Fast and Green Extraction Method Based on HS–SPME/GC–MS to Identify Chemical Markers of X-Ray Irradiated Hen Eggs
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Irradiation Procedure
2.3. Sample Preparation
2.4. Instrument and HS–SPME/GC–MS Method
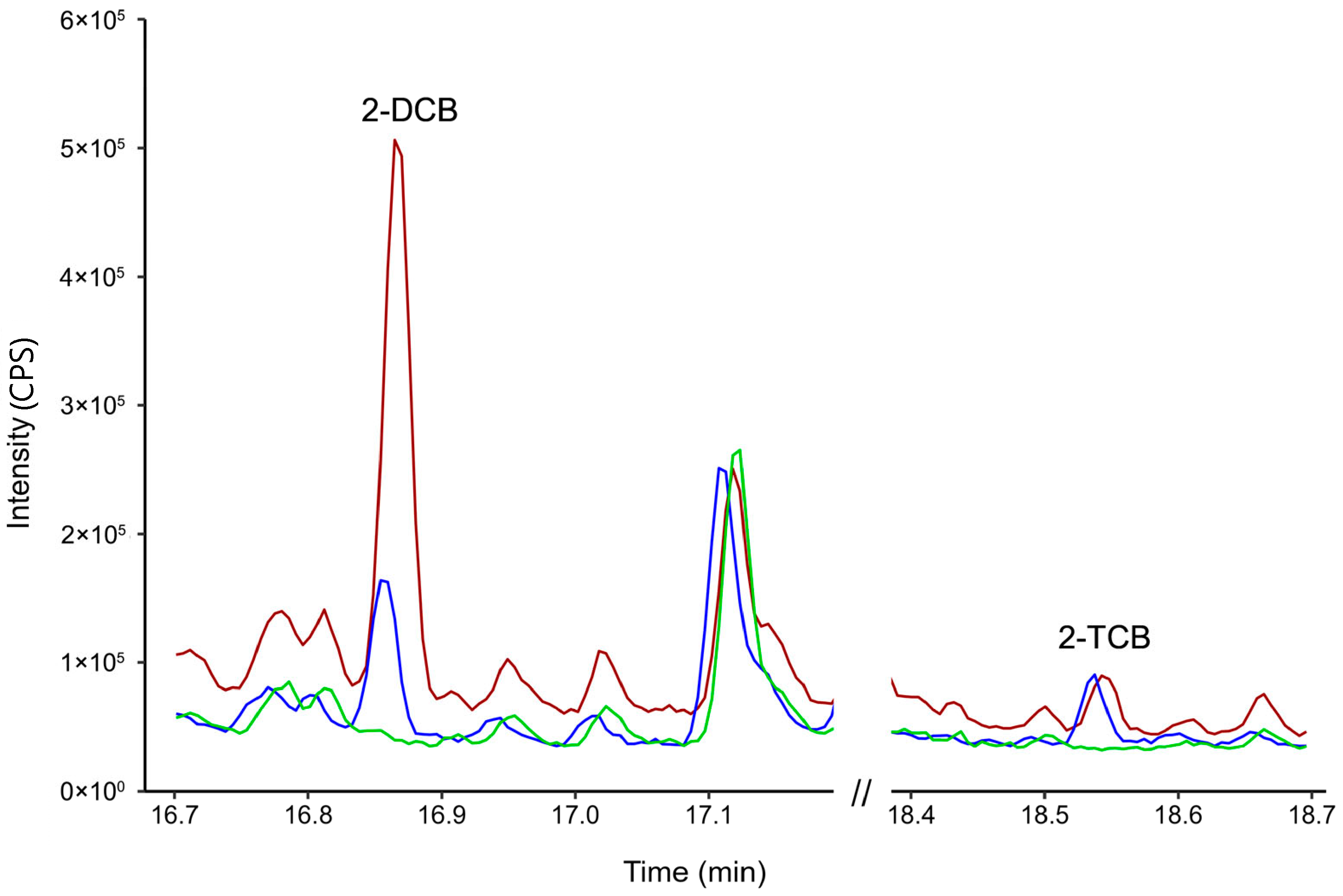
2.5. Assessment of 2–DCB and 2–TCB
2.6. Evaluation of Method Analytical Performances
2.7. WAC Evaluation: Red, Green and Blue Models (RGB12)
3. Results
3.1. Optimization of Sample Preparation
3.2. Analytical Performances of HS–SPME/GC–MS Method
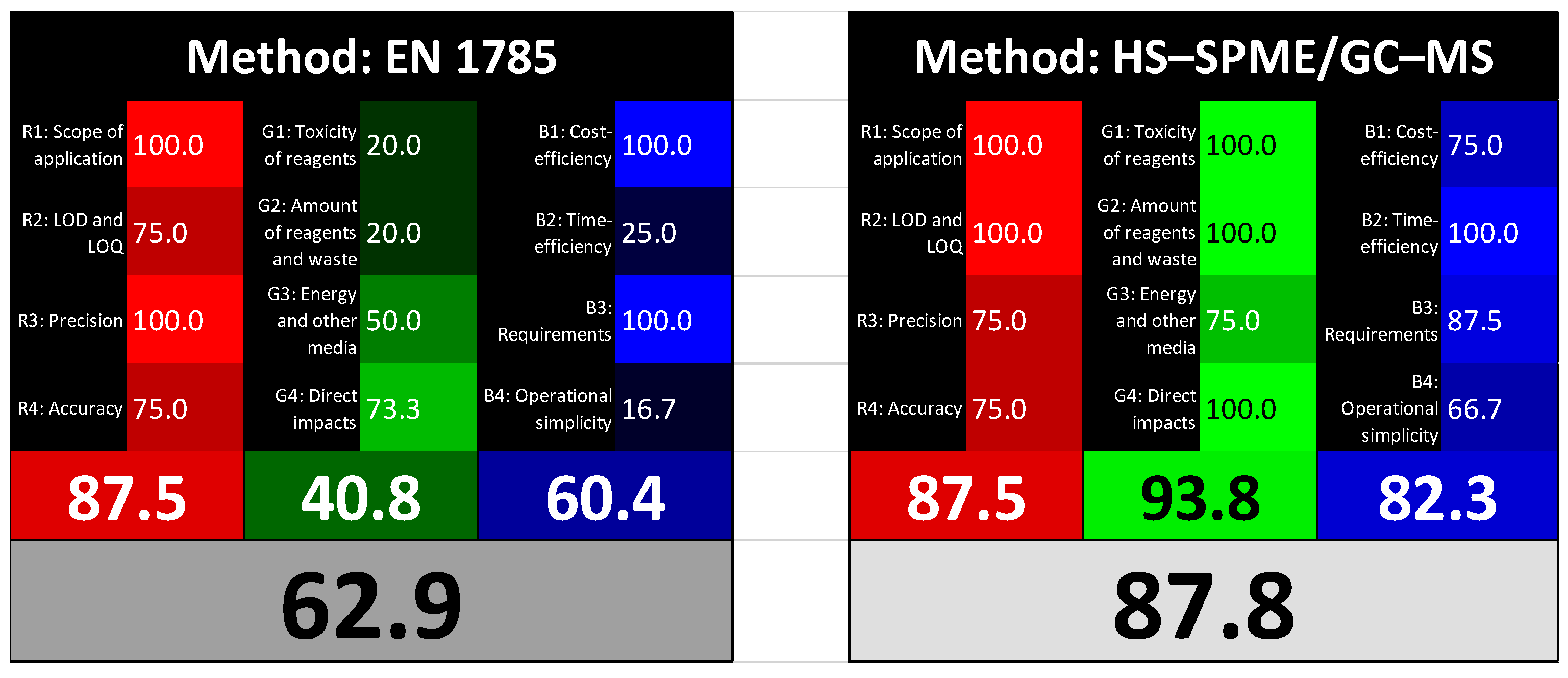
3.3. Red, Green and Blue Evaluation of HS–SPME/GC–MS Method: Comparison with the Standard Method EN 1785:2003
3.3.1. Red Principles
- R1: Scope of application
- R2: LOD and LOQLOD and LOQ were estimated more specifically in terms of the lower level of 2–DCB and 2–TCB in spiked and/or irradiated samples. In the EN 1785:2003, the detection of irradiated liquid whole egg has been validated for doses of approximately 1 kGy and above. The standardized method has been extensively studied and tested, and the literature data support its validity. Regarding the identification of radiation treatment, the LOQ is often expressed as the minimum irradiation dose that can be demonstrated. Kim and others [33], using a method close to the EN 1785:2003, reported low levels (14 μg kg−1) of 2–DCB in irradiated dried shrimp. In a previous work, the EN 1785:2003 method was applied to processed poultry meat products and it was proven that a dose of 0.5 kGy corresponds to 3.0 μg kg−1 of 2–DCB [17]. Based on these considerations, the analytical performances of the two methods were found to be comparable only for 2–DCB. For these reasons, we scored LOD and LOQ with 75 points because the EN 1785:2003 does not provide sufficient results. Conversely, the proposed HS–SPME/GC–MS method demonstrated a lower LOD of 2.50 μg kg−1, and a minimum detectable dose level of 0.5 kGy for both irradiation markers. Thus, a score of 100 was assigned.
