Quantitative Analysis Method for Full Lifecycle Aging Pathways of Lithium-Ion Battery Systems Based on Equilibrium Potential Reconstruction
Abstract
1. Introduction
- (1)
- An OCV curve analysis method under rest conditions is proposed. Using commercial NCM622/graphite power batteries as the research object, charge–discharge experiments were conducted to analyze aging modes, establishing a transferable experimental framework for investigating the degradation mechanisms of high-nickel NCM batteries.
- (2)
- When the actual properties of the cathode and anode materials were difficult to measure experimentally, we conducted this study based on the material property parameters (SOC-OCV) from COMSOL 6.0. By integrating the single-particle (SP) electrochemical model with the electrode equilibrium potentials, the OCV curve is reconstructed, thereby establishing a quantitative mapping from external battery characteristics to internal aging states.
- (3)
- To address the highly nonlinear relationship between the equilibrium potential and lithium intercalation concentration within the electrochemical model, an STA was introduced to resolve the high-dimensional, strongly coupled parameter identification problem. Finally, IC/DV analyses were synergistically integrated to analyze the complex degradation pathways within the battery.
2. Electrochemical Model-Based Identification of Battery-Aging Parameters Under Constant-Current (CC) Operation
2.1. Electrochemical Model for Lithium-Ion Batteries Under CC Conditions
- Li+ transport within solid-phase active particles is simplified as spherical diffusion;
- Infinite electrolyte conductivity is assumed, implying uniform lithium-ion distribution across all particles in both electrodes;
- Infinite solid/liquid phase conductivity is presumed, neglecting ohmic drops in these phases.
2.2. Identification of Active Material Loss in Lithium Batteries Under Rest Conditions
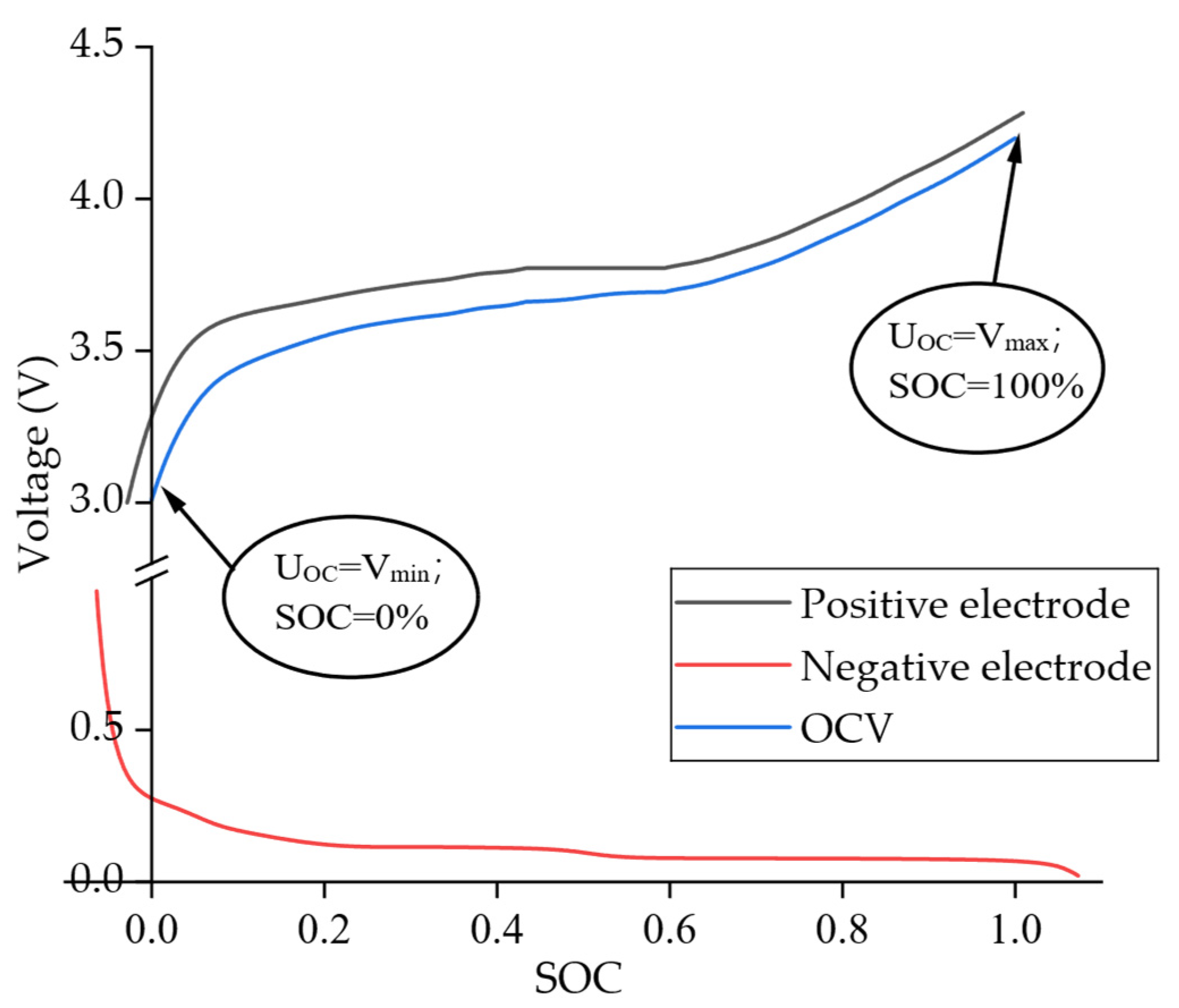
2.3. Electrochemical Parameter Identification Based on OCV Reconstruction
- SOC = 0% as the state where the relative equilibrium potential Uoc(t) reaches the lower cut-off voltage Vmin;
- SOC = 100% as the state where Uoc(t) reaches the upper cut-off voltage Vmax.
