Innovations in Orthotic Devices: Additive Manufacturing, Auxetic Materials and Smart Sensors for Enhanced Rehabilitation
Abstract
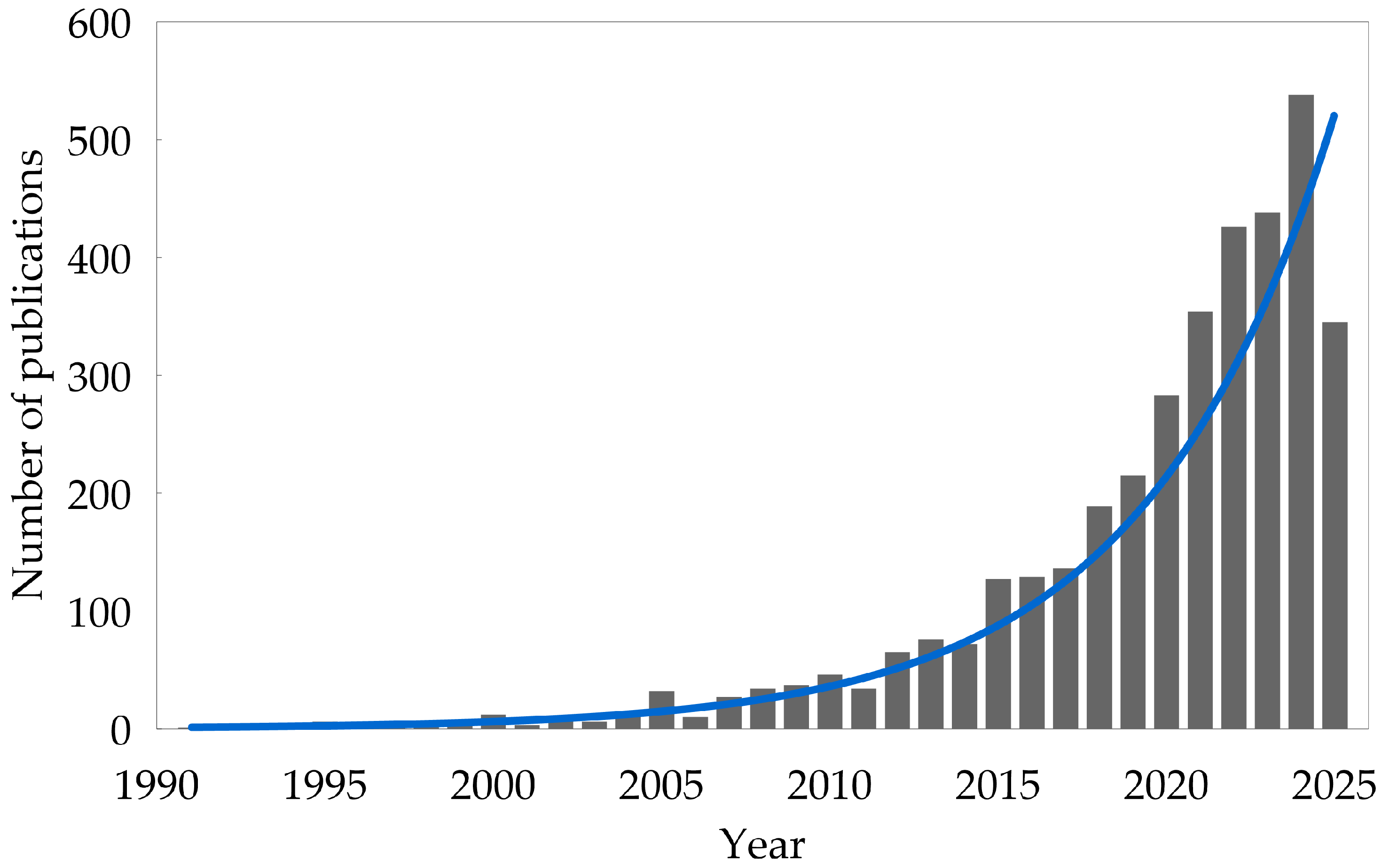
1. Introduction
- Showing how the use of auxetic metamaterials, AM, and sensors, improves orthoses performance (considering applications in any body district: upper limb, lower limb, and spinal) by analyzing the existing literature in the field;
- Highlighting the new possibilities and challenges for the full exploitation of the aforementioned technologies in the design of a new generation orthoses.
2. Joints
2.1. Anatomy
- Fibrous: These joints do not permit any movement. Bones are connected thanks to the presence of fibrous tissue, e.g., skull bones.
- Cartilaginous: In this case, the bones are joined by cartilage, e.g., intervertebral joints.
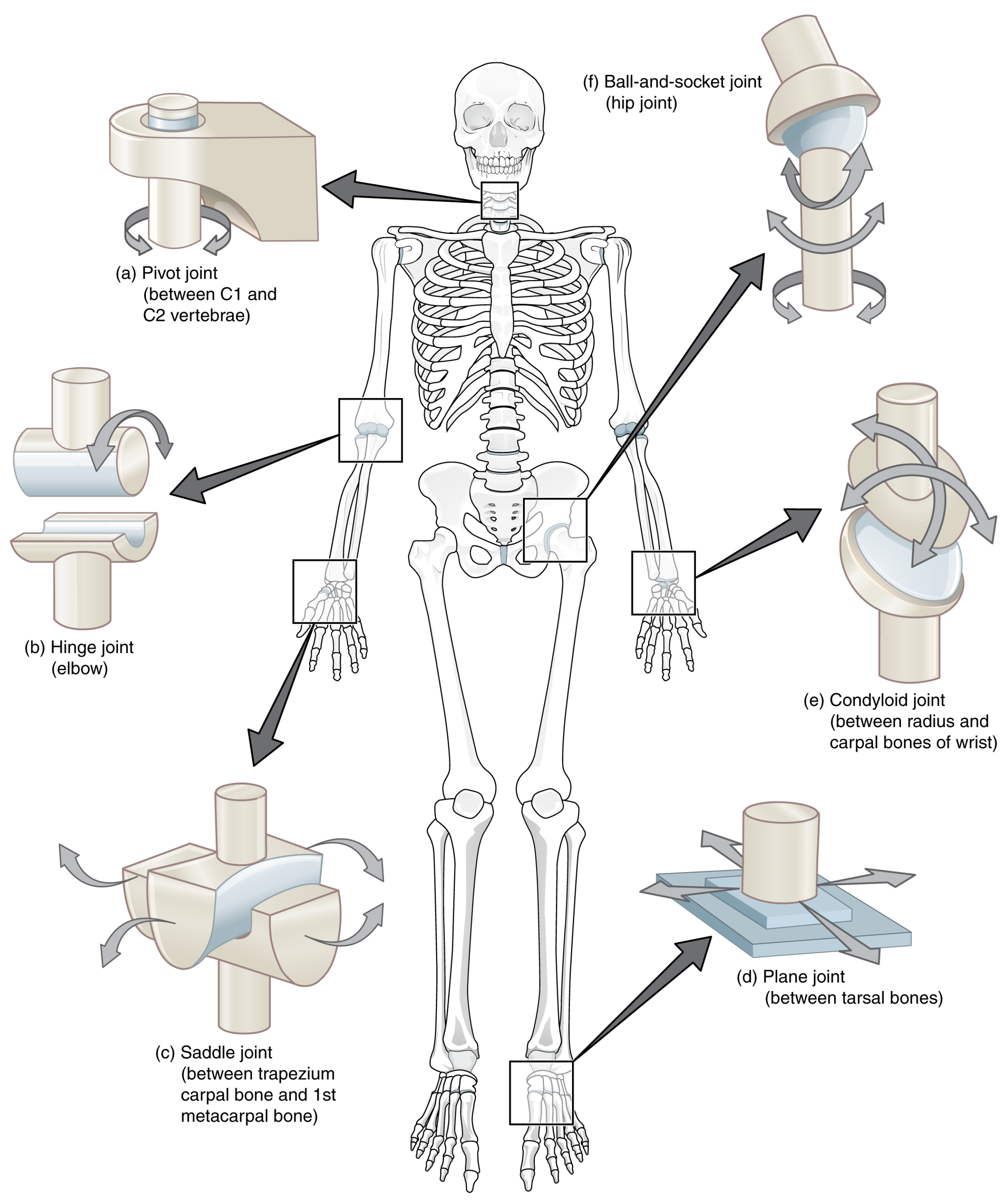
- Synovial: The most common type of joint. They make the reciprocal motion of bones possible with minimum friction. A joint capsule containing synovial fluid avoids direct contact between bones, reducing friction. There are six different types of synovial joints; see Figure 5.