- R3: PrecisionPrecision is expressed as repeatability and reproducibility of the results. The standard method was validated by inter-laboratory blind trials, so both repeatability and reproducibility were satisfied [34]. In the proposed new method, repeatability was only carried out during intra-laboratory validation. For these reasons, we assigned a value of 100 to the EN 1785:2003 and 75 to the HS–SPME/GC–MS method.
- R4: AccuracyThe R4 was evaluated in terms of relative error and recovery. The recovery was evaluated by Campaniello et al. (2025) [17] for the standard method applied to chicken meat, resulting in 67.5% for spiked samples at 40 μg kg−1. The EN 1785:2003 does not provide relative errors and recovery. Regarding the HS–SPME/GC–MS method, recovery was not calculated, since it is a qualitative method, while error of mean (σM), calculated with spiked samples analyzed for MMC, was near or below 0.30. Since both methods missed a parameter, a score of 75 was assigned for both.
3.3.2. Green Principles
- G1: Toxicity of reagentsThe RGB 12 algorithm determines the toxicity of reagents, i.e., their impact and biodegradation, by means of the total number of pictograms of reagents employed. For both methods tested, only the reagents were evaluated, excluding the 2–DCB and 2–TCB standards. The Soxhlet extraction which is very long (6 h) and uses a flammable solvent (hexane) [15] represents a major criticism of the EN 1785:2003 method. A further criticism concerns the clean-up via the Florisil® column, which requires hexane and 1% diethyl ether in hexane to clean-up and elute the 2–DCB. Considering these two steps, a total of six pictograms were recognized (score: 20), against none for the HS–SPME/GC–MS method (score: 100).
- G2: Amount of reagents and wasteAs described in the previous section, the EN 1785:2003 method shows an extensive use of solvents, which results in resource consumption and waste generation because during extraction and purification, approximately 500 mL of solvents were used and discarded. The HS–SPME/GC–MS method proposed, using only water for analyte extraction, reduces the use of solvents and the production of waste. The scores for the G2 parameter were the same as G1.
- G3: Consumption of energy and other mediaThis point considers the energy expenditure of the analytical method. We only evaluated the sample preparation, since the GC–MS step was identical for both methods. The HS–SPME/GC–MS method involves a headspace extraction that includes an enrichment time of 30 min, an extraction time of 60 min and fiber conditioning before and after each cycle of 30 and 20 min, respectively. Although this automated procedure takes more than two hours, it is less than the six hours required for Soxhlet extraction and the time needed for sample purification, without considering the additional time required for the solvent evaporation steps. For these reasons, we assigned a value of 50 to the EN 1785:2003 and 75 to the HS–SPME/GC–MS method.
- G4: Direct impactsThe principle “direct impacts” were determinate in terms of safety of users, use of animals and GMOs. As clarified in Section 2.7, the criteria related to the use of animals and GMOs were filled with zero because they were not used at any stage of the procedure. Even in this case, as occurred in the evaluation of the G1 and G2 parameters, the professional risk is linked to the use of solvents employed exclusively in the EN 1785:2003 standard, for which we attributed a score of 20 to the standardized method and a score of 100 to the HS–SPME/GC–MS method.
3.3.3. Blue Principles
- B1: Cost-efficiencyWhen investigating the application of different methods compared to the standard method EN 1785:2003, consideration of cost and availability of instrumentation is needed. Using alternative extraction procedures, such as automated HS–SPME, would be faster, but require a significant starting investment for equipment purchase (15,000.00 € cost presumed for the purchase of SPME autosampler). The Soxhlet method has a relatively low capital cost, estimated at around 1000.00 € for the instrumentation, without considering the cost of solvents used for each analysis, and the economic gap with sophisticated instruments cannot be evenly compensated by the costs of solvents (around 60.00 € for 500 mL). For the B1 parameter, the EN 1785:2003 received a score of 100, while 75 was assigned to that proposed in this study.
