Machine Learning in Stroke Lesion Segmentation and Recovery Forecasting: A Review
Abstract
1. Introduction
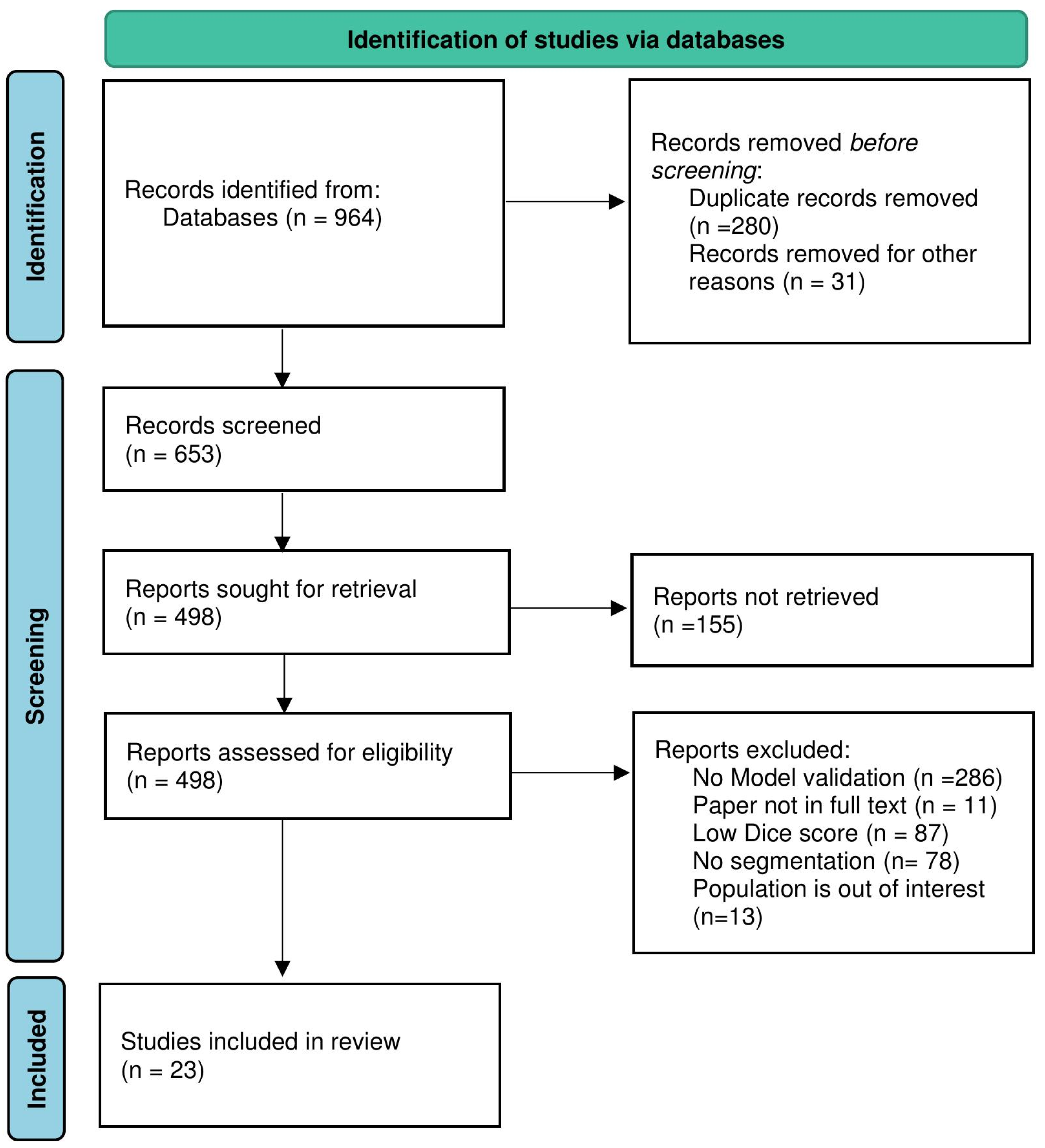
2. Methods
Study Search Strategy
3. Results
3.1. Machine Learning-Driven Stroke Lesion Segmentation with ATLAS Dataset
3.2. Post-Stroke Recovery Prediction Through Machine Learning Techniques
4. Discussion
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| ANTs | Advanced Normalisation Tools |
| ATLAS | Anatomical Tracings of Lesions After Stroke |
| AUC | Area Under the Curve |
| BCE | Binary Cross-Entropy Loss |
| CNN | Convolutional Neural Network |
| ConvNeXt | Convolutional Neural Network with Next-Generation Architecture |
| CSCA | Channel and Spatial Compound Attention |
| CST | Corticospinal Tracts |
| CT | Computed Tomography |
| DICE | Dice Loss |
| DNN | Deep Neural Network |
| DSC | Dice Similarity Coefficient |
| DTI | Diffusion Tensor Imaging |
| eDWI | Enhanced Diffusion-Weighted Imaging |
| eXGBoost | eXtreme Gradient Boosting |
| FAC | Functional Ambulation Category |
| fALFF | fractional Amplitude of Low-Frequency Fluctuations |
| FISRG | Fuzzy Information Seeded Region Growing |
| FLAIR | Fluid-Attenuated Inversion Recovery |
| FMA | Fugl–Meyer Assessment |
| FMA-LE | Fugl–Meyer Motor Assessment of the Lower Extremity |
| HCSM | Hybrid Contextual Semantic Module |
| HCSNet | Hybrid Contextual Semantic Network |
| ISLES’22 | Ischemic Stroke Lesion Segmentation Challenge 2022 |
| ISLES’24 | Ischemic Stroke Lesion Segmentation Challenge 2024 |
| LR | Linear Regression |
| MEP | Motor-Evoked Potentials |
| MICCAI | Medical Image Computing and Computer-Assisted Intervention |
| MI | Mutual Information |
| MIND | Modality Independent Neighborhood Descriptor |
| ML | Machine Learning |
| MLiRA-Net | Multi-scale Long-range Interactive and Regional Attention Network |
| MRI | Magnetic Resonance Imaging |
| QAB | Quick Aphasia Battery |
| RF | Random Forest |
| rsfMRI | Resting-State Functional Magnetic Resonance Imaging |
| SegResNet | Segmentation Residual Neural Network |
| SelfONN | Self-Organising Operational Neural Network |
| SQMLP-net | Simulated Quantum Mechanics-based Joint Learning Network |
| St-RegSeg | Stroke Unsupervised Registration and Segmentation Framework |
| SVM | Support Vector Machine |
| TICI | Thrombolysis in Cerebral Infarction |
| TMS | Transcranial Magnetic Stimulation |
| TransMorph | Transformer-Based Deformable Image Registration Model |
| U-Net | U-shaped Convolutional Neural Network |
| UL | Upper Limb |
| VNet | V-shaped Convolutional Neural Network |
| WMH | White Matter Hyperintensity |
| XAI | Explainable Artificial Intelligence |
References
- Mackay, J.; Mensah, G.A. The Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2004.
- Ling, X.; Zheng, Y.; Tao, J.; Zheng, Z.; Chen, L. Association study of polymorphisms in the ABO gene with ischemic stroke in the Chinese population. BMC Neurol. 2016, 16, 146. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar]
- Kakkar, P.; Kakkar, T.; Patankar, T.; Saha, S. Current Approaches and Advances in the Imaging of Stroke. Dis. Model. Mech. 2021, 14, dmm048785. [Google Scholar] [CrossRef] [PubMed]
- Zarahn, E.; Alon, L.; Ryan, S.; Lazar, R.; Vry, M.S.; Weiller, C.; Marshall, R.; Krakauer, J. Prediction of Motor Recovery Using Initial Impairment and fMRI 48 h Poststroke. Cereb. Cortex 2011, 21, 2712–2721. [Google Scholar] [CrossRef] [PubMed]
