Abstract
In addition to widely consumed dietary seeds, interest has been increasingly directed towards underutilised seeds, such as apricot kernels. These contain both beneficial nutrients and harmful compounds, such as amygdalin, a cyanogenic glycoside with still-debated effects. The aim of this study was to determine the proximate composition, antioxidant activity, and amygdalin content in apricot seeds of various origins. HPLC analyses were conducted to determine amygdalin content, together with the chemical composition (AOAC), antioxidant activity (ABTS), and the total polyphenols content of these seeds (Folin–Ciocalteu reagent). The apricot seed samples demonstrated considerable variability in their composition, revealing generally high levels of protein, fat, ash, carbohydrates, total polyphenols, and antioxidants. The amygdalin content was determined at the level of 89.1 mg/100 g in apricot seeds from Armenia compared to 879.6 mg/100 g in apricot seeds purchased at retail in Poland. In the tested apricot seeds, the highest content of polyphenols, the highest antioxidant activity, and, concurrently, the lowest content of amygdalin were found in apricot seeds from Armenia. Apricot seeds, especially those that originated from Armenia, might be a future sustainable source of crucial nutrients, characterised by satisfactory antioxidative properties and a low content of potentially toxic amygdalin.
1. Introduction
Both consumers and scientists have increasingly focused on alternative plant protein sources in response to the global challenge of protein shortage. This insufficiency affects approximately 25% of the population, resulting in malnutrition in nearly 2 billion people [1]. Among the commonly used seeds and grains in healthy diets—such as cereals, lentils, nuts, and oilseeds—there is increasing interest in underutilised kernels of stone fruits, such as apricot kernels. These kernels contain both nutrients and antinutritional compounds. A notable example is the presence of cyanogenic glycosides, particularly amygdalin, the biological role of which remains controversial. In general, cyanogenic glycosides are found in about 2000 plant species belonging to 110 families, mainly Rosaceae, Poaceae, Linaceae, Papilionaceae, Euphorbiaceae, and Crucuferae [2]. It is an organic compound of the glycoside family, primarily associated with the rose family (Rosaceae Juss.) and the passion fruit family (Passifloraceae Juss. Ex Kunth in Humb) [3]. It is found in more than 1200 plant species, especially in the seeds of commonly available fruits, e.g., apricots, plums, cherries, and almonds [1,2,3,4]. The substance is present in different concentrations in natural raw materials. The highest concentration of amygdalin has been detected in the kernels of apricot, at up to 5.2% (on a dry weight basis), as well as in bitter almonds and cherry kernels at 3–5%. It undertakes the function of chemical protection in plants against herbivores and insects, while its breakdown product, hydrogen cyanide, exhibits toxic properties; 59 mg HCN can be released from 1 g of amygdalin [2,3]. The consumption of products containing amygdalin may result in the ingestion of toxic levels of cyanide ions, which can consequently lead to poisoning [2,4]. Hydrogen cyanide is absorbed into the body not only through the gastrointestinal tract, but also through the skin and lungs [4]. Processes of hydrogen cyanide poisoning are a highly individual issue, exhibiting inter-species as well as inter-individual differences [5,6]. Cyanide can be removed from edible plant matrices through a range of conventional, natural, physical, and chemical methods, including washing, peeling, drying, fermentation, thermal processing, and chemical treatment. However, the application of these techniques in food processing remains limited due to several drawbacks. Chemical methods may introduce toxic residues, while natural and physical approaches often lack consistency, precision, and overall efficiency. As a result, their scalability and reliability are constrained. In recent years, the use of food-grade microorganisms to enzymatically degrade cyanogenic glycosides has emerged as the most promising, safe, and effective strategy for the detoxification of cyanide in edible plant products [7]. At the same time, apricot seeds, traditionally regarded as by-products of the fruit industry, are now being explored for their diverse applications within the food sector and beyond. They may be incorporated into a wide range of food products, including yoghurts, ice creams, biscuits, and cakes. Furthermore, apricot seed press cake can be effectively utilised in the formulation of protein-enriched, plant-based milk and milk powder alternatives. The physicochemical properties of apricot kernel oil can be affected by processing methods, including pressing techniques, which influence the quality and volatile compounds of the oil [8]. Beyond the food sector, apricot seeds are also being investigated for their potential applications in thermal energy storage systems, as well as in the cosmetics and pharmaceutical industries [1].
Additionally, there is also the potential benefit of the presence of amygdalin in kernels. Over time, this compound has been used in the unconventional treatment of various diseases, particularly cancer [2].
Recently, a considerable amount of research has been conducted on the effects of amygdalin on various cell lines, including cancer [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. The analyses of studies published to date on this subject reveal an apparent trend. Amygdalin inhibits the proliferation of cancer cells, induces their apoptosis, and reduces their ability to metastasise [3,9]. To date, in vitro studies have been conducted using the following human cell lines: bladder cancer (lines RT122, UMUC-3, TCCSUP) [10,11], non-small-cell lung cancer (lines H1299, PA, A549) [12,13], cervical cancer (line HeLa) [14], colon cancer (line SNU-C4) [15,16], prostate cancer (lines DU145 and LNCaP) [17,18], promyelocytic leukaemia (line HL-60) [19], breast cancer (lines MDA-MB-231, MCF-7, Hs578T) [21,22], kidney cancer (lines A498, Caki-1 and KTC 26) [22], gastric cancer and others (AGS line and others) [23,24] and pancreatic cancer (PANC-1 line) [25]. Moreover, according to the latest results, apricot seeds have potential as a valuable protein source with a content varying between 18% and 34% depending on their kind and origin, together with bioactive peptides that have antioxidative, anti-inflammatory, hypoglycaemic, and lowering blood pressure properties [1]. There are, however, limited studies in the literature on the content of macronutrients (protein, fat, ash, and carbohydrates) in apricot seeds originating from different cultivations, including conventional and ecological sources, together with their possible antioxidative properties and the amount of antinutrients such as amygdalin. Therefore, the aim of this study was to determine the proximate composition, total polyphenol content, amygdalin, and antioxidant activity of apricot seeds of different origins, including conventional and ecological cultivations.
