Abstract
In this study, the association of MMP-1 rs1799750 gene polymorphism, which plays an important role in collagen degradation, with open bite malocclusion was investigated. The study included 30 individuals diagnosed with open bite and 30 healthy control subjects aged 15–35 years. DNA isolation was performed from cells taken from cheek mucosa and MMP-1 rs1799750 polymorphism was analyzed by Real-Time PCR method. In the open bite malocclusion group, MMP-1 rs1799750 polymorphism 1G/1G genotype was detected in 43.3%, 1G/2G in 23.3% and 2G/2G in 33.3%. In the control group, 1G/1G was 36.7%, 1G/2G 43.3% and 2G/2G 20.0%. In individuals with overbite of −5 mm and above, 2G/2G genotype was observed in 50% of the individuals. The results showed that there was no statistically significant difference in genotype and allele distribution (p = 0.2269; p = 0.7125). However, the frequency of the 2G/2G genotype increased as the severity of open bite increased (p = 0.009). While no significant associations were detected, the findings point toward a potential role of MMP-1 rs1799750 polymorphism in open bite development through mechanisms involving collagen degradation, warranting further investigation. The results of this study may make an important contribution to understanding the genetic basis of malocclusion and may help in the development of personalized orthodontic treatment approaches.
1. Introduction
Occlusion refers to the proper contact between teeth that ensures a harmonious bite when the upper and lower jaws close. Malocclusion, or occlusal disorders, occurs due to the misalignment or incorrect positioning of teeth or jaws [1]. Open bite malocclusion is a common occlusal disorder characterized by a lack of contact between the maxillary (upper) and mandibular (lower) teeth during chewing [2]. This condition can impair masticatory function, lead to aesthetic concerns, and negatively affect dental health. The development of open bite results from a complex interaction between genetic and environmental factors [3]. Sleep disorders, mouth breathing, sucking habits, and obesity have been identified as important risk factors for the development of malocclusion. Identifying and managing these factors at an early age can prevent future orthodontic problems [4]. Genetic factors are known to increase the risk of malocclusion by influencing skeletal and dentoalveolar development [5].
Recent literature emphasizes that malocclusion reflects not only occlusal malalignment but also underlying craniofacial development patterns. Morphological changes in tooth anatomy have been associated with skeletal discrepancies, particularly in Class III cases, indicating a reciprocal relationship between tooth and skeletal development. Furthermore, a systematic analysis of differences in anterior tooth dimensions revealed consistent deviations from ideal Bolton ratios in individuals with Class II and III malocclusions. This may contribute to functional and aesthetic imbalances in occlusion [6,7].
When analyzing the genetic basis of open bite malocclusion, various genes have been identified as potential contributors to this condition. Fibroblast growth factor receptor 2 (FGFR2) and Alpha-actinin-3 (ACTN3) are among the key genes involved in facial bone development and jaw morphology. The FGFR2 rs2981582 polymorphism has been particularly associated with skeletal malocclusion types. Similarly, the ACTN3 rs1815739 polymorphism is thought to influence jaw muscle development and may affect jaw structure formation in open bite cases [8].
Additionally, studies suggest that genetic variations such as MMP-9 rs17576, TNF-α rs1799724 and MTRR rs1801394 polymorphisms may also contribute to open bite malocclusion. These genes have also been linked to oral health-related quality of life, particularly in children [9,10].
Matrix metalloproteinase 1 (MMP-1) gene is one of the main genes that affect this process by taking part in the destruction of connective tissue. In particular, MMP-1 rs1799750 polymorphism may contribute to the development of malocclusion by degrading collagen, which is the main component of ECM in the formation of tooth and jaw structures [11,12,13].
In this study, the association between the MMP-1 rs1799750 gene polymorphism and open bite malocclusion is examined in detail, and the findings are expected to highlight the importance of genetic factors in clinical dentistry and orthodontic treatment practices. Understanding the role of genetic polymorphisms in the development of malocclusion could contribute to personalized treatment approaches and represent an important step in predicting individuals’ responses to treatment.