3. STA-Based Battery Parameter Identification
3.1. STA Optimization Method
- (1)
- Rotation Operator
- (2)
- Translation Operator
- (3)
- Expansion Operator
- (4)
- Axial Search Operator
3.2. Experiment
- NCM622/graphite prismatic ternary lithium-ion power battery.
- Programmable battery test system (LTM200, Shenzhen Wright Energy Technology Co., Ltd, Shenzhen, China) with eight independent channels. Each channel provides
- Voltage sampling/output range: 0 to 5 V (voltage accuracy: ±0.01% FS, voltage stability: ±0.01% FS);
- Current output range: −100 A to +100 A (current accuracy: ±0.01% FS, current stability: ±0.01% FS).
- Programmable thermal chamber (JD-8001B, Dongguan Jiedong Testing Equipment Co., Ltd, Dongguan, China). The battery was maintained at 25.0 ± 0.5 °C throughout testing.
- Host computer controlling charge/discharge protocols and logging electrochemical data at 1 Hz sampling frequency.
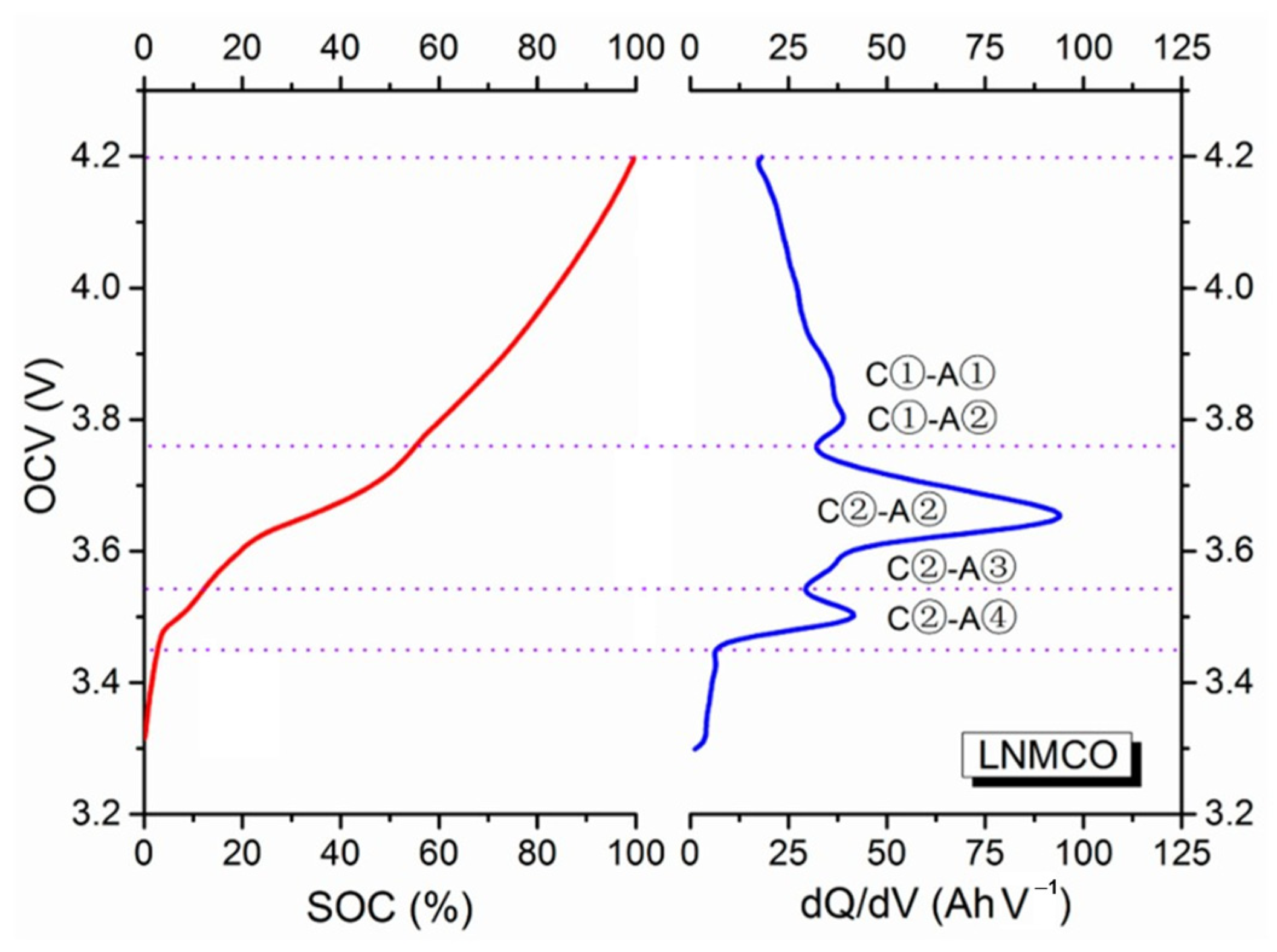
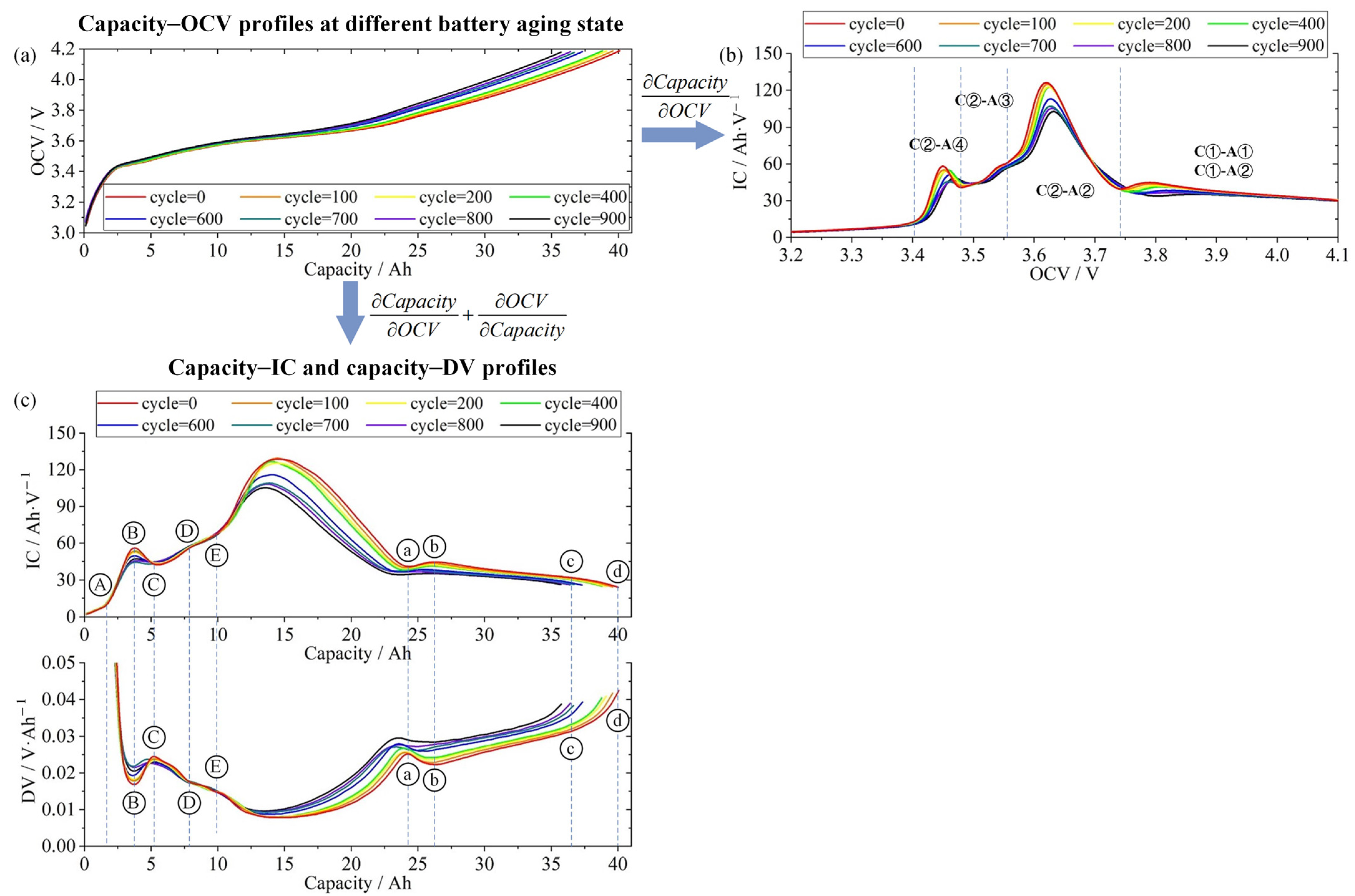
3.3. IC-Based Degradation Mechanism Analysis
- Reaction C① (voltage range of 4.2–3.75 V): Ni4+ ↔ Ni3+;
- Reaction C② (voltage range of 3.75–3.55 V): Ni3+ ↔ Ni2+.
