Abstract
The paper presents a hardware and software complex for monitoring the recurrent laryngeal nerve (RLN) with electromyography (EMG) as the primary tool, observing the RLN’s response to stimulation during intraoperative nerve monitoring (IONM). As a result of the analysis of available IONM tools using EMG, it was found that electromyography is an accurate and safe method for monitoring the bioelectric activity of the vocal cords. The article proposes a concept for monitoring the recurrent laryngeal nerve (RLN) by observing changes in the bioelectric activity of the vocal cords during RLN stimulation. A hardware and software complex was developed in accordance with the concept. The article presents the architecture of the hardware and software of this complex. A detailed description of all hardware parts, their purpose, and their interaction is given. Features of the software and tools used in its development are described. The results of the approval of the complex during thyroid surgery at the VITASANA Medical Center in the city of Ternopil are given. The complex could successfully register and record the change in the biometric potential of the vocal cord at the moment of stimulation of the RLN.
1. Introduction
Surgical interventions on the thyroid gland are performed to treat a wide range of medical conditions [1]. These procedures are considered complex and require significant surgical expertise due to the presence of vital blood vessels and nerves in the anterior neck region. One of the most serious risks during such surgeries is the potential damage to the recurrent laryngeal nerve (RLN) [2,3]. The likelihood of RLN injury is relatively high, as the nerve exhibits considerable anatomical variability among patients and is often not visually distinguishable by the surgeon [4,5].
To minimize the risk of RLN damage, intraoperative neural monitoring (IONM) is employed. IONM provides methods and tools to identify the RLN during surgery by observing physiological responses in real time.
Among the RLN identification techniques, one approach involves visual assessment of the patient’s laryngeal anatomy [6,7]. However, this method demands a high level of surgical expertise and is not considered an efficient IONM strategy. Another method involves the use of ultrasound imaging [7]. This approach relies on scanning the surgical field with an ultrasound scanner to visualize the surrounding tissues. While it enables visualization of the operative area, it requires either advanced interpretation skills on the part of the surgeon or the integration of auxiliary systems capable of automatically recognizing the RLN in the acquired images. Furthermore, the use of an ultrasound scanner—being an expensive piece of equipment—is not always feasible in thyroid surgeries and does not support continuous real-time monitoring of nerve integrity.
An additional technique for RLN identification involves electrical stimulation of the surgical field using either alternating or direct current [8,9]. This method utilizes electrodes inserted into the surgical site through various approaches depending on the specific technique. A constant current of 0.5–2 mA is applied to the electrodes, resulting in the contraction of the vocal cord muscles. The physiological response to electrical stimulation can be recorded using different technical means, with electromyography (EMG) sensors being among the most common.
The objective of this study is to present a technology for implementing a hardware–software system for RLN monitoring. This system employs electrical current to stimulate the tissues of the surgical site and uses electromyography to detect and monitor the physiological response to the stimulation.
2. Analysis of Existing Tools for IONM Based on EMG
Intraoperative neural monitoring is widely employed for the identification of the recurrent laryngeal nerve to prevent its injury during thyroid surgery. Damage to the RLN can lead to irreversible consequences; in some cases, such injury results in permanent vocal cord paralysis and loss of voice. Therefore, various technologies have been developed and applied to prevent or reduce the risk of RLN damage [8]. These technologies are fundamentally based on the principle of electrically stimulating the tissues surrounding the RLN—along with the nerve itself—and observing the physiological response to the stimulation [7,8].
The use of such techniques in RLN monitoring was first documented in 1965 [10]. The aim of the initial study was to record the physiological response to RLN stimulation. The experiment utilized a pressure sensor embedded in the cuff of an endotracheal tube; pressure changes occurred as the vocal cords contracted in response to electrical stimulation of the nerve [11]. This approach successfully identified the anatomical location of the RLN and validated the feasibility of using electrical stimulation for intraoperative nerve identification.
More advanced methods of electrical stimulation have since been proposed [12,13], relying on hardware–software monitoring systems. In these approaches, RLN stimulation is carried out using rectangular electrical pulses, and the physiological response is detected using acoustic sensors [14]. A significant advantage of such systems lies in their ability to identify the RLN in real time during surgery. Tissue classification is then conducted using mathematical models in the form of algebraic equations [13,15] or difference equations [16,17] that describe the characteristics of the received acoustic signal, such as signal amplitude or specific spectral components.
However, the effectiveness of these approaches is constrained by the performance of the microphone used in the system, the anatomical features of the larynx, and the physiological variability of the patient’s vocal cords. Additionally, in some cases, it may not be feasible to place an acoustic sensor inside the endotracheal tube due to the patient’s individual anatomical conditions. Consequently, the use of such systems may be limited in clinical practice.
An alternative and fundamentally different method for observing the RLN’s response to electrical stimulation is electromyography [18]. This approach involves the use of EMG electrodes (needles) inserted directly into the muscle tissue of the vocal cords. Upon stimulation, the muscles respond to the electrical activation of tissues adjacent to the RLN. The response can be recorded as a difference in electrical potential generated by muscle contraction, which is captured by the electrodes [18]. Notably, this method eliminates the need to place an acoustic sensor inside the endotracheal tube, which can be a critical constraint in some thyroid surgeries. Furthermore, EMG is also employed for predicting the recovery timeline in cases of vocal cord paralysis [19], thus supporting its clinical safety and utility.
Given the above, combining EMG with modern hardware–software systems provides the capability to identify the RLN in the surgical field in real time without the need for an acoustic sensor, thereby reducing the risk of false-positive detection [18,19].
Electromyography has been used since the 1950s to assess laryngeal muscle activity [20]. In 1970, this technology was adapted for use in surgical settings by Flisberg K. and Lindholm T. [21]. Initially, needle electrodes were inserted into the vocal muscles through the cricothyroid membrane; these are now referred to as thyroid cartilage (TC) electrodes. Later developments led to the use of laryngoscopically placed electrodes, which were positioned outside the surgical site [22,23]. Over the following 10–15 years, the electrodes were significantly miniaturized, allowing integration with endotracheal tubes. These are currently known as endotracheal (ET) electrodes. TC and ET electrodes are the two primary types used in EMG-based IONM, as illustrated in Figure 1.