2. Materials and Methods
2.1. Power Analysis Data
The sample size of our study was calculated using the G*Power 3.1.9.4 program, considering the significance level of the hypothesis and the effect size. In the study by Küchler et al. (2017), based on the Odds Ratio (OR) value, which indicates that having the GG genotype of the MMP-9 gene reduces the risk of open bite (OR = 0.18, 95% CI: 0.01–1.79) compared to the control group, with α = 0.05 and 1 − β = 0.85 (i.e., an error rate of 0.005 and a test power of 95%), the sample size was calculated as at least 30 individuals per group [9]. We note that this calculation was based on a previous study investigating the MMP-9 gene, which may not fully reflect the relevance to MMP-1 rs1799750.
2.2. Selection of Individuals
This study was conducted by Marmara University, Institute of Health Sciences, and Marmara University, Faculty of Dentistry, Basic Medical Sciences between September 2022 and September 2023. The protocol for our study was prepared in accordance with the Declaration of Helsinki 2 (2015) guidelines and was approved by the Marmara University Faculty of Medicine Clinical Research Ethics Committee (Protocol code: 09.2022.653). The study was conducted on individuals with open bite who applied for treatment at the Marmara University Faculty of Dentistry, Department of Orthodontics.
A total of 60 individuals participated in the study, including 30 patients diagnosed with open bite, aged 15–35 years, and 30 control group participants without open bite, who met clinically and radiologically determined criteria, and all participants were of Turkish origin. Signed informed consent forms were obtained from individuals aged 18 years and older, and from the parents of individuals under 18 years old. For the patient group, participants were classified as having a greater incisor separation than a head-to-head bite in centric occlusion. The control group consisted of individuals with a normal bite depth of less than 4 mm, measured from the head-to-head bite position. The mean gender and age distribution of the individuals included in the study are presented in Table 1.

Table 1.
Gender distribution and mean age of open bite patient and control groups.
To determine the degree of open bite and skeletal morphology, pretreatment initial cephalometric radiographs of individuals diagnosed with open bite on clinical examination and individuals included in the healthy group were examined using NemoStudioNX-Pro 10.4.2 software (Software Nemotec, Madrid, Spain). As a result of the cephalometric radiographs, overbite amounts were determined and recorded as patient and healthy groups. The individuals in the patient group were divided into 3 groups in millimeters according to their overbite amounts to be evaluated among themselves (Table 2).

Table 2.
Open bite patient groups according to overbite amount.
2.3. DNA Isolation
DNA isolation from buccal epithelial cells was performed according to the PureLink (Invitrogen, Van Allen Way, Carlsbad, CA, USA) Kit protocol. The quantity of isolated DNA was measured using the Qubit 4 fluorometer, following the manufacturer’s protocol.
Genotyping of MMP-1 rs1799750 polymorphism was performed using Real-Time PCR on StepOnePlus (Thermo Fisher Scientific, Inc., Santa Clara, CA, USA) and Taqman SNP Genotyping kit according to the manufacturers’ protocols (cat. no. 4351379, Thermo Fisher Scientific, Inc.). The sequence of the Real-Time PCR genotyping kit is given in Table 3.

Table 3.
Sequences of TaqMan probe used for genotyping of MMP-1 rs1799750 polymorphism.
2.4. Statistical Analysis
In the statistical analysis of the results obtained, χ2 (chi-square) analysis was performed using SPSS 25.0 (Statistical Packages of Social Sciences, IBM, Corp., Armonk, NY, USA) program. p < 0.05 was considered statistically significant. Hardy–Weinberg Equilibrium (HWE) was tested for the control group using a χ2 test. A p-value > 0.05 was considered consistent with HWE. In addition, odds ratios (ORs) with 95% confidence intervals were calculated to evaluate the association between genotypes and open bite occurrence under homozygote comparison, dominant and recessive models.
3. Results
Genotype and allelic distribution of MMP-1 rs1799750 polymorphism between the patient group and the control group are shown in Table 4. There was no statistically significant difference between the groups in terms of genotype distribution (p = 0.2269) and allelic distribution (p = 0.7125) of MMP-1 rs1799750 polymorphism. The Hardy–Weinberg Equilibrium (HWE) test showed that the control group was in equilibrium (p = 0.838), while the patient group showed a significant deviation from HWE expectations (p = 0.015).

Table 4.
Genotype and allelic distribution of MMP-1 rs1799750 polymorphisms between open bite patient and control groups.