- Ball and socket: This is a bone with a round end that fits with a convex bone. These joints are multi-axial and permit the maximum range of motion, an example being the shoulders and hip joints.
- Plane or gliding: Here, the only permitted movements are sliding between them and, in some cases, rotation. In this case, the motion is strictly limited by ligaments.
- Pivot: A rounded portion of a bone is enveloped by the presence of a ring formed by another bone and a ligament. The permitted motion of the bone is the rotation within this ring, hence being a uni-axial joint.
- Hinge: As its name suggests, this type of joint acts as a hinge. This joint works only for bending or straightening motions along a single axis, so they are considered uni-axial.
- Saddle: In this case, bones forming the joint have a saddle shape: concave in one direction and convex in the other. Thanks to their shape, they allow movements on two different axes to be performed, so they are classified as bi-axial joints.
- Condylar: Also known as condyloid joints, their structure resembles that of ball-and-socket joints. In this case, the joints are composed of an oval surface bone opposed to a round one. These are bi-axial joints that allow for movement to be made on the sagittal and frontal planes.
2.2. Traumatic Injuries in Joints
- Sprain [41,42]: It is a temporary dislocation with a spontaneous reduction, sometimes affecting the tendon as well. In some cases, damage to the ligamentous structure of the joint can occur. Based on the severity of the sprain, ligaments can be stretched or torn. Ligament injuries can be difficult to heal due to their poor vascularization.
- Fracture [43]: An external load applied to the bone or to the cartilage can create a discontinuity/crack in these structures. The fracture can affect the joint functionality if it happens very close to it. In such an event, usually, braces are used to immobilize bones in the correct position and to protect the damaged zone.
- Strain [44]: It can involve both muscles and tendons, and it happens when these structures are stretched in a severe way. In the case of the tendon, the action of the muscle is intense enough to elongate and damage the tendon.
3. New Technologies for Orthoses
3.1. Additive Manufacturing
- Binder jetting: A powder bed is created during the printing process, and the printing head jets the liquid binder on the powder according to the desired shape [47].
- Directed energy deposition: An electric arc or a laser is exploited to melt the feed materials locally, which is in the form of powder or wire [48].
- Material extrusion: A commonly used family of AM that involves the selective extrusion of material to obtain the printed object [49].
- Material jetting: This exploits piezo-based inkjet technology, i.e., the one used also by inkjet printers, to generate a single layer by depositing thousands of droplets at the same time. After the deposition of each layer, the entire structure is cured using UV light [52].
- Powder bed fusion: This exploits thermal energy to melt areas of the powder bed selectively. The difference between this technique and directed energy deposition is that, in this case, the powder is previously deposited on a bed, whilst the feed materials are melted as soon as they are deposited in directed energy deposition [53]. A common powder bed fusion process is Selective Laser Sintering. In this system, a thin, uniform layer of material in the form of powder is created, and a laser beam sinters the points which will compose the product locally.
- Sheet lamination: A machine deposits a sheet of materials and then heats, compresses, and cuts each layer to create the desired 3D object. It is considered one of the quickest AM techniques to create composite parts [54].
- Vat photopolymerization: This utilizes light to cure liquid photopolymers to build a 3D device [55].
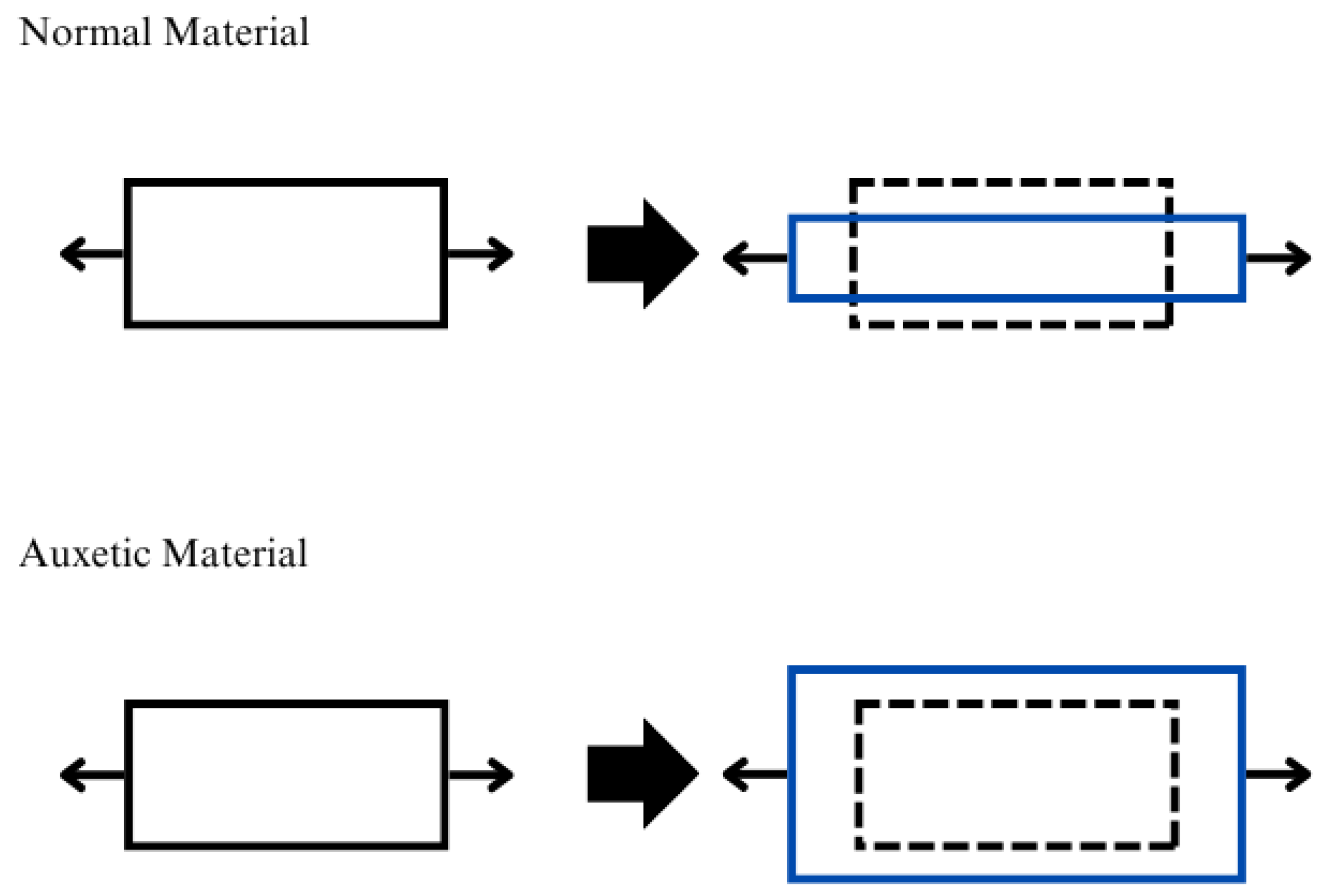
3.2. Auxetic Metamaterials
3.2.1. Properties
- Shear resistance: when loaded with shear forces, auxetic materials show more resistance than regular materials. This can be demonstrated by looking at the relation between the shear modulus (G), Poisson ratio (), and Young’s modulus (E) [68]:where it can be noted that G is inversely proportional to . In particular, when , then .
- Indentation resistance: In an indentation scenario, a conventional material would spread in the direction perpendicular to the applied load. Conversely, if the same compression acts on an auxetic material, it contracts laterally, drawing material inward toward the point of impact. This inward flow increases the local density and provides greater resistance to indentation, making auxetic materials particularly useful for applications requiring enhanced impact or puncture resistance. According to the classical theory of elasticity, the indentation resistance is correlated with material hardness (H), which is linked to the Poisson ratio () through [69]where is a parameter that considers the different indentation scenarios, i.e., for uniform pressure distribution, or is equal in value to 2/3 for Hertzian indentation. It is possible to see that when , then .