- Kalinosky, B.; Schmit, B.; Schindler-Ivens, S. Structurofunctional Resting-State Networks Correlate with Motor Function in Chronic Stroke. Neuroimage Clin. 2017, 16, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.K.; Gupta, V.; Khandelwal, N. Acute Stroke Imaging: Current Trends. Ann. Natl. Acad. Med. Sci. 2019, 55, 193–201. [Google Scholar]
- Halme, H.L.; Korvenoja, A.; Salli, E. ISLES (SISS) Challenge 2015: Segmentation of Stroke Lesions Using Spatial Normalization, Random Forest Classification and Contextual Clustering. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries; Lecture Notes in Computer Science; Crimi, A., Menze, B., Maier, O., Reyes, M., Handels, H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 9556, pp. 211–221. [Google Scholar]
- Kamnitsas, K.; Ledig, C.; Newcombe, V.F.J.; Simpson, J.P.; Kane, A.D.; Menon, D.K.; Rueckert, D.; Glocker, B. Efficient multi-scale 3D CNN with fully connected CRF for accurate brain lesion segmentation. Med. Image Anal. 2017, 36, 61–78. [Google Scholar]
- Abulnaga, S.M.; Rubin, J. Ischemic Stroke Lesion Segmentation in CT Perfusion Scans using Pyramid Pooling and Focal Loss. In International MICCAI Brainlesion Workshop; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Peters, H.; White, S.E.; Page, S.J. he NIH Stroke Scale Lacks Validity in Chronic Hemiparetic Stroke. Am. J. Occup. Ther. 2016, 70 (4_Supplement_1), 7011500008p1. [Google Scholar] [CrossRef]
- Marsh, E.B.; Lawrence, E.; Hillis, A.E.; Chen, K.; Gottesman, R.F. The NIH Stroke Scale Has Limited Utility in Accurate Daily Monitoring of Neurologic Status. Neurohospitalist. 2016, 6, 97–101. [Google Scholar] [CrossRef]
- Gi, C.; An, X.; Li, T.; Liu, S.; Ming, D. St RegSeg: An unsupervised registration based framework for multimodal magnetic resonance imaging stroke lesion segmentation. Quant. Imaging Med. Surg. 2024, 14, 9459–9476. [Google Scholar] [CrossRef]
- García-Salgado, B.P.; Almaraz-Damian, J.A.; Cervantes-Chavarria, O.; Ponomaryov, V.; Reyes-Reyes, R.; Cruz-Ramos, C.; Sadovnychiy, S. Enhanced Ischemic Stroke Lesion Segmentation in MRI Using Attention U-Net with Generalized Dice Focal Loss. Appl. Sci. 2024, 14, 8183. [Google Scholar] [CrossRef]
- Rahman, A.; Chowdhury, M.E.; Wadud, M.S.I.; Sarmun, R.; Mushtak, A.; Zoghoul, S.B.; Al-Hashimi, I. Deep learning driven segmentation of ischemic stroke lesions using multi-channel MRI. Biomed. Signal Process. Control 2025, 105, 107676. [Google Scholar]
- Siddiquee, M.M.R.; Yang, D.; He, Y.; Xu, D.; Myronenko, A. Automated ischemic stroke lesion segmentation from 3D MRI: ISLES 2022 challenge report. arXiv 2022, arXiv:2209.09546. [Google Scholar] [CrossRef]
- Jeong, H.; Lim, H.; Yoon, C.; Won, J.; Lee, G.Y.; de la Rosa, E.; Kirschke, J.S.; Kim, B.; Kim, N.; Kim, C.; et al. Robust Ensemble of Two Different Multimodal Approaches to Segment 3D Ischemic Stroke Segmentation Using Brain Tumor Representation Among Multiple Center Datasets. J. Imaging Inform. Med. 2024, 37, 2375–2389. [Google Scholar] [CrossRef]
- Maier, O.; Schröder, C.; Forkert, N.D.; Martinetz, T.; Handels, H. Classifiers for Ischemic Stroke Lesion Segmentation: A Comparison Study. PLoS ONE 2015, 10, e0145118. [Google Scholar]