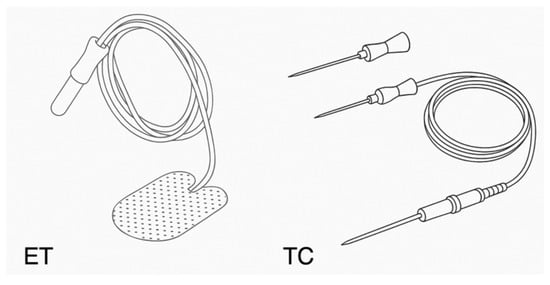
Figure 1.
The two main types of electrodes used in IONM are based on EMG [24].
One of the most widely used EMG monitoring systems during surgical procedures is the NIM-Response 3.0 developed by Medtronic [25], as illustrated in Figure 2a. The NIM-Response 3.0 system is a four-channel electromyographic (EMG) monitor designed for real-time tracking of muscle activity. It utilizes specialized NIM nerve monitoring electrodes, which are connected to the main monitoring unit. The system continuously monitors the EMG activity of muscles innervated by the stimulated nerve. When a specific nerve is activated or irritated, the NIM system alerts the surgeon and the operating room staff through both visual indicators on the color touchscreen display and auditory feedback, helping to minimize the risk of nerve injury.
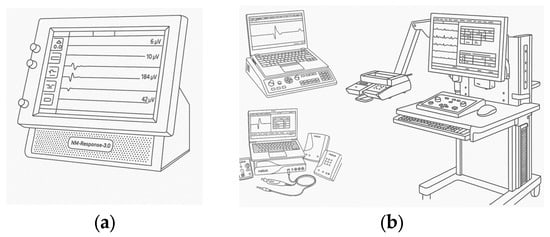
Figure 2.
The most popular EMG monitoring systems: (a)—NIM-Response 3.0 from Medtronic (b)—EMG/NCS/EP Systems from Natus [21,22].
The system offers several advantages: four EMG channels, real-time monitoring capability, an intuitive and user-friendly interface, automatic measurement, audio feedback, and commercial availability [26].
However, it also has notable limitations, including: large physical dimensions, lack of autonomous (standalone) operating mode, high cost due to its universal functionality, ranging from several thousand to tens of thousands of dollars.
Another widely used EMG monitoring solution is the EMG/NCS/EP system produced by Natus [27], also depicted in Figure 2b.
These systems are capable of providing EMG monitoring and measuring muscle activity across various regions of the patient’s body. Their advantages include: 3- to 8-channel amplifier, low input noise, electrode impedance measurement with LED-based feedback, and commercial availability.
However, they also exhibit several drawbacks: large physical dimensions, inability to operate autonomously (without external systems), high cost, primarily due to their multifunctionality and broad application scope.
As the analysis indicates, the main disadvantage of the reviewed systems is their high cost, which stems from their universal design and extensive feature set. Consequently, there is a clear need for the development of a specialized hardware–software system dedicated to EMG monitoring of the surgical wound area, particularly aimed at reducing the risk of recurrent laryngeal nerve (RLN) injury during thyroid surgery.
3. Technology of Electromyography Application for Recurrent Laryngeal Nerve Monitoring
The technology for utilizing an existing hardware–software system for the identification of the recurrent laryngeal nerve in the surgical field using an acoustic sensor is discussed in [13]. The core principle involves the electrical stimulation of the surgical site, with the physiological response being detected by an acoustic sensor. This sensor is placed within the endotracheal tube, and the response to stimulation arises from the contraction of the vocal folds, which causes a measurable change in the amplitude of the sound wave passing through the patient’s larynx.
Figure 3 schematically illustrates the main components of the hardware implementation of this technology. However, instead of relying on an acoustic sensor, it is proposed to record the tissue response to electrical stimulation using an electromyographic sensor. This substitution aims to enhance the reliability of RLN monitoring by providing a more direct and robust physiological signal.
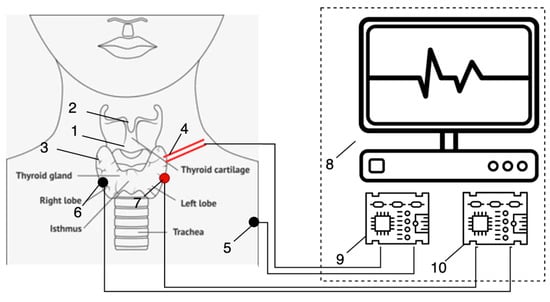
Figure 3.
Conception of complex RLN monitoring with electromyography as the primary tool, observing the RLN’s response to stimulation: (1)—surgical area; (2)—larynx; (3)—vocal cords; (4)—active probe; (5)—passive probe; (6)—negative electrode; (7)—positive electrode; (8)—hardware and software complex; (9)—electrical current generator (stimulation part); (10)—EMG sensor.
Let us consider the recurrent laryngeal nerve identification technology based on the proposed new concept.
Within the surgical field (Figure 3 (1)), active (Figure 3 (7)) and passive (Figure 3 (6)) electrodes are placed directly into the muscles responsible for controlling vocal fold movement. These electrodes are connected to an EMG sensor (Figure 3 (10)), which is part of the hardware–software system. This component now performs the function of recording the tissue response to electrical stimulation within the surgical area.
A current generator (Figure 3 (9)) is connected to another pair of electrodes: active (Figure 3 (4)) and passive (Figure 3 (5)). These electrodes are responsible for delivering electrical stimulation to the surgical tissues. The passive stimulation electrode is positioned on the patient’s shoulder, while the active electrode is used to stimulate tissues within the surgical site.
