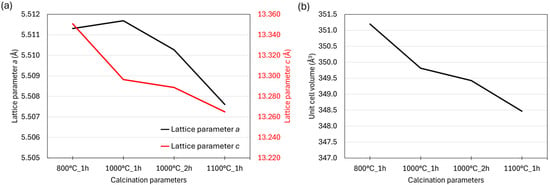
Figure 4.
Comparison of (a) lattice parameters and (b) unit cell volume of the main perovskite phase (space group R), as obtained from Rietveld refinement of the XRD data.
Microscopic analysis revealed that the particle morphology was fine-grained and heterogeneously distributed, consisting of loosely aggregated fragments ranging in size from a few micrometers up to several hundred micrometers (Figure 5). The particle surfaces appeared markedly irregular and rough. The intended chemical composition was equimolar; however, EDS analysis indicated a quasi-equimolar ratio of the transition metals (Table 1), which is within acceptable limits. Furthermore, EDS mapping confirmed a homogeneous elemental distribution across the analyzed area, demonstrating uniform dispersion of the constituent elements.
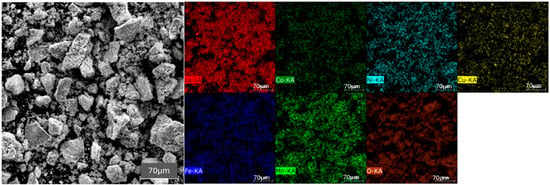
Figure 5.
SEM image of the HEPO powder calcined at 1000 °C for 1 h (secondary electron mode), accompanied by EDS elemental mapping of La, Co, Ni, Cu, Fe, Mn, and O.

Table 1.
Elemental composition of HEPO powder calcinated at 1000 °C for 1 h, as determined by EDS analysis. σ denotes standard deviation.
3.2. Electrochemical Characterization
To evaluate the electrochemical performance of the synthesized high-entropy perovskite oxides, cyclic voltammetry (CV) measurements were performed. For the sample calcined at 1100 °C for 1 h (Figure 6a), the first cycle exhibited a small cathodic peak at ~0.75 V, followed by a pronounced reduction peak at ~0.5 V, which is commonly associated with the formation of the solid electrolyte interphase (SEI) layer [24]. The corresponding anodic scan did not show a distinct peak but rather a broad anodic region between 0.2 and 1.0 V, indicating a continuous process of lithium extraction. In subsequent cycles, the initial reduction features diminished significantly, suggesting stabilization of the SEI layer. The close overlap of CV curves from the second to the sixth cycle demonstrates good electrochemical reversibility and cycling stability.
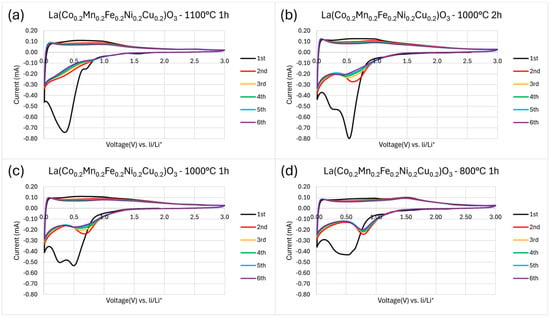
Figure 6.
Cyclic voltammetry (CV) curves recorded at a scan rate of 0.1 mV s−1 in the voltage window of 0.01–3.0 V vs. Li/Li+ for samples calcined at (a) 1100 °C for 1 h, (b) 1000 °C for 2 h, (c) 1000 °C for 1 h, (d) 800 °C for 1h.
In comparison, the material calcined at 1000 °C for 2 h (Figure 6b) showed a sharper and narrower main cathodic peak centered at ~0.55 V in the first cycle, with additional minor peaks at lower potentials, indicating a more complex lithiation mechanism. The sample calcined at 1000 °C for 1 h (Figure 6c) displayed a broader and more structured cathodic response, where the main reduction event appeared split into multiple narrower peaks, suggesting the occurrence of multi-step reduction processes, likely due to phase heterogeneity or incomplete crystallization.