- Su, J.; Luo, Z.; Lian, S.; Lin, D.; Li, S. Mutual Learning with Reliable Pseudo Label for Semi-Supervised Medical Image Segmentation. Med. Image Anal. 2024, 94, 103111. [Google Scholar] [CrossRef]
- Soh, W.; Rajapakse, J. Noise-Induced Self-Supervised Hybrid UNet Transformer for Ischemic Stroke Segmentation with Limited Data Annotations. Sci. Rep. 2025, 15, 19783. [Google Scholar] [CrossRef]
- Bai, W.; Chen, C.; Tarroni, G.; Duan, J.; Guitton, F.; Petersen, S.E.; Guo, Y.; Matthews, P.M.; Rueckert, D. Self-Supervised Learning for Cardiac MR Image Segmentation by Anatomical Position Prediction. In Proceedings of the Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), Shenzhen, China, 13–17 October 2019; Springer: Cham, Switzerland, 2019; Volume 11765, pp. 541–549. [Google Scholar] [CrossRef]
- Liew, S.; Lo, B.P.; Donnelly, M.R.; Zavaliangos-Petropulu, A.; Jeong, J.N.; Barisano, G.; Hutton, A.; Simon, J.P.; Juliano, J.M.; Suri, A.; et al. A large, curated, open-source stroke neuroimaging dataset to improve lesion segmentation algorithms. Sci. Data 2022, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Petzsche, M.R.; de la Rosa, E.; Hanning, U.; Wiest, R.; Valenzuela Pinilla, W.E.; Reyes, M.; Meyer, M.I.; Liew, S.-L.; Kofler, F.; Ezhov, I.; et al. ISLES 2022: A multi-center magnetic resonance imaging stroke lesion segmentation dataset. Sci. Data 2022, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Evamaria, O.R.; Ezequiel, d.R.; Anh, B.T.; Moritz, H.P.; Hakim, B.; Kaiyuan, Y.; Antonio, M.F.; Houjing, H.; David, R.; Oscar, S.J.; et al. ISLES’24—A Real-World Longitudinal Multimodal Stroke Dataset. arXiv 2025, arXiv:2408.11142. [Google Scholar] [CrossRef]
- Liew, S.L.; Zavaliangos-Petropulu, A.; Sondag, M. Anatomical Tracings of Lesions After Stroke (ATLAS) v2.0: A Curated Dataset of Stroke Lesions for Data Sharing and Machine Learning. Sci. Data 2023, 10, 51. [Google Scholar]
- Deb, P.; Baru, L.B.; Dadi, K.; S, B.R. BeSt-LeS: Benchmarking Stroke Lesion Segmentation using Deep Supervision. In Proceedings of the 9th International Workshop, BrainLes 2023, and 3rd International Workshop, SWITCH 2023, Held in Conjunction with MICCAI 2023, Vancouver, BC, Canada, 8–12 October 2023. [Google Scholar]
- Huo, J.; Chen, L.; Liu, Y.; Boels, M.; Granados, A.; Ourselin, S.; Sparks, R. MAPPING: Model Average with Post-processing for Stroke Lesion Segmentation. arXiv 2022, arXiv:2211.15486. [Google Scholar] [CrossRef]
- Mohapatra, S.; Gosai, A.; Shinde, A.; Rutkovskii, A.; Nouduri, S.; Schlaug, G. Meta-Analysis of Transfer Learning for Segmentation of Brain Lesions. arXiv 2023, arXiv:2306.11714. [Google Scholar] [CrossRef]
- Verma, K.; Kumar, S.; Paydarfar, D. Automatic Segmentation and Quantitative Assessment of Stroke Lesions on MR Images. Diagnostics 2022, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- González, M.P. Fuzzy Information Seeded Region Growing for Automated Lesions After Stroke Segmentation in MR Brain Images. arXiv 2023, arXiv:2311.11742. [Google Scholar] [CrossRef]
- Liu, L.; Chang, J.; Liu, Z.; Zhang, P.; Xu, X.; Shang, H. Hybrid Contextual Semantic Network for Accurate Segmentation and Detection of Small-Size Stroke Lesions From MRI. IEEE J. Biomed. Health Inform. 2023, 27, 4062–4073. [Google Scholar] [CrossRef]
- Liu, L.; Chang, J.; Liang, G.; Xiong, S. Simulated Quantum Mechanics-Based Joint Learning Network for Stroke Lesion Segmentation and TICI Grading. IEEE J. Biomed. Health Inform. 2023, 27, 3372–3383. [Google Scholar]