- B2: Time-efficiencyThe time-efficiency of a method is related to its speed of analysis. The longer extraction times required by the standardized method have already been commented in section “G3: Energy consumption and other media”. This characteristic, in addition to influencing energy consumption, also affects the number of samples that can be analyzed in a single batch. The HS–SPME/GC–MS method has also been proposed to reduce sample processing time. In fact, using the EN 1785:2003 method, it is possible to analyze an average of three samples per day (eight hours for each sample), while the method proposed in this work processes approximately ten samples per day (two and a half hours per sample), in a completely automatic way. For these reasons, we assigned a value of 25 to the EN 1785:2003 and 100 to the HS–SPME/GC–MS method.
- B3: RequirementsAnalytical methods should be characterized by the minimal practical requirements, including the amount of sample used, access to advanced equipment, personnel qualifications and laboratory infrastructure [20]. In our case, the amount of sample used in the two methods, although different, is irrelevant, since only a few grams are used. The difference is evident with regard to the use of sophisticated technologies and the employment of qualified personnel. The crucial point is once again the sample preparation which, in the case of the EN 1785:2003 method, requires simple instrumentation and unskilled personnel. In contrast, the HS–SPME extraction requires more expensive instrumentation (autosampler for headspace extraction) and adequately trained personnel. The B3 parameter was expressed as a mean value, resulting in 100 for the EN 1785:2003 and 87.5 for HS–SPME/GC–MS method.
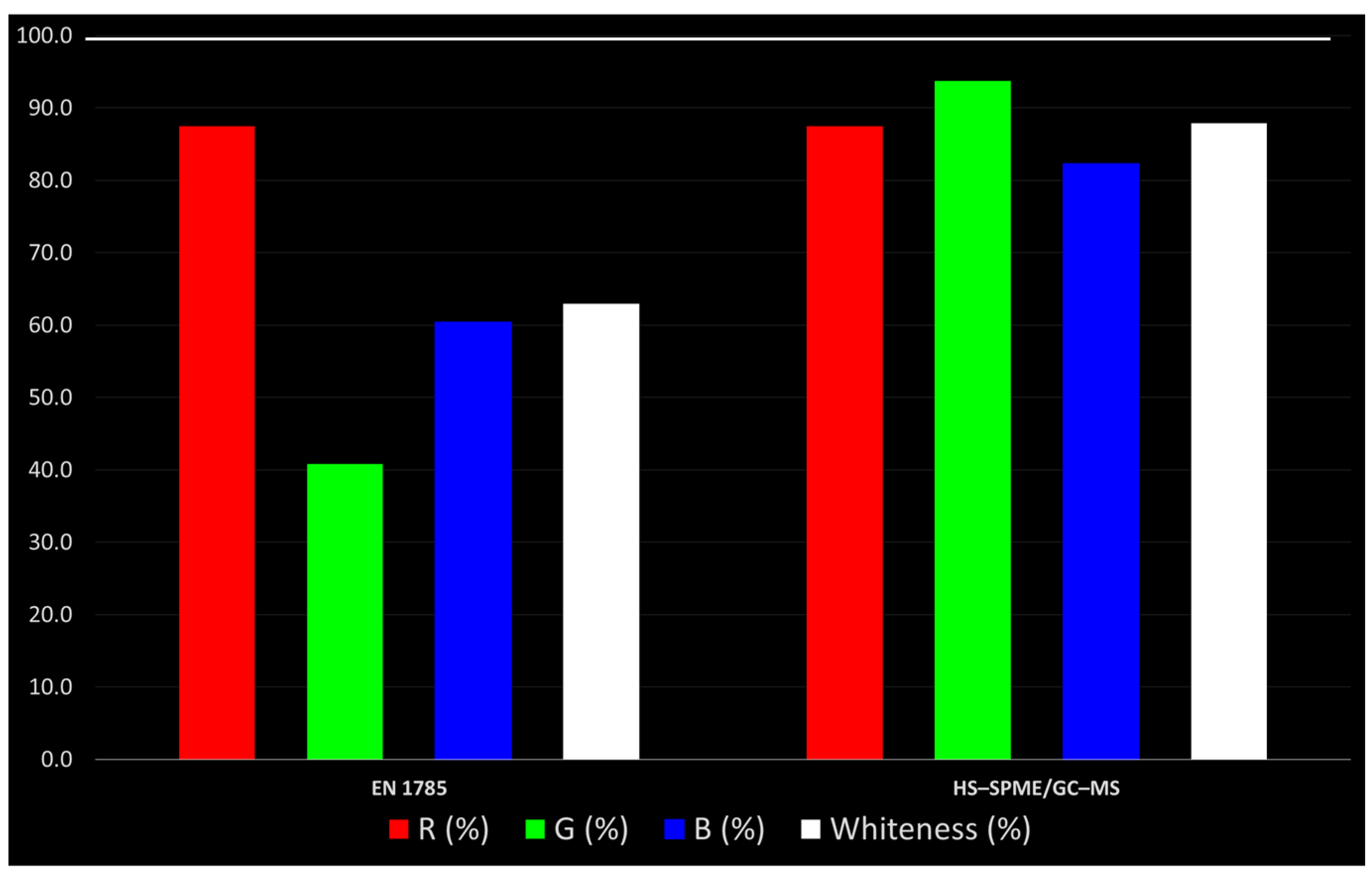
- B4: Operational simplicityThe overall evaluation of analytical methods should take into account their impact on the level of usability, resulting from the sum of miniaturization, integration, automation (online methods, artificial intelligence technologies) and portability (on-site measurements). Taking into account that the methods compared in this section are not portable, we evaluated this parameter as zero for both. The EN 1785:2003 cannot be considered miniaturizable, although Stewart et al. [35] demonstrated that the florisil cartridge with solid phase extraction (SPE) was a reliable method for 2–DCB purification in irradiated chicken meat, liquid whole eggs, minced beef and mango. Furthermore, the standard method cannot be defined as integrated and automated because the operator’s assistance is constantly required during the analytical flow. On the contrary, regarding the proposed method, the HS–SPME, together with the use of the autosampler, can be considered a miniaturization and automation of the extraction and purification steps, reducing manual labor and improving the speed and reproducibility of sample preparation and analysis. As with the B3, the B4 parameter was also expressed as mean value, resulting in 16.7 for the EN 1785:2003 and 66.7 for the HS–SPME/GC–MS method. The results were resumed in Figure 3.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2–ACBs | 2–Alkylcyclobutanones |
| 2–DCB | 2–Dodecylcyclobutanone |
| 2–TCB | 2–Tetradecylcyclobutanone |