- A①: LiC6 ↔ LiC12 at 0.10–0.08 V;
- A②: LiC12 ↔ LiC18 ↔ LiC36 at 0.14–0.11 V;
- A③: LiC36 ↔ LiC72 at 0.22–0.20 V;
- A④: LiC72 ↔ C6 above 0.30 V.
4. Experimental Results Analysis
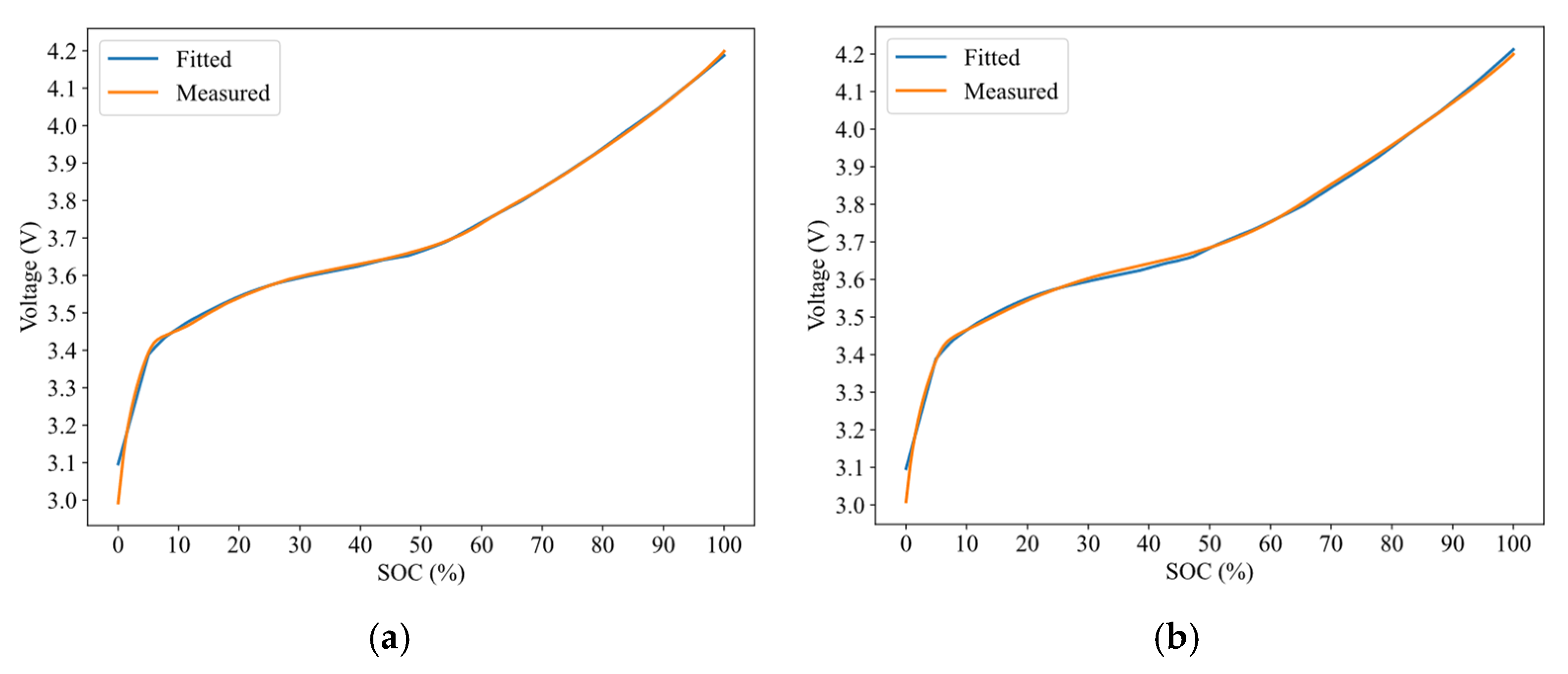
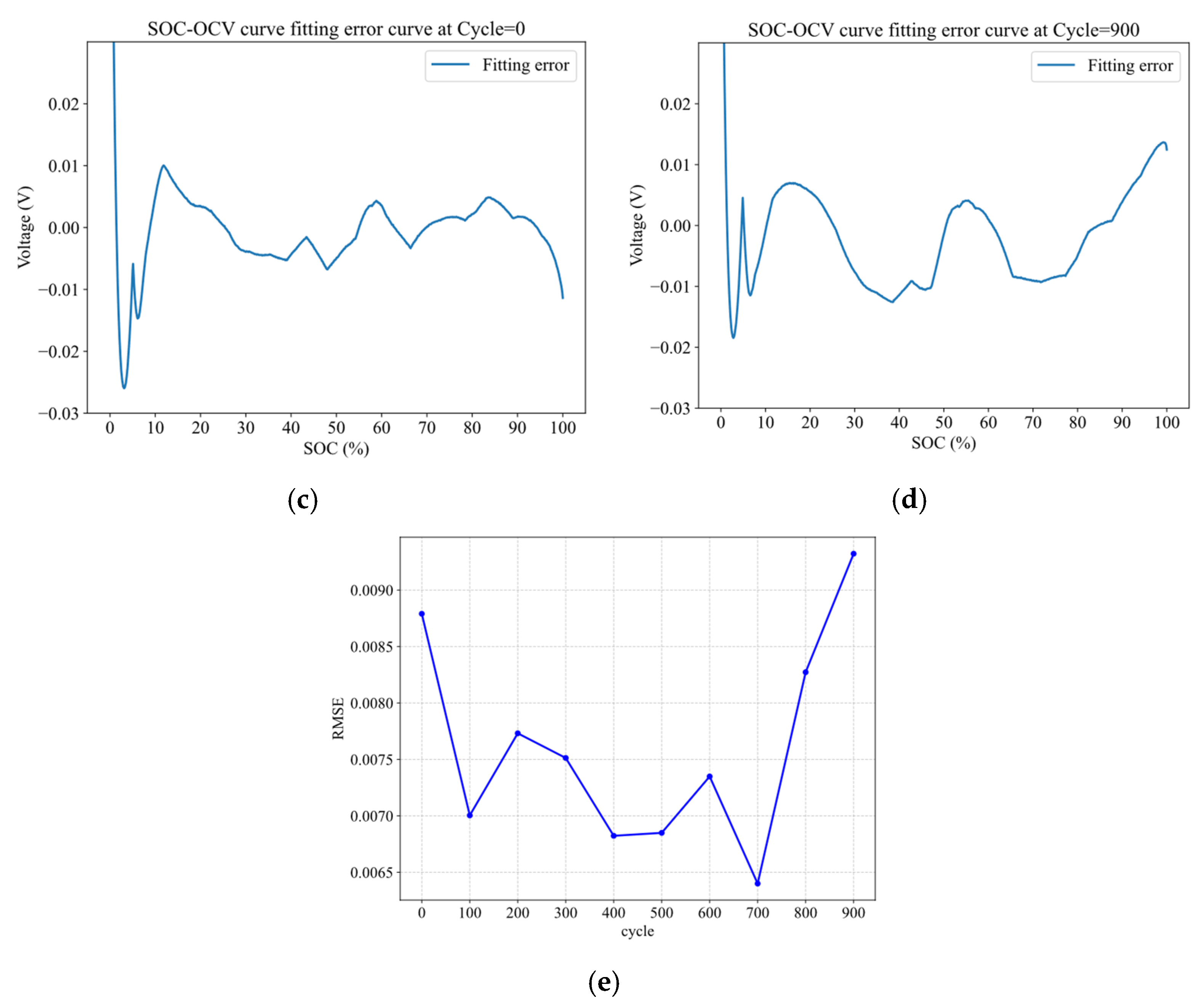
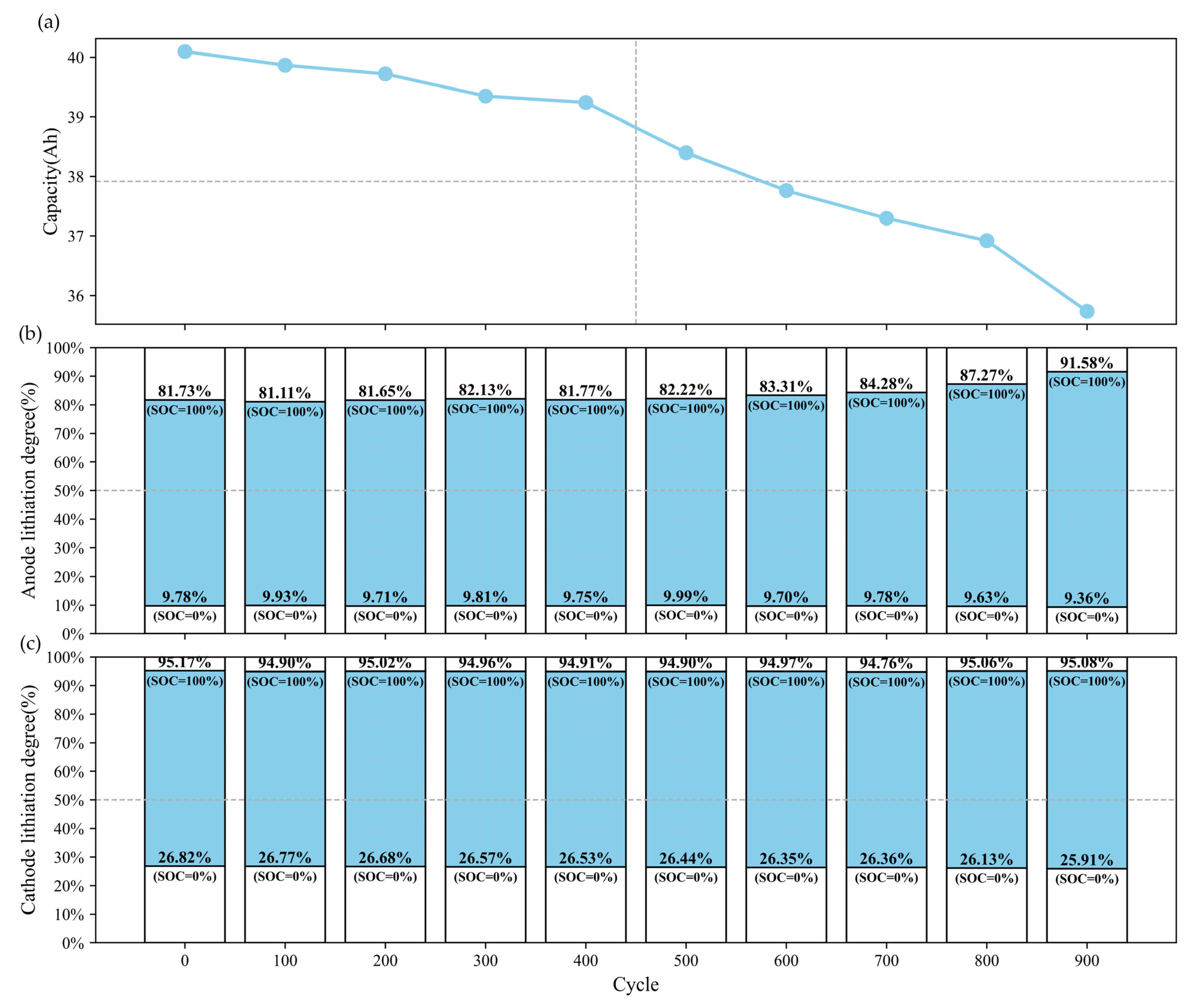
4.1. Evolution of Electrode Lithiation States with Cycling
4.2. IC-Based Degradation Mechanism Analysis Results
 to
to  , and the FOI of NCM622 (cathode) is labeled as
, and the FOI of NCM622 (cathode) is labeled as  to
to  . The location of the FOI is shown in Figure 7c, and its physical significance is shown in Table 2. The evolution of these FOIs (e.g., peak shift or attenuation) aligns with the degradation pathways inferred from the OCV reconstruction analysis.
. The location of the FOI is shown in Figure 7c, and its physical significance is shown in Table 2. The evolution of these FOIs (e.g., peak shift or attenuation) aligns with the degradation pathways inferred from the OCV reconstruction analysis.5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Variable/Symbol | Description | Units |
| V(t) | output voltage | V |
| Vmax | upper cut-off voltage | V |
| Vmin | lower cut-off voltage | V |
| E(t) | relative equilibrium potential | V |
| ηSEI(t) | SEI resistance drop | V |
| ηelectrolyte(t) | electrolyte ohmic drop | V |
| ηact(t) | activation overpotential | V |
| I | constant current | A |
| p | cathode parameter | - |
| n | anode parameter | - |
| Uref(t) | equilibrium potential of active materials | V |
| cs,surf(t) | surface Li-ion concentration | mol/m3 |
| cs,avg | average lithium-ion concentration | mol/m3 |
| cs,max | maximum intercalation concentration | mol/m3 |
| RSEI | SEI resistance | Ω |
| as | specific surface area of active particles | m−1 |
| local current density | A/m2 | |
| thickness of electrodes and separator | m | |
| Κeff | effective ionic conductivity of electrolyte | S/m |
| R | universal gas constant | J/(mol·K) |
| T | temperature | K |
| α | charge transfer coefficient | - |
| F | Faraday’s constant | C/mol |
| A | total equivalent surface area | m2 |
| Rs | active particle radius | m |