For the sample calcined at 800 °C for 1 h (Figure 6d), the cathodic peak was significantly broadened, likely reflecting lower crystallinity and the presence of multiple phases, which led to a wider distribution of lithiation potentials. The anodic scans of all samples resembled that of the 1100 °C sample; however, a distinct anodic peak at ~1.5 V emerged for the 800 °C sample, possibly corresponding to the oxidation of intermediate phases or electrochemically active transition-metal species absent in the more crystalline samples.
Rate-capability tests were performed by stepwise increasing the current density from 100 to 2000 mA g−1 in the sequence 100, 200, 500, 1000, and 2000 mA g−1. Among all tested samples, the material calcined at 1100 °C for 1 h consistently delivered the highest specific discharge capacities across the full range of current densities (Figure 7). A noticeable capacity drop occurred during the initial cycles, as is typical of conversion-type anode materials due to irreversible processes such as SEI formation and structural reorganization.
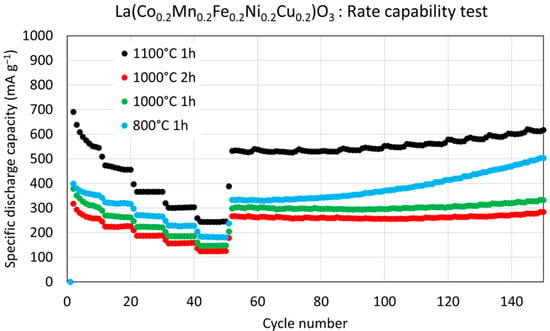
Figure 7.
Long-term rate capability of HEPO samples tested at different current densities (100, 200, 500, 1000, and 2000 mA g−1), with 10 cycles at each current density followed by regeneration at 100 mA g−1 for 100 cycles.
After the initial decline, the capacities remained relatively stable at each applied current density. When the current density was returned to the initial value of 100 mA g−1 in the regeneration step, all samples showed capacity recovery, with the 1100 °C sample nearly regaining its initial stable value. Notably, the discharge capacities of this sample were approximately twice those of the materials prepared at lower calcination temperatures at each corresponding current density.
Although the initial fade in capacity was less pronounced for the samples calcined at lower temperatures, their overall capacities were significantly lower. Nevertheless, all materials demonstrated excellent cycling stability during the regeneration phase, maintaining consistent performance up to 100 cycles.
The electrode surfaces before and after cycling were examined by SEM, as shown in Figure 8 for the representative sample calcined at 1100 °C for 1 h. The uncycled electrodes exhibited a relatively uniform surface morphology, with larger perovskite oxide particles homogeneously distributed across the electrode. Small cracks were already visible; they had likely formed during the drying process as a result of solvent evaporation and mismatched thermal expansion among the electrode components.
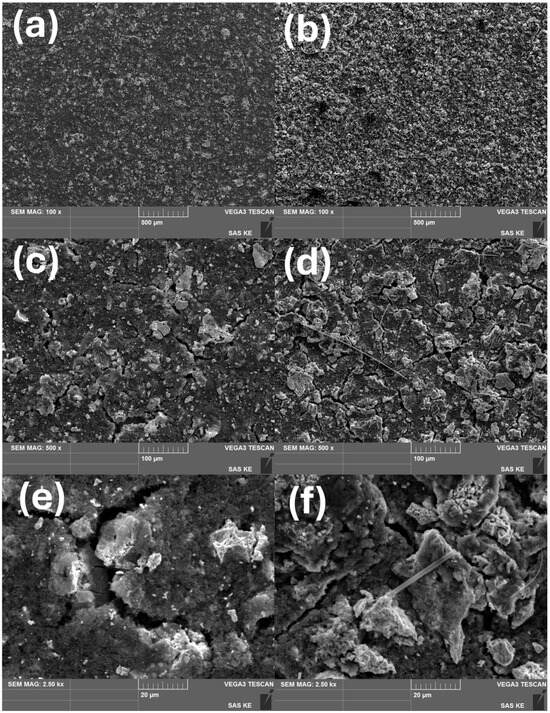
Figure 8.