- Wu, Z.; Zhang, X.; Li, F.; Wang, S.; Huang, L. Multi-scale long-range interactive and regional attention network for stroke lesion segmentation. Comput. Electr. Eng. 2022, 103, 108345. [Google Scholar]
- Tomita, N.; Jiang, S.; Maeder, M.E.; Hassanpour, S. Automatic post-stroke lesion segmentation on MR images using 3D residual convolutional neural network. Neuroimage Clin. 2020, 27, 102276. [Google Scholar]
- Alquhayz, H.; Tufail, H.Z.; Raza, B. The multi-level classification network (MCN) with modified residual U-Net for ischemic stroke lesions segmentation from ATLAS. Comput. Biol. Med. 2022, 151, 106332. [Google Scholar]
- Lin, D.J.; Cloutier, A.M.; Erler, K.S.; Cassidy, J.M.; Snider, S.B.; Ranford, J.; Parlman, K.; Giatsidis, F.; Burke, J.F.; Schwamm, L.H.; et al. Corticospinal Tract Injury Estimated From Acute Stroke Imaging Predicts Upper Extremity Motor Recovery After Stroke. Stroke 2019, 50, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Chang, W.H.; Kim, D.Y.; Lee, J.; Sohn, M.K.; Song, M.K.; Shin, Y.; Lee, Y.; Joo, M.C.; Lee, S.Y.; et al. Clustering and prediction of long-term functional recovery patterns in first-time stroke patients. Front. Neurol. 2023, 14, 1130236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Prasad, M.; Das, A.; Vibha, D.; Garg, A.; Goyal, V.; Srivastava, A.K. Utility of transcranial magnetic stimulation and diffusion tensor imaging for prediction of upper-limb motor recovery in acute ischemic stroke patients. Ann. Indian Acad. Neurol. 2022, 25, 54. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Entrup, J.L.; Schneck, S.M.; Onuscheck, C.F.; Levy, D.F.; Rahman, M.; Willey, E.; Casilio, M.; Yen, M.; Brito, A.C.; et al. Recovery from aphasia in the first year after stroke. Brain 2023, 146, 1021–1039. [Google Scholar] [CrossRef]
- Lundquist, C.B.; Nielsen, J.F.; Brunner, I.C. Prediction of Upper Limb use Three Months after Stroke: A Prospective Longitudinal Study. J. Stroke Cerebrovasc. Dis. 2021, 30, 106025. [Google Scholar] [CrossRef]
- Scrutinio, D.; Lanzillo, B.; Guida, P.; Mastropasqua, F.; Monitillo, V.; Pusineri, M.; Formica, R.; Russo, G.; Guarnaschelli, C.; Ferretti, C.; et al. Development and Validation of a Predictive Model for Functional Outcome After Stroke Rehabilitation: The Maugeri Model. Stroke 2017, 48, 3308–3315. [Google Scholar] [CrossRef]
- Scrutinio, D.; Guida, P.; Lanzillo, B.; Ferretti, C.; Loverre, A.; Montrone, N.; Spaccavento, S. Rehabilitation Outcomes of Patients With Severe Disability Poststroke. Arch. Phys. Med. Rehabil. 2019, 100, 520–529.e3. [Google Scholar] [CrossRef]
- Iorga, M.; Higgins, J.; Caplan, D.; Zinbarg, R.; Kiran, S.; Thompson, C.K.; Rapp, B.; Parrish, T.B. Predicting language recovery in post-stroke aphasia using behavior and functional MRI. Sci. Rep. 2021, 11, 8419. [Google Scholar] [CrossRef]
- Rivier, C.; Preti, M.G.; Nicolo, P.; Van De Ville, D.; Guggisberg, A.G.; Pirondini, E. Prediction of poststroke motor recovery benefits from measures of sub-acute widespread network damages. Brain Commun. 2023, 5, fcad055. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kim, J.; Chang, W.H.; Kim, Y.H. Multimodal Imaging Biomarker-Based Model Using Stratification Strategies for Predicting Upper Extremity Motor Recovery in Severe Stroke Patients. Neurorehabilit. Neural Repair 2022, 36, 217–226. [Google Scholar] [CrossRef]