| Ds | solid-phase Li+ diffusion coefficient | m2·s−1 |
| k | kinetic reaction constant | - |
| Uoc(t) | open-circuit voltage | V |
| εs | solid-phase volume fraction of active materials | - |
| nLi,useful | usable lithium-ion molar quantity | mol |
| Vs,j | effective volumes of anode/cathode active materials | m3 |
| ξ | lithiation degree | - |
References
- Wang, Z.; Zhao, X.; Fu, L.; Zhen, D.; Gu, F.; Ball, A.D. A review on rapid state of health estimation of lithium-ion batteries in electric vehicles. Sustain. Energy Technol. Assess. 2023, 60, 103457. [Google Scholar] [CrossRef]
- Wang, F.; Zhai, Z.; Zhao, Z.; Di, Y.; Chen, X. Physics-informed neural network for lithium-ion battery degradation stable modeling and prognosis. Nat. Commun. 2024, 15, 4332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, G.; Chen, K.; He, S.; Liu, K.; Xin, D.; Luo, Y.; Long, Z.; Wu, G. State-of-health prediction of lithium-ion batteries using feature fusion and a hybrid neural network model. Energy 2025, 319, 135163. [Google Scholar] [CrossRef]
- Barcellona, S.; Colnago, S.; Dotelli, G.; Latorrata, S.; Piegari, L. Aging effect on the variation of Li-ion battery resistance as function of temperature and state of charge. J. Energy Storage 2022, 50, 104658. [Google Scholar] [CrossRef]
- Xiao, Y.; Wen, J.; Yao, L.; Zheng, J.; Fang, Z.; Shen, Y. A comprehensive review of the lithium-ion battery state of health prognosis methods combining aging mechanism analysis. J. Energy Storage 2023, 65, 107347. [Google Scholar] [CrossRef]
- Sun, R.; Chen, J.; Li, B.; Piao, C. State of health estimation for Lithium-ion batteries based on novel feature extraction and BiGRU-Attention model. Energy 2025, 319, 134756. [Google Scholar] [CrossRef]
- Peng, S.; Sun, Y.; Liu, D.; Yu, Q.; Kan, J.; Pecht, M. State of health estimation of lithium-ion batteries based on multi-health features extraction and improved long short-term memory neural network. Energy 2023, 282, 128956. [Google Scholar] [CrossRef]
