Apricot Seeds as a Sustainable Source of Nutrients and Bioactive Compounds with Health-Relevant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Nutrient Composition
2.3. Phenolic Compounds Extraction
2.4. Total Phenolic Content
2.5. Antioxidant Capacity
2.6. High-Performance Liquid Chromatography (HPLC) Analyses of Amygdalin
2.6.1. Extraction
2.6.2. HPLC Quantification
2.7. Data Analysis
3. Results
3.1. Chemical Composition of Common and Ecological Apricot Seeds
3.2. Total Polyphenols and Antioxidant Activity of Common and Ecological Apricot Seeds
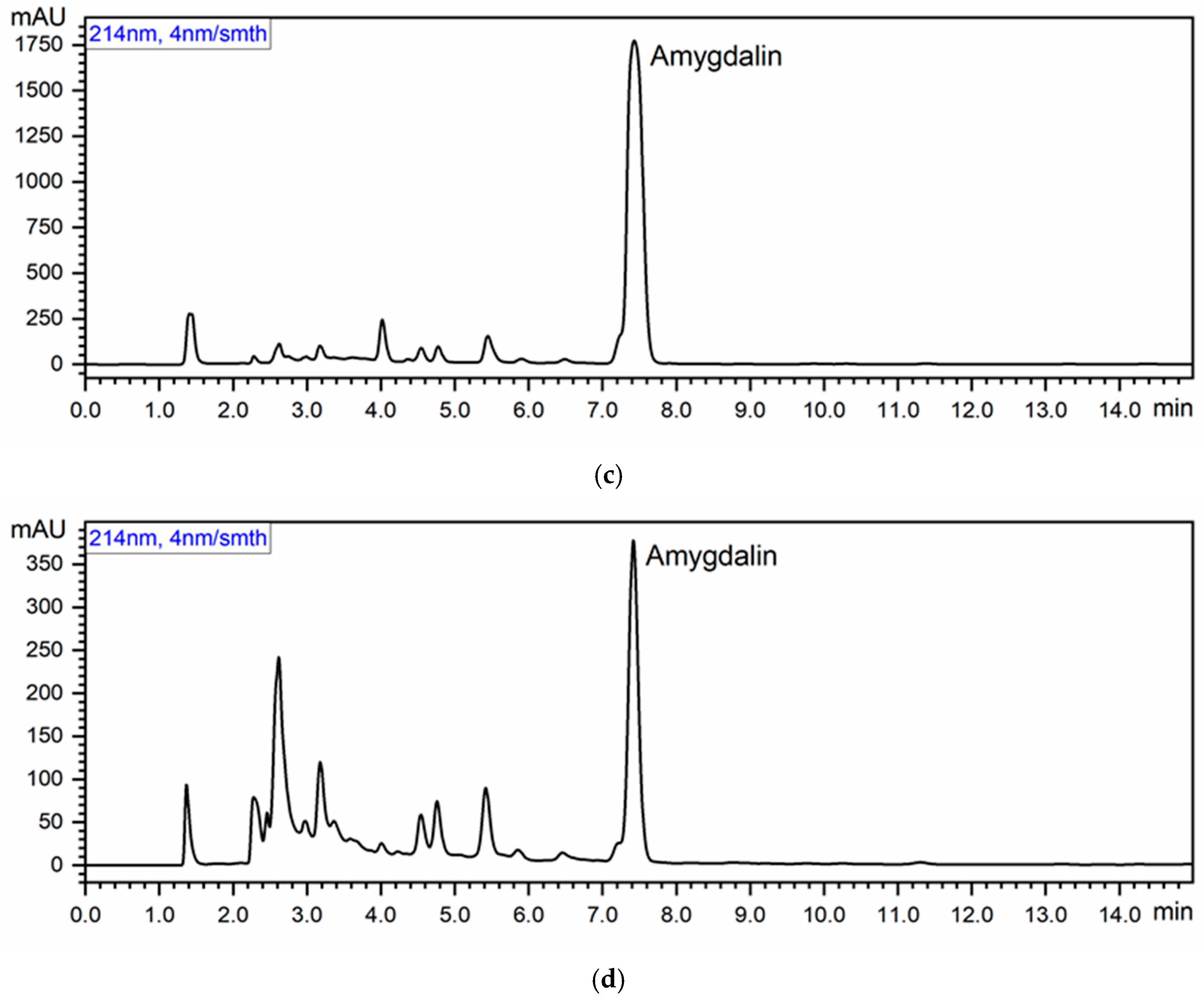
3.3. Amygdalin Content of Common and Ecological Apricot Seeds
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Meng, T.; Ren, F.; Ning, A.; Chen, B.; Liu, X.; Liu, H. A review on apricot kernel seed proteins and peptides: Biological functions and food applications. Int. J. Biol. Macromol. 2025, 292, 139053. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak-Wilke, E.; Polkowska, Z.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeuticand Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Kolesarova, A.; Baldovska, S.; Roychoudhury, S. The Multiple Actions of Amygdalin on Cellular Processes with an Emphasis on Female Reproduction. Pharmaceuticals 2021, 14, 881. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.J.; Cho, K.H.; Jung, W.S.; Moon, S.K.; Park, E.K.; Kim, D.H. Biotransformation of ginsenoside Rb1, crocin, amygdalin, geniposide, puerarin, ginsenoside Re, hesperidin, poncirin, glycyrrhizin and baicalin by human fecal microflora and its relation to cytotoxicity against tumor cells. J. Microbiol. Biotechnol. 2008, 18, 1109–1114. [Google Scholar]
- Song, Z.; Xu, X. Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 2014, 10, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Azi, F.; Li, Z.; Xu, P. Expanding Yarrowia lipolytica’s Metabolic Potential for Detoxification of Cyanogenic Glycosides in Edible Plants. Commun. Biol. 2025, 8, 188. [Google Scholar] [CrossRef]
- Zhang, C.X.; Guo, P.H.; Zhang, Q.A.; García Martín, J.F. Effects of Different Pressing Methods on Physicochemical Properties and Volatile Compounds of the Apricot Kernel Oil. J. Food Meas. Charact. 2024, 18, 8767–8781. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Q.; Xu, M.; Xia, Q.; Zheng, T.; Teng, J.; Li, M.; Fan, L. Recent updates and future perspectives about amygdalin as a potential anticancer agent: A review. Cancer Med. 2019, 8, 3004–3011. [Google Scholar] [CrossRef]
