Optimizing Knee MRI: Diagnostic Performance of a 3D PDW SPAIR-Based Short Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
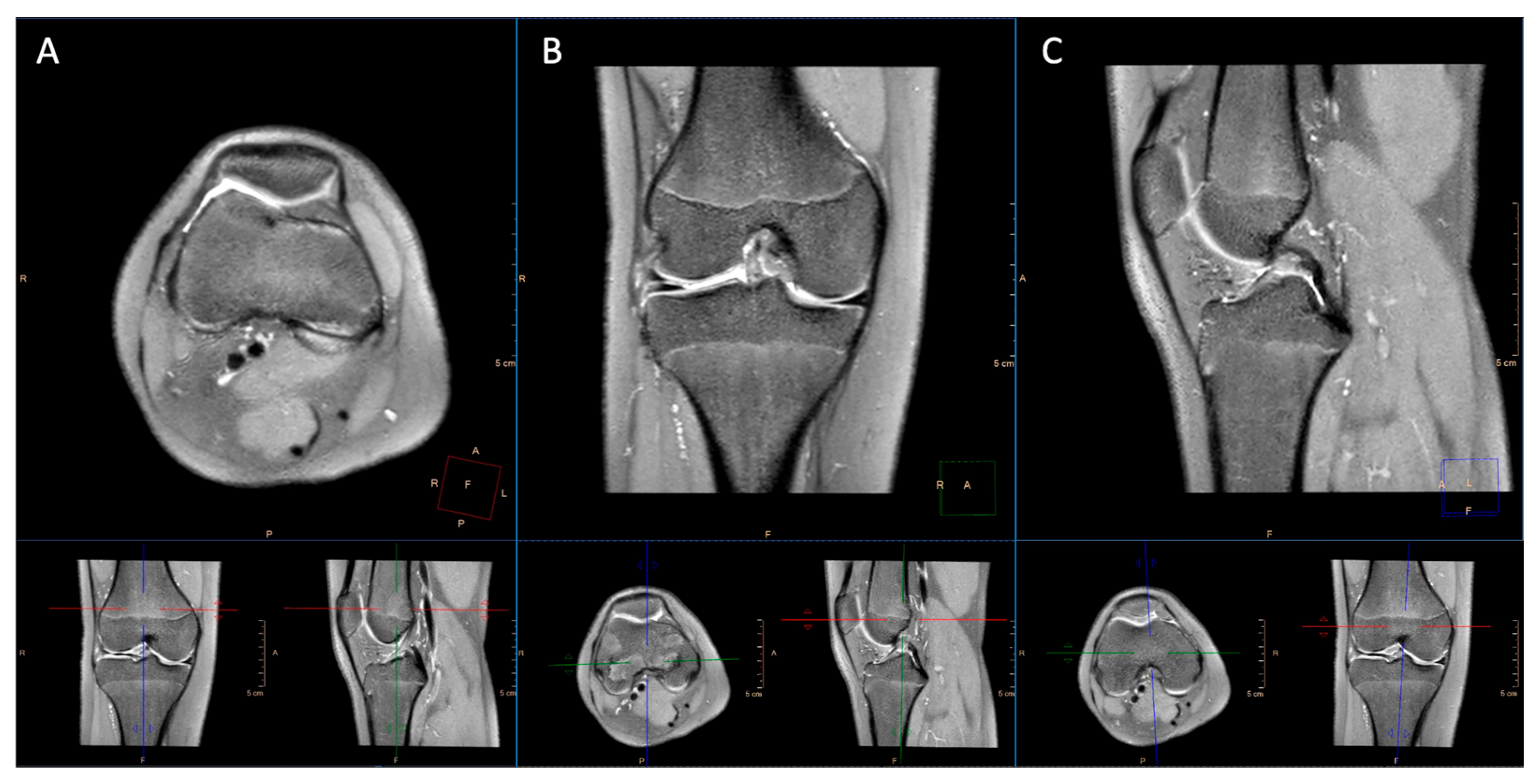
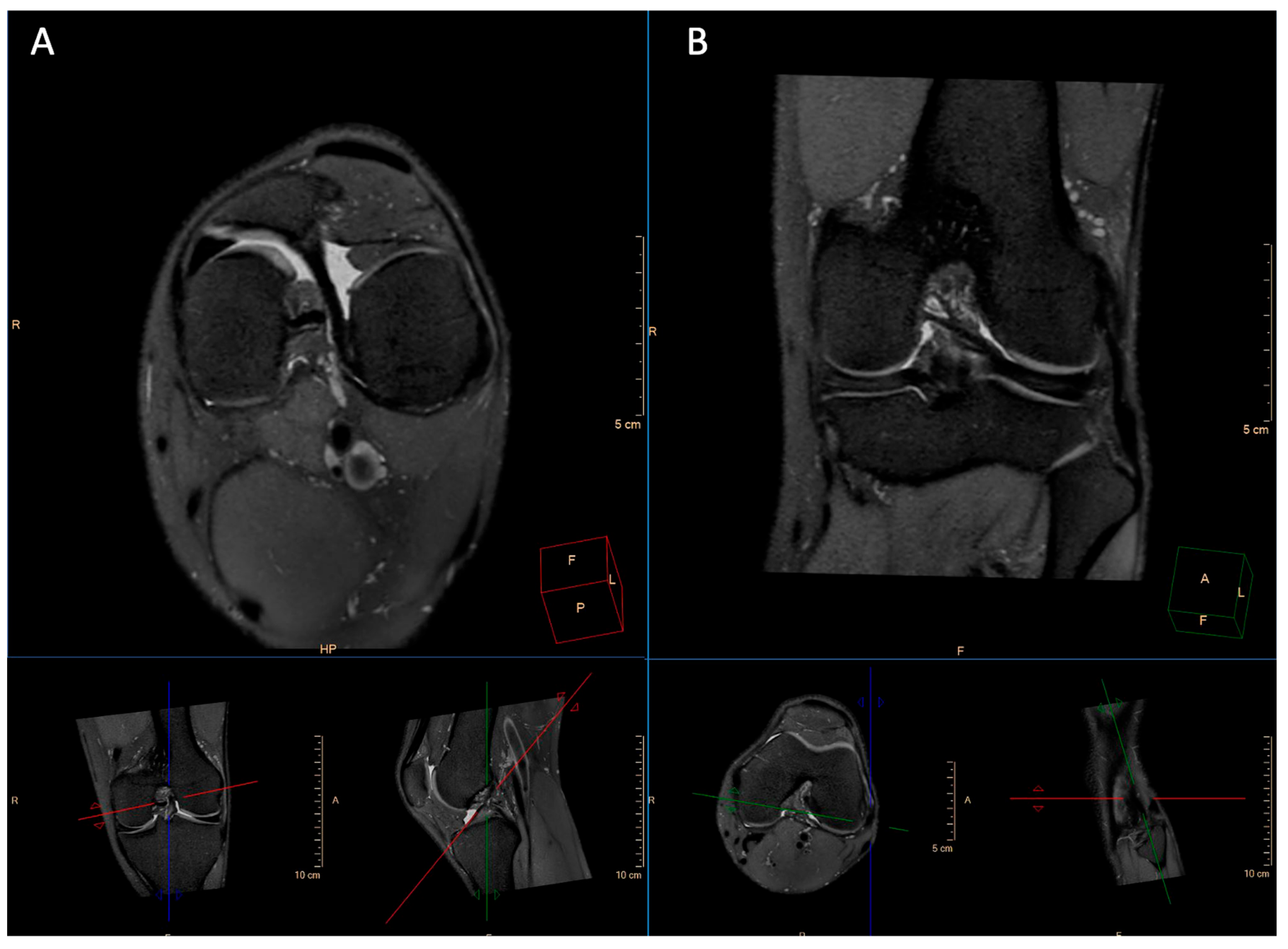
2.2. Imaging Technique
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
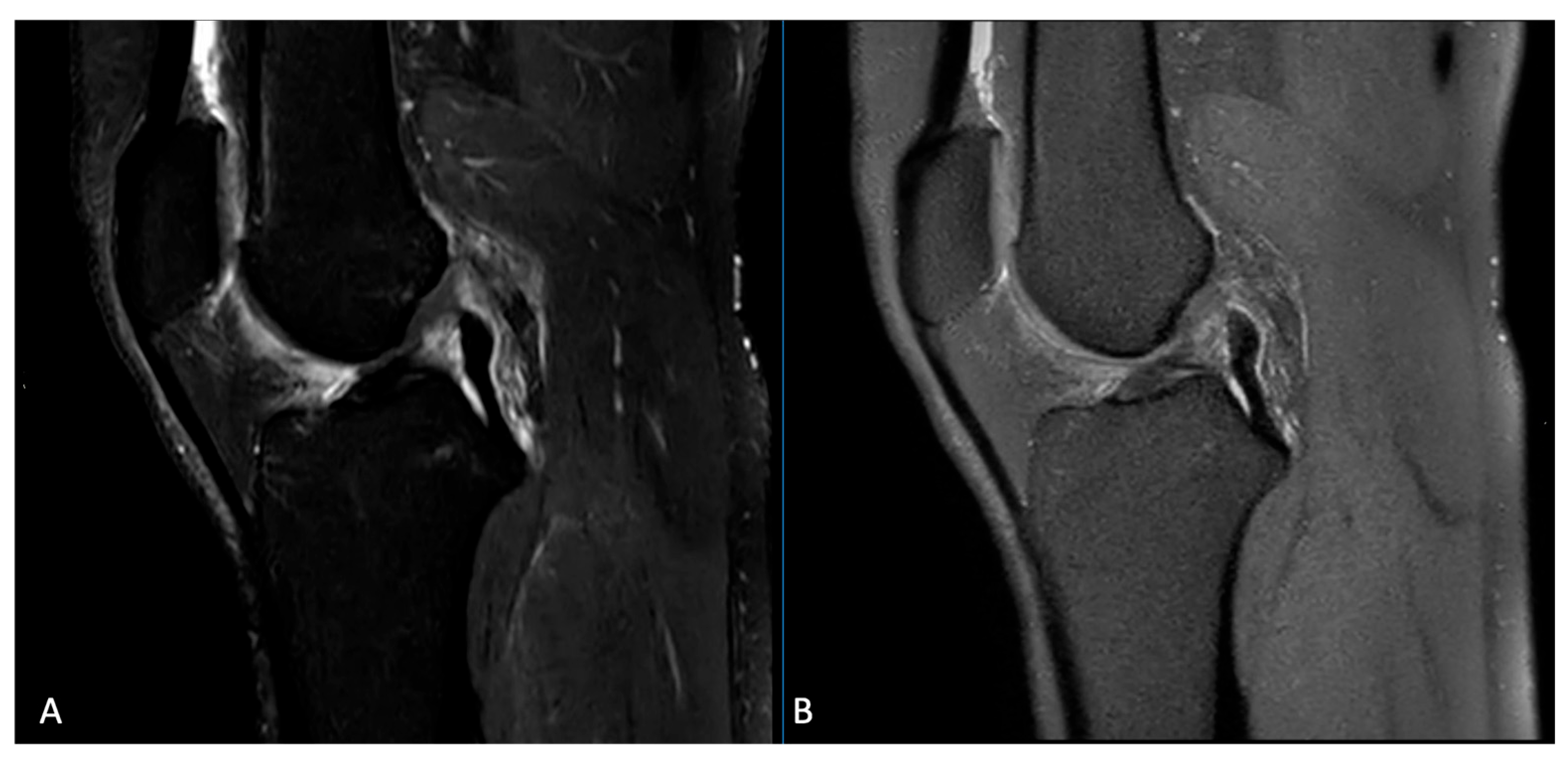
3.1. Image Quality
3.2. Artifacts
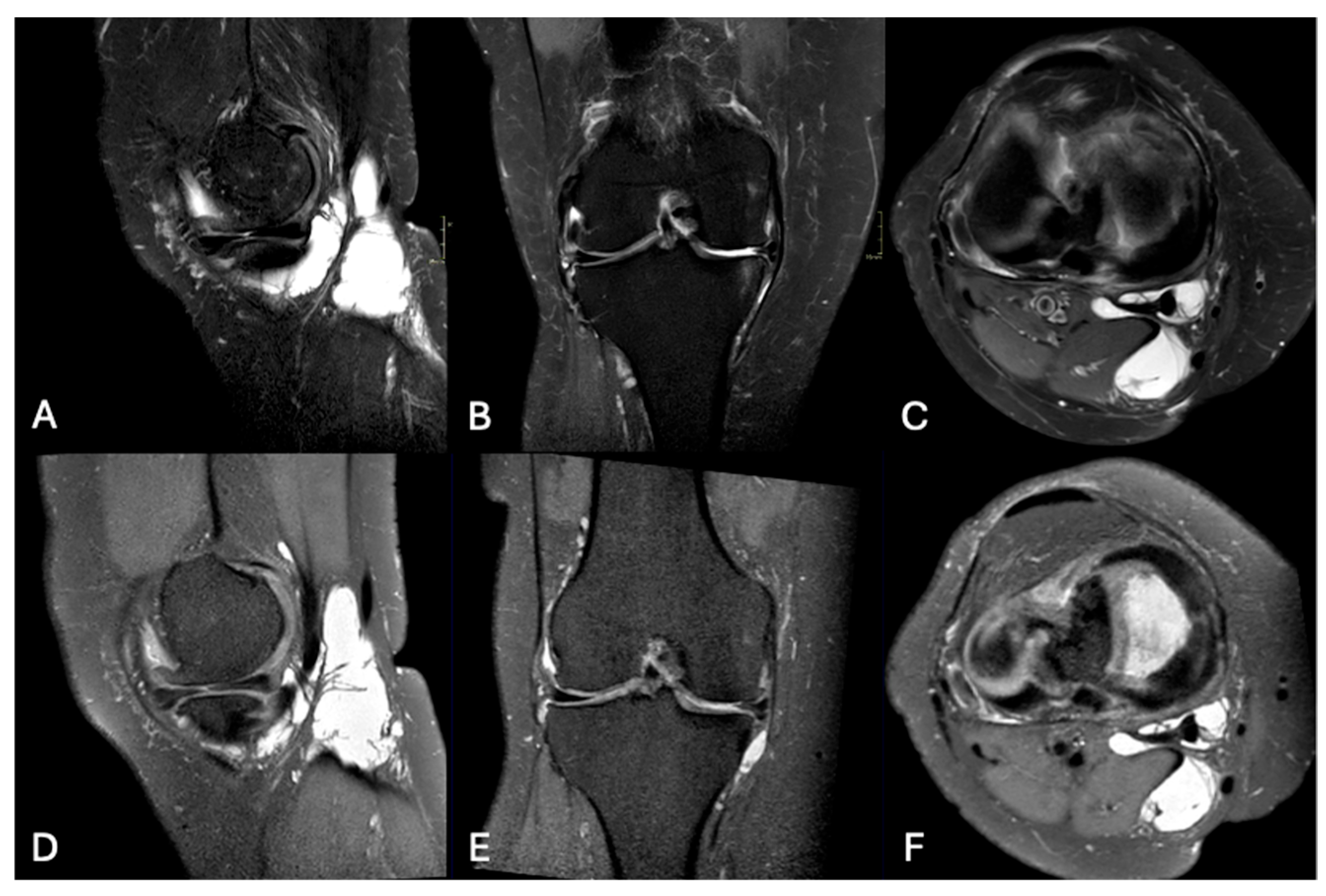
3.3. Pathological Entities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bull, A. Musculoskeletal biomechanics. J. Bone Joint Surg. Br. 2004, 86, 311. [Google Scholar] [CrossRef]
- Shelburne, K.B.; Torry, M.R.; Pandy, M.G. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. J. Orthop. Res. 2006, 24, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, K.B.; Torry, M.R.; Pandy, M.G. Muscle, Ligament, and Joint-Contact Forces at the Knee during Walking. Med. Sci. Sports Exerc. 2005, 37, 1948–1956. [Google Scholar] [CrossRef]
- Sanders, T.G.; Medynski, M.A.; Feller, J.F.; Lawhorn, K.W. Bone Contusion Patterns of the Knee at MR Imaging: Footprint of the Mechanism of Injury. RadioGraphics 2000, 20 (Suppl. S1), S135–S151. [Google Scholar] [CrossRef]
- Bencardino, J.T.; Rosenberg, Z.S.; Brown, R.R.; Hassankhani, A.; Lustrin, E.S.; Beltran, J. Traumatic Musculotendinous Injuries of the Knee: Diagnosis with MR Imaging. RadioGraphics 2000, 20 (Suppl. S1), S103–S120. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.H.; Deutsch, A.L. Occult cartilage and bone injuries of the knee: Detection, classification, and assessment with MR imaging. Radiology 1989, 170, 823–829. [Google Scholar] [CrossRef]
- Oei, E.H.G.; Ginai, A.Z.; Hunink, M.G.M. MRI for Traumatic Knee Injury: A Review. Semin. Ultrasound CT MRI 2007, 28, 141–157. [Google Scholar] [CrossRef]
- Carbonell, G.; Taouli, B. Abbreviated MR Protocols for Chronic Liver Disease and Liver Cancer. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 321–327. [Google Scholar] [CrossRef]