2. Materials and Methods
2.1. Material Preparation
The research material consisted of three types of apricot seeds obtained from fruits purchased in 2022 from different sources: (1) a locally situated market in Kraków, Poland, during the summer season, which is referred to as a common apricot; (2) a health food store, with fruits originating from Uzbekistan, which is referred to as an ecological apricot; and (3) a supermarket offering fruits grown and sold in Armenia, which is referred to as Armenia. All samples belonged to the species Prunus armeniaca L. However, due to the lack of certified varietal information provided by the vendors, the specific cultivars could not be identified. After the fruits were cut into, the fruit kernels were collected and stored in low-density polyethylene bags (PE-LD) with zip-lock closures to protect them from air exposure, and they were kept for approximately 14 days in a dry area without access to light at 4 °C until analyses. All the seeds for testing were used prior to their minimum shelf life expiring. Before the analyses, the appropriate amounts of seeds were ground in an electric grinder. The analyses of the apricot seeds were conducted using the seeds in their natural, unroasted state.
2.2. Nutrient Composition
The contents of dry matter (DM), protein, fat, and ash in the seeds were evaluated using standardised procedures in accordance with the guidelines provided by the Association of Official Analytical Chemists (AOAC) [26]. The moisture content was determined via the oven-drying technique (AOAC Method 940.26), using a laboratory dryer (SML 30/250, Zalmed, Warsaw, Poland) at 105 °C for 24 h. The ash content was measured by incinerating pre-weighed samples at 550 °C for 8 h in a muffle furnace (FCF5SH, Czylok, Jastrzębie-Zdrój, Poland), following the AOAC Method 930.05. The crude fat content was extracted with petroleum ether as the solvent using the automated Soxhlet method (AOAC Method 930.09) with the Soxtec Avanti 2050 Auto Extraction Unit (Tecator, Hillerød, Sweden). The protein content (calculated as nitrogen × 6.25) was quantified using the Kjeldahl method with an FOSS Digester and Kjeltec™ 8200 Autodistillation Unit (Tecator Foss, Hillerød, Sweden), in accordance with the AOAC Method 978.04. The carbohydrate content was estimated by difference using the following formula: 100 − (moisture + ash + protein + fat).
2.3. Phenolic Compounds Extraction
The ground seed samples (5 g) were subjected to extraction (by maceration) with 80 mL of a 70% methanol solution (Sigma-Aldrich, St. Louis, MO, USA) at 25 °C for 2 h, and then centrifugation in a laboratory centrifuge (MPW-352R, MPW Med. Instruments, Warsaw, Poland) (1500× g, 15 min). The supernatants were collected and stored at −20 °C until further analysis. Three extracts were prepared from each variety of the apricot kernels, which were subsequently used to analyse the total polyphenol content and antioxidant activity in three parallel replicates (n = 9).
2.4. Total Phenolic Content
The total polyphenol content in the seeds was determined using the method described by Swain and Hillis [27] with the use of Folin–Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO, USA). The previously prepared methanol extracts (Section 2.3) were diluted in the proportion (1:20), and 5 mL of the aliquots was incubated with Folin–Ciocalteu reagent at room temperature (25 °C). The absorbance was recorded at 760 nm in a spectrophotometer (Spectro 2000RS, Labomed Company, Inc., Los Angeles, CA, USA). The concentration of polyphenols was expressed in mg of chlorogenic acid per 100 g of the sample.
2.5. Antioxidant Capacity
The antioxidant activity of the seeds was assessed following the method developed by Re et al. [28], utilising ABTS (2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) (Sigma-Aldrich, St. Louis, MO, USA) as the radical source. A 0.5 mL aliquot of the methanolic extract (see Section 2.3) was diluted to 1 mL with methanol, after which 2 mL of the ABTS radical solution was added. The mixture was incubated at 30 °C for 6 min. The absorbance was then measured at 734 nm using a spectrophotometer (Spectro 2000RS, Labomed, Inc., Los Angeles, CA, USA). The values for each sample were compared to the concentration–response curve of the standard Trolox solution and expressed as micromoles of Trolox equivalent to the gram of fresh or dry weight (TEAC).
2.6. High-Performance Liquid Chromatography (HPLC) Analyses of Amygdalin
2.6.1. Extraction
Reflux extraction was performed on the defatted samples (2 g) using 50 mL of 98% ethanol at temperatures between 70 and 90 °C for 100 min After evaporating the solvent using a rotary evaporator (RE 100 PRO, CHEMLAND, Stargard, Poland), the residue was redissolved in 6 mL of distilled water. The water extract was filtered through a 0.22 µm filter and injected into an HPLC system.