- Li, X.; Yuan, C.; Wang, Z. State of health estimation for Li-ion battery via partial incremental capacity analysis based on support vector regression. Energy 2020, 203, 117852. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, Y.; Liu, S.; Li, J.; Tang, A. State of health estimation method for lithium-ion batteries based on multiple dynamic operating conditions. J. Power Sources 2023, 582, 233541. [Google Scholar] [CrossRef]
- Wu, J.; Fang, L.; Dong, G.; Lin, M. State of health estimation of lithium-ion battery with improved radial basis function neural network. Energy 2023, 262, 125380. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, X.; He, Y. Remaining useful life and state of health prediction for lithium batteries based on empirical mode decomposition and a long and short memory neural network. Energy 2021, 232, 121022. [Google Scholar] [CrossRef]
- Chang, C.; Wang, Q.; Jiang, J.; Wu, T. Lithium-ion battery state of health estimation using the incremental capacity and wavelet neural networks with genetic algorithm. J. Energy Storage 2021, 38, 102570. [Google Scholar] [CrossRef]
- Wang, W.; Yang, K.; Zhang, L.; Zhou, S.; Ren, B.; Lu, Y.; Tan, R.; Zhu, T.; Ma, B.; Yang, S.; et al. An end-cloud collaboration approach for online state-of-health estimation of lithium-ion batteries based on multi-feature and transformer. J. Power Sources 2024, 608, 234669. [Google Scholar] [CrossRef]
- Li, W.; Fan, Y.; Ringbeck, F.; Jöst, D.; Han, X.; Ouyang, M.; Sauer, D.U. Electrochemical model-based state estimation for lithium-ion batteries with adaptive unscented Kalman filter. J. Power Sources 2020, 476, 228534. [Google Scholar] [CrossRef]
- Lai, X.; He, L.; Wang, S.; Zhou, L.; Zhang, Y.; Sun, T.; Zheng, Y. Co-estimation of state of charge and state of power for lithium-ion batteries based on fractional variable-order model. J. Clean. Prod. 2020, 255, 120203. [Google Scholar] [CrossRef]
- Lai, X.; Weng, J.; Huang, Y.; Yuan, M.; Yao, Y.; Han, X.; Zheng, Y. A joint state-of-health and state-of-energy estimation method for lithium-ion batteries through combining the forgetting factor recursive least squares and unscented Kalman filter. Measurement 2022, 205, 112187. [Google Scholar] [CrossRef]
- Wang, T.; Pei, L.; Wang, T.; Lu, R.; Zhu, C. Capacity-loss diagnostic and life-time prediction in lithium-ion batteries: Part 1. Development of a capacity-loss diagnostic method based on open-circuit voltage analysis. J. Power Sources 2016, 301, 187–193. [Google Scholar] [CrossRef]