- Canellas, R.; Rosenkrantz, A.B.; Taouli, B.; Sala, E.; Saini, S.; Pedrosa, I.; Sahani, D.V. Abbreviated MRI Protocols for the Abdomen. RadioGraphics 2019, 39, 744–758. [Google Scholar] [CrossRef]
- Pozzi-Mucelli, R.M.; Rinta-Kiikka, I.; Wünsche, K.; Laukkarinen, J.; Labori, K.J.; Ånonsen, K.; Kartalis, N. Pancreatic MRI for the surveillance of cystic neoplasms: Comparison of a short with a comprehensive imaging protocol. Eur. Radiol. 2017, 27, 41–50. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Bulas, D. Techniques for minimizing sedation in pediatric MRI. J. Magn. Reson. Imaging 2019, 50, 1047–1054. [Google Scholar] [CrossRef]
- Jaimes, C.; Gee, M.S. Strategies to minimize sedation in pediatric body magnetic resonance imaging. Pediatr. Radiol. 2016, 46, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Kannikeswaran, N.; Sethuraman, U.; Sivaswamy, L.; Chen, X.; Mahajan, P.V. Children with and Without Developmental Disabilities. Pediatr. Emerg. Care. 2012, 28, 1036–1040. [Google Scholar] [CrossRef]
- Janos, S.; Schooler, G.R.; Ngo, J.S.; Davis, J.T. Free-breathing unsedated MRI in children: Justification and techniques. J. Magn. Reson. Imaging 2019, 50, 365–376. [Google Scholar] [CrossRef]
- Bjur, K.A.; Payne, E.T.; Nemergut, M.E.; Hu, D.; Flick, R.P. Anesthetic-Related Neurotoxicity and Neuroimaging in Children: A Call for Conversation. J. Child. Neurol. 2017, 32, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.; Nickerson, J.P.; Higgins, T.; Williams, R.K. Pediatric anesthesia and neurotoxicity: What the radiologist needs to know. Pediatr. Radiol. 2018, 48, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, G.B.; Babyn, P.S.; Vasanawala, S.S. Abdominal MR Imaging in Children: Motion Compensation, Sequence Optimization, and Protocol Organization. RadioGraphics 2013, 33, 703–719. [Google Scholar] [CrossRef]
- Ahmad, R.; Hu, H.H.; Krishnamurthy, R.; Krishnamurthy, R. Reducing sedation for pediatric body MRI using accelerated and abbreviated imaging protocols. Pediatr. Radiol. 2018, 48, 37–49. [Google Scholar] [CrossRef]