- Fracture resistance: Auxetic materials have a better fracture resistance and, generally, cracks propagate less than in bulk material [70].
- Synclastic behavior: When subjected to an out-of-plane bending moment, auxetic materials exhibit a dome shape [71]. Conversely, regular materials show a saddle shape. Exploiting this feature, it is possible to design sleeves that do not show wrinkles during limb movement [72] and also to build orthoses with a more complex and tailored shape [73], which allows them to adapt easily to the user’s body shape and to better follow joint movements.
- Variable permeability: Thanks to their porous microstructure, which changes when tensile or compressive stress is applied, auxetic materials can be suitable to build filters [74].
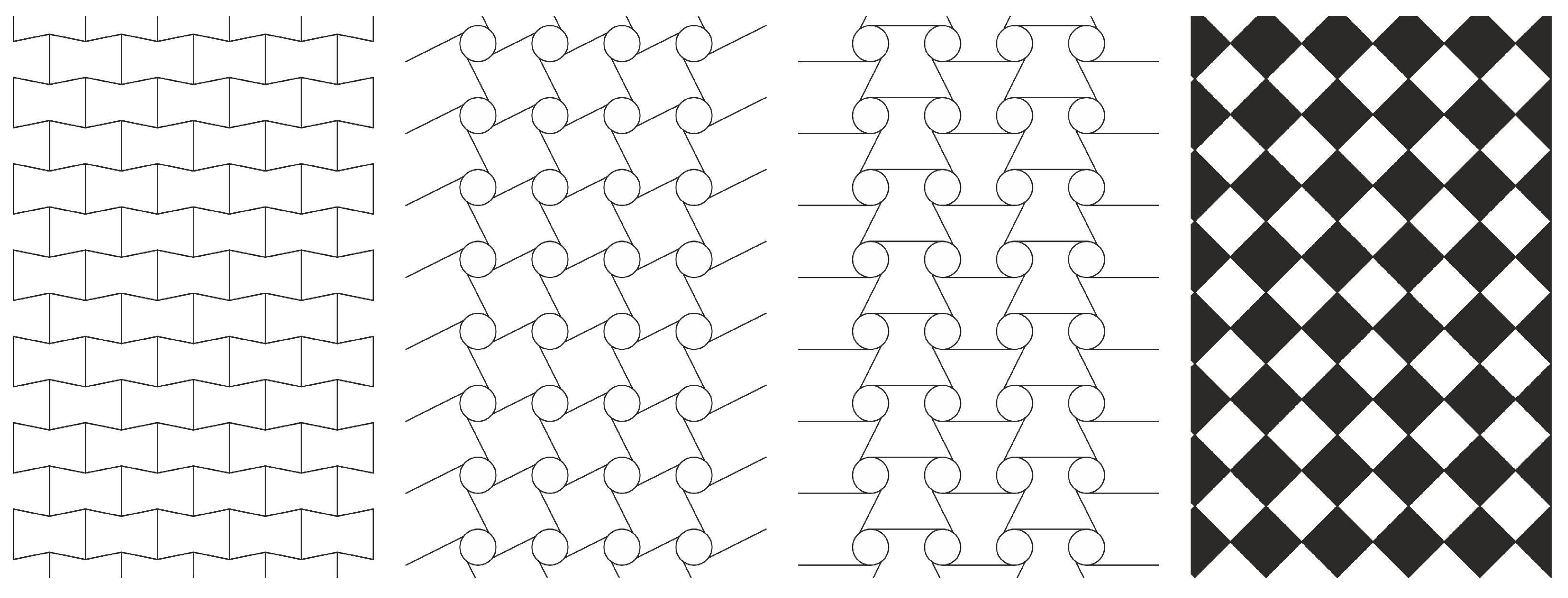
3.2.2. Auxetic Geometries
- Re-entrant structures: These structures present some ribs directed inward. In this case, the auxeticity is guaranteed by the realignment of ribs.
- Chiral structures: The unit cell is composed by a central cylinder with some ligaments connected tangentially to the cylinder. They can be constructed as right-hand or left-hand unit cells. If the lattice is composed of right-hand or left-hand cells only, then it is a proper chiral structure. Conversely, if right-hand cells are connected with left-hand cells, then it is an anti-chiral lattice. In this case latter case, the cylinder rotates when mechanically stressed, flexing in turn the ligaments. Hence, ligaments fold on the cylinder when facing a compression load, and they unfold during elongation.
- Rotating polygons: These are made by polygons connected on their vertexes by hinges. Here, the reciprocal rotation allows for an auxetic behavior to be obtained.
3.2.3. Auxetic Orthoses
- The KT by Meeusen et al. [76] shows the possibility of exploiting the negative Poisson ratio of an auxetic structure to obtain a tape that stimulates the skin in two directions. A re-entrant structure, i.e., the simplest and most studied auxhetic geometry, was here exploited. Furthermore, the synclastic characteristic of auxetic structures allows for obtaining a tape with interesting form-fitting properties, improving the adherence of the tape to the body. The tape was obtained by cutting a KT with a laser. Then, a layer of thermoplastic polyurethane (TPU) by fused filament fabrication (FFF) technology was deposited onto it.
- Hedayati et al. [77] studied the possibility of creating a tape capable of mimicking the Poisson ratio of the Achilles tendon, as it naturally shows an auxetic behavior [85]. Therefore, the authors designed a 2D auxetic structure with the same shape as the Achilles tendon. Then they discretized the tendon in many rectangular regions, and they calculated the average Poisson ratio for each of them. After that, they fine-tuned the Poisson ratio of the cell to mimic the characteristics of the tendon by changing the geometrical properties of the unit cell.
- Panico et al. [78] developed a device to aid neck muscles in performing their physiological functions, correct neck posture, and support the head. Auxetic materials were considered here due to their capability to fit the neck anatomy perfectly, and they allow for skin transpiration thanks to their perforated architecture. Furthermore, they provide support to the neck muscles while still allowing neck movement, an essential element for the healing process.
- Park et al. [79] designed a brace for people affected by carpal tunnel syndrome. It is a disease of symptomatic compression neuropathy characterized by pain, numbness, tingling, thus resulting in a reduction in hand function and grip capacity [86]. In this case, the auxetic material was used for its capability to absorb energy, and so to protect the patient from potential impacts.
- Hinrichs et al. [80] designed a heel pad to improve the Achilles tendon healing process. To reach this goal, they exploited auxetic materials to better redistribute pressure on the heel.
- Chen et al. [81]’s work consists of an insole composed of three areas. The forefoot and the heel areas were made of auxetic material, whilst the middle zone leveraged a honeycomb structure. In the middle zone, a foot arch support was inserted, which was designed to fit the patient’s foot.
- Leung et al. [82] designed a support for the heel with an auxetic material, whilst the remainder of the insole was composed of non-auxetic foam.
- Zhang et al. [83] proposed to design an insole completely made of auxetic materials.