2.6.2. HPLC Quantification
HPLC analysis was performed using a Prominence-i LC-2030C 3D Plus system (Shi-madzu, Kyoto, Japan), which was equipped with a diode array detector (DAD), as well as a Luna Omega 5 µm Polar C18, 100 Å, 250 × 10 mm column (Phenomenex, Torrance, CA, USA) [29,30]. The analysis was conducted with an isocratic elution. The mobile phase was a solution of methanol and water (HPLC grade) at a volume ratio of 25:75 (v/v). The other parameters of the chromatographic analysis were as follows: temperature 40 °C; flow rate 1 mL/min; injection volume 10 µL; and wavelength 214 nm. The amygdalin concentration was determined using a standard curve. An aqueous stock standard solution (TCI A0443 amygdalin >97%; TriMen Chemicals Sp. z o.o., Łódź, Poland) was prepared at a concentration of 100 µg/mL. A standard curve was created using amygdalin solutions with the following concentrations: 1, 5, 10, 20, 40, 50, and 100 µg/mL (R2 = 0.9999312). The lowest determined amygdalin value was 0.5 µg/mL. The equation of the calibration curve was y = 8439.11x.
2.7. Data Analysis
The results were presented as ranges of at least three parallel replicates with standard deviations around the mean. All values are given on a dry weight basis. The one-way analysis of variance was applied in order to assess the influence of different seeds on the tested parameters. Duncan’s test was used to assess the significance of differences at p < 0.05. The Pearson correlation coefficient (r) was used to assess the relationship between variables (total polyphenols and antioxidant activity together with total polyphenols and amygdalin or antioxidant activity and amygdalin). All calculations were performed using Statistica, v.13.1 software (Statsoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Chemical Composition of Common and Ecological Apricot Seeds
The dry matter content determined in the apricot seeds averaged 92.02 g/100 g of product (Table 1). Statistically significant, the highest amount of dry matter content was determined in the common apricot seeds (94.48 g/100 g), while the lowest amount of dry matter was found in the apricot seeds from Armenia (90.61 g/100 g). The highest amount of protein was determined in the ecological, bitter apricot seeds (26.96 g/100 g dm), and the lowest amount of protein was found in the seeds of Armenian fruits (23.51 g/100 g dm) (Table 1). The observed differences were statistically significant (p < 0.05). Conversely, there were no differences in the fat content of the tested products, although the highest total fat content was determined in the ecological, bitter apricot seeds (39.89 g/100 g dm) and the lowest fat content was found in the seeds from Armenia (36.81 g/100 g dm). The ash content determined in the apricot seeds was on average 2.82 g/100 g dm of the product (Table 1). The highest ash content with statistical significance was found in the apricot seeds from Armenia (3.00 g/100 g dm), and the lowest ash content was found in the common apricot seeds (2.71 g/100 g dm) (p < 0.05). There was no statistical difference between the values determined in the seeds of the common and ecological bitter apricot seeds, while the value for the apricot seeds from Armenia was statistically different from the other two values (p < 0.05). The average content of total carbohydrates calculated in the apricot seeds was 26.76 g/100 g dm (Table 1). Common apricot seeds had the highest total carbohydrate content (28.65 g/100 g dm), while the ecological, bitter samples had the lowest carbohydrate content (24.36 g/100 g dm). A statistical difference was found between the carbohydrate content of the ecological, bitter apricot seeds and that of the common apricot seeds and the apricot seeds from Armenia (p < 0.05).

Table 1.
Nutritional composition and amygdalin content of apricot kernels.
3.2. Total Polyphenols and Antioxidant Activity of Common and Ecological Apricot Seeds
The total polyphenol content determined in the apricot seeds was expressed in mg of chlorogenic acid, and averaged at 109.37 mg/100 g dm. A statistically higher content of polyphenols was identified in the seeds from Armenia (171.21 mg/100 g dm), while the lowest content of polyphenols was found in the common apricot seeds (65.08 mg/100 g dm) (Table 2) (p < 0.05). The average antioxidant activity determined by the ABTS method in the apricot seeds was 13.22 µmol Trolox/g dm (1.32 mmol/100 g dm) (Table 2). The highest value with statistically significant antioxidant activity was identified in the apricot seeds from Armenia (15.04 µmol Trolox/g dm), and the lowest antioxidant activity was found in common apricot seeds (12.05 µmol Trolox/g dm) (p < 0.05). The antioxidant activities of the common and ecological bitter apricot seeds were not statistically different (p > 0.05).

Table 2.
Total polyphenols and antioxidant activity of the tested apricot seeds.
3.3. Amygdalin Content of Common and Ecological Apricot Seeds
The retention time resulting from the chromatograms was approximately 7.5 min (Figure 1). The amygdalin content of the tested products averaged 509.12 mg/100 g dm (Table 1). The highest amygdalin content was found in the common apricot seeds (879.62 mg/100 g dm), and the lowest amygdalin content was found in the apricot seeds from Armenia (89.10 mg/100 g dm). A statistically significant difference was found between all determined values (p < 0.05).
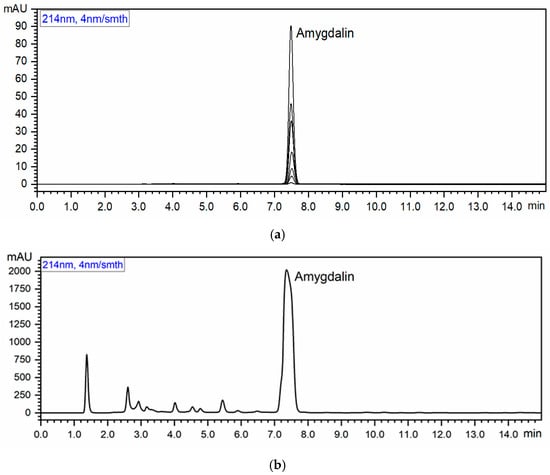
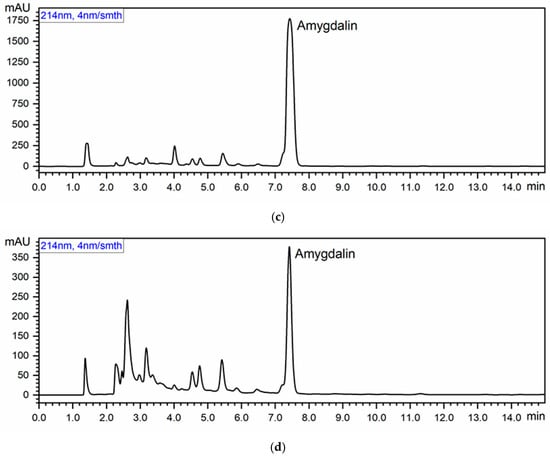
Figure 1.