- Sale, P.; Ferriero, G.; Ciabattoni, L.; Cortese, A.M.; Ferracuti, F.; Romeo, L.; Masiero, S. Predicting Motor and Cognitive Improvement Through Machine Learning Algorithm in Human Subject that Underwent a Rehabilitation Treatment in the Early Stage of Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 2962–2972. [Google Scholar]
- White, A.; Saranti, M.; d’Avila Garcez, A.; Hope, T.M.H.; Price, C.J.; Bowman, H. Predicting recovery following stroke: Deep learning, multimodal data and feature selection using explainable AI. Neuroimage Clin. 2024, 43, 103638. [Google Scholar] [PubMed]
- Tang, Z.; Su, W.; Liu, T.; Lu, H.; Liu, Y.; Li, H.; Zhang, H. Prediction of poststroke independent walking using machine learning: A retrospective study. BMC Neurol. 2024, 24, 332. [Google Scholar] [CrossRef] [PubMed]
- Jabal, M.S.; Joly, O.; Kallmes, D.; Harston, G.; Rabinstein, A.; Huynh, T.; Brinjikji, W. Interpretable Machine Learning Modeling for Ischemic Stroke Outcome Prediction. Front. Neurol. 2022, 13, 884693. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.K.; Tavenner, B.P.; Barisano, G.; Brodtmann, A.; Buetefisch, C.M.; Conforto, A.B.; Liew, S.L. Modulation of the association between corticospinal tract damage and outcome after stroke by white matter hyperintensities. Neurology 2024, 102, e209387. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Stewart, R.; Park, M.S.; Bae, K.Y.; Kim, S.W.; Kim, J.M.; Shin, I.S.; Cho, K.H.; Yoon, J.S. White Matter Hyperintensities and Functional Outcomes at 2 Weeks and 1 Year after Stroke. Cerebrovasc. Dis. 2013, 35, 138–145. [Google Scholar] [CrossRef]
- Giese, A.K.; Schirmer, M.D.; Dalca, A.V.; Sridharan, R.; Donahue, K.L.; Nardin, M.; Rost, N.S. White Matter Hyperintensity Burden in Acute Stroke Patients Differs by Ischemic Stroke Subtype: A Multicenter Study. Neurology 2020, 95, e79–e88. [Google Scholar] [CrossRef]
- Peng, Y.; Luo, D.; Zeng, P.; Zeng, B.; Xiang, Y.; Wang, D.; Luo, T. Impact of white matter hyperintensity location on outcome in acute ischemic stroke patients: A lesion symptom mapping study. Brain Imaging Behav. 2025, 19, 269–278. [Google Scholar] [CrossRef]
- Liew, S.L.; Schweighofer, N.; Cole, J.H.; Zavaliangos-Petropulu, A.; Tavenner, B.P.; Han, L.K.; Thompson, P. Association of brain age, lesion volume, and functional outcome in patients with stroke. Neurology 2023, 100, e2103–e2113. [Google Scholar] [CrossRef]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated Learning in Medicine: Facilitating Multi-Institutional Collaborations without Sharing Patient Data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef]
- Rangel, E.; Martinez, F. Federative Ischemic Stroke Segmentation as an Alternative to Overcome Domain-Shift Multi-Institution Challenges. arXiv 2025, arXiv:2508.18296. [Google Scholar] [CrossRef]
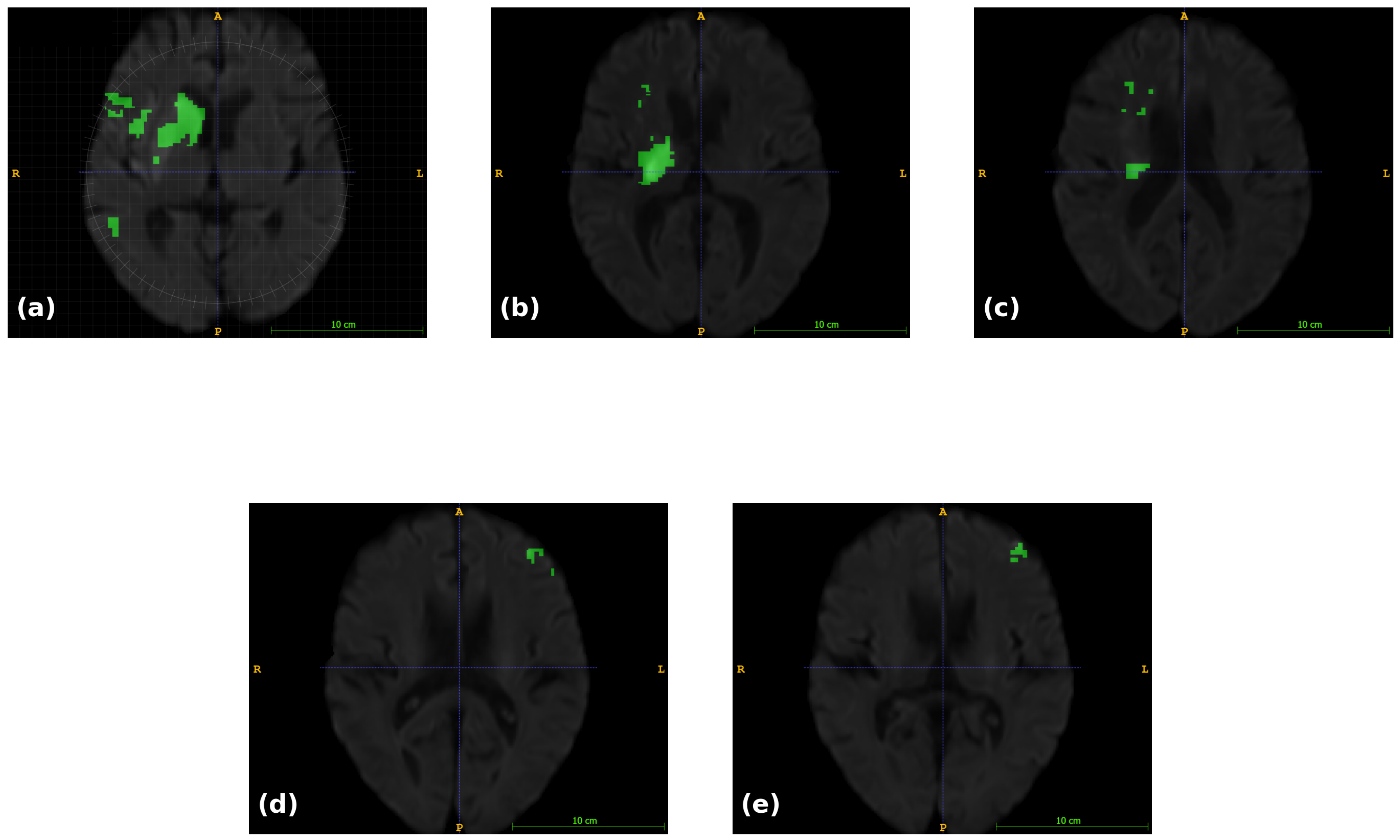
| Study (Author, Year) | Year | Modalities Used | Method | Result |
|---|---|---|---|---|
| Rahman et al., 2025 [15] | 2025 | DWI, ADC, and eDWI | DenseNet121 encoder + SelfONN-CSCA (Channel and Spatial Compound Attention)-UNet decoder | DWI only: 83.88, DWI + ADC: 85.86, DWI + ADC + eDWI: 87.49 |
| Siddiquee et al., 2022 [16] | 2022 | DWI and ADC | SegResNet + Deep Supervision + Auto3DSeg | DSC = 0.824 |