- Parkar, A.P.; Adriaensen, M.E.A.P.M. ESR essentials: MRI of the knee—Practice recommendations by ESSR. Eur. Radiol. 2024, 34, 6590–6599. [Google Scholar] [CrossRef]
- Del Grande, F.; Santini, F.; Herzka, D.A.; Aro, M.R.; Dean, C.W.; Gold, G.E.; Carrino, J.A. Fat-Suppression Techniques for 3-T MR Imaging of the Musculoskeletal System. RadioGraphics 2014, 34, 217–233. [Google Scholar] [CrossRef]
- Dalto, V.F.; Assad, R.L.; Lorenzato, M.M.; Crema, M.D.; Louzada-Junior, P.; Nogueira-Barbosa, M.H. Comparison between STIR and T2-weighted SPAIR sequences in the evaluation of inflammatory sacroiliitis: Diagnostic performance and signal-to-noise ratio. Radiol. Bras. 2020, 53, 223–228. [Google Scholar] [CrossRef]
- Chien, A.; Weaver, J.S.; Kinne, E.; Omar, I. Magnetic resonance imaging of the knee. Pol. J. Radiol. 2020, 85, 509–531. [Google Scholar] [CrossRef]
- American College of Radiology. ACR–SPR–SSR Practice Parameter for the Performance and Interpretation of Magnetic Resonance (MRI) of the Knee. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Knee.pdf (accessed on 15 June 2025).
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Guermazi, A. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research. RadioGraphics 2011, 31, 37–61. [Google Scholar] [CrossRef]
- Kijowski, R.; Davis, K.W.; Woods, M.A.; Lindstrom, M.J.; De Smet, A.A.; Gold, G.E.; Busse, R.F. Knee Joint: Comprehensive Assessment with 3D Isotropic Resolution Fast Spin-Echo MR Imaging—Diagnostic Performance Compared with That of Conventional MR Imaging at 3.0 T. Radiology 2009, 252, 486–495. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Gold, G.E.; Busse, R.F.; Beehler, C.; Han, E.; Brau, A.C.; Beatty, P.J.; Beaulieu, C.F. Isotropic MRI of the Knee with 3D Fast Spin-Echo Extended Echo-Train Acquisition (XETA): Initial Experience. Am. J. Roentgenol. 2007, 188, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Naraghi, A.; White, L.M. Three-Dimensional MRI of the Musculoskeletal System. Am. J. Roentgenol. 2012, 199, W283–W293. [Google Scholar] [CrossRef]