- Makarevic, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Reiter, M.; Tsaur, I.; Bartsch, G.; Haferkamp, A.; Blaheta, R. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS ONE 2014, 9, e105590. [Google Scholar] [CrossRef]
- Makarevic, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Tsaur, I.; Nelson, K.; Pfitzenmaier, J.; Haferkamp, A.; Blaheta, R. Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS ONE 2014, 9, e110244. [Google Scholar] [CrossRef]
- Qian, L.; Xie, B.; Wang, Y.; Qian, J. Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 5363–5370. [Google Scholar] [PubMed]
- Lin, S.; Wen, J.; Xu, X.; Shi, J.; Zhang, W.; Zheng, T.; Hou, Y.; Zhang, Y.; Li, Z.; Wang, K.; et al. Amygdalin Induced Mitochondria-Mediated Apoptosis of Lung Cancer Cells via Regulating NFκB-1/NFκB Signaling Cascade in Vitro and in Vivo. Am. J. Chin. Med. 2022, 50, 1361–1386. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Wang, F.; Hu, J.; Cui, A.; Wei, C.; Yang, Q.; Li, F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol. 2013, 35, 43–51. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, S.H.; Han, L.S.; Zheng, L.T.; Jung, K.H.; Uhm, Y.K.; Lee, J.H.; Jeong, J.S.; Joo, W.S.; Yim, S.V.; et al. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol. 2005, 11, 5156–5161. [Google Scholar] [CrossRef]
- Dimitrov, M.; Iliev, I.; Bardarov, K.; Georgieva, D.; Todorova, T. Phytochemical characterization and biological activity of apricotkernels’ extract in yeast-cell based tests and hepatocellular and colorectal carcinoma cell lines. J. Ethnopharmacol. 2021, 279, 114333. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Shin, M.S.; Yang, H.Y.; Lee, J.W.; Kim, Y.S.; Lee, M.H.; Kim, J.; Kim, K.H.; Kim, C.J. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expression in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. 2006, 29, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, I.; Thomas, A.; Monecke, M.M.; Zugelder, M.; Rutz, J.; Grein, T.; Maxeiner, S.; Xie, H.; Chun, F.K.-H.; Florian Rothweiler, F.; et al. Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells. Cancers 2022, 14, 3111. [Google Scholar] [CrossRef] [PubMed]
- Hee-Young, K.; Seon-Pyo, H.; Dong-Hoon, H.; Jeong Hee, K. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch. Pharm. Res. 2003, 26, 157–161. [Google Scholar] [CrossRef]
- Lee, H.M.; Moon, A. Amygdalin regulates apoptosis and adhesion in Hs578T triplenegative breast cancer cells. Biomol. Ther. 2016, 24, 62–66. [Google Scholar] [CrossRef]
- Cecarini, V.; Salima Selmi, S.; Cuccioloni, M.; Gong, C.; Bonfili, L.; Zheng, Y.; Cortese, M.; Angeletti, M.; Kilani, S.; Eleuteri, A.M. Targeting Proteolysis with Cyanogenic Glycoside Amygdalin Induces Apoptosis in Breast Cancer Cells. Molecules 2022, 27, 7591. [Google Scholar] [CrossRef]