| Gi et al., 2024 [13] | 2024 | ADC + FLAIR vs. DWI + FLAIR | integrates an unsupervised registration model, ConvNXMorph, and a segmentation model, nnUNet-v2 | DSC = 0.84 |
| Jeong et al., 2024 [17] | 2024 | DWI and ADC | Base model: nnU-Net | Dataset I: 60.35% with ensemble model. Dataset II: 74.12% with ensemble model. ISLES’22 Challenge: Achieved first rank overall, with an average Dice = 78.69% across test cases |
| García-Salgado et al., 2024 [14] | 2024 | FLAIR, DWI, and T2 | U-Net architecture | F1-Scores over 0.7 in FLAIR, DWI, and T2 |
| Ref. | Preprocessing | Dataset | Segmentation Method | Loss Function | Performance Metric | Gap/Limitations |
|---|---|---|---|---|---|---|
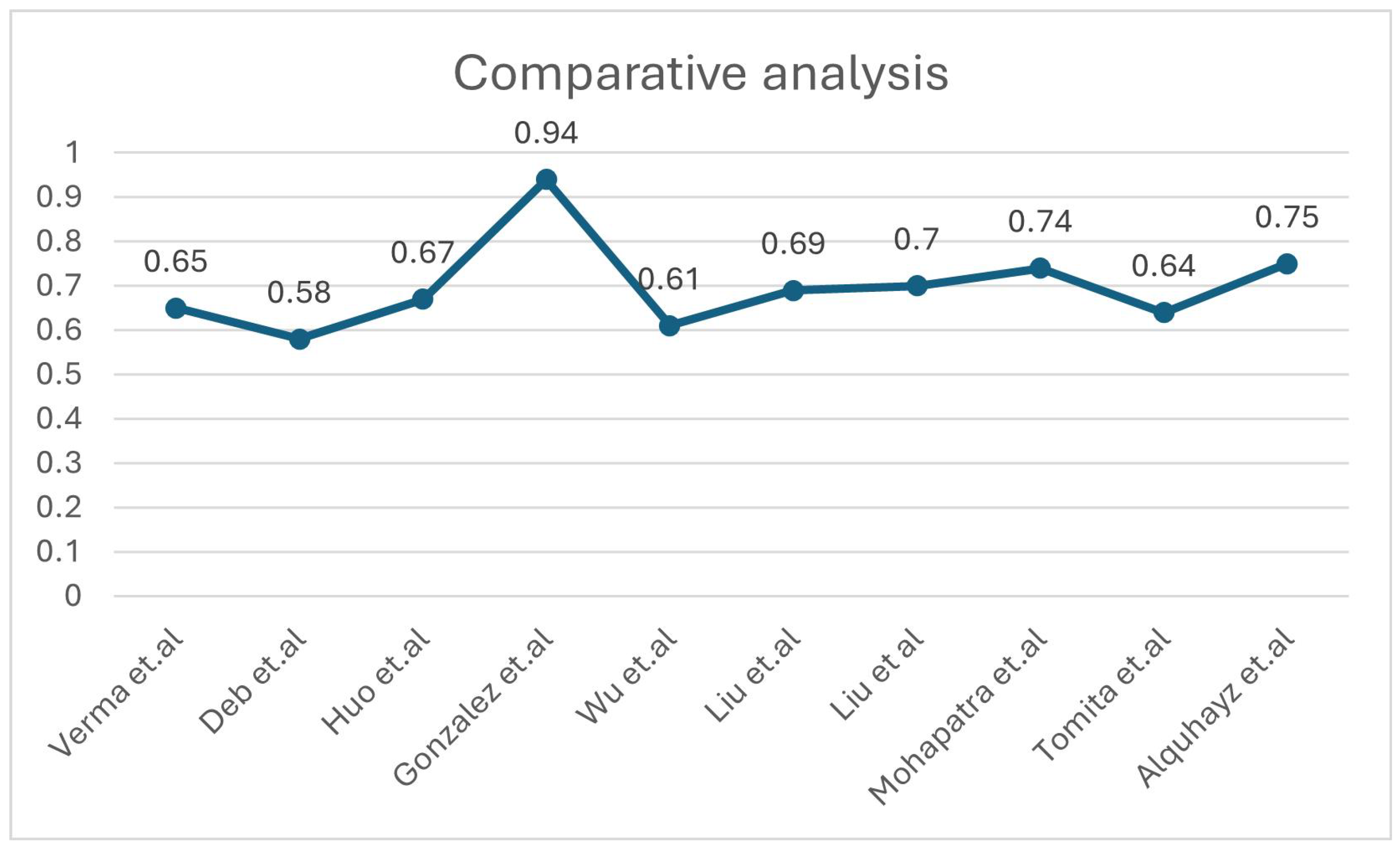
| [29] | Intensity normalisation, registration and defacing, brain extraction, skull removing | ATLAS V2.0 | Deep Neural Network (DNN) using 3D-UNet with 5-fold cross-validation | NM | DSC = 0.65 | Although potential biases from the manual segmentation process could influence the model, and the reliance on a subset for testing may limit generalisability, the scarcity of publicly accessible stroke datasets with manual segmentation labels makes independent validation. |
| [26] | Z-score Norma, slicing for 2D modality | ATLAS V2.0 | Multiple U Net variants | Proportionate weightage for Dice loss (DICE) and Binary Cross-Entropy loss (BCE) | Dice: 0.583 | There is a need for better data augmentation, U-Net models, supervised learning, handling small lesions, and decision-making uncertainty. |
| [27] | Normalisation and registration | ATLAS V2.0 | nnU-Net | Compound loss (Dice plus cross-entropy), TopK10 loss | Dice: 0.6667 | Small stroke lesions are hard to segment, especially with artifacts or similar intensities to surrounding tissue. Training schemes often predict unconnected lesions as continuous grey matter due to similar intensities. |
| [30] | Gaussian denoising | ATLAS V2.0 | Fuzzy Information Seeded Region Growing (FISRG) algorithm | DiNM | Dice: 0.94 | The algorithm struggles with abrupt lesion topology changes, misclassifies regions with similar intensities, and has increased computational time. Intensity-based classification causes errors, especially with variable lesion textures. |
| [33] | Image slicing, cropping, patch partitioning, patch embedding, normalisation, and augmentation | ATLAS v1 | MLiRA-Net (Multi-scale Long-range Interactive and Regional Attention Network) | Dice loss + weighted binary cross-entropy loss | Dice: 0.6119 | It comes at the cost of increased computational complexity. Additionally, the current implementation is limited to two-dimensional segmentation. |
| [31] | Matrix complement and clipping method | ATLAS V2.0 | Hybrid Contextual Semantic Network (HCSNet) | Mixing-loss function | Dice: 0.69 | Segmenting small lesions is challenging due to the heavy reliance on training data quality and quantity. Additionally, the model’s ability to generalise to real-world clinical settings needs further validation. |
| [32] | Intensity correction, MNI-152 template registration | ATLAS V2.0 | Simulated Quantum Mechanics-based joint Learning Network (SQMLP-net) | The joint loss function incorporates the segmentation and classification losses | Dice: 0.7098 | Finding the right balance between the trade-offs of multi-task learning weights is crucial for optimising task performance. |