Chromatograms: (a) Amygdalin standards at the following concentrations (µg/mL): 1, 5, 10, 20, 40, 50, 100; (b) Polish common apricot seeds; (c) Uzbekistan ecological apricot seeds of bitter taste; and (d) common apricot seeds from Armenia.
3.4. Correlation Analysis
Pearson correlation coefficient (r) analysis indicated a strong positive correlation between the polyphenol content and antioxidant activity (r = 0.996437), confirming that polyphenols were major contributors to antioxidant potential. By contrast, strong negative correlations were observed between the amygdalin content and antioxidant activity (r = −0.97754) and between the amygdalin content and polyphenol content (r = −0.99183) (Table 3). These findings suggest that a higher amygdalin content is associated with lower levels of antioxidant compounds and lower antioxidant capacity in apricot seeds.

Table 3.
Pearson correlation coefficients (r) between selected chemical components and antioxidant activity in apricot seeds.
4. Discussion
To date, only a few publications have investigated the proximate composition, total polyphenol content, and antioxidant activity of apricot seeds. Consequently, the results of this study were compared with those of similar products within the same food group and with previously published data on apricot seeds.
The dry matter content of groundnuts was 91.70 g/100 g [31], and that of almonds was 97.70 g/100 g [32]. Both results were similar to those presented in this paper, and the slight variation between them was likely due to interspecific differences. A lower dry matter content was reported by Alajil et al. [33] in six apricot kernel samples obtained from India, which ranged from 50.04 to 62.44%; however, this might be due to the fact that the kernels were analysed immediately after deseeding, with the omission of a drying phase. By contrast, our results were similar to the dry matter of apricot seeds grown in Turkey (from 96.75 to 97.82%) [34] and Pakistan (between 94.27 and 96.34%) [35].
The total protein content of the apricot seeds ranged from 23.52 to 26.96 g/100 g dm, depending on the type. The values were in concordance with the data recorded by other authors from India (from 17.23% to 24.10%) [33], Serbia (from 14.1 to 45.3%) [36], China (between 23 and 27%) [37], and Turkey (from 15.7 to 18.3%) [34]. According to the Tables of composition and nutritional value of foods [38], the total protein content was 20.0 g/100 g in almonds, 25.70 g/100 g in peanuts, 14.40 g/100 g in hazelnuts, and 16.00 g/100 g in walnuts. In another study, peanuts contained a protein content of 23.10 g/100 g [31], while Cieniewska and Ratusz [39] in a study conducted on three hazelnut varieties determined the total protein content to be 12.59 g/100 g (variety “Catalan”), 13.02 g/100 g (variety “Webba Cenny”), and 12.99 g/100 g (variety “Cosford”). Overall, the reported values were similar to those determined in this study.
In this study, the total fat content of the apricot kernels ranged from 36.81 g/100 g dm to 39.89 g/100 g dm, which is comparable to the values reported by Alajil et al. [33] for apricot kernels from India containing 15.74 to 51.63 g/100 g dry matter. In the same study [33], major fatty acids found in the apricot kernel were oleic acid (C18:1), linoleic acid (C18:2), and palmitic acid (C16:0), and the amount of these three fatty acids varied considerably in the seeds, indicating that the total unsaturated fatty acid content ranged from 97.1% to 98.3%, significantly exceeding the levels found in sunflower oil (85–91%), cottonseed oil (70–75%), and olive oil (81–91%). Hence, apricot kernel oil might be a suitable alternative to almond kernel oil for both culinary and industrial uses, including the production of cosmetics, paints, and varnishes. According to the literature data on other dry fruits, the total fat content in apricot seeds was lower than that in almonds at 52.00 g/100 g and 49.42 g/100 g; peanuts at 46.10 g/100 g, and 49.24 g/100 g; and walnuts at 60.30 g/100 g, and 65.21 g/100 g, as reported by Kunachowicz et al. [38] and Ciemniewska-Żytkiewicz et al. [40], respectively. Ciemniewska and Ratusz [39], in a study conducted on three different hazelnut varieties, determined the total fat content as ranging from 61.16 to 63.16 g/100 g. These results differed slightly from the literature reports on products similar to apricot kernels, which may be attributed to interspecific variations. Additionally, the differences in the total fat content were observed even among the varieties of the same nut species, such as hazelnuts [39]. A study by Ciemniewska and Ratusz [39] demonstrated that the varieties of the same nut species (hazelnuts) were characterised by different total fat content. This trend was also observed in the apricot seeds from different origins. The ash content of the apricot seeds ranged from 2.71 g/100 g dm to 3.00 g/100 g dm. A similar ash content (2.70 g/100 g) was determined in peanuts [31] and in different varieties of hazelnuts (2.05–2.33 g/100 g) [39]. In almonds, 6.76 g/100 g of ash was measured [32]. The calculated total carbohydrate content of the apricot seeds ranged from 24.35 g/100 g dm to 28.65 g/100 g dm g. These values varied considerably (p < 0.05) and were significantly lower compared to the carbohydrate content detected in almond nuts (54.87 g/100 g) [32], but comparable to the values reported for almond seeds (20.50 g/100 g), peanuts (19.50 g/100 g), hazelnuts (14.90 g/100 g), and walnuts (18.0 g/100 g) [38].