| HS–SPME/GC–MS | Headspace Solid Phase Micro-Extraction/Gas Chromatography—Mass Spectrometry |
| LOD | Limit of Detection |
| MDL | Minimum Detectable dose Level |
| GAC | Green Analytical Chemistry |
| WAC | White Analytical Chemistry |
| RGB | Red, Green and Blue |
| IS | Internal Standard |
| QC | Quality Control |
| SMC | Solvent-Matched Calibration curve |
| MMC | Matrix-Matched Calibration curve |
| NI | Non-Irradiated |
| PDMS | Polydimethylsiloxane |
| PTV | Programmed Temperature Vaporizer |
| RA | Ratio Areas |
| EICs | Extracted Ion Chromatograms |
| NDCB | Normalized value of 2–DCB |
| NTCB | Normalized value of 2–TCB |
References
- Brosnan, J.T.; Brosnan, M.E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef]
- Serrano, L.E.; Murano, E.A.; Shenoy, K.; Olson, D.G. D Values of Salmonella Enteritidis Isolates and Quality Attributes of Shell Eggs and Liquid Whole Eggs Treated with Irradiation. Poult. Sci. 1997, 76, 202–206. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Biological Hazards (BIOHAZ) Related to the Microbiological Risks on Washing of Table Eggs. EFSA J. 2005, 3, 269. [Google Scholar] [CrossRef][Green Version]
- Badr, H.M. Effect of Gamma Radiation and Cold Storage on Chemical and Organoleptic Properties and Microbiological Status of Liquid Egg White and Yolk. Food Chem. 2006, 97, 285–293. [Google Scholar] [CrossRef]
- Sokovnin, S.Y.; Donnik, I.M.; Shkuratova, I.A.; Krivonogova, A.S.; Isaeva, A.G.; Balezin, M.E.; Vazirov, R.A. The Use of Nanosecond Electron Beam for the Eggs Surface Disinfection in Industrial Poultry. J. Phys. Conf. Ser. 2018, 1115, 022034. [Google Scholar] [CrossRef]
- Liu, X.D.; Han, R.X.; Yun, H.; Jung, K.C.; Jin, D.I.; Lee, B.D.; Min, T.S.; Jo, C. Effect of Irradiation on Foaming Properties of Egg White Proteins. Poult. Sci. 2009, 88, 2435–2441. [Google Scholar] [CrossRef]
- European Commission. Directive 1999/2/EC of the European Parliament and of the Council of 22 February 1999 on the approximation of the laws of the member states concerning foods and food ingredients treated with ionizing radiation. Off. J. Eur. Communities 1999, L 66, 16–22. [Google Scholar]
- European Commission. Directive 1999/3/EC of the European Parliament and of the Council of 22 February 1999 on the establishment of a Community list of the laws of foods and food ingredients treated with ionizing radiation. Off. J. Eur. Communities 1999, L 66, 24–25. [Google Scholar]
- Mangiacotti, M.; Marchesani, G.; Floridi, F.; Siragusa, G.; Chiaravalle, A.E. Official Checks by an Accredited Laboratory on Irradiated Foods at an Italian Market. Food Control. 2013, 33, 307–312. [Google Scholar] [CrossRef]
- Marozzi, S.; Condoleo, R.; Saccares, S.; Campagna, M.C.; Mangiacotti, M.; Chiaravalle, E. Irradiation of Food of Animal Origin: Analytical Checks to Verify Compliance with EU Legislation in Lazio Region. Ital. J. Food Saf. 2013, 2, 53–54. [Google Scholar] [CrossRef][Green Version]
- Campagna, M.C.; Schiavi, M.T.D.; Foti, M.; Mosconi, M.C.; Mattiolo, G.; Cavallina, R. Application of Microbiological Method Direct Epifluorescence Filter Techique/Aerobic Plate Count Agar in the Identification of Irradiated Herbs and Spices. Ital. J. Food Saf. 2014, 3, 140–142. [Google Scholar] [CrossRef][Green Version]