Genotype-based odds ratio analyses (Table 5) indicated a trend toward higher risk for open bite in individuals carrying the 2G/2G genotype, particularly under the recessive model (OR = 2.00) and when compared to heterozygotes (OR = 3.10).

Table 5.
The chi-square test for genotypic dominance.
Genotype and allelic distribution of MMP-1 rs1799750 polymorphism in female open bite patients and female controls are shown in Table 6. Similarly to the overall analysis, there was no statistically significant difference between the groups with respect to genotype distribution (p = 0.6929) or allelic distribution (p = 0.6135).

Table 6.
Genotype and allelic distribution of MMP-1 rs1799750 polymorphism between open bite patients and controls in female.
Genotype and allelic distribution of MMP-1 rs1799750 polymorphism in male open bite patients and male controls are shown in Table 7. No statistically significant difference was observed between the groups with respect to genotype distribution (p = 0.1711) or allelic distribution (p = 0.8095).

Table 7.
Genotype and allelic distribution of MMP-1 rs1799750 polymorphism between open bite patients and controls in male.
The amount of overbite in the patient group was found to be statistically significant in terms of genotype distribution between groups (p = 0.009). In the group with “0 and −0.9” open bite, there were more individuals (76.9%) with 1G/1G genotype than expected compared to the other groups (Table 8, Figure 1).

Table 8.
Classification of MMP-1 rs1799750 polymorphism genotype and open bite assessment for the patient group.
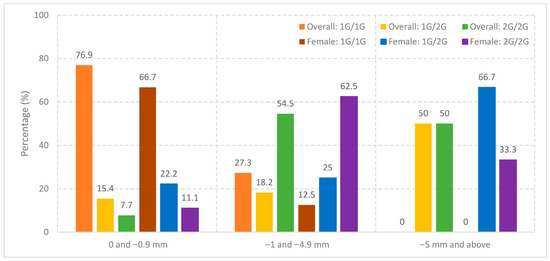
Figure 1.
Distribution of MMP-1 rs1799750 genotypes by open bite severity in the overall cohort and in females.
The amount of overbite in the female patient group was also found to be statistically significant in terms of genotype distribution (p = 0.0482). In particular, individuals with the 2G/2G genotype were more frequent in the subgroup with −1 to −4.9 mm overbite (71.4%), whereas the 1G/1G genotype was predominant in the subgroup with 0 to −0.9 mm overbite (85.7%) (Table 9, Figure 1).

Table 9.
Classification of MMP-1 rs1799750 polymorphism genotype and open bite assessment for the patient group in female (n = 20).
The allelic distribution of overbite in the patient group was statistically significant (p < 0.001). The percentage of 1G allele was higher in the group with “0 and −0.9” overbite (84.6%); 2G allele was higher in the group with “−1 and −4.9” overbite (63.6%); and 2G allele was higher in the group with “−5 and above” overbite (75.0%). According to the results of our study, the percentage of 2G allele increases as the amount of overbite increases (Table 10, Figure 2).

Table 10.
Classification of MMP-1 rs1799750 polymorphism allelic distribution and open bite assessment for the patient group.
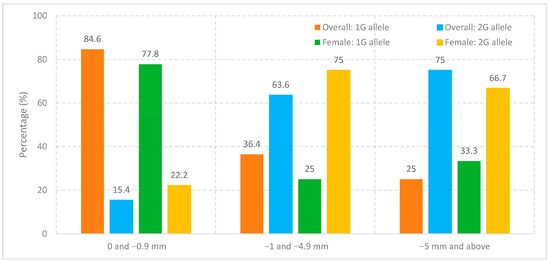
Figure 2.
Distribution of MMP-1 rs1799750 alleles by open bite severity in the overall cohort and in females.
The allelic distribution of overbite in the female patient group was statistically significant (p = 0.006). The percentage of the 1G allele was higher in the group with 0 to −0.9 mm overbite (77.8%), whereas the 2G allele was more frequent in the groups with −1 to −4.9 mm (75.0%) and −5 mm and above (66.7%). These results indicate that, similar to the overall patient group, the proportion of the 2G allele increases with the severity of open bite in female patients (Table 11, Figure 2).

Table 11.
Classification of MMP-1 rs1799750 polymorphism allelic distribution and open bite assessment for the patient group in female.