In the apricot seeds, the polyphenol content ranged from 65.08 mg/100 g dm to 171.21 mg/100 g dm depending on the origin. These values are in line with Korekar et al. [41], Chen et al. [35] and Alalij [33], who reported the total polyphenol values of apricot kernels as ranging from 92.20 to 162.1 mg/100 g of dry matter, between 10.60 and 209.40 mg/100 g of dry matter, and in the range of 39.71–130.87 mg/100 g of dry matter, respectively. According to the literature data, the major phenolic compounds in apricot are catechin, caffeic acid, epicatechin, gallic acid, quercetin, p-coumaric acid, chlorogenic acid, and kaempferol [33]. Furthermore, in comparison to other dry fruits, the total polyphenolic compounds were similar to almonds without peel at 47.00 mg/100 g, peanuts at 112.00 mg/100 g, and lower than in almonds with peel at 239.00 mg/100 g, hazelnuts at 291.00 mg/100 g, or in walnuts at 1625.00 mg/100 g [40]. According to John and Shahidi [42], the content of phenolic compounds in Brazil nuts (methanolic extracts) was 331.39 mg/100 g, which is the highest among the nuts studied.
The antioxidant activity of apricot seeds determined in this study ranged from 12.05 to 15.04 µmol Trolox/g dm, depending on the origin, which is in line with the study by Alalij et al. [33]. In their study, apricot seeds from India were analysed using three in vitro assays: CUPRAC (Cupric Reducing Antioxidant Capacity) which resulted in a range between 10.21 and 23.49 µmol Trolox/g dry matter, and DPPH (2,2-diphenyl-1-picrylhydrazyl) and FRAP (Ferric Reducing Antioxidant Power) produced ranges between 4.59 and 9.01 µmol Trolox/g dry matter and 5.77 to 14.27 µmol Trolox/g dry matter, respectively. According to the literature, independent of the assay applied, the seeds contain higher levels of lipophilic than hydrophilic antioxidants [41]. With regard to other dried fruits, John and Shahidi [42] reported antioxidant activity in Brazil nuts without peel at 14.04 µmol Trolox/g and for nuts with peel at 36.28 µmol Trolox/g. Abe et al. [43] evaluated the antioxidant activity of various types of nuts using the DPPH method. Walnuts showed the highest antioxidant activity (120 µmol Trolox/g), while almonds had the lowest antioxidant activity (1.2 µmol Trolox/g). Hazelnuts, as noted earlier, displayed an antioxidant activity of 4.20 µmol Trolox/g. In a study conducted on two kinds of apricot seed varieties from Turkey, sweet (Hasanbey) seeds showed higher antioxidant activity, expressed as a percentage of inhibition of fat oxidation (68.6%/100 µg of product), whereas bitter (Zerdali) seeds exhibited markedly lower activity (20.2%/100 µg), approximately three-fold lower [42]. The same authors [44] also conducted some analyses of antioxidant potential using the DPPH method. This study was conducted at two concentration levels of the apricot seed extract: 100 µg/mL and 300 µg/mL; both aqueous and methanolic extracts were prepared. The sweet variety of apricot seeds exhibited free radical neutralising ability at 100 µg/mL, with values of 89.9 for the aqueous extract and 87.7% for the methanolic extract, respectively. In a solution with a concentration of 300 µg/100 mL, the results were as follows: 89.9 and 92.2%. For the bitter apricot seed variety, no free radical scavenging activity was detected in either extract [44]. The correlation analysis provided additional insight into the biochemical composition of the apricot seeds. A strong positive correlation between polyphenols and antioxidant activity aligns with the existing literature, confirming that polyphenolic compounds significantly contribute to the antioxidant properties of plant materials [33,36,43,44]. However, a strong negative correlation between amygdalin and both antioxidant activity and polyphenol content may suggest a metabolic or varietal trade-off, in which cultivars richer in amygdalin tend to accumulate fewer antioxidant-related compounds. This observation may be relevant when selecting apricot seed varieties for nutritional or therapeutic purposes, particularly when balancing health benefits with potential toxicity risks associated with amygdalin.
In addition to the comparative analysis, it is important to consider the nutritional and functional relevance of the values obtained. The fat content observed in the apricot seeds (36.81–39.89 g/100 g) is slightly lower than that in other commonly consumed nuts, such as almonds or walnuts, but still provides a considerable amount of unsaturated fatty acids, which are associated with cardioprotective effects. Therefore, apricot seeds may contribute positively to dietary fat intake. Regarding antioxidant activity, although the levels observed (12.05–15.04 μmol Trolox/g) are moderate in comparison to walnuts (120 μmoL Trolox/g) [43], they still indicate the presence of biologically active compounds capable of reducing oxidative stress. This supports the potential use of apricot seeds in the development of functional foods or nutraceuticals.