- Wang, F.; Tang, S.; Han, X.; Lu, L.; Yu, C.; Zhao, C.; Gao, Y.; Zhu, G.; Zhuang, K.; Ouyang, M. A voltage reconstruction model for lithium-ion batteries considering the polarization process. J. Power Sources 2023, 588, 233744. [Google Scholar] [CrossRef]
- Pan, B.; Dong, D.; Wang, J.; Nie, J.; Liu, S.; Cao, Y.; Jiang, Y. Aging mechanism diagnosis of lithium ion battery by open circuit voltage analysis. Electrochim. Acta 2020, 362, 137101. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, C.; Jiang, J.; Zhang, W.; Gao, Y.; Zhang, L. A voltage reconstruction model based on partial charging curve for state-of-health estimation of lithium-ion batteries. J. Energy Storage 2021, 35, 102271. [Google Scholar] [CrossRef]
- Schaltz, E.; Stroe, D.I.; Nørregaard, K.; Ingvardsen, L.S.; Christensen, A. Incremental Capacity Analysis Applied on Electric Vehicles for Battery State-of-Health Estimation. IEEE Trans. Ind. Appl. 2021, 57, 1810–1817. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Zhang, G.; Takyi-Aninakwa, P.; Fernandez, C.; Blaabjerg, F. A review of data-driven whole-life state of health prediction for lithium-ion batteries: Data preprocessing, aging characteristics, algorithms, and future challenges. J. Energy Chem. 2024, 97, 630–649. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Zhang, L.; Liu, S.; Wang, L.; Loh, P.C. A Generalized SOC-OCV Model for Lithium-Ion Batteries and the SOC Estimation for LNMCO Battery. Energies 2016, 9, 900. [Google Scholar] [CrossRef]


| Electrode | C①-A① | C①-A② | C②-A② | C②-A③ | C②-A④ |
|---|---|---|---|---|---|
| Cathode | C① Ni4+ ↔ Ni3+ | C② Ni3+ ↔ Ni2+ | |||
| Anode | A① LiC6 ↔ LiC12 | A② LiC12 ↔ LiC18 ↔ LiC36 | A③ LiC36 ↔ LiC72 | A④ LiC72 ↔ C6 | |
| Electrode | FOI | Physical Meaning | Remark |
|---|---|---|---|
| graphite | 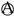 | Start of phase transformation LiC72 ↔ C6 | Corresponds to the starting voltage plateau of lithium deintercalation. |