4. Discussion
Malocclusion is a complex dental anomaly characterized by incompatible positioning of the teeth and jaws, and can negatively affect the quality of life of individuals both functionally and aesthetically. Open bite malocclusion is characterized by the anterior teeth not coming into contact with each other during bite and is thought to develop as a result of the interaction of genetic and environmental factors [14]. In this study, in order to understand the genetic background of open bite cases, the possible association of MMP-1 rs1799750 polymorphism, which plays an important role in the remodeling of the extracellular matrix, with this condition was examined. MMP-1 is one of the collagenase enzymes that regulate the degradation of connective tissues and may play a decisive role in jaw and tooth development by changing ECM dynamics [15]. There are limited number of studies in the literature investigating the association of this polymorphism with open bite malocclusion. In this context, by examining the MMP-1 rs1799750 polymorphism, the role of genetic factors in the etiology of open bite was discussed more comprehensively with the aim of contributing to the understanding of genetic aspects of malocclusion.
However, the effects of malocclusions on the quality of life of individuals also stand out as an important research topic. In a study by Sabouni et al., (2022), although no significant effect of open bite on quality of life was found directly, the need for comprehensive diagnostic evaluation was emphasized in order to determine the most appropriate corrective treatment. This finding suggests that the impact of open bite on individuals should be evaluated not only in terms of physical but also psychosocial factors [16]. Accordingly, the biological and environmental factors underlying open bite malocclusion should be addressed in a holistic approach. Factors such as growth patterns, orofacial muscle activity and skeletal–oral incompatibilities are known to be effective in the development of open bite. However, the role of genetic factors in this process is still not fully elucidated.
Beyond MMP-1, other genes involved in craniofacial development may also contribute to open bite etiology. Notably, polymorphisms in the fibroblast growth factor receptor 2 (FGFR2) gene have been associated with altered craniofacial morphology and skeletal growth patterns, given FGFR2′s critical role in osteogenesis and bone remodeling. Variations in this gene may disrupt maxillary or mandibular development, thereby predisposing individuals to vertical skeletal discrepancies such as open bite [17]. These findings emphasize the polygenic nature of malocclusion and underscore the importance of future multi-gene analyses that integrate collagen degradation pathways with craniofacial growth regulators to better predict susceptibility and long-term treatment outcomes [18].
Collagen degradation metabolism is a determining factor in the formation of malocclusion. At this point, it is critical to understand the effect of polymorphisms in matrix metalloproteinases, which are responsible for collagen degradation metabolism, on the development of malocclusion.
The MMP-1 gene plays a critical role in the degradation of fibrillar collagens, particularly collagen types I and III, which are abundant in bone, periodontal ligament and craniofacial connective tissues. The rs1799750 polymorphism involves the addition of an extra guanine nucleotide (2G allele) to the promoter region, creating a binding site for Ets transcription factors. This functional alteration increases the transcriptional activity of the MMP-1 gene, leading to increased mRNA and protein expression in individuals carrying the 2G allele, particularly in 2G/2G homozygotes [19,20].
High MMP-1 expression contributes to excessive collagen turnover and ECM degradation, which may compromise the structural stability of the periodontium and alveolar bone. Such dysregulation may disrupt craniofacial bone growth and remodeling, predisposing individuals to skeletal malocclusions such as open bite. The rs1799750 polymorphism corresponds to the −1607 1G/2G insertion; the 2G allele creates a functional Ets transcription factor binding site that cooperates with AP-1 elements, resulting in higher MMP-1 transcriptional activity and protein expression. The presence of this binding motif enhances the recruitment of Ets proteins to the promoter, thereby increasing transcriptional activation of MMP-1 [13,21].