Another possible explanation for the considerable antioxidative properties, which still requires further investigation, is the presence of peptides in apricot seeds that act synergistically with non-peptide antioxidants. Numerous studies have demonstrated that the enzymatic hydrolysis of proteins from bitter apricot kernels using alkaline protease produces peptides with markedly enhanced antioxidant activity. The antioxidative efficacy of peptides is intrinsically linked to their amino acid composition and sequence. Aromatic amino acids, in particular, may have a key role in the scavenging of lipid-derived free radicals through proton donation mechanisms. Consequently, peptides enriched with aromatic residues are capable of effectively suppressing lipid peroxidation processes [1].
The amygdalin (D-Mandelonitrile-ß-glucoside or D-man-delonitrile-ß-gentiobioside) content of the apricot seeds was 89.10 mg/100 g dm in the seeds from Armenia, 558.65 mg/100 g dm in the ecological bitter apricot seeds from Uzbekistan, and 879.62 mg/100 g dm in the seeds purchased from Poland’s retail market. According to Bolarinwa et al. [29], the content of amygdalin in the seeds of individual plant species of the Rosaceae family is as follows: black cherry 268 mg/100 g; red cherry 389 mg/100 g; peach 681 mg/100 g; plum (depending on the species) from 44 mg/100 g to 1749 mg/100 g; apple (Royal Gala) 296 mg/100 g; and pear (Conference) 129 mg/100 g. The amygdalin content of apricot seeds (type not specified) was 1437 mg/100 g. Our data on the amygdalin content are consistent with Chen et al. [35] and Alalij et al. [33], who reported a range of amygdalin content between 32.14 and 1145 mg/100 g dry matter and between 0 and 1705 mg/100 g of dry matter, respectively. In a study by Gunther et al. [30], the concentration of amygdalin in apricot seed extracts prepared under the same conditions as this work (extraction time 100 min, temperature 97.5 °C) was 239.58 mg per 10 g of biological material. Since about 2 g of material was used in this work, the above amygdalin value was recalculated to this amount for comparison. Recalculated to 2 g of material, the amygdalin content of apricot kernels was 47.92 mg. The authors used apricot seeds acquired commercially in Israel. Considering the above, it can be assumed that the amygdalin content in apricot seeds and other fruits may vary greatly depending on various factors. Previous studies have shown that white-skinned apricot kernels tend to be sweeter than those with brown skins, indicating lower levels of amygdalin [45]. As a result, they pose little toxicological risk. By contrast, seeds with substantially higher amygdalin content may present a toxicological risk if consumed, and those available commercially should carry an appropriate warning on the packaging.
In some countries, apricot seeds are widely consumed due to their low amygdalin content, as confirmed in this study. The maximum tolerated intravenous dose of amygdalin in humans is estimated at approximately 0.07 g/kg body weight [33]. When administered orally at low doses, amygdalin has been reported to exhibit health-promoting properties, including the stimulation of respiratory function and enhancement of digestive processes, with potential applications in cancer treatment, marked as vitamin B17 or laetrile [1,33,35,36]. Additionally, amygdalin has been associated with hepatoprotective effects, including the prevention of carbon tetrachloride-induced liver damage and the treatment of hepatocellular carcinoma induced by nitrosodiethylamine in experimental animal models [1,33]. In addition to this, the analgesic activity of a hydro-ethanolic extract from bitter apricot kernels at a dosage of 100 mg/kg of body weight was recorded in the course of experiments using different animal models alongside the remarkable healing of wounds and burns in rats when applied daily in a formulated ointment with 10% addition of an extract from bitter apricot kernels [46].
5. Conclusions
This study demonstrates that apricot seeds from different geographic origins exhibit significant variations in their macronutrient composition, antioxidant capacity, and amygdalin content. These differences are most likely due to both genetic factors and agroclimatic conditions in their region of origin. Among the samples, the Armenian apricot seeds showed the most favourable profile—combining the highest total polyphenol content and antioxidant activity with the lowest amygdalin concentration (p < 0.05). This finding is particularly important given the potential health risks associated with high levels of amygdalin, which can release toxic hydrogen cyanide during digestion. The data suggest that apricot seeds from selected regions may offer health-promoting properties due to their antioxidant potential, provided that their amygdalin content remains within safe limits. These results are consistent with the traditional consumption of low-amygdalin apricot seeds, for example, in Armenian apricot seeds, while also highlighting the importance of origin-based evaluations when considering apricot seeds for food or nutraceutical applications. However, due to the known toxicity of amygdalin at higher doses, the seeds with elevated amygdalin content should not be recommended for consumption. The products containing such seeds should carry appropriate warnings on their labels to inform consumers of the potential health risks. Further research is needed to better understand the bioactive potential of apricot seed components beyond polyphenols, including peptides and polypeptides with potential hypoglycaemic, lipotropic, and anti-inflammatory effects. Additionally, future studies should investigate the biological effects of amygdalin, considering not only its toxic effects but also any potential pharmacological properties under controlled conditions.