SEM images of electrode surfaces from the HEPO calcinated at 1100 °C 1 h, shown before (a,c,e) and after (b,d,f) six charge–discharge cycles at different magnifications (secondary electron mode).
After cycling, the electrode surface displayed crater-like features, indicative of the formation of new electrochemically active areas; these correlate with the increased capacity observed in the galvanostatic cycling tests. The initial cracks propagated further, although the overall electrode integrity remained intact. In addition, the larger oxide particles appeared to undergo pulverization into smaller fragments, a process that may also have contributed to the enhanced specific capacity.
Post-mortem XRD analysis of the electrodes after six CV cycles revealed a pronounced phase transformation (Figure 9). The characteristic diffraction peaks of the main perovskite phase disappeared, while a broad amorphous feature emerged along with the diffraction peaks of the copper-foil current collector. These observations indicate that the material undergoes a conversion-type reaction in which oxide phases are reduced to metallic states during discharge and re-oxidized upon charging.
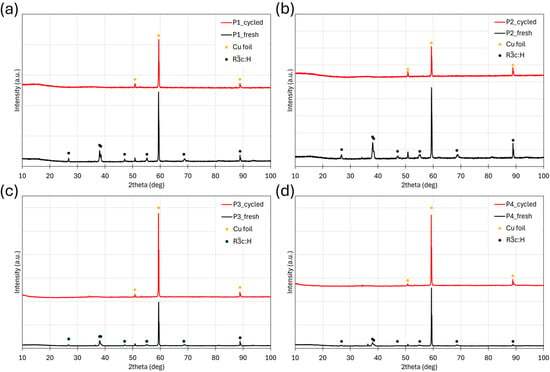
Figure 9.
XRD patterns of the electrodes before and after six CV cycles for HEPO samples calcined at (a) 1100 °C for 1 h, (b) 1000 °C for 2 h, (c) 1000 °C for 1 h, and (d) 800 °C for 1 h.
4. Discussion
The results of this study demonstrate that the preparation route and its parameters strongly influence both the phase composition and the electrochemical properties of the synthesized materials. In this work, we employed spray drying to prepare a high-entropy oxide with a perovskite structure, La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3. Although this material has previously been synthesized by other groups using different routes [10], to the best of our knowledge, this is the first report of its preparation via spray drying. This technique is low-cost, relatively fast, and highly scalable, making it suitable for producing materials with unique morphologies [25]. Estimated operational costs for various synthesis approaches are summarized in Table S4 (see Supplementary Information). Spray drying followed by calcination is an industrially relevant method and one of the few wet-chemical pathways that can be scaled from the laboratory to large-scale production [26]. Its main advantages include the use of inexpensive precursors, atomic-level cation mixing that ensures high compositional homogeneity, and tunability of morphology and phase purity through careful control of drying and calcination conditions [27]. Importantly, this specific high-entropy perovskite has not previously been investigated as an anode material for lithium-ion batteries, making these batteries a new potential application avenue.