- Marrone, R.; Carosielli, L.; Mangiacotti, M.; Chiaravalle, E.; Smaldone, G.; Anastasio, A. Monitoring of Irradiated Food Products Marketed in Italy and Evaluation of Electron Spin Resonance Signal Sensitivity of Experimentally Irradiated Fish Scales. Ital. J. Food Saf. 2014, 3, 73–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vazirov, R.A.; Sokovnin, S.Y.; Agdantseva, E.N.; Tsmokalyuk, A.N. Investigation of Radiation-Induced Electron Paramagnetic Resonance Signal of an Eggshell after Electron Beam Irradiation. Radiat. Phys. Chem. 2022, 192, 109882. [Google Scholar] [CrossRef]
- EN 1785:2003; Detection of Irradiated Food Containing Fat—Gas Chromatographic Analysis of 2-Alkylcyclobutanones. European Committee for Standardisation: Brussels, Belgium, 1997.
- Crews, C.; Driffield, M. Literature Review and Practical Investigation of the Potential Formation of 2-Alkylcyclobutanones in Non-Irradiated Food (FS231029 (A05021)); The Food and Environment Research Agency: York, UK, 2011.
- Campaniello, M.; Zianni, R.; Chiappinelli, A.; Mentana, A.; Nardelli, V. EN 1785:2003 Method for the Identification of Ionizing Treatment in Poultry Meat Products: Optimization of the Accelerated Solvent Extraction Procedure. Ital. J. Food Saf. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Campaniello, M.; Marchesani, G.; Zianni, R.; Tarallo, M.; Mangiacotti, M.; Chiaravalle, A.E. Validation of an Alternative Method for the Identification of 2-dodecylcyclebutanone (2-DCB) of Irradiated Meats by Solid-phase Microextraction (SPME) Gas Chromatography–Mass Spectrometry (GC-MS). Int. J. Food Sci. Tech. 2020, 55, 961–969. [Google Scholar] [CrossRef]
- Zianni, R.; Mentana, A.; Campaniello, M.; Chiappinelli, A.; Tomaiuolo, M.; Chiaravalle, A.E.; Marchesani, G. An Investigation Using a Validated Method Based on HS-SPME-GC-MS Detection for the Determination of 2-Dodecylcyclobutanone and 2-Tetradecylcyclobutanone in X-Ray Irradiated Dairy Products. LWT 2022, 153, 112466. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC Trend Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trend Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Fuente-Ballesteros, A.; Ares, A.M.; Bernal, J. Paving the Way towards Green Contaminant Analysis: Strategies and Considerations for Sustainable Analytical Chemistry. Green Anal. Chem. 2025, 12, 100221. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wojnowski, W.; Manousi, N.; Samanidou, V.; Płotka-Wasylka, J. Red Analytical Performance Index (RAPI) and Software: The Missing Tool for Assessing Methods in Terms of Analytical Performance. Green Chem. 2025, 27, 5546–5553. [Google Scholar] [CrossRef]
- Mousa, H.S.; Gouda, A.A.; El-Zahry, M.R.; ElHosary, M.M.I.; Hassan, M.H. Greenness-Integrated Analytical Quality by Design-Compliant Development of a Quasi-HDES-DLLME Method for Patent Blue V Preconcentration from Various Food Products, and Environmental Water Samples. Microchem. J. 2025, 210, 113052. [Google Scholar] [CrossRef]
- Caja, M.M.; Ruiz Del Castillo, M.L.; Blanch, G.P. Solid Phase Microextraction as a Methodology in the Detection of Irradiation Markers in Ground Beef. Food Chem. 2008, 110, 531–537. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories 2017. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66912.html (accessed on 25 July 2025).