- Juengel, E.; Afschar, M.; Makarevic, J.; Rutz, J.; Tsaur, I.; Mani, J.; Nelson, K.; Haferkamp, A.; Blaheta, R. Amygdalin blocks in vitro adhesion and invasion of renal cel carcinoma cells by an integrin-dependent mechanism. Int. J. Mol. Med. 2016, 37, 843–850. [Google Scholar] [CrossRef]
- Arshi, A.; Hosseini, S.M.; Hosseini, F.S.K.; Amiri, Z.Y.; Hosseini, F.S.; Lavasani, M.S.; Kerdarian, H.; Dehkordi, M.S. The anti-cancer effect of amygdalin on human cancer cell lines. Mol. Biol. Rep. 2019, 46, 2059–2066. [Google Scholar] [CrossRef]
- Mamdouh, A.M.; Khodeer, D.M.; Tantawy, M.A.; Moustafa, Y.M. In-vitro and in-vivo investigation of amygdalin, metformin, andcombination of both against doxorubicin on hepatocellular carcinoma. Life Sci. 2021, 285, 119961. [Google Scholar] [CrossRef] [PubMed]
- Aamazadeh, F.; Ostadrahimi, A.; Saadat, Y.R.; Barar, J. Bitter apricot ethanolic extract induces apoptosis through increasing expression of Bax/Bcl-2 ratio and caspase-3 in PANC-1 pancreatic cancer cells. Mol. Biol. Rep. 2020, 47, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Swain, T.; Hillis, W.E. The phenolic constituens of Prunus domestica. The quantitative analysis of phenolic constituents. J. Agric. Food Chem. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bolarinwa, I.; Orfila, C.; Morgan, M. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gunther, A.; Bednarczyk-Cwyna, R.B.; Turała, A. Usprawniona metoda ekstrakcji amigdaliny z nasion pestek moreli (Improved method for extracting amigdalin from apricot kernel seeds.). Apar. Badaw. Dydakt. 2018, 4, 164–167. [Google Scholar]
- Sikora, E.; Liszka, P. Składniki odżywcze i nie odżywcze w surowych i przetworzonych orzeszkach ziemnych (Arachis hipogea) (Nutrients and non-nutrients in raw and processed peanuts (Arachis hypogea). Bromatol. Chem. Toksykol. 2011, 4, 1047–1053. [Google Scholar]
- Agunbiade, S.O.; Olanlokun, J.O. Evaluation of some nutritional characteristics of Indian almond (Prunus amygdalus) nut. Pak. J. Nutr. 2006, 5, 316–318. [Google Scholar] [CrossRef]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Vasudev, S.; Chandran, D.; Lorenzo, J.M. Chemical characterization of apricot kernel: Nutraceutical composition, amino acid, and fatty acid profile. Food Anal. Methods. 2022, 15, 2594–2604. [Google Scholar] [CrossRef]
- Gezer, I.; Hacıseferoğulları, H.; Özcan, M.M.; Arslan, D.; Asma, B.M.; Ünver, A. Physico-chemical properties of apricot (Prunus armeniaca L.) kernels. South-West. J. Horticult. Biol. Environ. 2011, 2, 1–13. [Google Scholar]