- Smith, J.P.; Barrett, G.R. Medial and Lateral Meniscal Tear Patterns in Anterior Cruciate Ligament-Deficient Knees. Am. J. Sports Med. 2001, 29, 415–419. [Google Scholar] [CrossRef]
- Endler, C.H.J.; Faron, A.; Isaak, A.; Katemann, C.; Mesropyan, N.; Kupczyk, P.A.; Luetkens, J.A. Fast 3D Isotropic Proton Density-Weighted Fat-Saturated MRI of the Knee at 1.5 T with Compressed Sensing: Comparison with Conventional Multiplanar 2D Sequences. In RöFo—Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgebenden Verfahren; Georg Thieme Verlag KG: Stuttgart, Germany, 2021. [Google Scholar] [CrossRef]
- Kijowski, R. Three-Dimensional Magnetic Resonance Imaging of Joints. Top. Magn. Reson. Imaging 2010, 21, 297–313. [Google Scholar] [CrossRef]
- Nguyen, J.C.; De Smet, A.A.; Graf, B.K.; Rosas, H.G. MR Imaging–based Diagnosis and Classification of Meniscal Tears. RadioGraphics 2014, 34, 981–999. [Google Scholar] [CrossRef]
- Shakoor, D.; Guermazi, A.; Kijowski, R.; Fritz, J.; Roemer, F.W.; Jalali-Farahani, S.; Demehri, S. Cruciate ligament injuries of the knee: A meta-analysis of the diagnostic performance of 3D MRI. J. Magn. Reson. Imaging 2019, 50, 1545–1560. [Google Scholar] [CrossRef]
- Chagas-Neto, F.A.; Nogueira-Barbosa, M.H.; Lorenzato, M.M.; Salim, R.; Kfuri-Junior, M.; Crema, M.D. Diagnostic performance of 3D TSE MRI versus 2D TSE MRI of the knee at 1.5 T, with prompt arthroscopic correlation, in the detection of meniscal and cruciate ligament tears. Radiol. Bras. 2016, 49, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kijowski, R.; Blankenbaker, D.G.; Klaers, J.L.; Shinki, K.; De Smet, A.A.; Block, W.F. Vastly Undersampled Isotropic Projection Steady-State Free Precession Imaging of the Knee: Diagnostic Performance Compared with Conventional MR. Radiology 2009, 251, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Del Grande, F.; Delcogliano, M.; Guglielmi, R.; Raithel, E.; Stern, S.E.; Papp, D.F.; Fritz, J. Fully Automated 10-Minute 3D CAIPIRINHA SPACE TSE MRI of the Knee in Adults. Investig. Radiol. 2018, 53, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Altahawi, F.; Pierce, J.; Aslan, M.; Li, X.; Winalski, C.S.; Subhas, N. 3D MRI of the Knee. Semin. Musculoskelet. Radiol. 2021, 25, 455–467. [Google Scholar] [CrossRef]