- Cantwell, H. (Ed.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 3rd ed.; Eurachem: Bucharest, Romania, 2025. [Google Scholar]
- Dutson, T.R.; Orcutt, M.W. Chemical Changes in Proteins Produced by Thermal Processing. J. Chem. Educ. 1984, 61, 303. [Google Scholar] [CrossRef][Green Version]
- Available online: https://www.alimentinutrizione.it/tabelle-nutrizionali/181100 (accessed on 25 July 2025).
- Mentana, A.; Palermo, C.; Tomaiuolo, M.; Campaniello, M.; Centonze, D.; Zianni, R. Evaluation of Aroma Fingerprint in X-Ray Irradiated Robiola Cheese over Time by Volatolomic and Chemometric Approaches. Int. Dairy. J. 2025, 163, 106182. [Google Scholar] [CrossRef]
- Baykalir, Y.; Simsek, U.G.; Yilmaz, O. Age-Related Changes in Egg Yolk Composition between Conventional and Organic Table Eggs. Agric. Food Sci. 2020, 29, 307–317. [Google Scholar] [CrossRef]
- Kim, K.-S.; Seo, H.-Y.; Lee, J.-M.; Park, E.-R.; Kim, J.-H.; Hong, C.-H.; Byun, M.-W. Analysis of Radiation-Induced Hydrocarbons and 2-Alkylcyclobutanones from Dried Shrimps (Penaeus aztecus). J. Food Prot. 2004, 67, 142–147. [Google Scholar] [CrossRef]
- Tewfik, I. Inter-Laboratory Trial to Validate the Direct Solvent Extraction Method for the Identification of 2-Dodecylcyclobutanone in Irradiated Chicken and Whole Liquid Egg. Food Sci. Technol. Int. 2008, 14, 277–283. [Google Scholar] [CrossRef]
- Stewart, E.M.; McRoberts, W.C.; Hamilton, J.T.G.; Graham, W.D. Isolation of Lipid and 2-Alkylcyclobutanones from Irradiated Foods by Supercritical Fluid Extraction. J. AOAC Int. 2001, 84, 976–986. [Google Scholar] [CrossRef]

| The 12 Principles of White Analytical Chemistry (WAC) | ||
|---|---|---|
| RED PRINCIPLES (analytical performance) | GREEN PRINCIPLES (green chemistry) | BLUE PRINCIPLES (practical side) |
| R1: Scope of application | G1: Toxicity of reagents (impact and biodegradation) | B1: Cost-efficiency |
| R2: LOD and LOQ | G2: Amounts of reagents and waste | B2: Time-efficiency |
| R3: Precision | G3: Consumption of energy and other media | B3: Requirements |
| R4: Accuracy | G4: Direct impacts (safety, use of animals and GMOs) | B4: Operational simplicity |
| Parameters | 2–DCB and 2–TCB (Concentration or Dose) | Number of Samples |
|---|---|---|
| Linearity | SMC: 5.0–10.0–20.0–40.0 (μg kg−1) MMC: 1.25–2.50–12.5–25.0 (μg kg−1) | 12 12 |
| Matrix effect | Irradiated samples: 0.5–1.0–3.0 (kGy) | 9 |
| Selectivity | Non-irradiated samples | 9 |
| LOD | Spiked samples: 1.25–2.50–12.5–25.0 (μg kg−1) | 12 |
| MDL | Irradiated samples: 0.5–1.0–3.0 (kGy) | 9 |
| Diagnostic sensitivity and diagnostic specificity | Non-irradiated samples Irradiated samples: 0.5–1.0–3.0 (kGy) | 9 9 |