- Chen, Y.; Al-Ghamdi, A.A.; Elshikh, M.S.; Shah, M.H.; Al-Dosary, M.A.; Abbasi, A.M. Phytochemical profiling, antioxidant and HepG2 cancer cells’ antiproliferation potential in the kernels of apricot cultivars. Saudi J. Biol. Sci. 2020, 27, 163–172. [Google Scholar] [CrossRef]
- Mesarović, J.; Trifković, J.; Tosti, T.; Akšić, M.F.; Milatović, D.; Ličina, V.; Milojković-Opsenica, D. Relationship between ripen-ing time and sugar content of apricot (Prunus armeniaca L.) kernels. Acta Physiol. Plant 2018, 40, 157. [Google Scholar] [CrossRef]
- Han, Z.P.; Liu, R.L.; Cui, H.Y.; Zhang, Z.Q. Microwave-assisted extraction and LC/MS analysis of phenolic antioxidants in sweet apricot (Prunus armeniaca L.) kernel skins. J. Liquid Chromatogr. Relat. Technol. 2013, 36, 2182–2195. [Google Scholar] [CrossRef]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Składu I Wartości Odżywczej Żywności (Tables of Composition and Nutritional Value of Foods); Wydawnictwo Lekarskie PZWL (Medical Publishing House PZWL): Warszawa, Poland, 2020. [Google Scholar]
- Ciemniewska, H.; Ratusz, K. Charakterystyka orzechów laskowych trzech odmian leszczyny uprawianej w Polsce (Characteristics of hazelnuts from three hazelnut varieties grown in Poland). Rośl. Oleiste 2012, 33, 273–283. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Krygier, K.; Bryś, J. Wartość odżywcza orzechów oraz ich znaczenie w diecie (Nutritional value of nuts and their importance in the diet). Technol. Prog. Food Process. 2014, 1, 90–96. [Google Scholar]
- Korekar, G.; Stobdan, T.; Arora, R.; Yadav, A.; Singh, S.B. Antioxidant capacity and phenolics content of apricot (Prunus armeniaca L.) kernel as a function of genotype. Plant Foods Hum. Nutr. 2011, 66, 376–383. [Google Scholar] [CrossRef] [PubMed]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods. 2010, 2, 196–209. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. LWT-Food Sci. Technol. 2010, 30, 254–259. [Google Scholar] [CrossRef]
- Yigit, D.; Yigit, N.; Mavi, D. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunas armeniaca L.) kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Angmo, P.; Angmo, S.; Upadhyay, S.S.; Targais, K.; Kumar, B.; Stobdan, T. Apricots (Prunus armeniaca L.) of trans-Himalayan Ladakh: Potential candidate for fruit quality breeding programs. Sci. Hortic. 2017, 218, 187–192. [Google Scholar] [CrossRef]