When the tissues near the RLN are stimulated, a bioelectric potential is generated in the vocal fold muscles. This potential is captured by the EMG sensor. A working hypothesis is proposed: the closer the stimulating electrode is to the RLN, the greater the amplitude of the potential difference recorded between electrodes (Figure 3 (6) and (7)). This difference is then processed by the hardware–software system.
The processed signal is visualized on a video monitoring device (Figure 3 (8)) and is accompanied by an auditory alert. This approach allows for direct detection of the neuromuscular response to stimulation without the need for indirect acoustic signal mediation. However, the implementation of this new EMG-based technology requires significant modifications to the existing hardware–software system.
4. Technology of Electromyography Application for Recurrent Laryngeal Nerve Monitoring
To implement and validate the proposed concept, an advanced system for recurrent laryngeal nerve (RLN) identification during thyroid surgery has been developed. This system utilizes the monitoring of the bioelectric potential of vocal fold muscle tissue as the primary means of observing the RLN’s response to stimulation. The components of the system are illustrated in Figure 4.
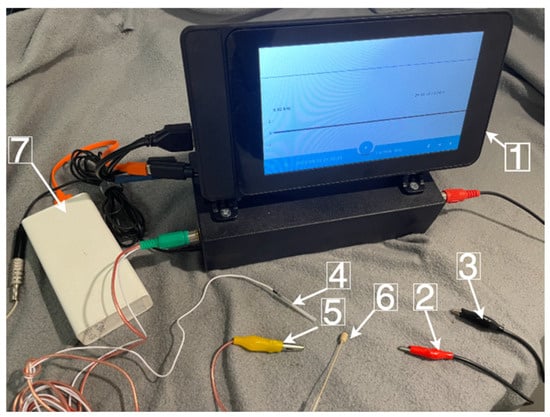
Figure 4.
Hardware and software complex RLN monitoring with electromyography as the primary tool, observing the RLN’s response to stimulation: (1)—hardware and software complex identification RLN; (2)—active probe; (3)—passive probe; (4)—positive electrode; (5)—negative electrode (6)—microphone; (7)—power supply.
This system is capable of performing the following functions:
- Stimulating the recurrent laryngeal nerve (RLN) with rectangular electrical pulses;
- Manually adjusting the stimulation frequency and current intensity (as an additional option);
- Monitoring the muscle response of the vocal folds to electrical stimulation of the surgical site via electromyography;
- Monitoring the response via an acoustic sensor to electrical stimulation of the surgical tissues (as an optional feature);
- Recording the procedure results into a file system.
The software component integrates the operation of all subsystems into a unified framework. It provides an intuitive user interface for device interaction and real-time monitoring of the RLN’s response to stimulation. The main screen of the software interface is presented in Figure 5.
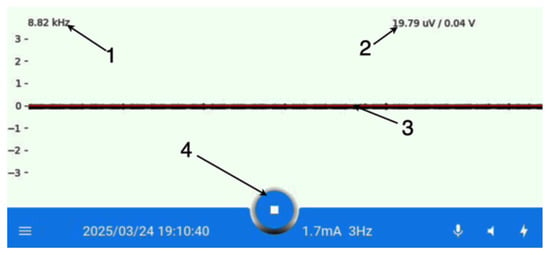
Figure 5.
The main screen of the application: (1)—frequency of a signal measurement; (2)—current voltage; (3)—EMG signal; (4)—navigation menu.
As shown in Figure 5, the majority of the screen is occupied by a window displaying the EMG sensor readings as a continuous waveform. This enables the surgeon to monitor the recurrent laryngeal nerve response to stimulation in real time. The remaining portion of the screen is allocated to supplementary information and the navigation panel.
5. Hardware of a Complex
Among the three main hardware components, the system includes an electric current generator, an EMG sensor board, and a high-performance microcontroller.
The electric current generator performs the essential function of delivering a constant current amplitude regardless of the conductive properties of the surgical tissue. This ensures a stable and reliable stimulation signal for the surgical site. The generator is built around the analog INA132 integrated circuit [28] and is capable of producing rectangular pulses with a frequency ranging from 1 to 20 Hz and a current amplitude adjustable between 0.5 and 2 mA.
The EMG sensor is responsible for detecting the bioelectric potential generated by the vocal folds. The sensor board is based on low-power dual operational amplifiers AU2904 [29] and provides a voltage gain of approximately 60 dB, enabling amplification of the input signal from 100 µV.
In Figure 6, the principal schematic diagram of the operational amplifier of the EMG sensor signal is presented, while Figure 7 illustrates the amplitude–frequency response of the EMG sensor signal amplifier. In the graph, the measurement results of the gain coefficient at different frequencies are shown as blue dots. As can be observed, the response remains relatively linear from 300 Hz to several kilohertz. The primary objective in the process of recurrent laryngeal nerve detection is the determination of the maximum amplitude of the EMG signal, since this amplitude reflects the distance between the site of stimulation on the surgical wound and the recurrent laryngeal nerve. As noted in [30], when needle EMG sensors implanted into the muscle tissue of the vocal folds are used, the main portion of the spectral power of this signal is concentrated within the range of 300 Hz to 1 kHz (and above). This finding was also confirmed by the authors during the course of preliminary studies. Therefore, the frequency response of the amplifier within this range can be considered satisfactory, ensuring effective signal amplification.
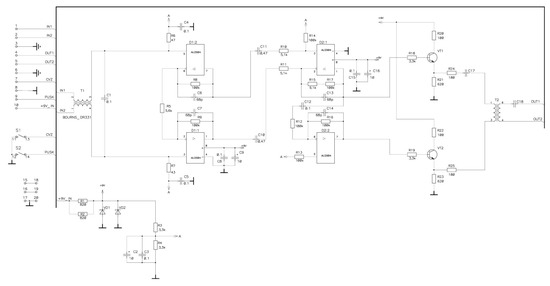
Figure 6.
The principal schematic diagram of the operational amplifier designed for the EMG sensor signal.