In the dentoalveolar complex, MMP-1 degrades fibrillar collagens (types I and III) in the periodontal ligament and alveolar bone. Upregulated MMP-1 expression, as seen in 2G/2G carriers, may accelerate collagen turnover, compromise ligament stiffness, and disturb bone remodeling balance—factors that could exacerbate anterior open bite severity [22]. Taken together, these mechanistic insights support our findings that MMP-1 rs1799750 acts less as a primary susceptibility factor and more as a genetic modifier influencing the severity of the open bite phenotype. Recent studies in periodontal and craniofacial biology have shown that MMP-1 overexpression is associated with alterations in osteoblast and osteoclast activity, as well as imbalances in bone resorption and apposition, particularly in the context of orthodontic movement or inflammatory stimuli [22,23]. Importantly, our findings suggest that the MMP-1 rs1799750 polymorphism may not act as a primary susceptibility factor for the occurrence of open bite, but rather as a genetic modifier influencing its severity. The higher frequency of the 2G/2G genotype in more severe cases supports this modulation hypothesis. In addition, genotype-based odds ratio (OR) analyses indicated that individuals carrying the 2G/2G genotype showed higher odds of open bite compared to 1G/1G homozygotes (OR = 1.41), to the combined group of 1G/1G and 1G/2G carriers (recessive model, OR = 2.00), and particularly to heterozygotes (OR = 3.10). Although none of these associations reached statistical significance (p > 0.05), the consistent direction of these OR values supports the modulatory role of the 2G/2G genotype. Furthermore, the Hardy–Weinberg Equilibrium (HWE) test demonstrated that the control group was in equilibrium (p = 0.838), confirming the validity of the control cohort, whereas the patient group deviated from equilibrium (p = 0.015). This deviation may reflect a genetic contribution of the MMP-1 rs1799750 variant to the open bite phenotype. However, we note that the power calculation for this study was based on a previous investigation of the MMP-9 gene, which may not fully reflect the relevance to MMP-1 rs1799750. In addition, the stratified subgroup analysis included only six individuals in the most severe category, which limits statistical robustness and indicates that these findings should be interpreted with caution.
In addition to the overall analysis, genotype and allelic distributions were also examined separately in female-only and male-only patient versus control comparisons; however, none of these subgroup analyses revealed statistically significant differences. While detailed tables are presented for the overall cohort and female subgroup, the corresponding male subgroup analyses were not reported in detail due to the relatively small number of male participants (n = 10 in the open bite group and n = 13 in the control group), which limited statistical power and interpretability. Since subgroup comparisons generally require larger sample sizes to ensure reliable inference, presenting these male data in detail could have been misleading. Therefore, we focused on the overall and female subgroup results, while emphasizing that future studies with larger, sex-balanced populations are required to clarify potential sex-specific effects of the MMP-1 rs1799750 polymorphism on open bite severity. In craniofacial development, maintaining the balance between ECM synthesis and degradation is crucial. Therefore, increased transcription activity of MMP-1 in 2G carriers may serve as a genetic susceptibility factor for open bite, especially in individuals exposed to additional risk factors such as parafunctional habits or altered muscle forces.
This mechanistic insight is supported by several studies in the literature that have investigated the relationship between matrix metalloproteinase gene polymorphisms and the risk of developing open bite malocclusion. In the study by Küchler et al., (2017), MMP-9 rs17576 polymorphism was found to have a protective effect against open bite. In the analysis of 219 children with open bite malocclusion and 253 children with normal malocclusion as a control group, it was reported that MMP-9 is an enzyme that degrades denatured collagen and type IV collagen and therefore is a protective factor against open bite malocclusion by affecting the remodeling of the extracellular matrix [9].
Teixeira et al., (2022) examined the effect of open bite on oral health-related quality of life (OHRQoL) in children and evaluated whether Methionine Synthase (MTR), Methionine synthase reductase (MTRR), Transforming Growth Factor Beta 1 (TGFβ1) and Tumor Necrosis Factor (TNF-α) genes were potential biomarkers. In the study, TNF-α rs1799724 and MTRR rs1801394 polymorphisms were found to be important biomarkers for OHRQoL in children with open bite. However, it was concluded that these genetic polymorphisms are associated with psychosocial factors such as depression and stress rather than directly affecting open bite malocclusion [10].
Lin et al., (2013) and Leal et al., (2020) examined the interaction of genetic and environmental factors in the development of open bite malocclusion. Lin et al. stated that long-term finger sucking habit during childhood is an important environmental factor, but facial skeleton and jaw structure are greatly influenced by genetic factors. Leal et al. showed that genes such as GHR, TNF-α, OPG and ACTN3 may be associated with open bite malocclusion [24,25].
Zebrick et al. (2014) addressed the effect of muscle forces on jaw development and showed that the mechanical load on the bone during muscle contractions alters the skeletal structure. The study suggested that genetic polymorphisms in muscle structure may be associated with certain types of malocclusions [26].