| [28] | Resampling and normalisation, skull stripping, slicing, and augmentation | ATLAS V2.0 | Transfer learning and mixed data approaches | NM | Dice: 0.736 | The ensemble methods’ accuracy may change by the chosen parameters, which may require further adjustment for different datasets or lesion types. The ensemble method tends to overpredict lesions by approximately 10%. |
| [34] | Normalisation, cropping, a zoom-in and out training strategy | ATLAS v1 | 3D U-Net architecture with residual learning | Binary cross entropy (BCE) + Dice loss | Dice: 0.64 | The small dataset limits generalisability, and the model struggles with smaller lesions. Further validation with diverse scanners is needed. Manual tracing introduces variability, and the dataset only focuses on embolic strokes, leaving other types untested. |
| [35] | Slice selection, patch extraction, data augmentation | ATLAS v1 | U-Net architecture with Xception as the backbone- XU-Net | BCE and Jaccard coefficient | Dice: 0.754 | The study struggles with accurately segmenting small stroke lesions and reducing false positives. The model’s generalisability requires further validation on diverse datasets. Additionally, the approach increases computational complexity, limiting real-time application. |
| Ref. | Study Design | Sample Size | Data Modality | Method | Focus Area | Performance Metrics | Gap/Limitations |
|---|---|---|---|---|---|---|---|
| [43] | Longitudinal design | 57 patients | Resting-state functional MRI; behavioural language measures | Elastic net regression models | Language recovery | R2 = 0.948 | Small sample size and lack of validation set. |
| Use of LOOCV (Leave-One-Out Cross-Validation)—a less robust and reliable model selection method. | |||||||
| Recruitment constraints and minimal fMRI filtering resulted in lower accuracy and generalisability. | |||||||
| [44] | Longitudinal design | 37 patients | T2 and diffusion-weighted MRI; Fugl–Meyer Assessment (FMA) scores | Ridge regression | Motor recovery | R2 = 0.68 | Small sample size and no age-matching; manual lesion masking; larger, diverse cohorts required to confirm findings. |
| [36] | Longitudinal design | 48 patients | Magnetic resonance diffusion-weighted images and CT (one case) | Logistic and linear regression models | Upper extremity motor recovery | AUC = ranging from 0.70 to 0.8 | The study sample was younger, predominantly male, and ethnically homogeneous compared to national averages, with a small sample size of 48 participants. |
| [37] | Longitudinal design | 7858 patients | Demographic and clinical characteristicss | K-means clustering | Functional recovery | Ischemic: 0.926; hemorrhagic: 0.887 | Survivor bias; design limitations—absence of functional MRI or dynamic nomograms. |
| [38] | Prospective cohort study | 29 patients | Transcranial magnetic stimulation or diffusion tensor imaging parameters or their combinations | Multivariate logistic regression analysis | The upper-limb motor function | Predictive ability: 93.3% | Rigorous inclusion criteria: Only 29 patients enrolled, limiting generalisability; exclusion criteria: participants with medical issues were excluded, potentially skewing the results. |