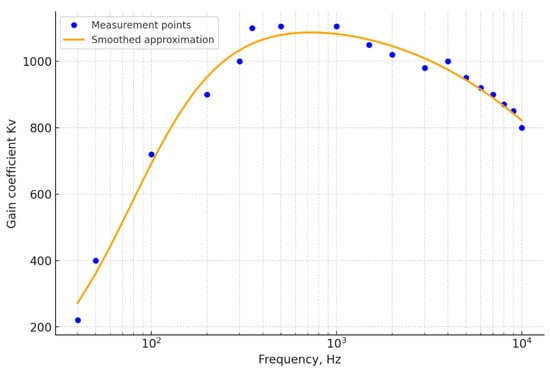
Figure 7.
The amplitude–frequency response of the EMG sensor signal amplifier.
The physical design and circuit layout of these components are shown in Figure 8a and Figure 8b, respectively.

Figure 8.
Physical implementation on printed circuit boards: (a) EMG sensor board; (b) electric current generator.
The central hardware component of the system is a high-performance Raspberry Pi 5 microcontroller (Raspberry Pi Ltd., Cambridge, UK). It features a quad-core 64-bit Arm Cortex-A76 processor (Arm Ltd., Cambridge, UK) operating at 2.4 GHz [31] and includes 40 GPIO (General-Purpose Input/Output) pins for controlling the electronic subsystems.
To interface with the electric current generator and the EMG sensor board, an additional analog-to-digital converter (ADC) is connected to the Raspberry Pi microcontroller. This is necessary because Raspberry Pi provides only digital I/O ports and lacks native analog input capability. To convert analog signals from various hardware components into digital values, the MCP3008 ADC [32] was selected. The sampling frequency is set to 44 kHz. This value is justified by the fact that the specified ADC can be used not only with the EMG sensor, but also with an acoustic sensor, which requires a higher sampling frequency than that needed for the EMG signal. The Raspberry Pi communicates with the ADC via the SPI (Serial Peripheral Interface) protocol, while the ADC receives analog signals directly from the EMG sensor.
To coordinate and manage the interaction between all hardware components, a dedicated hardware architecture for the system was developed, as illustrated in Figure 9.
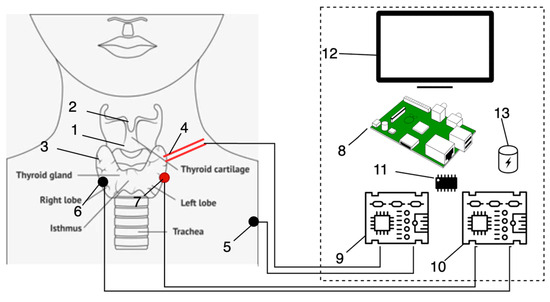
Figure 9.
Hardware architecture of complex: (1)—surgical area; (2)—larynx; (3)—vocal cords; (4)—active probe; (5)—passive probe; (6)—negative electrode; (7)—positive electrode; (8)—Raspberry Pi 5; (9) electrical current generator; (10)—EMG sensor; (11)—ADC; (12)—Led screen; (13)—UPS.
To ensure autonomous operation of the system, an uninterruptible power supply (UPS) module was integrated. Specifically, the UPS Module 3S [33] was used. It is powered by three 18650 Li-ion batteries (Samsung SDI, Yongin, South Korea) and provides an output voltage of 5 V at 5 A and 3.3 V at 300 mA. The module also supports the I2C bus protocol for monitoring battery voltage. This UPS enables the system to operate in standalone mode for up to 4 h without recharging. Additionally, the system can operate while connected to the main power supply.
In addition to the aforementioned components, several auxiliary elements were employed. A complete list of all hardware components, along with their approximate cost estimates, is presented in Table 1.

Table 1.
Hardware components specification.
Table 1 presents the average price of each component as of the year 2025. The total average production cost of the system does not exceed $350, which is significantly lower than that of existing commercial counterparts.
6. Software of a Complex
For the correct functioning of the RLN identification software complex, it must solve the following tasks:
- Control the frequency of the rectangular pulses generated for the electrical current generator.
- Obtaining the information signal from an EMG sensor.
- Record the information signal into a file.
- Visualization of the EMG Signal.
Since the software is intended for the high-performance microcontroller Raspberry Pi, Python 3.11. was chosen as the main programming language. Since software should launch as a desktop application, the main framework, Kivy 2.3.0 [34], was chosen. Let us consider the implementation of each of the tasks set for the software more deeply.
Control the frequency of the rectangular pulses generated for the electrical current generator. To be able to control the frequency of the rectangular pulses, there should be a possibility to write values to the GPIO of the Raspberry Pi. For this, the gpiozero [35] library was chosen. The functioning of the electrical current generator is controlled using the LowFrequencyGenerator module. This is a small Node.js v22.12.0 script that is run from the main program as a subprocess and [36] transmits the required frequency to the main program. A detailed description of the functioning of the LowFrequencyGenerator can be found in the article [37].
Obtaining the information signal from the EMG sensor. To work with analog sensors, such as the EMG sensor, Raspberry Pi uses the SPI protocol [38] and ADC. On a hardware level, MCP3008 was chosen as the ADC. On the software level, gpiozero.MCP3008 [39] library is used to read values from ADC output pins. In addition to reading the values obtained from the EMG sensor, it is also necessary to ensure the periodicity of this reading. This is the main task of the EMGSubprocess module. Its job is to read EMG sensor value in some period of time based on the stated frequency (each 10 ms, for example) and generate a vector of values for future processing. To achieve this, a subprocess starts reading the EMG sensor value, saves it into an array, and sleeps for a set time. When the array reaches a certain limit, it generates an event. A fragment of the code that implements the logic described above is given in Appendix A.
Record the information signal into a file. To enable further monitoring of the results of RLN stimulation, the ability to record the information signal into a file was added. The ReportEMG module is responsible for this. Each time the EMGSubprocess module stores enough EMG sensor measurements, it transfers them to ReportEMG for recording into a file. Communication between the modules occurs via event subscription communication [36]. All files are saved in .wav format with the timestamp of the recording in the name of the file.