Cunha et al., (2019) conducted studies examining the relationship between muscle forces and maxillofacial morphology. In their study, it was stated that ACTN2 and ACTN3 genes may be associated with the development of open bite. In particular, it was reported that the XX (TT) genotype of ACTN3 rs1815739 polymorphism was found to be more highly expressed in individuals with open bite and less expressed in individuals with deep bite [27].
A similar result to this study was reported by Yaylacı et al.. In their study, they reported that XX (TT) genotype in ACTN3 rs1815739 polymorphism was found at a higher rate in open bite cases, and XX genotype was more common in individuals with increased severity of open bite. However, while there was no significant difference between the control and case groups in terms of genotype distribution, it was observed that the XX genotype was significantly higher in individuals with an open bite malocclusion of −5 mm or more [28].
In a study investigating the relationship between COL1A1 rs1800012 polymorphism associated with collagen production and open bite malocclusion, a total of 60 individuals, including 30 individuals with open bite malocclusion and 30 individuals with normal malocclusion, were evaluated. There was no statistically significant difference in the genotype and allelic distribution of COL1A1 rs1800012 polymorphism between the patient and healthy groups (p > 0.05). In addition, no significant difference was found in internal comparisons within the patient group. These results suggest that COL1A1 rs1800012 polymorphism, which is associated with collagen production, may not be a determining genetic factor in the development of open bite malocclusion [29].
This study has several limitations that should be considered when interpreting the findings. The relatively small sample size (30 patients and 30 controls) may restrict the statistical power and generalizability of the results. Furthermore, all participants were recruited from a single population in Turkey, without representation of different ethnicities, which limits extrapolation to broader populations. In addition, potential environmental and lifestyle factors that could interact with genetic predisposition were not evaluated.
Future research should address these limitations by including larger sample sizes to enhance statistical reliability. Multicenter studies across different geographic regions and ethnic groups would provide a broader understanding of the role of MMP-1 polymorphism in open bite malocclusion. Moreover, incorporating analyses of multiple genes and their potential interactions, alongside environmental and lifestyle factors, could offer a more comprehensive view of the genetic and non-genetic determinants of collagen degradation and craniofacial development. Such integrative approaches will be essential to better elucidate the complex etiology of open bite malocclusion and to guide potential personalized prevention or treatment strategies.
In addition to genotypic findings, bioinformatic approaches such as haplotype analysis, in silico transcription factor binding predictions, and eQTL data could provide deeper insight into the functional role of the MMP-1 rs1799750 variant. For example, the GTEx database offers the opportunity to examine whether this polymorphism influences MMP-1 gene expression in relevant tissues such as skeletal muscle and fibroblasts. Such analyses may strengthen the observed genotype–phenotype association by linking the 2G allele to increased extracellular matrix degradation, further supporting its modulatory effect on open bite severity.
5. Conclusions
In this study, the relationship between open bite malocclusion and MMP-1 rs1799750 polymorphism, which plays a critical role in connective tissue remodeling, was investigated. The results show that MMP-1 rs1799750 polymorphism may be associated with the severity of open bite. In particular, the 2G/2G genotype of the MMP-1 rs1799750 polymorphism was found to be higher in individuals with severe open bite, suggesting that the collagen degradation process may be a determining factor in the development of malocclusion. This pattern indicates that MMP-1 rs1799750 may not directly predispose individuals to developing open bite, but instead modulates the severity of the phenotype. Therefore, our results should be interpreted as exploratory evidence of a gene–phenotype modulation effect, which warrants confirmation in larger studies. This finding suggests that MMP-1, which regulates connective tissue remodeling, may play an important role in jaw and facial skeletal development. In conclusion, this study emphasizes the potential contribution of genetic factors in the formation of this malocclusion by revealing that collagen degradation mechanisms are more effective in open bite malocclusion rather than collagen production process.