By optimizing synthesis parameters, we successfully obtained a single-phase high-entropy oxide with a perovskite structure of La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3. Comparable high-entropy perovskites have been reported in the literature [14,28,29,30]. For instance, LaMn0.2Fe0.2Co0.2Ni0.2Cu0.2O3–δ was synthesized in [9] after calcination at 950 °C for 3 h as a single-phase perovskite with space group Rc:H and lattice parameters a = 5.502 Å and c = 13.235 Å. In our case, a pure perovskite phase was obtained only after calcination at 1100 °C for 1 h, as treatment at 1000 °C for 2 h proved insufficient. Nevertheless, the lattice parameters of our material were similar (a = 5.508 Å, c = 13.265 Å). In contrast, Li0.3La0.57TiO3, as reported in [31], required calcination at 900 °C for 6 h followed by sintering at 1300 °C for 12 h to achieve a single-phase perovskite. On the other hand, ref. [14] reported the same composition, La(Ni0.2Mn0.2Cu0.2Fe0.2Co0.2)O3–δ, synthesized by ball milling of hydrate precursors and subsequent calcination, yielding a nanocrystalline single-phase cubic perovskite (Pmm, a = 3.854 Å) already at 500 °C. These comparisons highlight the critical role of synthesis conditions—particularly temperature, dwelling time, and synthesis method—in dictating the structural evolution and phase stability of high-entropy perovskites, which in turn govern their functional properties.
High-entropy perovskite oxides hold promise for a wide range of applications [7,32,33], including use as anode materials in lithium-ion batteries [6,24,26,34]. Anode materials are generally classified into three categories according to their lithiation/delithiation mechanisms [35]: intercalation, conversion, and alloying. Perovskite oxides have been successfully explored in all three categories, though their mechanisms can differ significantly. For example, [(Bi,Na)1/5(La,Li)1/5(Ce,K)1/5Ca1/5Sr1/5]TiO3, as reported in [24], preserved its crystal structure during cycling, with diffraction peak shifts indicating lattice expansion due to lithium intercalation, resulting in a reversible capacity of ~80 mAh g−1. Conversely, Na0.5Bi0.5TiO3 [36] exhibited a biphasic conversion mechanism: the perovskite structure decomposed into oxides and metallic Bi, which subsequently alloyed with Li to form Li–Bi alloys, yielding ~200 mAh g−1 with a Coulombic efficiency of 98.7%.
In our study, the synthesized high-entropy perovskite delivered substantially higher specific capacities of ~530 mAh g−1, with capacity gradually increasing upon cycling. This enhancement can be attributed to electrochemical activation and structural rearrangements, which are typical of conversion-type high-entropy oxides [37,38,39]. SEM analysis (Figure 8) further supports this interpretation: after cycling, the electrode surface exhibited crater-like features and particle pulverization, leading to smaller fragments and increased surface area. Such morphological evolution likely facilitates improved electrolyte penetration and creates new electrochemically active sites, thereby enhancing specific capacity [24,40]. Additionally, crack propagation during cycling can expose fresh surfaces and promote more complete utilization of the active material. Post-mortem XRD confirmed amorphization of the perovskite phase, consistent with a conversion-type mechanism [9,41], in which oxides are reduced to metallic species during lithiation and reoxidized upon delithiation, though predominantly in amorphous structures. While these results exclude a simple intercalation process, further advanced characterization is necessary to distinguish between conversion and alloying contributions. In particular, in-operando XRD and XAS will be required to fully elucidate the lithiation mechanism, while electrochemical impedance spectroscopy (EIS) will help clarify the evolution of interfacial kinetics during cycling.
5. Conclusions
In this work, we demonstrated for the first time the synthesis of the high-entropy perovskite oxide La(Co0.2Mn0.2Fe0.2Ni0.2Cu0.2)O3 by spray drying of metal nitrates followed by calcination at different temperatures and its application as an anode material for lithium-ion batteries. Structural analysis confirmed that a single-phase perovskite (space group Rc, a = 5.508 Å, c = 13.265 Å) was obtained only after calcination at 1100 °C for 1 h, whereas lower temperatures resulted in multiphase compositions. This single-phase sample exhibited the most favorable electrochemical performance, delivering a high specific capacity of ~530 mAh g−1 with progressive activation upon cycling. Post-mortem SEM and XRD analyses revealed particle pulverization, crack formation, and amorphization, which generated new electrochemically active surfaces and accounted for the capacity increase that occurred during cycling.