Display EMG signal in the form of a wave. The received information signal is presented in the form of a sinusoidal graph or, more simply, in “wave form”. To build the graph, the library Matplotlib [40] was used. Matplotlib 3.9.4 is a comprehensive library for creating static, animated, and interactive visualizations. The EMGWidget module receives data from the EMGSubprocess module and uses the Matplotlib library to build the graph. Each time new chunks of information signal are received, the graph is rebuilt in a specific way. The last chunk goes on the head of the chart, and previously collected chunks are shifting to the tail. This ensures continuous monitoring of EMG sensor indicators. To integrate Matplotlib into a Kivy screen, kivy-garden.matplotlib library [41] was used.
The relationship between software modules is presented in the form of a UML class diagram, shown in Figure 10.
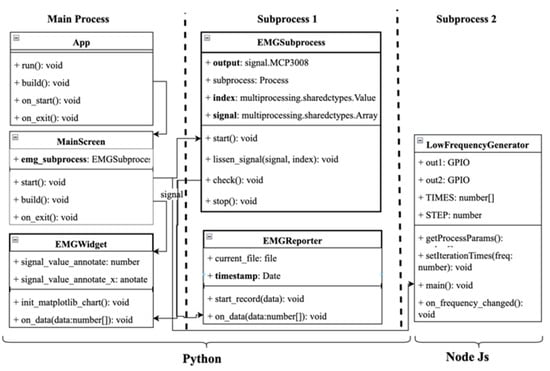
Figure 10.
The class diagram of software.
The developed software is launched as a desktop application automatically when the microcontroller Raspberry Pi is started. Thus, the software and hardware function as a single unit, ensuring the operation of the RLN identification complex during thyroid surgery.
7. Results and Discussion
The developed system was applied during a thyroid surgery at the VITASANA Medical Center in Ternopil, Ukraine. Electrical stimulation of the surgical site in the region proximal to the recurrent laryngeal nerve (RLN) was performed using rectangular pulses at a frequency of 4 Hz and a current amplitude of 1.7 mA.
The stimulation results demonstrated that the EMG sensor recorded a sharp increase in the amplitude of the potential difference across the electrodes inserted into the vocal fold muscle tissue at the moment of RLN stimulation. Figure 11 presents the visualization of the stimulation response.
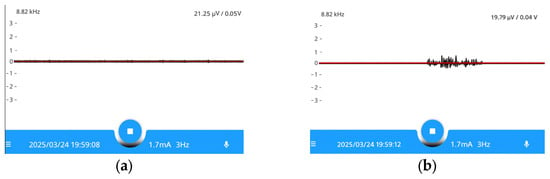
Figure 11.
The main screen of the application at the moment of RLN simulation: (a)—EMG signal; (b)—EMG signal at the moment of RLN simulation. The color in the figure shows the change of EMG signal.
Subsequent to the initial application, clinical trials of the developed system were conducted. The evaluation of the system’s performance took place during surgeries on 10 patients. During the trial, EMG signal amplitude was first recorded without stimulation, followed by measurements taken during electrical stimulation of the surgical tissues at varying distances from the recurrent laryngeal nerve (RLN).
As shown in the table:
- -
- On 24 March 2025, patient No. 1 underwent thyroidectomy for a diagnosis of medullary carcinoma;
- -
- On the same day, patient No. 2 underwent hemithyroidectomy for multinodular euthyroid goiter (stage III) with bilateral nodules and compression syndrome;
- -
- Patient No. 3 underwent thyroidectomy for recurrent multinodular goiter, nodules in the left lobe, and compression syndrome.
Thus, the patients had varying thyroid pathologies, requiring different surgical approaches and manipulations by the operating surgeon. The full list of clinical cases for all 10 patients is presented in Table 2 and additionally in Appendix B. The measured values of the maximum EMG signal amplitude for all patients are also summarized in Table 2.

Table 2.
EMG Signal Amplitude Values for Different Patients During RLN Stimulation at Varying Distances.
It should be noted that although the study involved 10 patients with various thyroid pathologies, the data presented reflect averaged results in terms of the number of surgical site stimulations. During each operation, between 20 and several hundred stimulations were performed per patient, which substantially increased the overall sample size used to verify the effectiveness of the proposed hardware–software system.
By relying on a large number of stimulations rather than the relatively small number of patients, this approach allows for a more statistically robust evaluation of the system’s performance. It ensures a reliable assessment of the accuracy, reproducibility, and robustness of the developed method under diverse intraoperative conditions.
It should be emphasized that, based on the analysis of the recorded information signals obtained from the EMG sensor, the average noise level was determined using dedicated software tools. The measured values varied within the range of −29.43 dB to −29.61 dB. According to the data presented in Table 2, the mean value of the signal-to-noise ratio (SNR) was subsequently calculated using the following formula:
where is the mean value of the EMG signal during stimulation (638.62 µV), is the mean value of the EMG signal without stimulation (25.9 µV). SNR is equal to 13.91 dB.
To assess how effectively the EMG sensor amplifies the signal, the common-mode rejection ratio (CMRR) was calculated according to the following formula:
where is the mean value of the EMG signal without stimulation (25.9 µV), is the output value of the EMG sensor (3.36 V). CMRR is equal to 102.2 dB.
The results of these studies are also presented in the table in Appendix B. As evidenced by the collected data, the amplitude of the EMG signal during electrical stimulation of the surgical site increases significantly depending on the distance between the stimulation point and the recurrent laryngeal nerve (RLN). The closer the stimulation point is to the RLN, the higher the amplitude of the EMG response. The average EMG amplitude values for the patient group at varying distances from the RLN are summarized in Table 3.

Table 3.
Average EMG Signal Amplitude Obtained During RLN Stimulation at Varying Distances from the Nerve in a Group of Patients.