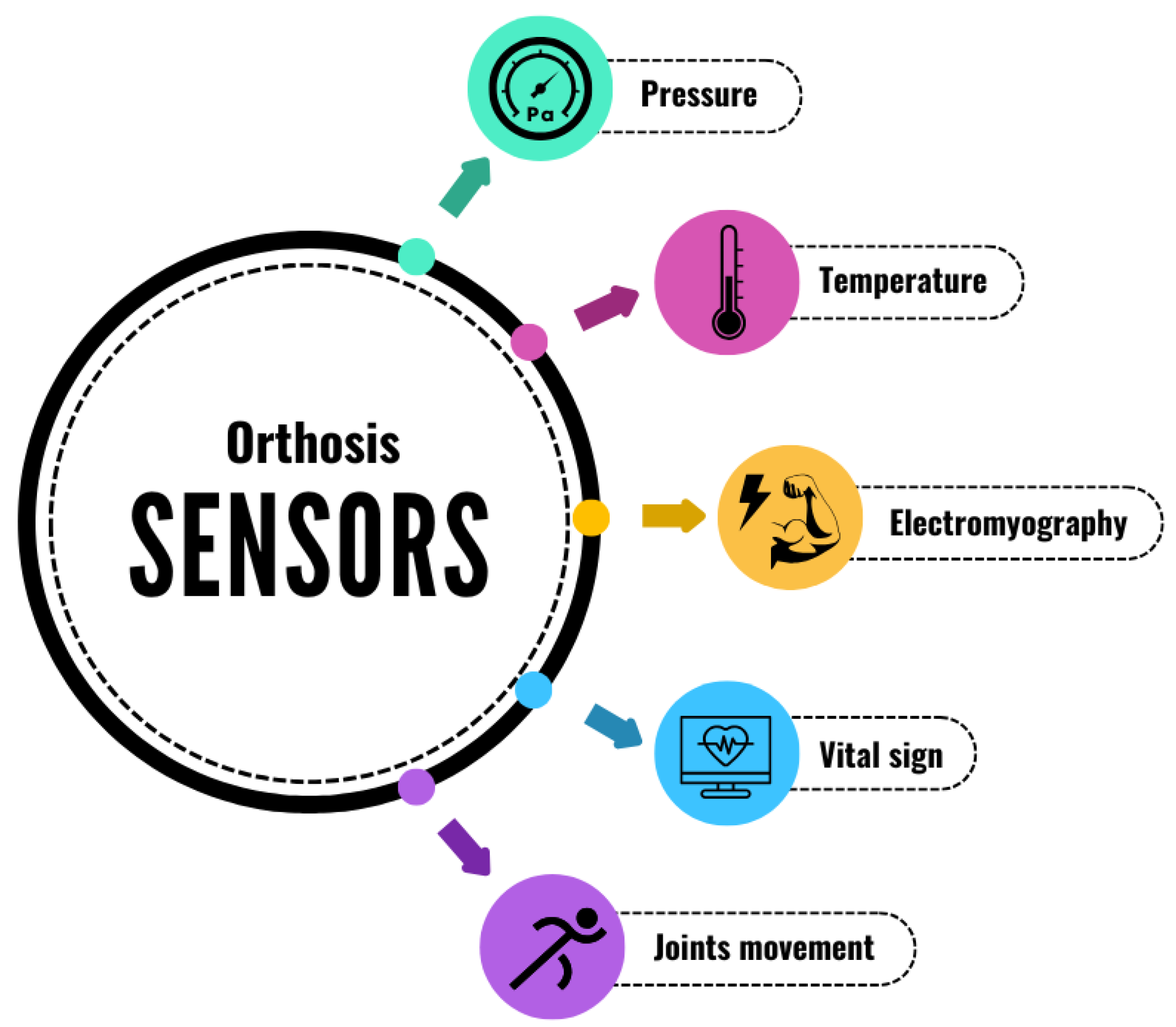
3.3. Sensors
3.3.1. Monitoring Parameters
- i.
- Range of motion (ROM): It is the measure of the maximum amplitude of movement that a joint can reach during a particular movement. Every joint has its particular ROM. This parameter is also influenced by many other individual characteristics like age, sex, physical structure, and daily activity. ROM can be altered due to injuries, joint pathologies, or incorrect posture. A good rehabilitation process aims to restore the complete ROM of the articulation.
- ii.
- Joint motion: It is conceptually similar to the ROM, but it considers how the joint moves in 3D space, therefore describing more complex movements.
- iii.
- Skeletal tracking: It is a technique that allows for the reconstruction of bone positions during movement to be obtained so that clinicians can exploit it to analyze the body posture. The study and knowledge of the body posture are of the utmost importance—an incorrect body posture can cause (or can be caused by) some joint morbidities.
3.3.2. Monitoring Tools
- Optical sensors: devices based on an optical fiber. Thanks to a laser system, light is conveyed into the optic fiber, while a detector receives the transmitted signal. It is then possible to detect the motion of the joint by post-processing the acquired data. The pros of this type of sensor are the small size (grating length, typically 1–20 mm), high resolution, flexibility, light weight, and immunity to electromagnetic noise. In recent years, many different types of monitoring systems have been introduced. For example, Pedro et al. [88] developed a device capable of measuring knee flexion. In this case, a fiber Bragg Grating (FBG) sensor was used. The FBG sensor is an optic fiber that has a grating inscribed, working in turn as a filter reflecting waves with wavelengths satisfying Bragg’s condition:where is the Bragg wavelength, is the effective refraction index of the fiber core, and finally is the grating pitch. Stretching or compressing the sensor affects the grating pitch, causing a shift in . In this case, this sensor was embedded in a polyvinyl chloride (PVC) rectangular sheet, and the knee movement during walking or running was measured by it, assessing the capability of the sensing tool. A Fabry–Pérot interferometer was used by Domingues et al. [89] to develop a sensor for ankle rehabilitation. This type of sensor exploits the presence of micro-voids, which create a resonant system reflecting light differently depending on the wavelength. When subject to a mechanical load, a change of the void shapes occurs, provoking the reflected interference to change as well. Here, the optic fiber was embedded in a polymeric matrix and then fixed on a Kinesio tape.Another type of optic sensor is a fiber optic curvature sensor. To obtain these sensors, an optic fiber is etched, usually forming teeth. These teeth are present on a single side of the fiber and impinge both the cladding and the core layer of the fiber. When they bend, the obtained gaps can be either on the convex or on the concave side. If they are on the convex side, the teeth will be more open, decreasing the capability of the fiber to transfer light. On the other hand, if the etchings are on the concave side, they will be closed, increasing in turn the intensity of the transmitted light. This kind of sensor was used by Stupar et al. [90] to develop a knee monitoring system. When using this type of technology, laser sources for the interrogating system can be avoided, opening room for employing simple and low-cost LED chips. Thanks to this feature, the authors were able to develop a wireless monitoring system.
- Textile-based sensors: In this case, an electronic sensor or a net of sensors is embedded in a textile material. These kinds of sensors are based on electromechanical sensor technology, and they can be divided into four groups based on the transduction systems [91].Firstly, piezoresistive sensors convert deformations into an electric signal. The working principle is based on the second Ohm law, i.e., the relation between electrical resistance and the shape of the conducting fiber. Therefore, any change in the conducting fiber shape will trigger a change of the resistance value. An innovative system of wearable goniometers was developed by Tognetti et al. [92] using this technology. In their study, they assessed the possibility of obtaining a goniometer that, for quasi-static measurement, shows performance that is comparable with those accepted for goniometry applications but is still worse than that of the commercial electro-goniometers. From a dynamic point of view, this device was compared with an inertial measurement unit (IMU) system, and a complete correspondence was found. Another example was shown by Ge et al. [93]. The idea behind this study was to mimic the sensing apparatus of the human skin. It highlighted the form-fitting capability of this sensor, and the possibility of monitoring the posture and joint movement was shown by applying it onto a finger. Capacitive sensors, on the other hand, take advantage of capacitors made of properly designed elastic materials to correlate strain changes with capacitance variation. Yao et al. [94] designed this kind of sensor to detect multiple stimuli, i.e., they were able to track the flexion of the thumb and the flexion of the knee when the patient was subjected to the patellar reflex test. Instead of using silver or gold nanoparticles to produce a capacitive sensor, Sheng et al. [95] employed a gallium (Ga)–indium (In)–tin (Sn) alloy. This metal is liquid at room temperature, so they painted a thin layer of metal on tape (3M VHB 4905) to build the sensor, and this was used to measure finger and wrist bending. Piezoelectric sensors exploit the piezoelectric phenomena—when the sensor is stretched, it generates an electric potential that can be measured and correlated to the imposed deformation. Guo et al. [96] investigated the opportunity of creating a device capable of communicating wirelessly by Bluetooth technology. This tool can acquire motion data and send it to the smartphone of the patient. It was tested by analyzing human motions, i.e., walking, running, and elbow flexion. Kim et al. [97] developed a sensing system inspired by ligament anatomic structure. Its capability to acquire biometric data was tested by creating a system of seven sensors that collected the movement of the entire left upper limb. A data processing algorithm was then exploited to produce an avatar that mimics the patient’s motion. The triboelectric effect is a contact electrification phenomenon. In this case, a transfer of charges between two materials when contact is made is involved. A voltage signal is formed and detected, and it is possible to store it in batteries. As a result, this type of sensor can be self-powered. C. Li et al. [98] designed a triboelectric sensor to monitor different human joints in real time. This study aimed to develop a system that could be used to monitor the spine during daily activity to prevent the occurrence of disease, to help during rehabilitation, or for diagnoses. They obtained a wireless sensor with high precision, high measurement repeatability, and high robustness to environmental noise. W. Li [99] designed a sweat-resistant triboelectric sensor. To this aim, they produced two superhydrophobic and self-cleaning triboelectric layers, taking inspiration from the hierarchical organization of the lotus leaf. This device showed excellent humidity resistance.