Author Contributions
Conceptualization, E.S. and B.B.; methodology, K.D.; software, K.D.; validation, K.D.; formal analysis, K.D.; investigation, E.S., B.B., K.D. and J.K.-D.; resources, B.B., E.S. and J.K.-D.; data curation, E.S., B.B. and K.D.; writing—original draft preparation, B.B. and J.K.-D.; writing—review and editing, B.B.; visualisation, J.K.-D.; supervision, E.S.; funding acquisition, E.S., J.K.-D. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, X.; Meng, T.; Ren, F.; Ning, A.; Chen, B.; Liu, X.; Liu, H. A review on apricot kernel seed proteins and peptides: Biological functions and food applications. Int. J. Biol. Macromol. 2025, 292, 139053. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak-Wilke, E.; Polkowska, Z.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeuticand Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Kolesarova, A.; Baldovska, S.; Roychoudhury, S. The Multiple Actions of Amygdalin on Cellular Processes with an Emphasis on Female Reproduction. Pharmaceuticals 2021, 14, 881. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.J.; Cho, K.H.; Jung, W.S.; Moon, S.K.; Park, E.K.; Kim, D.H. Biotransformation of ginsenoside Rb1, crocin, amygdalin, geniposide, puerarin, ginsenoside Re, hesperidin, poncirin, glycyrrhizin and baicalin by human fecal microflora and its relation to cytotoxicity against tumor cells. J. Microbiol. Biotechnol. 2008, 18, 1109–1114. [Google Scholar]
- Song, Z.; Xu, X. Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 2014, 10, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Azi, F.; Li, Z.; Xu, P. Expanding Yarrowia lipolytica’s Metabolic Potential for Detoxification of Cyanogenic Glycosides in Edible Plants. Commun. Biol. 2025, 8, 188. [Google Scholar] [CrossRef]
- Zhang, C.X.; Guo, P.H.; Zhang, Q.A.; García Martín, J.F. Effects of Different Pressing Methods on Physicochemical Properties and Volatile Compounds of the Apricot Kernel Oil. J. Food Meas. Charact. 2024, 18, 8767–8781. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Q.; Xu, M.; Xia, Q.; Zheng, T.; Teng, J.; Li, M.; Fan, L. Recent updates and future perspectives about amygdalin as a potential anticancer agent: A review. Cancer Med. 2019, 8, 3004–3011. [Google Scholar] [CrossRef]
- Makarevic, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Reiter, M.; Tsaur, I.; Bartsch, G.; Haferkamp, A.; Blaheta, R. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS ONE 2014, 9, e105590. [Google Scholar] [CrossRef]
- Makarevic, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Tsaur, I.; Nelson, K.; Pfitzenmaier, J.; Haferkamp, A.; Blaheta, R. Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS ONE 2014, 9, e110244. [Google Scholar] [CrossRef]
- Qian, L.; Xie, B.; Wang, Y.; Qian, J. Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 5363–5370. [Google Scholar] [PubMed]
- Lin, S.; Wen, J.; Xu, X.; Shi, J.; Zhang, W.; Zheng, T.; Hou, Y.; Zhang, Y.; Li, Z.; Wang, K.; et al. Amygdalin Induced Mitochondria-Mediated Apoptosis of Lung Cancer Cells via Regulating NFκB-1/NFκB Signaling Cascade in Vitro and in Vivo. Am. J. Chin. Med. 2022, 50, 1361–1386. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Wang, F.; Hu, J.; Cui, A.; Wei, C.; Yang, Q.; Li, F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol. 2013, 35, 43–51. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, S.H.; Han, L.S.; Zheng, L.T.; Jung, K.H.; Uhm, Y.K.; Lee, J.H.; Jeong, J.S.; Joo, W.S.; Yim, S.V.; et al. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol. 2005, 11, 5156–5161. [Google Scholar] [CrossRef]
- Dimitrov, M.; Iliev, I.; Bardarov, K.; Georgieva, D.; Todorova, T. Phytochemical characterization and biological activity of apricotkernels’ extract in yeast-cell based tests and hepatocellular and colorectal carcinoma cell lines. J. Ethnopharmacol. 2021, 279, 114333. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Shin, M.S.; Yang, H.Y.; Lee, J.W.; Kim, Y.S.; Lee, M.H.; Kim, J.; Kim, K.H.; Kim, C.J. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expression in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. 2006, 29, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, I.; Thomas, A.; Monecke, M.M.; Zugelder, M.; Rutz, J.; Grein, T.; Maxeiner, S.; Xie, H.; Chun, F.K.-H.; Florian Rothweiler, F.; et al. Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells. Cancers 2022, 14, 3111. [Google Scholar] [CrossRef] [PubMed]
- Hee-Young, K.; Seon-Pyo, H.; Dong-Hoon, H.; Jeong Hee, K. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch. Pharm. Res. 2003, 26, 157–161. [Google Scholar] [CrossRef]
- Lee, H.M.; Moon, A. Amygdalin regulates apoptosis and adhesion in Hs578T triplenegative breast cancer cells. Biomol. Ther. 2016, 24, 62–66. [Google Scholar] [CrossRef]
- Cecarini, V.; Salima Selmi, S.; Cuccioloni, M.; Gong, C.; Bonfili, L.; Zheng, Y.; Cortese, M.; Angeletti, M.; Kilani, S.; Eleuteri, A.M. Targeting Proteolysis with Cyanogenic Glycoside Amygdalin Induces Apoptosis in Breast Cancer Cells. Molecules 2022, 27, 7591. [Google Scholar] [CrossRef]