As observed, there is a clear correlation between the distance from the stimulation point and the EMG signal amplitude recorded by the sensor. This indicates the generation of a bioelectric potential in the vocal fold muscles at the moment of tissue stimulation within a specific zone surrounding the recurrent laryngeal nerve (RLN).
This relationship for the patient group is illustrated in Figure 12.
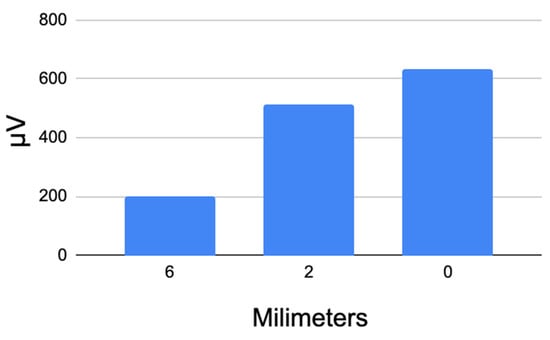
Figure 12.
Average potential difference values recorded by the EMG sensor during RLN stimulation at varying distances from the nerve.
The results of the trials indicate that the developed RLN identification system is capable of detecting the potential difference in the vocal folds at the moment of RLN stimulation. This enables the identification of both the nerve itself and the surrounding area of its anatomical location within the surgical field.
The results indicate the success of applying EMG for the hardware and software complex as a primary means of monitoring RLN during thyroid surgery. The developed EMG sensor was able to detect, and the software was able to display the change in EMG signal in the vocal cord at the moment of RLN stimulation. This proves the feasibility of using electromyography as part of the hardware and software complexes of IONM.
It should be emphasized that the developed system is specifically designed for the identification of the recurrent laryngeal nerve (RLN). This is determined by the physiological characteristics of vocal fold function. In particular, an electrical stimulus applied to the site of surgical intervention propagates along the RLN and induces contraction of the vocal folds. Consequently, an electromyographic (EMG) response is generated in the muscle tissue responsible for vocal fold movement, which is subsequently detected by the EMG sensor.
Another limitation of the proposed approach relates to the distance between the stimulation point within the surgical field and the RLN. Specifically, if this distance exceeds approximately 2 cm, the attenuation of the electrical signal within the tissues becomes significant, resulting in insufficient stimulation of the nerve fibers. As a result, the EMG sensor is unable to register a reliable response. This constraint highlights the importance of precise electrode placement and careful intraoperative monitoring to ensure the validity and reproducibility of the obtained signals.
The presented complex still lacks the function of automatic location determination of RLN based on EMG. In previous versions, machine learning has proven itself as a means of identifying RLN based on a sound signal. Therefore, the implementation of machine learning for processing EMG signals remains one of the main tasks related to developing this work. Overall, it should be noted that all signals generated by the system are of a purely advisory nature, and the surgeon may discontinue the use of the hardware–software complex at any point during the surgical procedure. This implies that the system functions solely as an auxiliary tool and cannot substitute for the surgeon’s professional expertise in the course of the operation.
8. Conclusions
Thus, the preliminary clinical trials of the developed hardware–software system designed for the identification of the recurrent laryngeal nerve (RLN) during thyroid surgery—utilizing an electromyographic (EMG) sensor—provide sufficient evidence to conclude that the proposed system is suitable for locating the RLN within the surgical field. A key advantage of the developed system is its low cost (a few hundred US dollars) combined with its relatively high effectiveness across various types of thyroid procedures.
The use of an EMG sensor for detecting RLN functionality presents a significant advantage over acoustic sensors, as it eliminates the dependency of the response signal on individual patient laryngeal physiology. Another important finding is the observed consistent relationship between muscle activity in the vocal folds and the distance from the stimulation point to the RLN. However, this phenomenon requires further investigation, taking into account additional factors such as the varying electrical conductivity of the surgical tissue and the specific nature of thyroid diseases in different patients.
The developed system is comprehensively described in this article. All hardware components used in the design are listed and detailed. A thorough description of the EMG sensor—considered the core hardware element of the system—is provided. Additionally, the functions and interconnections of all supporting components are explained. Custom software, written in Python and deployed on a Raspberry Pi microcontroller, was developed to ensure the system’s functionality. The software architecture was designed in accordance with the functional requirements of the system and is also described in detail, along with the key technologies and approaches applied during its development.
All of the above substantiates the feasibility of scaling the production of such systems for broader clinical application.
Author Contributions
Conceptualization, M.D., V.T., A.D. and A.M.; methodology, M.D., V.T. and A.M.; software, V.T. and A.M.; validation, V.T., A.D., A.M., A.B., K.P. and M.W.; investigation, M.D. and A.M.; writing—original draft preparation, M.D., A.M., V.T., A.D., A.B., K.P. and M.W.; writing—review and editing, M.D., A.M. and V.T.; funding acquisition, A.B., K.P. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
Financed by a subvention from the Ministry of Science and Higher Education, Poland, to the Academy of Silesia in Katowice.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of the Ivan Horbachevsky Ternopil National Medical University, Ministry of Health of Ukraine, under protocol No. 76 dated 15 January 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RLN | Recurrent Laryngeal Nerve |
| EMG | Electromyography |
| IONM | Intraoperative Nerve Monitoring |
| CT | Thyroid Cartilage electrode |
| ET | Endotracheal tube electrode |
| ADC | An analog-to-digital converter |
| GPIO | General-purpose input/output |
| UPS | Uninterruptible Power Supply |
| SPI | Serial Peripheral Interface |
| SNR | Signal-to-noise ratio |
| CMRR | Common-mode rejection ratio |
Appendix A
A Fragment of the Code for the Module for Obtaining an Information Signal from the EMG Sensor
def lissen_signal(self, signal, index):
while True:
if(index.value == 0):
for i,v in enumerate(signal):
signal[i] = 0
if(index.value < len(signal)):
signal[index.value] = self.get_signal_plus_value()
index.value = index.value + 1
time.sleep(1 / EMG_SIGNAL_FREQUENCY)
def check(self, frame):
if(self.index.value):
sigmal = [x for x in self.signal_plus if x != 0]
self.report_emg_ch1.on_data(sigmal)
em.emit(‘data’, [x * SUPPLY_VOLTAGE for x in sigmal])
self.index.value = 0
signal = Array(c_double, signal_length, lock=lock)
subprocess = Process(target=lissen_signal, args=(signal, indx)
self.subprocess.start()
Clock.schedule_interval (check, 0.1).