Author Contributions
Conceptualization, B.T.A. and Ö.Ö.Y.; methodology, B.T.A., Ö.Ö.Y. and T.P.; validation, B.T.A. and E.Ö.Ö.; formal analysis, Ö.Ö.Y. and T.P.; investigation, Ö.Ö.Y.; resources, E.Ö.Ö.; data curation, Ö.Ö.Y.; writing—original draft preparation, Ö.Ö.Y.; writing—review and editing, B.T.A. and E.Ö.Ö.; supervision, E.Ö.Ö.; project administration, B.T.A.; funding acquisition, B.T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was derived from the master’s thesis of Özlem Özge Yılmaz (under the supervision of Beste Tacal Aslan) submitted to the University of Marmara, Institute of Health Sciences, Oral Biology master programme. This research was funded by Marmara University, Scientific Research Projects Department, grant number 10934.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Marmara University Faculty of Medicine Clinical Research (protocol code: 09.2022.653; date of approval: 20 June 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable requests. Owing to institutional policies and ethical restrictions, the dataset is not publicly available.
Acknowledgments
The authors would like to express their sincere gratitude to Elif Aslıhan Yaylacı for her valuable support during the data collection process. We also extend our heartfelt thanks to Korkut Ulucan for his insightful guidance, knowledge, and supervision throughout the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hassan, R.; Rahimah, A.K. Occlusion, malocclusion and method of measurements—An overview. Arch. Orofac. Sci. 2007, 2, 3–9. [Google Scholar]
- Proffit, W.R.; Fields, H.; Larson, B.; Sarver, D.M. Contemporary Orthodontics, 6th ed.; South Asia Edition; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Shapiro, P.A. Stability of open bite treatment. Am. J. Orthod. Dentofac. Orthop. 2002, 121, 566–568. [Google Scholar] [CrossRef]
- Granja, G.L.; Leal, T.R.; Lima, L.C.M.; Silva, S.E.D.; Neves, É.T.B.; Ferreira, F.M. Predictors associated with malocclusion in children with and without sleep disorders: A cross-sectional study. Braz. Oral Res. 2023, 37, e106. [Google Scholar] [CrossRef]
- Teittinen, M.; Tuovinen, V.; Tammela, L.; Schätzle, M.; Peltomäki, T. Long-term stability of anterior open bite closure corrected by surgical-orthodontic treatment. Eur. J. Orthod. 2012, 34, 238–243. [Google Scholar] [CrossRef]
- Guarnieri, R.; Squillace, F.; Podda, R.; Monterossi, A.S.; Galluccio, G.; Di Giorgio, R.; Barbato, E. Three-Dimensional Dental Analysis in Subjects with Skeletal Malocclusion: A Retrospective Observational Study. Dent. J. 2025, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, N.D.; Romanec, C.; Cernei, E.R.; Karvelas, N.; Nastri, L.; Zetu, I.N. Scoping Review—The Effectiveness of Clear Aligners in the Management of Anterior Open Bite in Adult Patients. Medicina 2025, 61, 1113. [Google Scholar] [CrossRef] [PubMed]
- Moreno Uribe, L.M.; Miller, S.F. Genetics of the dentofacial variation in human malocclusion. Orthod. Craniofac. Res. 2015, 18, 91–99. [Google Scholar] [CrossRef]
- Küchler, E.C.; Barreiros, D.; Silva, R.O.D.; Abreu, J.G.B.; Teixeira, E.C.; Silva, R.A.B.; Silva, L.A.B.; Romano, F.L.; Granjeiro, J.M.; Antunes, L.A.A.; et al. Genetic polymorphism in MMP9 may be associated with anterior open bite in children. Braz. Dent. J. 2017, 28, 277–280. [Google Scholar] [CrossRef]
- Teixeira, E.C.; Das Neves, B.M.; Castilho, T.; Silva Ramos, T.; Flaviana, A.; Carelli, J.; Kuchler, E.C.; Germano, F.N.; Alves Antunes, L.A.; Antunes, L.S. Evidence of association between MTRR and TNF-α gene polymorphisms and oral health-related quality of life in children with anterior open bite. J. Clin. Pediatr. Dent. 2022, 46, 249–258. [Google Scholar] [CrossRef]
- Nagase, H.; Woessner, J.F. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E.; Rutter, J.L.; Benbow, U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 2000, 6, 4823–4830. [Google Scholar]