The novelty of this study lies in combining spray drying with high-temperature calcination to obtain a single-phase high-entropy perovskite oxide and in demonstrating its potential as a conversion-type anode for lithium-ion batteries. These findings provide new insights into the relationship between synthesis conditions, structural evolution, and electrochemical functionality of high-entropy oxides and open promising pathways toward their further optimization for advanced energy-storage applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app151810171/s1, additional references [42,43,44,45,46,47,48,49,50] are cited in the Supplementary Materials are listed in the References section.
Author Contributions
Conceptualization, M.H. and D.C.; methodology, M.H.; validation, M.H., D.C. and R.D.; formal analysis, M.H., D.C., F.K. and G.S.; investigation, M.H., D.C., K.G., R.D. and G.S.; resources, A.F., K.S. and K.G.; data curation, M.H., D.C. and K.S.; writing—original draft preparation, M.H. and D.C.; writing—review and editing, M.H., D.C., A.F., G.S. and K.S.; visualization, M.H., D.C., G.S. and R.D.; supervision, K.S.; project administration, K.S.; funding acquisition, A.F., K.G. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovak Research and Development Agency under Contract no. APVV-23-0030, No. VV-MVP-24-0264, VEGA project No. 1/0122/25 and KEGA project No. 011TUKE-4/2025. This research was funded in part by the international project M-ERA.NET 3/2022/235/H2MobilHydride.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
During the preparation of this manuscript, the author(s) used ChatGPT–5 to assist with translation and rephrasing of text. The authors have carefully reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, X.; Liu, G.; Tang, C.; Tang, H.; Zhang, W.; Ju, Z.; Jiang, J.; Zhuang, Q.; Cui, Y. A novel high entropy perovskite fluoride anode with 3D cubic framework for advanced lithium-ion battery. J. Alloys Compd. 2023, 934, 167889. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-specific-interest/critical-raw-materials_en (accessed on 24 May 2025).
- Zhao, W.; Zhao, C.; Wu, H.; Li, L.; Zhang, C. Progress, challenge and perspective of graphite-based anode materials for lithium batteries: A review. J. Energy Storage 2024, 81, 110409. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Huang, H. The concept of high entropy for rechargeable batteries. Prog. Mater. Sci. 2025, 148, 101382. [Google Scholar] [CrossRef]
- Amiri, A.; Shahbazian-Yassar, R. Recent progress of high-entropy materials for energy storage and conversion. J. Mater. Chem. A 2021, 9, 782–823. [Google Scholar] [CrossRef]
- Ma, J.; Liu, T.; Ye, W.; He, Q.; Chen, K. High-entropy perovskite oxides for energy materials: A review. J. Energy Storage 2024, 90, 111890. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Song, Y.; Yu, J.; Tian, Y.; Robson, M.J.; Wang, J.; Zhang, Z.; Lin, X.; Zhou, G.; et al. High-Entropy Perovskites for Energy Conversion and Storage: Design, Synthesis, and Potential Applications. Small Methods 2023, 7, 2201138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Robson, M.J.; Manzotti, A.; Ciucci, F. High-entropy perovskites materials for next-generation energy applications. Joule 2023, 7, 848–854. [Google Scholar] [CrossRef]