- Subhas, N. Abbreviated Musculoskeletal MRI Protocols: Point—Improved Patient Access and Reduced Health Care Costs. Am. J. Roentgenol. 2021, 216, 33–34. [Google Scholar] [CrossRef]
- Ippolito, D.; Giandola, T.; Maino, C.; Gandola, D.; Ragusi, M.; Brambilla, P.; Sironi, S. Diagnostic Value of Whole-Body MRI Short Protocols in Bone Lesion Detection in Multiple Myeloma Patients. Diagnostics 2021, 11, 1053. [Google Scholar] [CrossRef]
- Puhr-Westerheide, D.; Froelich, M.F.; Solyanik, O.; Gresser, E.; Reidler, P.; Fabritius, M.P.; Kazmierczak, P.M. Cost-effectiveness of short-protocol emergency brain MRI after negative non-contrast CT for minor stroke detection. Eur. Radiol. 2022, 32, 1117–1126. [Google Scholar] [CrossRef]
- Peters, S.; Gärtner, F.; Austein, F.; Wodarg, F.; Jansen, O.; Hensler, J. Evaluation of an Ultra-Short MRI Protocol for Cerebral Staging Examinations in Melanoma Patients. In RöFo—Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgebenden Verfahren; Georg Thieme Verlag KG: Stuttgart, Germany, 2022; Volume 194, pp. 409–415. [Google Scholar] [CrossRef]
- Kuhl, C.K. Abbreviated Breast MRI: State of the Art. Radiology 2024, 310, e221822. [Google Scholar] [CrossRef]
- van de Braak, C.; Willemssen, F.E.J.A.; de Man, R.A.; van der Lugt, A.; Uyl-de Groot, C.A.; Bos, D.; Dwarkasing, R.S. Non-contrast short MRI surveillance for HCC screening: The study protocol of the SMS-HCC prospective multicenter study. Eur. Radiol. Exp. 2024, 8, 29. [Google Scholar] [CrossRef]
- Ghorra, C.; Pommier, R.; Piveteau, A.; Rubbia-Brandt, L.; Vilgrain, V.; Terraz, S.; Ronot, M. The diagnostic performance of a simulated “short” gadoxetic acid-enhanced MRI protocol is similar to that of a conventional protocol for the detection of colorectal liver metastases. Eur. Radiol. 2021, 31, 2451–2460. [Google Scholar] [CrossRef]