- El-Hajjaji, M.A.; Fikri-Benbrahim, K.; Soulo, N.; Nouioura, G.; Laaroussi, H.; Ferreira-Santos, P.; Lyoussi, B.; Benziane Ouaritini, Z. Analgesic, Antioxidant, Anti-Inflammatory, and Wound-Treating Actions of Bitter Apricot Kernel Extract. Adv. Pharm. Pharm. Sci. 2024, 2024, 5574259. [Google Scholar] [CrossRef] [PubMed]

| Analysed Parameter [g/100 g] | Polish Common Apricot Seeds | Uzbekistan Ecological Apricot Seeds | Armenian Common Apricot Seeds |
|---|---|---|---|
| Dry matter | 94.48 ± 0.03 c | 93.97 ± 0.03 b | 90.62 ± 0.11 a |
| Protein | 25.24 ± 0.06 b | 26.96 ± 0.46 c | 23.52 ± 0.02 a |
| Fat | 37.87 ± 0.47 a | 39.89 ± 1.53 a | 36.81 ± 0.22 a |
| Ash | 2.71 ± 0.00 a | 2.76 ± 0.02 a | 3.00 ± 0.00 b |
| Total Carbohydrates | 28.65 ± 0.69 b | 27.29 ± 0.40 a | 24.36 ± 1.71 ab |
| [mg/100 g] | |||
| Amygdalin | 879.62 ± 0.15 c | 558.65 ± 0.89 b | 89.10 ± 1.68 a |
| Analysed Parameter | Polish Common Apricot Seeds | Uzbekistan Ecological Apricot Seeds | Armenian Common Apricot Seeds |
|---|---|---|---|
| Polyphenol content [mg/100 g] | 65.08 ± 0.81 a | 91.81 ± 0.54 b | 171.21 ± 1.62 c |
| Antioxidant activity | |||
| ABTS | |||
| [µmol Trolox/g] | 12.05 ± 0.12 a | 12.56 ± 0.33 a | 15.04 ± 0.35 b |
| [mmol Trolox/100 g] | 1.21 ± 0.012 a | 1.26 ± 0.033 a | 1.50 ± 0.035 b |
| Variables Compared | Pearson Correlation Coefficient (r) | Correlation Strength | Direction |
|---|---|---|---|
| Polyphenol content vs. Antioxidant activity | 0.996437 | Very strong | Positive |
| Amygdalin content vs. Antioxidant activity | −0.977540 | Very strong | Negative |
| Amygdalin content vs. Polyphenol content | −0.991830 | Very strong | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borczak, B.; Kapusta-Duch, J.; Dziadek, K.; Sikora, E. Apricot Seeds as a Sustainable Source of Nutrients and Bioactive Compounds with Health-Relevant Properties. Appl. Sci. 2025, 15, 10154. https://doi.org/10.3390/app151810154
Borczak B, Kapusta-Duch J, Dziadek K, Sikora E. Apricot Seeds as a Sustainable Source of Nutrients and Bioactive Compounds with Health-Relevant Properties. Applied Sciences. 2025; 15(18):10154. https://doi.org/10.3390/app151810154
Chicago/Turabian StyleBorczak, Barbara, Joanna Kapusta-Duch, Kinga Dziadek, and Elżbieta Sikora. 2025. "Apricot Seeds as a Sustainable Source of Nutrients and Bioactive Compounds with Health-Relevant Properties" Applied Sciences 15, no. 18: 10154. https://doi.org/10.3390/app151810154
APA StyleBorczak, B., Kapusta-Duch, J., Dziadek, K., & Sikora, E. (2025). Apricot Seeds as a Sustainable Source of Nutrients and Bioactive Compounds with Health-Relevant Properties. Applied Sciences, 15(18), 10154. https://doi.org/10.3390/app151810154