- Juengel, E.; Afschar, M.; Makarevic, J.; Rutz, J.; Tsaur, I.; Mani, J.; Nelson, K.; Haferkamp, A.; Blaheta, R. Amygdalin blocks in vitro adhesion and invasion of renal cel carcinoma cells by an integrin-dependent mechanism. Int. J. Mol. Med. 2016, 37, 843–850. [Google Scholar] [CrossRef]
- Arshi, A.; Hosseini, S.M.; Hosseini, F.S.K.; Amiri, Z.Y.; Hosseini, F.S.; Lavasani, M.S.; Kerdarian, H.; Dehkordi, M.S. The anti-cancer effect of amygdalin on human cancer cell lines. Mol. Biol. Rep. 2019, 46, 2059–2066. [Google Scholar] [CrossRef]
- Mamdouh, A.M.; Khodeer, D.M.; Tantawy, M.A.; Moustafa, Y.M. In-vitro and in-vivo investigation of amygdalin, metformin, andcombination of both against doxorubicin on hepatocellular carcinoma. Life Sci. 2021, 285, 119961. [Google Scholar] [CrossRef] [PubMed]
- Aamazadeh, F.; Ostadrahimi, A.; Saadat, Y.R.; Barar, J. Bitter apricot ethanolic extract induces apoptosis through increasing expression of Bax/Bcl-2 ratio and caspase-3 in PANC-1 pancreatic cancer cells. Mol. Biol. Rep. 2020, 47, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Swain, T.; Hillis, W.E. The phenolic constituens of Prunus domestica. The quantitative analysis of phenolic constituents. J. Agric. Food Chem. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bolarinwa, I.; Orfila, C.; Morgan, M. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gunther, A.; Bednarczyk-Cwyna, R.B.; Turała, A. Usprawniona metoda ekstrakcji amigdaliny z nasion pestek moreli (Improved method for extracting amigdalin from apricot kernel seeds.). Apar. Badaw. Dydakt. 2018, 4, 164–167. [Google Scholar]
- Sikora, E.; Liszka, P. Składniki odżywcze i nie odżywcze w surowych i przetworzonych orzeszkach ziemnych (Arachis hipogea) (Nutrients and non-nutrients in raw and processed peanuts (Arachis hypogea). Bromatol. Chem. Toksykol. 2011, 4, 1047–1053. [Google Scholar]
- Agunbiade, S.O.; Olanlokun, J.O. Evaluation of some nutritional characteristics of Indian almond (Prunus amygdalus) nut. Pak. J. Nutr. 2006, 5, 316–318. [Google Scholar] [CrossRef]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Vasudev, S.; Chandran, D.; Lorenzo, J.M. Chemical characterization of apricot kernel: Nutraceutical composition, amino acid, and fatty acid profile. Food Anal. Methods. 2022, 15, 2594–2604. [Google Scholar] [CrossRef]
- Gezer, I.; Hacıseferoğulları, H.; Özcan, M.M.; Arslan, D.; Asma, B.M.; Ünver, A. Physico-chemical properties of apricot (Prunus armeniaca L.) kernels. South-West. J. Horticult. Biol. Environ. 2011, 2, 1–13. [Google Scholar]
- Chen, Y.; Al-Ghamdi, A.A.; Elshikh, M.S.; Shah, M.H.; Al-Dosary, M.A.; Abbasi, A.M. Phytochemical profiling, antioxidant and HepG2 cancer cells’ antiproliferation potential in the kernels of apricot cultivars. Saudi J. Biol. Sci. 2020, 27, 163–172. [Google Scholar] [CrossRef]
- Mesarović, J.; Trifković, J.; Tosti, T.; Akšić, M.F.; Milatović, D.; Ličina, V.; Milojković-Opsenica, D. Relationship between ripen-ing time and sugar content of apricot (Prunus armeniaca L.) kernels. Acta Physiol. Plant 2018, 40, 157. [Google Scholar] [CrossRef]
- Han, Z.P.; Liu, R.L.; Cui, H.Y.; Zhang, Z.Q. Microwave-assisted extraction and LC/MS analysis of phenolic antioxidants in sweet apricot (Prunus armeniaca L.) kernel skins. J. Liquid Chromatogr. Relat. Technol. 2013, 36, 2182–2195. [Google Scholar] [CrossRef]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Składu I Wartości Odżywczej Żywności (Tables of Composition and Nutritional Value of Foods); Wydawnictwo Lekarskie PZWL (Medical Publishing House PZWL): Warszawa, Poland, 2020. [Google Scholar]
- Ciemniewska, H.; Ratusz, K. Charakterystyka orzechów laskowych trzech odmian leszczyny uprawianej w Polsce (Characteristics of hazelnuts from three hazelnut varieties grown in Poland). Rośl. Oleiste 2012, 33, 273–283. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Krygier, K.; Bryś, J. Wartość odżywcza orzechów oraz ich znaczenie w diecie (Nutritional value of nuts and their importance in the diet). Technol. Prog. Food Process. 2014, 1, 90–96. [Google Scholar]
- Korekar, G.; Stobdan, T.; Arora, R.; Yadav, A.; Singh, S.B. Antioxidant capacity and phenolics content of apricot (Prunus armeniaca L.) kernel as a function of genotype. Plant Foods Hum. Nutr. 2011, 66, 376–383. [Google Scholar] [CrossRef] [PubMed]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods. 2010, 2, 196–209. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. LWT-Food Sci. Technol. 2010, 30, 254–259. [Google Scholar] [CrossRef]
- Yigit, D.; Yigit, N.; Mavi, D. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunas armeniaca L.) kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Angmo, P.; Angmo, S.; Upadhyay, S.S.; Targais, K.; Kumar, B.; Stobdan, T. Apricots (Prunus armeniaca L.) of trans-Himalayan Ladakh: Potential candidate for fruit quality breeding programs. Sci. Hortic. 2017, 218, 187–192. [Google Scholar] [CrossRef]
- El-Hajjaji, M.A.; Fikri-Benbrahim, K.; Soulo, N.; Nouioura, G.; Laaroussi, H.; Ferreira-Santos, P.; Lyoussi, B.; Benziane Ouaritini, Z. Analgesic, Antioxidant, Anti-Inflammatory, and Wound-Treating Actions of Bitter Apricot Kernel Extract. Adv. Pharm. Pharm. Sci. 2024, 2024, 5574259. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).