- Inertial measurement unit (IMU): It is a combination of three sensors (accelerators, gyroscopes, and magnetometers) and it can be used to measure 3D displacements, velocity, magnetic field vector, and energy consumption. Thanks to their high accuracy, low-cost design, and portability, they are very interesting in the orthoses framework. Nevertheless, they are sensitive to electromagnetic fields and affected by drift effect and high rigidity, which can limit their use in everyday life [91]. Bonnet et al. [100] developed an IMU-based device to monitor hip and knee joint angle during lower limb rehabilitation exercise. They proposed a novel algorithm to estimate joint dynamics using the data acquired by IMU sensors. They obtained good accuracy, though some limitations were present due to some approximation necessary to simplify calculations. Ianculescu et al. [101] introduced a novel system called “re.flex”. This system is based on two IMU sensors and a mobile application that uses sensor data to visualize real-time joint motion.
3.3.3. Biofeedback
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO:8549-3:2020; Prosthetics and Orthotics—Vocabulary—Part 3: Terms Relating to Orthoses. Technical Report; International Organization for Standardization: Geneva, Switzerland, 2020.
- World Health Organization. Priority Assistive Products List: Improving Access to Assistive Technology for Everyone, Everywhere; Technical Report; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Department of Health and Social Care. Research and Development Work Relating to Assistive Technology; Technical Report; UK Government: London, UK, 2023.
- Loor, R.B.S.; Martínez-Gómez, J.; Anchundia, J.S. Material Selection for the Development of Orthoses Using Multicriteria Methods (MCDMs) and Simulation. Processes 2025, 13, 1796. [Google Scholar] [CrossRef]
- Devanand, D.B.; Kedgley, A.E. Objective Methods of Monitoring Usage of Orthotic Devices for the Extremities: A Systematic Review. Sensors 2023, 23, 7420. [Google Scholar] [CrossRef]
- van Opstal, S.H.; van den Elzen, Y.; Jansen, M.; Willemsen, M.; Cup, E.; Groot, I.D. Facilitators and Barriers to Wearing Hand Orthoses by Adults with Duchenne Muscular Dystrophy: A Mixed Methods Study Design. J. Neuromuscul. Dis. 2020, 7, 467–475. [Google Scholar] [CrossRef]
- Philipsen, A.B.; Ellitsgaard, N.; Krogsgaard, M.R.; Sonne-Holm, S. Patient compliance and effect of orthopaedic shoes. Prosthetics Orthot. Int. 1999, 23, 59–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Boer, I.G.; Peeters, A.J.; Ronday, H.K.; Mertens, B.J.A.; Huizinga, T.W.J.; Vlieland, T.P.M.V. Assistive devices: Usage in patients with rheumatoid arthritis. Clin. Rheumatol. 2009, 28, 119–128. [Google Scholar] [CrossRef]
- Bartsch, A.; Witt, E.; Sahm, G.; Schneider, S. Correlates of objective patient compliance with removable appliance wear. Am. J. Orthod. Dentofac. Orthop. 1993, 104, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Gargiulo, P. Poor compliance with ankle-foot-orthoses in Charcot-Marie-Tooth disease. Eur. J. Phys. Rehabil. Med. 2008, 44, 27–31. [Google Scholar]
- Koyuncu, E.; Yüzer, G.F.N.; Çam, P.; Özgirgin, N. Investigating the status of using lower extremity orthoses recommended to patients with spinal cord injury. Spinal Cord 2016, 54, 996–1000. [Google Scholar] [CrossRef]
- Devanand, D.B.; Gardiner, M.D.; Kedgley, A.E. A Compact Orthosis Compliance Monitoring Device Using Pressure Sensors and Accelerometers: Design and Proof-of-Concept Testing. Sensors 2025, 25, 1352. [Google Scholar] [CrossRef] [PubMed]
- Lakes, R. Foam Structures with a Negative Poisson’s Ratio. Science 1987, 235, 1038–1040. [Google Scholar] [CrossRef]
- Jin, Y.; Qi, C.; Tian, H.; Dai, S.; Wang, X.; Ning, H.; Jiang, D. Optimal design of a novel nested metamaterial with hybrid auxetic-locally resonant band gap for suppressing robotic grinding vibration. Mech. Adv. Mater. Struct. 2024, 1–12. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, Z.; Yang, X.; Zhang, Y.; Chen, T.; Chen, Y.; Wang, Y. Multistable Soft Robots Assembled from Bistable Auxetic Building Blocks. Adv. Intell. Syst. 2024, 7, 2400529. [Google Scholar] [CrossRef]
- Giubilini, A.; Minetola, P. Multimaterial 3D printing of auxetic jounce bumpers for automotive suspensions. Rapid Prototyp. J. 2023, 29, 131–142. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Xu, C.; Ren, J.; Zhong, J. Auxetic meta-materials and their engineering applications: A review. Eng. Res. Express 2023, 5, 042003. [Google Scholar] [CrossRef]
- Yang, X.; Ma, J.; Wen, D.; Yang, J. Crashworthy design and energy absorption mechanisms for helicopter structures: A systematic literature review. Prog. Aerosp. Sci. 2020, 114, 100618. [Google Scholar] [CrossRef]
- Ghavidelnia, N.; Bodaghi, M.; Hedayati, R. Femur Auxetic Meta-Implants with Tuned Micromotion Distribution. Materials 2020, 14, 114. [Google Scholar] [CrossRef]
- Amin, F.; Ali, M.N.; Ansari, U.; Mir, M.; Minhas, M.A.; Shahid, W. Auxetic Coronary Stent Endoprosthesis: Fabrication and Structural Analysis. J. Appl. Biomater. Funct. Mater. 2015, 13, 127–135. [Google Scholar] [CrossRef]
- Mehmood, S.; Ali, M.N.; Ansari, U.; Mir, M.; Khan, M.A. Auxetic polymeric bone plate as internal fixator for long bone fractures: Design, fabrication and structural analysis. Technol. Health Care 2015, 23, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Flamourakis, G.; Spanos, I.; Vangelatos, Z.; Manganas, P.; Papadimitriou, L.; Grigoropoulos, C.; Ranella, A.; Farsari, M. Laser-made 3D Auxetic Metamaterial Scaffolds for Tissue Engineering Applications. Macromol. Mater. Eng. 2020, 305, 2000238. [Google Scholar] [CrossRef]
- Betz, J.; Klingspor, C.; Seel, T. IMU-based Assessment of Ankle Inversion Kinematics and Orthosis Migration. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6395–6400. [Google Scholar] [CrossRef]
- Xiao, M.; Yao, Y.; Fan, C.; Xu, Z.; Liu, Y.; Liu, B.; Li, J.; Zhang, X.; Jin, X.; Yang, J.; et al. Multiple H-bonding chain extender-based polyurethane: Ultrastiffness, hot-melt adhesion, and 3D printing finger orthosis. Chem. Eng. J. 2022, 433, 133260. [Google Scholar] [CrossRef]
- Cantu, E.; Fapanni, T.; Giorgi, G.; Narduzzi, C.; Sardini, E.; Serpelloni, M.; Tonello, S. Printed Multi-EMG Electrodes on the 3D Surface of an Orthosis for Rehabilitation: A Feasibility Study. IEEE Sens. J. 2021, 21, 14407–14417. [Google Scholar] [CrossRef]
- Menz, H.B.; Bonanno, D.R. Objective measurement of adherence to wearing foot orthoses using an embedded temperature sensor. Med. Eng. Phys. 2021, 88, 19–24. [Google Scholar] [CrossRef]
- Ngui, I.R.Y.; Bowden, J.; Jones, S.L.; Daebeler, R.; Causby, R.S. Measurement of plantar pressure differences in the contralateral limb when using offloading modalities for diabetic foot ulcerations. J. Foot Ankle Res. 2025, 18, e70028. [Google Scholar] [CrossRef]