| [39] | Longitudinal design | 334 patients | Demographic factors; MRI or CT imaging | Linear model | Language recovery | QAB overall: 59.5% of the variance | Smaller group sizes and missing data points. Lesions identified through acute clinical imaging may not accurately reflect irreversible tissue damage. QAB limitation: Does not assess written word comprehension or writing. |
| [45] | Retrospective cohort study | 104, 42 patients | Structural, diffusion, and functional magnetic resonance imaging | Multiple linear regression algorithm | Upper extremity motor recovery | R2 = 0.853 | Small sample size; single-centre study. |
| [42] | Retrospective cohort study | 1265 patients | Data from electronic records | Multivariable logistic regression model | Motor impairment | AUC = 0.833 | Retrospective design may introduce biases. No control group. Dynamic motor changes: study does not account for rehabilitation-related variations. |
| [41] | Retrospective cohort study | 717 patients | Clinical and demographic data | Multivariable logistic regression model | Physical functioning | AUC = 0.883 | Retrospective design introduces biases. Exclusion of mild stroke patients. Did not assess the prognostic role of neuroimaging. |
| [40] | Observational prospective cohort design | 87 patients | Clinical, demographic, and statistical data; accelerometer data | Multivariate regression model | Upper-limb | AUC = 0.86 | Limited accelerometer use, and visible devices could have led to overestimation. |
| [46] | Longitudinal cohort design | 55 patients | Demographic, clinical, biochemical, and hematological parameters; health status data at discharge | SVM | Motor and cognitive improvement | Correlation ranged from 0.75 to 0.81 | Single hospital data. Reliance on common inflammatory biomarkers, which can vary between individuals, and short follow-up period. |
| [47] | Cross-sectional design | 758 patients | T1-weighted structural MRI, demographic and clinical characteristics | CNN | Aphasia | Classification accuracy: 0.854 | Restricted to research-quality MRI scanners, and the measure of initial severity is relatively crude. Overfitting risk. Simplifies the Comprehensive Aphasia Test T-scores. |
| [48] | Retrospective cohort study | 778 patients | Demographic and clinical information; functional scores at admission. | Machine learning models | Walking independence | LR:AUC: 0.891, XGBoost:AUC: 0.880, SVM:AUC: 0.659, RF:AUC: 0.713 | Retrospective, single-centre design. No external validation. Only clinical admission data were used. No long-term outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasidharan, S.M.; Mdletshe, S.; Wang, A. Machine Learning in Stroke Lesion Segmentation and Recovery Forecasting: A Review. Appl. Sci. 2025, 15, 10082. https://doi.org/10.3390/app151810082
Sasidharan SM, Mdletshe S, Wang A. Machine Learning in Stroke Lesion Segmentation and Recovery Forecasting: A Review. Applied Sciences. 2025; 15(18):10082. https://doi.org/10.3390/app151810082
Chicago/Turabian StyleSasidharan, Simi Meledathu, Sibusiso Mdletshe, and Alan Wang. 2025. "Machine Learning in Stroke Lesion Segmentation and Recovery Forecasting: A Review" Applied Sciences 15, no. 18: 10082. https://doi.org/10.3390/app151810082
APA StyleSasidharan, S. M., Mdletshe, S., & Wang, A. (2025). Machine Learning in Stroke Lesion Segmentation and Recovery Forecasting: A Review. Applied Sciences, 15(18), 10082. https://doi.org/10.3390/app151810082