Appendix B
Voltage Values of Vocal Fold Potential Difference During RLN Stimulation at Various Distances in a Patient Group
| Patient Number | Sex | Age | Distance from the Point of Stimulation to the RLN (mm) | The Value of the EMG Signal During Stimulation (µV) |
| 1 | Female | 65 | 6 | 224 |
| 2 | 481 | |||
| 0 | 635 | |||
| 2 | Female | 65 | 6 | 234 |
| 2 | 467 | |||
| 0 | 672 | |||
| 3 | Female | 51 | 6 | 136 |
| 2 | 191 | |||
| 0 | 574 | |||
| 4 | Female | 52 | 6 | 235 |
| 2 | 423 | |||
| 0 | 626 | |||
| 5 | Male | 62 | 6 | 156 |
| 2 | 235 | |||
| 0 | 562 | |||
| 6 | Female | 54 | 6 | 162 |
| 2 | 456 | |||
| 0 | 617 | |||
| 7 | Female | 63 | 6 | 231 |
| 2 | 567 | |||
| 0 | 673 | |||
| 8 | Female | 33 | 6 | 178 |
| 2 | 278 | |||
| 0 | 534 | |||
| 9 | Female | 46 | 6 | 145 |
| 2 | 245 | |||
| 0 | 546 | |||
| 10 | Female | 77 | 6 | 256 |
| 2 | 357 | |||
| 0 | 576 |
References
- Malcharek, M.; Priebe, H.J. Anesthesia and IONM. In Intraoperative Neuromonitoring: Fundamentals, Possibilities, Limitations; Springer International Publishing: Cham, Switzerland, 2024; pp. 143–157. [Google Scholar]
- Valenzuela-Fuenzalida, J.J.; Baeza-Garrido, V.; Navia-Ramírez, M.F.; Cariseo-Ávila, C.; Bruna-Mejías, A.; Becerra-Farfan, Á.; Lopez, E.; Orellana Donoso, M.; Loyola-Sepulveda, W. Systematic Review and Meta-Analysis: Recurrent Laryngeal Nerve Variants and Their Implication in Surgery and Neck Pathologies, Using the Anatomical Quality Assurance (AQUA) Checklist. Life 2023, 13, 1077. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Rovira, A.; Quer, M.; Sanabria, A.; Guntinas-Lichius, O.; Zafereo, M.; Hartl, D.M.; Coca-Pelaz, A.; Shaha, A.R.; Marie, J.-P.; et al. Recurrent Laryngeal Nerve Intraoperative Neuromonitoring Indications in Non-Thyroid and Non-Parathyroid Surgery. J. Clin. Med. 2024, 13, 2221. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Fahim, D.K.; Gemechu, J.M. Anatomical Variations of the Recurrent Laryngeal Nerve and Implications for Injury Prevention during Surgical Procedures of the Neck. Diagnostics 2020, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Gumus, S.; Yuksel, C.; Pulat, H.; Akyuz, C.; Gul, M.O. Inferior-to-Superior Dissection for Recurrent Laryngeal Nerve Identification in Redo Thyroid Surgery: Enhanced Safety and Reduced Injuries. J. Clin. Med. 2024, 13, 7364. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, N.; Muehlbeyer, S.; Weyhe, D.; Uslar, V. Risk Factors for Recurrent Laryngeal Nerve Palsy in Thyroid Surgery: A Single Center Experience of 1147 Procedures with Intermittent Intraoperative Neuromonitoring. J. Pers. Med. 2024, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Han, L.; He, Y.; Xu, J.; Ravikumar, N.; Mann, R.; Frangi, A.F.; Yap, P.-T.; Huang, Y. Localizing the Recurrent Laryngeal Nerve via Ultrasound with a Bayesian Shape Framework. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2022; Wang, L., Dou, Q., Fletcher, P.T., Speidel, S., Li, S., Eds.; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2022; Volume 13434. [Google Scholar] [CrossRef]
- Randolph, G.W.; Dralle, H.; Abdullah, H.; Barczynski, M.; Bellantone, R.; Brauckhoff, M.; Carnaille, B.; Cherenko, S.; Chiang, F.-Y.; Dionigi, G.; et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: International standards guideline statement. Laryngoscope 2011, 121 (Suppl. S1), S1–S16. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, T.; Tomosugi, T.; Okada, M.; Futamura, K.; Goto, N.; Narumi, S.; Watarai, Y.; Tominaga, Y.; Ichimori, T. Intraoperative recurrent laryngeal nerve monitoring using endotracheal electromyography during parathyroidectomy for secondary hyperparathyroidism. J. Int. Med. Res. 2021, 49, 03000605211000987. [Google Scholar] [CrossRef] [PubMed]
- Shedd, D.P.; Durham, C. Electrical identification of the recurrent laryngeal nerve. I. Response of the canine larynx to electrical stimulation of the recurrent laryngeal nerve. Ann. Surg. 1966, 163, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Shedd, D.P.; Burget, G.C. Identification of the recurrent laryngeal nerve. Arch. Surg. 1966, 92, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Dyvak, M.; Kasatkina, N.; Pukas, A.; Padletska, N. Spectral analysis of the information signal in the task of identifying the recurrent laryngeal nerve in thyroid surgery. Przegląd Elektrotech. 2013, 89, 275–277. [Google Scholar]
- Dyvak, M.; Kozak, O.; Pukas, A. Interval model for identification of laryngeal nerves. Przegląd Elektrotech. 2010, 86, 139–140. [Google Scholar]
- Dyvak, M.; Tymets, V.; Sheketa, V. Adaptive information technology for recurrent laryngeal nerve identification based on electrophysical method of Its stimulation. Przegląd Elektrotech. 2020, 96, 28–34. [Google Scholar] [CrossRef]