- Han, X.; Yang, Y.; Fan, Y.; Ni, H.; Guo, Y.; Chen, Y.; Ou, X.; Ling, Y. New approach to enhance Sr-free cathode performance by high-entropy multi-component transition metal coupling. Ceram. Int. 2021, 47, 17383–17390. [Google Scholar] [CrossRef]
- Nie, S.; Wu, L.; Zhao, L.; Zhang, P.F. Enthalpy-change driven synthesis of high-entropy perovskite nanoparticles. Nano Res. 2022, 15, 4867–4872. [Google Scholar] [CrossRef]
- Meng, Z.; Gong, X.; Xu, J.; Sun, X.; Zeng, F.; Du, Z.; Hao, Z.; Shi, W.; Yu, S.; Hu, X.; et al. A General Strategy for Preparing Hollow Spherical Multilayer Structures of Oxygen-Rich Vacancy Transition Metal Oxides, Especially High Entropy Perovskite Oxides. Chem. Eng. J. 2023, 457, 141242. [Google Scholar] [CrossRef]
- Liu, X.; Ding, L.; Li, K.; Lv, J.; Wen, J.; Zhang, H.; Wang, Y.; Yao, Y.; Lei, W. The role of oxygen defects in high entropy perovskite for lithium-ion batteries. Acta Mater. 2025, 287, 120812. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, S.; Shao, X.; Cheng, J.; Lin, N.; Fang, D.; Mao, A.; Li, C. Preparation and High-Performance Lithium-Ion Storage of Cobalt-Free Perovskite High-Entropy Oxide Anode Materials. Acta Chim. Sin. 2023, 81, 486–495. [Google Scholar]
- Jia, Y.; Chen, S.; Shao, X.; Chen, J.; Fang, D.-L.; Li, S.; Mao, A.; Li, C. Synergetic Effect of Lattice Distortion and Oxygen Vacancies on High-Rate Lithium-Ion Storage in High-Entropy Perovskite Oxides. J. Adv. Ceram. 2023, 12, 1214–1227. [Google Scholar] [CrossRef]
- Shao, X.; Jia, Y.; Cheng, J.; Fang, D.; Mao, A.; Tan, J. Preparation and Electrochemical Properties of Perovskite-Type La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 High-Entropy Oxide. J. Process Eng. 2023, 23, 771–780. [Google Scholar]
- Bao, M.; Chen, S.; Shao, X.; Deng, H.; Mao, A.; Tan, J. Preparation and High-Rate Lithium-Ion Storage of Hollow Sphere Perovskite High-Entropy Oxides Assisted by Deep Eutectic Solvents. Acta Chim. Sin. 2024, 82, 303–313. [Google Scholar] [CrossRef]
- Wei, J.; Rong, K.; Li, X.; Wang, Y.; Qiao, Z.-A.; Fang, Y.; Dong, S. Deep Eutectic Solvent Assisted Facile Synthesis of Low-Dimensional Hierarchical Porous High-Entropy Oxides. Mater. Horiz. 2022, 15, 2756–2763. [Google Scholar] [CrossRef]
- Jia, Y.; Shao, X.; Cheng, J.; Wang, P.; Mao, A. Preparation and Lithium Storage Performance of Pseudocapacitance-Controlled Chalcogenide High-Entropy Oxide La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 Anode Materials. Chem. J. Chin. Univ. 2022, 43, 20220157. [Google Scholar]
- Rivas-Murias, B.; Fagnard, J.-F.; Vanderbemden, P.; Traianidis, M.; Henrist, C.; Cloots, R.; Vertruyen, B. Spray Drying: An Alternative Synthesis Method for Polycationic Oxide Compounds. J. Phys. Chem. Solids 2011, 72, 158–163. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Cryst. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Luc, P.-M.; Bauer, S.; Kowal, J. Reproducible Production of Lithium-Ion Coin Cells. Energies 2022, 15, 7949. [Google Scholar] [CrossRef]