- Rutter, J.L.; Mitchell, T.I.; Butticè, G.; Meyers, J.; Gusella, J.F.; Ozelius, L.J.; Brinckerhoff, C.E. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998, 58, 5321–5325. [Google Scholar]
- Artese, F.; Fernandes, L.Q.P.; De Oliveira Caetano, S.R.; Miguel, J.A.M. Early treatment for anterior open bite: Choosing adequate treatment approaches. Semin. Orthod. 2023, 29, 207–215. [Google Scholar] [CrossRef]
- Evrosimovska, B.; Dimova, C.; Popovska, L.; Zabokova-Bilbilova, E. Matrix Metalloproteinase-8 gene polymorphism in chronic periapical lesions. Prilozi 2015, 36, 217–224. [Google Scholar] [CrossRef][Green Version]
- Sabouni, W.; Hansa, I.; Al Ali, S.M.; Adel, S.M.; Vaid, N. Invisalign treatment with mandibular advancement: A retrospective cohort cephalometric appraisal. J. Clin. Imaging Sci. 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Chehaibi, K.; Hrira, M.Y.; Nouira, S.; Maatouk, F.; Hamda, K.B.; Slimane, M.N. Matrix metalloproteinase-1 and matrix metalloproteinase-12 gene polymorphisms and the risk of ischemic stroke in a Tunisian population. J. Neurol. Sci. 2014, 342, 107–113. [Google Scholar] [CrossRef]
- Loo, W.T.; Wang, M.; Jin, L.J.; Cheung, M.N.; Li, G.R. Association of matrix metalloproteinase (MMP-1, MMP-3 and MMP-9) and cyclooxygenase-2 gene polymorphisms and their proteins with chronic periodontitis. Arch. Oral Biol. 2011, 56, 1081–1090. [Google Scholar] [CrossRef]
- Dey, S.; Ghosh, N.; Saha, D.; Kesh, K.; Gupta, A.; Swarnakar, S. Matrix metalloproteinase-1 (MMP-1) promoter polymorphisms are well linked with lower stomach tumor formation in eastern Indian population. PLoS ONE 2014, 9, e88040. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Nemec, M.; Weissinger, F.; Rausch, M.A.; Andrukhov, O.; Jonke, E. MMPs and TIMPs expression levels in the periodontal ligament during orthodontic tooth movement: A systematic review of in vitro and in vivo studies. Int. J. Mol. Sci. 2021, 22, 6967. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Huang, G.W.; Chen, C.S. Etiology and treatment modalities of anterior open bite malocclusion. J. Exp. Clin. Med. 2013, 5, 1–4. [Google Scholar] [CrossRef]
- Leal, F.; Lemos, A.R.B.; Costa, G.F.; Cardoso, I.L. Genetic and environmental factors involved in the development of oral malformations such as cleft lip/palate in non-syndromic patients and open bite malocclusion. Eur. J. Med. Health Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Zebrick, B.; Teeramongkolgul, T.; Nicot, R.; Horton, M.J.; Raoul, G.; Ferri, J.; Vieira, A.R.; Sciote, J.J. ACTN3 R577X genotypes associate with Class II and deepbite malocclusions. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 603–611. [Google Scholar] [CrossRef]
- Cunha, A.; Nelson-Filho, P.; Marañón-Vásquez, G.A.; Ramos, A.G.C.; Dantas, B.; Sebastiani, A.M.; Silvério, F.; Omori, M.A.; Rodrigues, A.S.; Teixeira, E.C.; et al. Genetic variants in ACTN3 and MYO1H are associated with sagittal and vertical craniofacial skeletal patterns. Arch. Oral Biol. 2019, 97, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yaylacı, E.A.; Önem Özbilen, E.; Aslan, B.T.; Polat, T. Investigation of the relationship between ACTN3 rs1815739 polymorphism and openbite cases: A prospective study. Orthod. Craniofac. Res. 2025, 28, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Polat, T. Investigation of the Relationship Between Collagen Type 1 Alpha Chain Gene rs1800012 Polymorphism and Openbite Cases. Master’s Thesis, Marmara University, İstanbul, Türkiye, 2024. [Google Scholar]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2002, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Mei, L.; Zou, Y.; Ding, Q.; Cannon, R.D.; Chen, H.; Li, H. Genetic Polymorphisms in FGFR2 Underlie Skeletal Malocclusion. J. Dent. Res. 2019, 98, 1340–1347. [Google Scholar] [CrossRef]
- Gershater, E.; Li, C.; Ha, P.; Chung, C.H.; Tanna, N.; Zou, M.; Zheng, Z. Genes and Pathways Associated with Skeletal Sagittal Malocclusions: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 13037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).