- Resch, S.; Schauer, J.; Schwind, V.; Völz, D.; Sanchez-Morillo, D. Improving Social Acceptance of Orthopedic Foot Orthoses Through Image-Generative AI in Product Design. Appl. Sci. 2025, 15, 4132. [Google Scholar] [CrossRef]
- Mikołajewska, E.; Mikołajewski, D.; Mikołajczyk, T.; Paczkowski, T. A Breakthrough in Producing Personalized Solutions for Rehabilitation and Physiotherapy Thanks to the Introduction of AI to Additive Manufacturing. Appl. Sci. 2025, 15, 2219. [Google Scholar] [CrossRef]
- Kumar, A.; Chhabra, D. Parametric topology optimization approach for sustainable development of customized orthotic appliances using additive manufacturing. Mech. Adv. Mater. Struct. 2024, 31, 5276–5289. [Google Scholar] [CrossRef]
- Ariyasinghe, N.; Dahanayake, U.; Samarasinghe, K.; Samarakoon, D.; Krishara, J.; Weerathunga, I. Enhancing Diabetic Shoe Insole Customization with AI-Driven Approaches. In Proceedings of the 2023 5th International Conference on Advancements in Computing (ICAC), Colombo, Sri Lanka, 7–8 December 2023; pp. 149–154. [Google Scholar] [CrossRef]
- Tang, D. Hybridized Hierarchical Deep Convolutional Neural Network for Sports Rehabilitation Exercises. IEEE Access 2020, 8, 118969–118977. [Google Scholar] [CrossRef]
- Adans-Dester, C.; Hankov, N.; O’Brien, A.; Vergara-Diaz, G.; Black-Schaffer, R.; Zafonte, R.; Dy, J.; Lee, S.I.; Bonato, P. Enabling precision rehabilitation interventions using wearable sensors and machine learning to track motor recovery. Npj Digit. Med. 2020, 3, 121. [Google Scholar] [CrossRef]
- Zhu, Z.A.; Lu, Y.C.; You, C.H.; Chiang, C.K. Deep Learning for Sensor-Based Rehabilitation Exercise Recognition and Evaluation. Sensors 2019, 19, 887. [Google Scholar] [CrossRef] [PubMed]
- Shefa, F.R.; Sifat, F.H.; Uddin, J.; Ahmad, Z.; Kim, J.M.; Kibria, M.G. Deep Learning and IoT-Based Ankle–Foot Orthosis for Enhanced Gait Optimization. Healthcare 2024, 12, 2273. [Google Scholar] [CrossRef]
- Bogusz, A. Zintegrowana Platforma Edukacyjna Ministerstwa Edukacji Narodowej. Available online: https://zpe.gov.pl/a/jak-sie-poruszamy/D16e6tMZE (accessed on 1 April 2025).
- Sawhney, S.; Aggarwal, A. Pediatric Rheumatology A Clinical Viewpoint, 1st ed.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Betts, J.G.; Young, K.A.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H.; Korol, O.; Johnson, J.E.; Womble, M.; DeSaix, P. Anatomy and Physiology; OpenStax: Houston, TX, USA, 2013. [Google Scholar]
- Lange, J.; Menefee, W.; Jenks, J.; Mazzasette, C.; Nguyen, K.L. Synovial Joints. Available online: https://med.libretexts.org/Bookshelves/Anatomy_and_Physiology/Human_Anatomy_%28Lange_et_al.%29/07%3A_Joints/7.03%3A_Synovial_Joints (accessed on 1 April 2025).
- Sokoloff, L. Joint Disease. Available online: https://www.britannica.com/science/joint-disease (accessed on 1 April 2025).
- Kelly, B.M.; Patel, A.T.; Dodge, C. Upper Limb Orthotic Devices. In Braddom’s Physical Medicine and Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 209–228. [Google Scholar] [CrossRef]
- Bundy, M.; Leaver, A. Musculoskeletal injury. In A Guide to Sports and Injury Management; Elsevier: Amsterdam, Netherlands, 2010; pp. 77–102. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Elmagd, M.A. Common sports injuries. Int. J. Phys. Educ. Sports Health 2016, 3, 142–148. [Google Scholar]
- Kiran, A.S.K.; Veluru, J.B.; Merum, S.; Radhamani, A.V.; Doble, M.; Kumar, T.S.S.; Ramakrishna, S. Additive manufacturing technologies: An overview of challenges and perspective of using electrospraying. Nanocomposites 2018, 4, 190–214. [Google Scholar] [CrossRef]
- ISO/ASTM 52900:2021(en); Additive Manufacturing—General Principles—Fundamentals and Vocabulary. Technical report; American Society for Testing and Materials; International Organization for Standardization: Geneva, Switzerland, 2021.
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Svetlizky, D.; Das, M.; Zheng, B.; Vyatskikh, A.L.; Bose, S.; Bandyopadhyay, A.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Directed energy deposition (DED) additive manufacturing: Physical characteristics, defects, challenges and applications. Mater. Today 2021, 49, 271–295. [Google Scholar] [CrossRef]
- Lotfizarei, Z.; Mostafapour, A.; Barari, A.; Jalili, A.; Patterson, A.E. Overview of debinding methods for parts manufactured using powder material extrusion. Addit. Manuf. 2023, 61, 103335. [Google Scholar] [CrossRef]
- Diegel, O.; Nordin, A.; Motte, D. Additive Manufacturing Technologies. In A Practical Guide to Design for Additive Manufacturing; Springer: Singapore, 2019; pp. 19–39. [Google Scholar] [CrossRef]
- Rouf, S.; Malik, A.; Singh, N.; Raina, A.; Naveed, N.; Siddiqui, M.I.H.; Haq, M.I.U. Additive manufacturing technologies: Industrial and medical applications. Sustain. Oper. Comput. 2022, 3, 258–274. [Google Scholar] [CrossRef]
- Chen, K.J.; Elkaseer, A.; Scholz, S.G.; Hagenmeyer, V. On the correlation between pre-processing workflow and dimensional accuracy of 3D printed parts in high-precision Material Jetting. Addit. Manuf. 2024, 91, 104335. [Google Scholar] [CrossRef]
- Friel, R. Power ultrasonics for additive manufacturing and consolidating of materials. In Power Ultrasonics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 313–335. [Google Scholar] [CrossRef]
- Frketic, J.; Dickens, T.; Ramakrishnan, S. Automated manufacturing and processing of fiber-reinforced polymer (FRP) composites: An additive review of contemporary and modern techniques for advanced materials manufacturing. Addit. Manuf. 2017, 14, 69–86. [Google Scholar] [CrossRef]
- Arshad, Q.; Saqib, M.; Arshad, M.A.; Raza, M.; Hussain, M.I.; Asghar, A.; Luo, X.; Chen, Z. Progress in vat photopolymerisation additive manufacturing of ceramic lattice structures and applications. Thin-Walled Struct. 2025, 209, 112918. [Google Scholar] [CrossRef]
- Dal Maso, A.; Cosmi, F. ScienceDirect 3D-printed ankle-foot orthosis: A design method. Mater. Today Proc. 2019, 12, 252–261. [Google Scholar] [CrossRef]
- Oud, T.; Kerkum, Y.; Groot, P.; Gijsbers, H.; Nollet, F.; Brehm, M. Production time and user satisfaction of 3-dimensional printed orthoses for chronic hand conditions compared with conventional orthoses: A prospective case series. J. Rehabil. Med.-Clin. Commun. 2021, 4, jrmcc00049. [Google Scholar] [CrossRef]
- Vasiliauskaite, E.; Paepegem, W.V.; Deckers, J.P.; Vermandel, M.; Forward, M.; Plasschaert, F. Additive Manufacturing of Ankle-Foot Orthosis with Predefined Ankle Stiffness—A Case Report. Jpo J. Prosthetics Orthot. 2020, 32, 310–318. [Google Scholar] [CrossRef]
- Popișter, F.; Dragomir, M.; Ciudin, P.; Ștefan Goia, H. Empowering Rehabilitation: Design and Structural Analysis of a Low-Cost 3D-Printed Smart Orthosis. Polymers 2024, 16, 1303. [Google Scholar] [CrossRef] [PubMed]
- Banga, H.K.; Belokar, R.M.; Kalra, P.; Kumar, R. Fabrication and stress analysis of ankle foot orthosis with additive manufacturing. Rapid Prototyp. J. 2018, 24, 301–312. [Google Scholar] [CrossRef]