- Guerreiro, H.M.; Melnikov, P.; Arkhangelsky, I.; de Oliveira, L.C.S.; Wandekoken, G.A.; do Nascimento, V.A. Thermal decomposition of lanthanum nitrate hexahydrate La(NO3)3∙6H2O. Int. J. Dev. Res. 2021, 11, 43318–43321. [Google Scholar]
- Yan, J.; Wang, D.; Zhang, X.; Li, J.; Du, Q.; Liu, X.; Zhang, J.; Qi, X. A high-entropy perovskite titanate lithium-ion battery anode. J. Mater. Sci. 2020, 55, 6942–6951. [Google Scholar] [CrossRef]
- Wen, S.; Li, G.; Ren, R.; Li, C. Preparation of Spherical Li4Ti5O12 Anode Materials by Spray Drying. Mater. Lett. 2015, 148, 130–133. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Wang, Y.; Li, Z.; Larring, Y.; Cai, N. Industry-Scale Production of a Perovskite Oxide as Oxygen Carrier Material in Chemical Looping. Chem. Eng. J. 2022, 431, 134006. [Google Scholar] [CrossRef]
- Stunda-Zujeva, A.; Irbe, Z.; Berzina-Cimdina, L. Controlling the Morphology of Ceramic and Composite Powders Obtained via Spray Drying—A Review. Ceram. Int. 2017, 43, 11543–11551. [Google Scholar] [CrossRef]
- Zhou, S.; Pu, Y.; Zhang, Q.; Shi, R.; Guo, X.; Wang, W.; Ji, J.; Wei, T.; Ouyang, T. Microstructure and Dielectric Properties of High Entropy Ba(Zr0.2Ti0.2Sn0.2Hf0.2Me0.2)O3 Perovskite Oxides. Ceram. Int. 2020, 46, 7430–7437. [Google Scholar] [CrossRef]
- Sharma, Y.; Zheng, Q.; Mazza, A.R.; Skoropata, E.; Heitmann, T.; Gai, Z.; Musico, B.; Miceli, P.F.; Sales, B.C.; Keppens, V.; et al. Magnetic anisotropy in single-crystal high-entropy perovskite oxide La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 films. Phys. Rev. Mater. 2020, 4, 014410. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A New Class of High-Entropy Perovskite Oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Mena, M.F.; Vasquez, F.A.; Florentin, O.; Mosa, J.; Aparicio, M.; Calderon, J.A.; Rosero-Navarro, N.C. Electrochemical performance enhancement of perovskite-type Li0.3La0.57TiO3 ceramic electrolyte by controlling synthesis parameters. J. Eur. Ceram. Soc. 2025, 45, 116972. [Google Scholar] [CrossRef]
- Cao, J.; Wu, S.; He, J.; Zhou, Y.; Ma, P. Research progress of high-entropy perovskite oxides in energy and environmental applications: A review. Particuology 2024, 95, 62–81. [Google Scholar] [CrossRef]
- Nan, H.-S.; Hu, X.-Y.; Tian, H.-W. Recent Advances in Perovskite Oxides for Anion-Intercalation Supercapacitor: A Review. Mater. Sci. Semicond. Process. 2019, 94, 35–50. [Google Scholar] [CrossRef]
- Yazhou, K.; Zhiren, Y. Synthesis, Structure and Electrochemical Properties of Al Doped High Entropy Perovskite Lix(LiLaCaSrBa)Ti1−xAlxO3. Ceram. Int. 2022, 48, 5035–5039. [Google Scholar] [CrossRef]
- Palacín, M.R. Recent Advances in Rechargeable Battery Materials: A Chemist’s Perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Chintha, S.; Atif, S.; Chaupatnaik, A.; Golubnichiy, A.; Abakumov, A.M.; Barpanda, P. Na0.5Bi0.5TiO3 perovskite anode for lithium-ion batteries. Sustain. Energy Fuels 2024, 8, 5058–5064. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Dong, Y.; Dong, K.; Wang, Z.; Luo, S.; Liu, Y.; Qi, X. Spinel-Structured High Entropy Oxide (FeCoNiCrMn)\3O4 as Anode towards Superior Lithium Storage Performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, G.; Wang, T.; Wei, Z.; Sui, Y.; Shen, B.; Qi, J.; Wei, F.; Zheng, J. High-Entropy Oxides as Advanced Anode Materials for Long-Life Lithium-Ion Batteries. Nano Energy 2022, 95, 106962. [Google Scholar] [CrossRef]