- Potter, H.G.; Linklater, I.J.M.; Allen, A.A.; Hannafin, J.A.; Haas, S.B. Magnetic Resonance Imaging of Articular Cartilage in the Knee. An Evaluation with Use of Fast-Spin-Echo Imaging. J. Bone Joint Surg. 1998, 80, 1276–1284. [Google Scholar] [CrossRef]
- Mohr, A. The value of water-excitation 3D FLASH and fat-saturated PDw TSE MR imaging for detecting and grading articular cartilage lesions of the knee. Skelet. Radiol. 2003, 32, 396–402. [Google Scholar] [CrossRef]
- Schaefer, F.K.W.; Kurz, B.; Schaefer, P.J.; Fuerst, M.; Hedderich, J.; Graessner, J.; Heller, H. Accuracy and precision in the detection of articular cartilage lesions using magnetic resonance imaging at 1.5 tesla in an in vitro study with orthopedic and histopathologic correlation. Acta Radiol. 2007, 48, 1131–1137. [Google Scholar] [CrossRef]
- Fritz, J.; Ahlawat, S.; Fritz, B.; Thawait, G.K.; Stern, S.E.; Raithel, E.; Lee, R.J. 10-Min 3D Turbo Spin Echo MRI of the Knee in Children: Arthroscopy-Validated Accuracy for the Diagnosis of Internal Derangement. J. Magn. Reson. Imaging 2019, 49, e139–e151. [Google Scholar] [CrossRef]
| Sequence | Thickness | FOV | Matrix | TE (ms) | TR (ms) | FA (°) | Time |
|---|---|---|---|---|---|---|---|
| Standard protocol | |||||||
| T2W SPAIR sag | 3 | 160*160*92 | 308*238 | 60 | 3585 | 90 | 3.57 |
| PDW SPAIR cor | 3 | 79*160*160 | 268*255 | 30 | 3000 | 90 | 3.42 |
| T1W TSE cor | 3 | 86*160*160 | 380*257 | 17 | 500 | 90 | 3.17 |
| PDW SPAIR ax | 3 | 160*79*160 | 268*256 | 30 | 3000 | 90 | 3.18 |
| T2W FFE | 3 | 160*92*160 | 324*259 | 18.42 | Shortest | 30 | 3.15 |
| Short protocol | |||||||
| 3D PD SPAIR | 0.6*0.6*0.6 | 157*140*165 | 232*262 | 31 | 1100 | 90 | 5.54 |
| T1W TSE cor | 3 | 86*160*160 | 380*257 | 17 | 500 | 90 | 3.17 |
| T2W FFE | 3 | 160*92*160 | 324*259 | 18.42 | Shortest | 30 | 3.15 |
| Pathological Alteration | Description |
|---|---|
| Anterior cruciate ligament injury | Total or partial |
| Posterior cruciate ligament injury | Total or partial |
| Medial meniscal tear | Type of tear |
| Lateral meniscal tear | Type of tear |