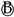 | Phase transformation LiC72 ↔ C6 | Point of phase transformation completion, reflecting the integrity of the phase transformation in the high SOC region of the anode. | |
 | Start of phase transformation LiC36 ↔ LiC72 | Corresponds to phase transformation in the medium–high SOC region. | |
 | Phase transformation LiC36 ↔ LiC72 | Point of phase transformation completion, coupled with the cathode C② reaction. | |
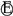 | Start of phase transformation LiC12 ↔ LiC18 ↔ LiC36 | Corresponds to multi-stage phase transformation in the medium SOC region. | |
| NCM622 |  | Start of phase transformation Ni4+ ↔ Ni3+ | Corresponds to the redox reaction in the high-voltage region. |
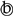 | Phase transformation Ni4+ ↔ Ni3+ and LiC6 ↔ LiC12 | The IC peaks of C① and A① overlap. As the lithium-ion battery ages, the IC peak of C① gradually shifts left, making FOI increasingly inaccurate. | |
 | Convexity of the IC curve | The peak area reflects the amount of available lithium ions in the high-voltage region; peak attenuation during aging indicates LLI. | |
 | ≈4.27 V | During phase transformation A① ≈ 4.27 V. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Guo, Y.; Zhang, W. Quantitative Analysis Method for Full Lifecycle Aging Pathways of Lithium-Ion Battery Systems Based on Equilibrium Potential Reconstruction. Appl. Sci. 2025, 15, 10079. https://doi.org/10.3390/app151810079
Yu J, Guo Y, Zhang W. Quantitative Analysis Method for Full Lifecycle Aging Pathways of Lithium-Ion Battery Systems Based on Equilibrium Potential Reconstruction. Applied Sciences. 2025; 15(18):10079. https://doi.org/10.3390/app151810079
Chicago/Turabian StyleYu, Jiaqi, Yanjie Guo, and Wenjie Zhang. 2025. "Quantitative Analysis Method for Full Lifecycle Aging Pathways of Lithium-Ion Battery Systems Based on Equilibrium Potential Reconstruction" Applied Sciences 15, no. 18: 10079. https://doi.org/10.3390/app151810079
APA StyleYu, J., Guo, Y., & Zhang, W. (2025). Quantitative Analysis Method for Full Lifecycle Aging Pathways of Lithium-Ion Battery Systems Based on Equilibrium Potential Reconstruction. Applied Sciences, 15(18), 10079. https://doi.org/10.3390/app151810079