| Stability | Irradiated samples T0: 0.5–1.0–3.0 (kGy) Irradiated samples T1: 0.5–1.0–3.0 (kGy) | 9 9 |
| Treatment | Time Elapsed from Irradiation | Normalized Value of 2–DCB (NDCB) | Mean ± St. dev of NDCB | Normalized Value of 2–TCB (NTCB) | Mean ± St. dev of NTCB | (NDCB)/(NTCB) |
|---|---|---|---|---|---|---|
| 0.5 kGy | Immediately after irradiation | 0.094 | 0.122 ± 0.024 | 0.013 | 0.013 ± 0.001 | 7.1 |
| 0.137 | 0.013 | 10.1 | ||||
| 0.135 | 0.012 | 11.0 | ||||
| 1.0 kGy | 0.216 | 0.262 ± 0.045 | 0.027 | 0.029 ± 0.003 | 8.1 | |
| 0.265 | 0.032 | 8.4 | ||||
| 0.306 | 0.028 | 10.9 | ||||
| 3.0 kGy | 1.061 | 1.073 ± 0.029 | 0.091 | 0.099 ± 0.011 | 11.6 | |
| 1.051 | 0.094 | 11.1 | ||||
| 1.106 | 0.112 | 9.9 | ||||
| 0.5 kGy | One month after irradiation | 0.157 | 0.167 ± 0.009 | 0.014 | 0.017 ± 0.004 | 10.9 |
| 0.170 | 0.021 | 8.0 | ||||
| 0.174 | 0.015 | 11.7 | ||||
| 1.0 kGy | 0.256 | 0.290 ± 0.029 | 0.025 | 0.028 ± 0.003 | 10.3 | |
| 0.307 | 0.031 | 10.0 | ||||
| 0.307 | 0.029 | 10.4 | ||||
| 3.0 kGy | 1.218 | 1.099 ± 0.136 | 0.104 | 0.100 ± 0.009 | 11.7 | |
| 0.950 | 0.089 | 10.7 | ||||
| 1.129 | 0.106 | 10.6 | ||||
| Mean | 10.1 | |||||
| St. dev | 1.4 | |||||
| CV% | 13.4 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappinelli, A.; Iammarino, M.; Tomaiuolo, M.; Nardelli, V.; Boniglia, C.; Bortolin, E.; Pastorelli, A.A.; Gargiulo, R.; Di Giacomo, S.; Rosetti, M.; et al. Fast and Green Extraction Method Based on HS–SPME/GC–MS to Identify Chemical Markers of X-Ray Irradiated Hen Eggs. Appl. Sci. 2025, 15, 10044. https://doi.org/10.3390/app151810044
Chiappinelli A, Iammarino M, Tomaiuolo M, Nardelli V, Boniglia C, Bortolin E, Pastorelli AA, Gargiulo R, Di Giacomo S, Rosetti M, et al. Fast and Green Extraction Method Based on HS–SPME/GC–MS to Identify Chemical Markers of X-Ray Irradiated Hen Eggs. Applied Sciences. 2025; 15(18):10044. https://doi.org/10.3390/app151810044
Chicago/Turabian StyleChiappinelli, Andrea, Marco Iammarino, Michele Tomaiuolo, Valeria Nardelli, Concetta Boniglia, Emanuela Bortolin, Augusto Alberto Pastorelli, Raffaella Gargiulo, Silvia Di Giacomo, Matteo Rosetti, and et al. 2025. "Fast and Green Extraction Method Based on HS–SPME/GC–MS to Identify Chemical Markers of X-Ray Irradiated Hen Eggs" Applied Sciences 15, no. 18: 10044. https://doi.org/10.3390/app151810044
APA StyleChiappinelli, A., Iammarino, M., Tomaiuolo, M., Nardelli, V., Boniglia, C., Bortolin, E., Pastorelli, A. A., Gargiulo, R., Di Giacomo, S., Rosetti, M., & Campaniello, M. (2025). Fast and Green Extraction Method Based on HS–SPME/GC–MS to Identify Chemical Markers of X-Ray Irradiated Hen Eggs. Applied Sciences, 15(18), 10044. https://doi.org/10.3390/app151810044