| Lateral collateral ligament injury | Grade I, Grade II, Grade III |
| Medial collateral ligament injury | Grade I, Grade II, Grade III |
| Patellofemoral chondromalacia | Grade I, Grade II, Grade III, Grade IV |
| Tibiofemoral chondromalacia | Grade I, Grade II, Grade III, Grade IV |
| Bone edema | |
| Fracture |
| Likert Scale Value | Reader 1 | Reader 2 | ||
|---|---|---|---|---|
| 2D Sequences | 3D Sequences | 2D Sequences | 3D Sequences | |
| 1 (n, %) | 0 | 0 | 0 | 0 |
| 2 (n, %) | 1 (1.3) | 2 (2.6) | 0 | 2 (2.6) |
| 3 (n, %) | 15 (19.7) | 5 (6.6) | 27 (35.5) | 10 (13.1) |
| 4 (n, %) | 40 (52.6) | 14 (18.4) | 42 (55.3) | 26 (34.2) |
| 5 (n, %) | 20 (26.4) | 55 (72.4) | 7 (9.2) | 38 (50.1) |
| Pathological Alteration | 2D Sequences | 3D Sequences | p-Value |
|---|---|---|---|
| Artifacts (n, %) | 17 (22.4) | 18 (23.7) | 0.801 |
| Anterior cruciate ligament injury (n, %) | 13 (17.1) | 13 (17.1) | 1.0 |
| Posterior cruciate ligament injury (n, %) | 0 | 0 | - |
| Medial meniscal tear (n, %) | 27 (35.5) | 37 (48.7) | < 0.001 |
| Lateral meniscal tear (n, %) | 12 (15.8) | 17 (22.4) | < 0.001 |
| Lateral collateral ligament injury (n, %) | 2 (2.6) | 3 (3.9) | 0.990 |
| Medial collateral ligament injury (n, %) | 8 (10.5) | 8 (10.5) | 0.890 |
| Femoropatellar chondropathy (n, %) | 44 (57.9) | 40 (52.6) | 0.620 |
| Tibiofemoral chondropathy (n, %) | 35 (46.1) | 37 (48.7) | 0.500 |
| Bone edema (n, %) | 38 (50) | 39 (51.3) | 0.977 |
| Fracture (n, %) | 3 (3.9) | 3 (3.9) | 1.0 |
| Pathological Alteration | 2D Sequences | 3D Sequences | p-Value | |
|---|---|---|---|---|
| Anterior cruciate ligament injury | Complete (n, %) | 8 (10.5) | 8 (10.5) | 1.0 |
| Partial (n, %) | 5 (6.6) | 5 (6.5) | ||
| Medial meniscus injury | Vertical (n, %) | 2 (2.6) | 1 (1.3) | <0.001 |
| Horizontal (n, %) | 16 (21) | 21 (27.6) | ||
| Radial (n, %) | 1 (1.3) | 4 (5.2) | ||
| Complex (n, %) | 8 (10.4) | 11 (14.3) | ||
| Lateral meniscus injury | Vertical (n, %) | 3 (3.9) | 4 (5.2) | 0.006 |
| Horizontal (n, %) | 4 (5.2) | 5 (6.5) | ||
| Radial (n, %) | 1 (1.3) | 4 (5.2) | ||