- Marques, O.J.B.J.; Walter, M.D.; Timofeeva, E.V.; Segre, C.U. Effect of Initial Structure on Performance of High-Entropy Oxide Anodes for Li-Ion Batteries. Batteries 2023, 9, 115. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.; Yoon, J.; Um, J.H.; Lee, W.; Kim, J.; Cabana, J.; Yoon, W.-S. Exploring Anomalous Charge Storage in Anode Materials for Next-Generation Li Rechargeable Batteries. Chem. Rev. 2020, 120, 6934–6976. [Google Scholar] [CrossRef]
- Ran, B.; Li, H.; Cheng, R.; Yang, Z.; Zhong, Y.; Qin, Y.; Yang, C.; Fu, C. High-Entropy Oxides for Rechargeable Batteries. Adv. Sci. 2024, 11, 2401034. [Google Scholar] [CrossRef]
- Schweidler, S.; Tang, Y.; Lin, L.; Karkera, G.; Alsawaf, A.; Bernadet, L.; Breitung, B.; Hahn, H.; Fichtner, M.; Tarancón, A.; et al. Synthesis of Perovskite-Type High-Entropy Oxides as Potential Candidates for Oxygen Evolution. Front. Energy Res. 2022, 10, 983979. [Google Scholar] [CrossRef]
- Krawczyk, P.A.; Wyrwa, J.; Kubiak, W.W. Synthesis and Catalytic Performance of High-Entropy Rare-Earth Perovskite Nanofibers: (Y0.2La0.2Nd0.2Gd0.2Sm0.2)CoO3 in Low-Temperature Carbon Monoxide Oxidation. Materials 2024, 17, 1883. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, C.; Niu, S.; Wang, C.; Sun, Y.; Su, W.; Liu, M.; Deng, Z.; Zhang, X. Effect of Calcination Temperature on the Microstructure, Composition and Properties of Nanometer Agglomerated 8YSZ Powders for Plasma Spray-Physical Vapor Deposition (PS-PVD) and Coatings Thereof. Ceram. Int. 2021, 47, 16632–16640. [Google Scholar] [CrossRef]
- Panchal, J. Cost-Benefit Analysis: Investing in a Spray Dryer. SprayDryer.com. [Online]. 2025. Available online: https://spraydryer.com/cost-benefit-analysis-investing-in-spray-dryer/ (accessed on 10 September 2025).
- KROHNE Group. 3 Ways in Which KROHNE Can Significantly Improve the Efficiency of Your Drying Process. KROHNE.com. [Online]. 2025. Available online: https://www.krohne.com/en-kg/trends/sustainability/saving-energy-in-spray-drying-applications (accessed on 10 September 2025).
- Ai, S.; Wang, B.; Li, X.; Shi, W. Analysis of a Heat Recovery System of the Spray-Drying Process in a Soy Protein Powder Plant. Appl. Therm. Eng. 2016, 103, 1022–1030. [Google Scholar] [CrossRef]
- Baker, C.G.J.; McKenzie, K.A. Energy Consumption of Industrial Spray Dryers. Drying Technol. 2005, 23, 365–386. [Google Scholar] [CrossRef]
- Fuzhou Xing Shun Da Refrigeration Facility Project Co., Ltd. The Ultimate Guide to Understanding Industrial Freeze Dryer Prices. GoodFreezeDryer.com. [Online]. 2025. Available online: https://goodfreezedryer.com/the-ultimate-guide-to-understanding-industrial-freeze-dryer-prices/ (accessed on 10 September 2025).
- KEMOLO. FD Industrial Freeze-Dryer. KEMOLO.com. [Online]. 2025. Available online: https://www.kemolo.com/products/industrial-freeze-dryer?srsltid=AfmBOoreJRVadTKnpNx3R4fp7lDvHZ3SFhUax2WX1MydjzK0G7RZzwNO&utm (accessed on 10 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).