- Portnoy, S.; Barmin, N.; Elimelech, M.; Assaly, B.; Oren, S.; Shanan, R.; Levanon, Y. Automated 3D-printed finger orthosis versus manual orthosis preparation by occupational therapy students: Preparation time, product weight, and user satisfaction. J. Hand Ther. 2020, 33, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, Y.; Ruan, K.; Guo, H.; He, M.; Shi, X.; Guo, Y.; Kong, J.; Gu, J. Advances in 3D printing for polymer composites: A review. InfoMat 2024, 6, e12568. [Google Scholar] [CrossRef]
- Valentine, A.D.; Busbee, T.A.; Boley, J.W.; Raney, J.R.; Chortos, A.; Kotikian, A.; Berrigan, J.D.; Durstock, M.F.; Lewis, J.A. Hybrid 3D Printing of Soft Electronics. Adv. Mater. 2017, 29, 1703817. [Google Scholar] [CrossRef]
- Veselago, V.G. The electrodynamics of substances with simultaneously negative values of epsilon and mu. Sov. Phys. Uspekhi 1968, 10, 509–514. [Google Scholar] [CrossRef]
- Pendry, J.B.; Holden, A.J.; Stewart, W.J.; Youngs, I. Extremely Low Frequency Plasmons in Metallic Mesostructures. Phys. Rev. Lett. 1996, 76, 4773–4776. [Google Scholar] [CrossRef]
- Ren, X.; Das, R.; Tran, P.; Ngo, T.D.; Xie, Y.M. Auxetic metamaterials and structures: A review. Smart Mater. Struct. 2018, 27. [Google Scholar] [CrossRef]
- Lee, J.H.; Singer, J.P.; Thomas, E.L. Micro-/nanostructured mechanical metamaterials. Adv. Mater. 2012, 24, 4782–4810. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.M.; Shi, W.; Xie, B.H.; Yang, M.B. Review on auxetic materials. J. Mater. Sci. 2004, 39, 3269–3279. [Google Scholar] [CrossRef]
- Lakes, R.; Elms, K. Indentability of Conventional and Negative Poisson’s Ratio Foams. J. Compos. Mater. 1993, 27, 1193–1202. [Google Scholar] [CrossRef]
- Bezazi, A.; Boukharouba, W.; Scarpa, F. Mechanical properties of auxetic carbon/epoxy composites: Static and cyclic fatigue behaviour. Phys. Status Solidi (B) 2009, 246, 2102–2110. [Google Scholar] [CrossRef]
- Lakes, R.S.; Witt, R. Making and Characterizing Negative Poisson’s Ratio Materials. Int. J. Mech. Eng. Educ. 2002, 30, 50–58. [Google Scholar] [CrossRef]
- Hedayati, R.; Güven, A.; Zwaag, S.V.D. 3D gradient auxetic soft mechanical metamaterials fabricated by additive manufacturing. Appl. Phys. Lett. 2021, 118, 141904. [Google Scholar] [CrossRef]
- Konaković, M.; Crane, K.; Deng, B.; Bouaziz, S.; Piker, D.; Pauly, M. Beyond developable: Computational design and fabrication with auxetic materials. In Proceedings of the ACM Transactions on Graphics. Assoc. Comput. Mach. 2016, 35, 89. [Google Scholar] [CrossRef]
- Alderson, A.; Rasburn, J.; Ameer-Beg, S.; Mullarkey, P.G.; Perrie, W.; Evans, K.E. An auxetic filter: A tuneable filter displaying enhanced size selectivity or defouling properties. Ind. Eng. Chem. Res. 2000, 39, 654–665. [Google Scholar] [CrossRef]
- Kolken, H.M.; Zadpoor, A.A. Auxetic mechanical metamaterials. RSC Adv. 2017, 7, 5111–5129. [Google Scholar] [CrossRef]
- Meeusen, L.; Candidori, S.; Micoli, L.L.; Guidi, G.; Stanković, T.; Graziosi, S. Auxetic structures used in kinesiology tapes can improve form-fitting and personalization. Sci. Rep. 2022, 12, 13509. [Google Scholar] [CrossRef]
- Hedayati, R.; Yousefi, A.; Dezaki, M.L.; Bodaghi, M. Analytical relationships for 2D Re-entrant auxetic metamaterials: An application to 3D printing flexible implants. J. Mech. Behav. Biomed. Mater. 2023, 143, 105938. [Google Scholar] [CrossRef]
- Panico, M.; Langella, C.; Santulli, C. Development of a biomedical neckbrace through tailored auxetic shapes. Emerg. Sci. J. 2017, 1, 105–117. [Google Scholar] [CrossRef]
- Park, Y.E.; Lee, H.; Jung, I.; Lee, S. Characterization of 3D Printed Wrist Brace with Various Tilting Angles of Re-entrant Pattern Using Thermoplastic Elastomer. J. Korean Soc. Cloth. Text. 2022, 46, 1074–1087. [Google Scholar] [CrossRef]
- Hinrichs, A.; Malukhina, K.; Sharma, I.; Vierra, M. Active Auxetic Heel Support for Achilles Tendon Therapy. Master’s Thesis, Santa Clara University, Santa Clara, CA, USA, 2018. [Google Scholar]
- Chen, T.; Tian, M.; Wang, X. A Novel Porous Structural Design of the Orthotic Insole for Diabetic Foot. In Proceedings of the 2021 International Conference on Computer, Control and Robotics (ICCCR), Shanghai, China, 8–10 January 2021; pp. 188–192. [Google Scholar] [CrossRef]
- Leung, M.S.h.; lun Yick, K.; Sun, Y.; Chow, L.; pui Ng, S. 3D printed auxetic heel pads for patients with diabetic mellitus. Comput. Biol. Med. 2022, 146, 105582. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Lin, Y.; Wang, Y.; Yi, X.; Fang, W. Pressure-Reducing Design of 3D-Printed Diabetic Shoe Midsole Utilizing Auxetic Lattice Structure. Appl. Sci. 2024, 14, 5291. [Google Scholar] [CrossRef]
- Lishnevsky, S. Supporting corset for use in wounds and disorders of the lower abdomen and lumbar region. Gospital’noe delo 1945, 12, 49. [Google Scholar]
- Gatt, R.; Vella Wood, M.; Gatt, A.; Zarb, F.; Formosa, C.; Azzopardi, K.M.; Casha, A.; Agius, T.P.; Schembri-Wismayer, P.; Attard, L.; et al. Negative Poisson’s ratios in tendons: An unexpected mechanical response. Acta Biomater. 2015, 24, 201–208. [Google Scholar] [CrossRef]
- Burton, C.; Chesterton, L.S.; Davenport, G. Diagnosing and managing carpal tunnel syndrome in primary care. Br. J. Gen. Pract. 2014, 64, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.I.; Majumder, S.; Mondal, T.; Cowan, D.; Naseh, S.; Deen, M.J. Monitoring methods of human body joints: State-of-the-art and research challenges. Sensors 2019, 19, 2629. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.P.; da Silva, A.F. FBG in PVC Foils for Monitoring the Knee Joint Movement During the Rehabilitation Process. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, Ma, USA, 30 August–3 September 2011; pp. 458–461. [Google Scholar]
- Domingues, M.D.F.; Rosa, V.; Nepomuceno, A.C.; Tavares, C.; Alberto, N.; Andre, P.; Radwan, A.; Antunes, P.F.D.C. Wearable Devices for Remote Physical Rehabilitation Using a Fabry-Perot Optical Fiber Sensor: Ankle Joint Kinematic. IEEE Access 2020, 8, 109866–109875. [Google Scholar] [CrossRef]
- Stupar, D.Z.; Bajic, J.S.; Manojlovic, L.M.; Slankamenac, M.P.; Joza, A.V.; Zivanov, M.B. Wearable low-cost system for human joint movements monitoring based on fiber-optic curvature sensor. IEEE Sens. J. 2012, 12, 3424–3431. [Google Scholar] [CrossRef]
- De Fazio, R.; Mastronardi, V.M.; De Vittorio, M.; Visconti, P. Wearable Sensors and Smart Devices to Monitor Rehabilitation Parameters and Sports Performance: An Overview. Sensors 2023, 23, 1856. [Google Scholar] [CrossRef]
- Tognetti, A.; Lorussi, F.; Mura, G.; Carbonaro, N.; Pacelli, M.; Paradiso, R.; Rossi, D. New generation of wearable goniometers for motion capture systems. J. Neuroeng. Rehabil. 2014, 11, 56. [Google Scholar] [CrossRef]