| Complex (n, %) | 4 (5.2) | 4 (5.2) | ||
| Lateral collatarl ligament injury | Grade 1 (n, %) | 2 (2.6) | 3 (3.9) | 0.990 |
| Grade 2 (n, %) | 0 | 0 | ||
| Grade 3 (n, %) | 0 | 0 | ||
| Medial collatarl ligament injury | Grade 1 (n, %) | 4 (5.2) | 4 (5.2) | 1.0 |
| Grade 2 (n, %) | 3 (3.9) | 3 (3.9) | ||
| Grade 3 (n, %) | 1 (1.3) | 1 (1.3) | ||
| Femoropatellar chondropathy | Grade 1 (n, %) | 6 (7.8) | 3 (3.9) | 0.620 |
| Grade 2 (n, %) | 13 (16.9) | 12 (15.6) | ||
| Grade 3 (n, %) | 12 (15.6) | 13 (16.9) | ||
| Grade 4 (n, %) | 13 (16.9) | 12 (15.6) | ||
| Tibiofemoral chondromalacia | Grade 1 (n, %) | 2 (2.6) | 2 (2.6) | 0.530 |
| Grade 2 (n, %) | 6 (7.8) | 7 (9.1) | ||
| Grade 3 (n, %) | 4 (5.2) | 5 (6.5) | ||
| Grade 4 (n, %) | 23 (29.9) | 23 (29.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinnizzotto, M.; Ragusi, M.; Maino, C.; Allegranza, P.; Talei Franzesi, C.; Pellegatta, S.; Gandola, D.; Turati, M.; Corso, R.; Ippolito, D. Optimizing Knee MRI: Diagnostic Performance of a 3D PDW SPAIR-Based Short Protocol. Appl. Sci. 2025, 15, 8870. https://doi.org/10.3390/app15168870
Pinnizzotto M, Ragusi M, Maino C, Allegranza P, Talei Franzesi C, Pellegatta S, Gandola D, Turati M, Corso R, Ippolito D. Optimizing Knee MRI: Diagnostic Performance of a 3D PDW SPAIR-Based Short Protocol. Applied Sciences. 2025; 15(16):8870. https://doi.org/10.3390/app15168870
Chicago/Turabian StylePinnizzotto, Marco, Maria Ragusi, Cesare Maino, Pietro Allegranza, Cammillo Talei Franzesi, Stefania Pellegatta, Davide Gandola, Marco Turati, Rocco Corso, and Davide Ippolito. 2025. "Optimizing Knee MRI: Diagnostic Performance of a 3D PDW SPAIR-Based Short Protocol" Applied Sciences 15, no. 16: 8870. https://doi.org/10.3390/app15168870
APA StylePinnizzotto, M., Ragusi, M., Maino, C., Allegranza, P., Talei Franzesi, C., Pellegatta, S., Gandola, D., Turati, M., Corso, R., & Ippolito, D. (2025). Optimizing Knee MRI: Diagnostic Performance of a 3D PDW SPAIR-Based Short Protocol. Applied Sciences, 15(16), 8870. https://doi.org/10.3390/app15168870