- Dyvak, M.; Pukas, A.; Oliynyk, I.; Melnyk, A. Selection the “Saturated” Block from Interval System of Linear Algebraic Equations for Recurrent Laryngeal Nerve Identification. In Proceedings of the 2018 IEEE Second International Conference on Data Stream Mining & Processing (DSMP), Lviv, Ukraine, 21–25 August 2018; pp. 444–448. [Google Scholar] [CrossRef]
- Dyvak, M.; Porplytsya, N. Formation and Identification of a Model for Recurrent Laryngeal Nerve Localization During the Surgery on Neck Organs. In Advances in Intelligent Systems and Computing III. CSIT 2018; Springer: Cham, Switzerland, 2019; Volume 871, pp. 391–404. [Google Scholar]
- Porplytsya, N.; Dyvak, M.; Spivak, I.; Voytyuk, I. Mathematical and algorithmic foundations for implementation of the method for structure identification of interval difference operator based on functioning of bee colony. In Proceedings of the 2015 13th International Conference on The Experience of Designing and Application of CAD Systems in Microelectronics, Lviv, Ukraine, 24–27 February 2015; IEEE: New York, NY, USA, 2015; pp. 196–199. [Google Scholar] [CrossRef]
- Conte, A.; Maselli, M.; Nacci, A.; Manti, M.; Galli, J.; Paludetti, G.; Ursino, F.; Cianchetti, M. A biorobotic simulator of vocal folds for the reproduction and analysis of electroglottographic signals. In Proceedings of the 2021 IEEE 4th International Conference on Soft Robotics (RoboSoft), New Haven, CT, USA, 12–16 April 2021; pp. 90–96. [Google Scholar] [CrossRef]
- Paniello, R.C.; Park, A.M.; Bhatt, N.K.; Al-Lozi, M. Recurrent laryngeal nerve recovery patterns assessed by serial electromyography. Laryngoscope 2016, 126, 651–656. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, F. Movement of the larynx induced by electrical stimulation of the laryngeal nerves. In Research Potentials in Voice Physiology; Brewer, D.W., Ed.; State University of New York Press: Albany, NY, USA, 1964; pp. 129–140. [Google Scholar]
- Flisberg, K.; Lindholm, T. Electrical stimulation of the human recurrent laryngeal nerve during thyroid operation. Acta Otolaryngol. 1969, 263, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.J.; McCaffrey, T.V.; Litchy, W.J. Intraoperative electrophysiologic monitoring of laryngeal muscle during thyroid surgery. Laryngoscope 1988, 98, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Maloney, R.W.; Murcek, B.W.; Steehler, K.W.; Sibly, D.; Maloney, R.E. A new method for intraoperative recurrent laryngeal nerve monitoring. Ear Nose Throat J. 1994, 73, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Ambu® Neuroline 710. Available online: https://www.ambuaustralia.com.au/neurology/surface-electrodes/product/ambu-neuroline-710 (accessed on 28 June 2025).
- Medtronic. Available online: https://www.medtronic.com/ (accessed on 28 June 2025).
- Medtronic—Mini Nerve Monitoring System. Available online: https://www.medtronic.com/it-it/operatori-sanitari/products/ear-nose-throat/nerve-monitoring/nim-nerve-monitoring-systems.html (accessed on 28 June 2025).
- Natus. Available online: https://natus.com/neuro/emg/emg-ncs-ep-systems/ (accessed on 28 June 2025).
- NA132UA Datasheet. Available online: https://www.digchip.com/datasheets/parts/datasheet/477/INA132.php (accessed on 28 June 2025).
- AU2904. Available online: https://www.alldatasheet.com/view.jsp?Searchword=AU2904 (accessed on 28 June 2025).
- Kamen, G.; Gabriel, D. Essentials of Electromyography; Human Kinetics: Champaign, IL, USA, 2010. [Google Scholar]
- BCM2712-Processor. Available online: https://www.notebookcheck.net/Broadcom-BCM2712-Processor-Benchmarks-and-Specs.873280.0.html (accessed on 28 June 2025).
- MCP3008. Available online: https://cdn-shop.adafruit.com/datasheets/MCP3008.pdf (accessed on 28 June 2025).
- UPS Module 3S. Available online: https://www.waveshare.com/wiki/UPS_Module_3S (accessed on 28 June 2025).
- Kivy Documentation. Available online: https://kivy.org/doc/stable/ (accessed on 28 June 2025).
- Gpiozero. Available online: https://gpiozero.readthedocs.io/en/latest/ (accessed on 28 June 2025).
- Stack, M. Event-Driven Architecture in Golang Building Complex Systems with Asynchronicity and Eventual Consistency; Packt Publishing: Birmingham, UK, 2022; ISBN 9781803232188. [Google Scholar]
- Dyvak, M.; Tymets, V.; Dyvak, A. Laryngeal Nerve Identification during Thyroid Surgery with Automatic Adjustment of Electrical Signal Parameters. Int. J. Comput. 2024, 23, 552–562. [Google Scholar] [CrossRef]
- What Is Serial Peripheral Interface (SPI)? Available online: https://www.geeksforgeeks.org/what-is-serial-peripheral-interface-spi/ (accessed on 28 June 2025).
- Gpiozero mcp3008. Available online: https://gpiozero.readthedocs.io/en/latest/api_spi.html#mcp3008 (accessed on 28 June 2025).
- Matplot. Available online: https://matplotlib.org/stable/ (accessed on 28 June 2025).
- Kivy-Garden Matplotlib Flower. Available online: https://pypi.org/project/kivy-garden.matplotlib/ (accessed on 28 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).