- Ge, J.; Sun, L.; Zhang, F.; Zhang, Y.; Shi, L.; Zhao, H.; Zhu, H.; Jiang, H.; Yu, S. A Stretchable Electronic Fabric Artificial Skin with Pressure-, Lateral Strain-, and Flexion-Sensitive Properties. Adv. Mater. 2016, 28, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhu, Y. Wearable multifunctional sensors using printed stretchable conductors made of silver nanowires. Nanoscale 2014, 6, 2345–2352. [Google Scholar] [CrossRef]
- Sheng, L.; Teo, S.; Liu, J. Liquid-Metal-Painted Stretchable Capacitor Sensors for Wearable Healthcare Electronics. J. Med Biol. Eng. 2016, 36, 265–272. [Google Scholar] [CrossRef]
- Guo, W.; Tan, C.; Shi, K.; Li, J.; Wang, X.X.; Sun, B.; Huang, X.; Long, Y.Z.; Jiang, P. Wireless piezoelectric devices based on electrospun PVDF/BaTiO3 NW nanocomposite fibers for human motion monitoring. Nanoscale 2018, 10, 17751–17760. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Jo, G.; Kim, J.S.; Lim, M.T.; Cha, Y. Tendon-Inspired Piezoelectric Sensor for Biometric Application. IEEE/ASME Trans. Mechatron. 2021, 26, 2538–2547. [Google Scholar] [CrossRef]
- Li, C.; Liu, D.; Xu, C.; Wang, Z.; Shu, S.; Sun, Z.; Tang, W.; Wang, Z.L. Sensing of joint and spinal bending or stretching via a retractable and wearable badge reel. Nat. Commun. 2021, 12, 2950. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, L.; Kottapalli, A.G.P.; Pei, Y. Bioinspired sweat-resistant wearable triboelectric nanogenerator for movement monitoring during exercise. Nano Energy 2022, 95, 107018. [Google Scholar] [CrossRef]
- Bonnet, V.; Joukov, V.; Kulić, D.; Fraisse, P.; Ramdani, N.; Venture, G. Monitoring of Hip and Knee Joint Angles Using a Single Inertial Measurement Unit during Lower Limb Rehabilitation. IEEE Sens. J. 2016, 16, 1557–1564. [Google Scholar] [CrossRef]
- Ianculescu, M.; Andrei, B.; Alexandru, A. A Smart Assistance Solution for Remotely Monitoring the Orthopaedic Rehabilitation Process Using Wearable Technology: re.flex System. Stud. Inform. Control 2019, 28, 317–326. [Google Scholar] [CrossRef]
- Campanella, C.E.; Cuccovillo, A.; Campanella, C.; Yurt, A.; Passaro, V.M.N. Fibre Bragg Grating Based Strain Sensors: Review of Technology and Applications. Sensors 2018, 18, 3115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, S.; Xu, G.; Wang, C.; Tan, C. Joint angle measurement of manipulator and error compensation based on an IMU sensor. J. Eng. 2019, 2019, 9001–9005. [Google Scholar] [CrossRef]
- Merkle, T.P.; Hofmann, N.; Knop, C.; Silva, T.D. Biofeedback’s Effect on Orthosis Use: Insights from Continuous Six-Week Monitoring of Ankle Fracture Loading. Sensors 2025, 25, 825. [Google Scholar] [CrossRef]
- Eldemir, K.; Eldemir, S.; Ozkul, C.; Guclu-Gunduz, A. The immediate efficacy of the spinomed orthosis and biofeedback posture orthosis on balance and gait in older people with thoracic hyperkyphosis. Gait Posture 2024, 111, 136–142. [Google Scholar] [CrossRef]
- Hsu, C.C.; Huang, Y.K.; Kang, J.H.; Ko, Y.F.; Liu, C.W.; Jaw, F.S.; Chen, S.C. Novel design for a dynamic ankle foot orthosis with motion feedback used for training in patients with hemiplegic gait: A pilot study. J. Neuroeng. Rehabil. 2020, 17, 112. [Google Scholar] [CrossRef]
- Christanell, F.; Hoser, C.; Huber, R.; Fink, C.; Luomajoki, H. The influence of electromyographic biofeedback therapy on knee extension following anterior cruciate ligament reconstruction: A randomized controlled trial. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2012, 4, 41. [Google Scholar] [CrossRef] [PubMed]

| Synovial Joint | Anatomic Reference | Number of Rotation Axis | Permitted Movement |
|---|---|---|---|
| Ball and socket | Shoulder (glenohumeral), hip (acetabulofemoral) | 3 | sagittal, frontal, horizontal |
| Plane | Intercarpal, acromioclavicular | none | varies |
| Pivot | Radioulnar, atlantoaxial | 1 | horizontal |
| Hinge | Elbow (humeroulnar), knee (tibiofemoral) | 2 | sagital, frontal |
| Saddle | Carpometacarpal, sternoclavicular | 2 | sagittal, frontal |
| Condylar | Wrist (radiocarpal), atlanto-occipital | 2 | sagittal, forntal |
| Year | Device | Geometry | Reference |
|---|---|---|---|
| 2017 | Neck Brace | Fractal unit cell | Panico et al. [78] |
| 2018 | Heel Pads | Re-entrant | Hinrichs et al. [80] |
| 2021 | Insole | Re-entrant | Zhang et al. [83] |
| 2021 | Insole | Rotating Square | Chen et al. [81] |
| 2022 | Insole | Re-entrant | Leung et al. [82] |
| 2022 | Kinesio Tape | Re-entrant | Meeusen et al. [76] |
| 2022 | Wrist Brace | Re-entrant | Park et al. [79] |
| 2023 | Kinesio Tape | Re-entrant | Hedayati et al. [77] |
| Sensor | Dimensions | Connection | Reference |
|---|---|---|---|
| Optical sensor | 125 m | Physicalconnection with an interrogator | [88,89,90] |
| Textile-based sensor | Dimensions depend on a sensor design—a sensor net is embedded in a device | Physical or wireless connection with processing unit | [92,93,94,95,96,97,98,99] |
| IMU | 40 × 30 × 15 mm | Physical or wireless connection with processing unit | [100,101] |
| Aspect | Orthotic Devices | |
|---|---|---|
| Traditional | Innovative | |
| Technology Used | Conventional materials (plastics, metals, foams) and handmade (casts) | Auxetic materials, smart sensors, additive manufacturing |
| Customization | Standardized sizes with some manual adjustments or handmade casts | Highly personalized through 3D scanning and additive manufacturing |
| Manufacturing Process | Labor intensive, requires skilled professionals for customized ones, industrial process for the others | Automated with additive manufacturing |
| Cost | Often lower due to mass production | Potentially higher due to advanced materials and technology |
| Effectiveness | Proven through decades of clinical use | Promising but requires more clinical validation |
| Availability | Widely available, covered by insurance in many cases | Limited availability, adoption still in progress |
| Patient Comfort | May lack ergonomic design, less adaptive materials | Improved fit, lighter, breathable materials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroni, R.C.; Majewska, K. Innovations in Orthotic Devices: Additive Manufacturing, Auxetic Materials and Smart Sensors for Enhanced Rehabilitation. Appl. Sci. 2025, 15, 10167. https://doi.org/10.3390/app151810167
Moroni RC, Majewska K. Innovations in Orthotic Devices: Additive Manufacturing, Auxetic Materials and Smart Sensors for Enhanced Rehabilitation. Applied Sciences. 2025; 15(18):10167. https://doi.org/10.3390/app151810167
Chicago/Turabian StyleMoroni, Riccardo Carlo, and Katarzyna Majewska. 2025. "Innovations in Orthotic Devices: Additive Manufacturing, Auxetic Materials and Smart Sensors for Enhanced Rehabilitation" Applied Sciences 15, no. 18: 10167. https://doi.org/10.3390/app151810167
APA StyleMoroni, R. C., & Majewska, K. (2025). Innovations in Orthotic Devices: Additive Manufacturing, Auxetic Materials and Smart Sensors for Enhanced Rehabilitation. Applied Sciences, 15(18), 10167. https://doi.org/10.3390/app151810167








