Enhanced Cyclic Stability of Composite-Modified Iron-Based Oxygen Carriers in Methane Chemical Looping Combustion: Mechanistic Insights from Chemical Calculations
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Software
2.3. Principles
2.4. Steps
2.4.1. Definition of Reaction System and Components
2.4.2. Construction of Thermodynamic Database
2.4.3. Set Model Constraints
2.4.4. Selection of Solution Algorithm
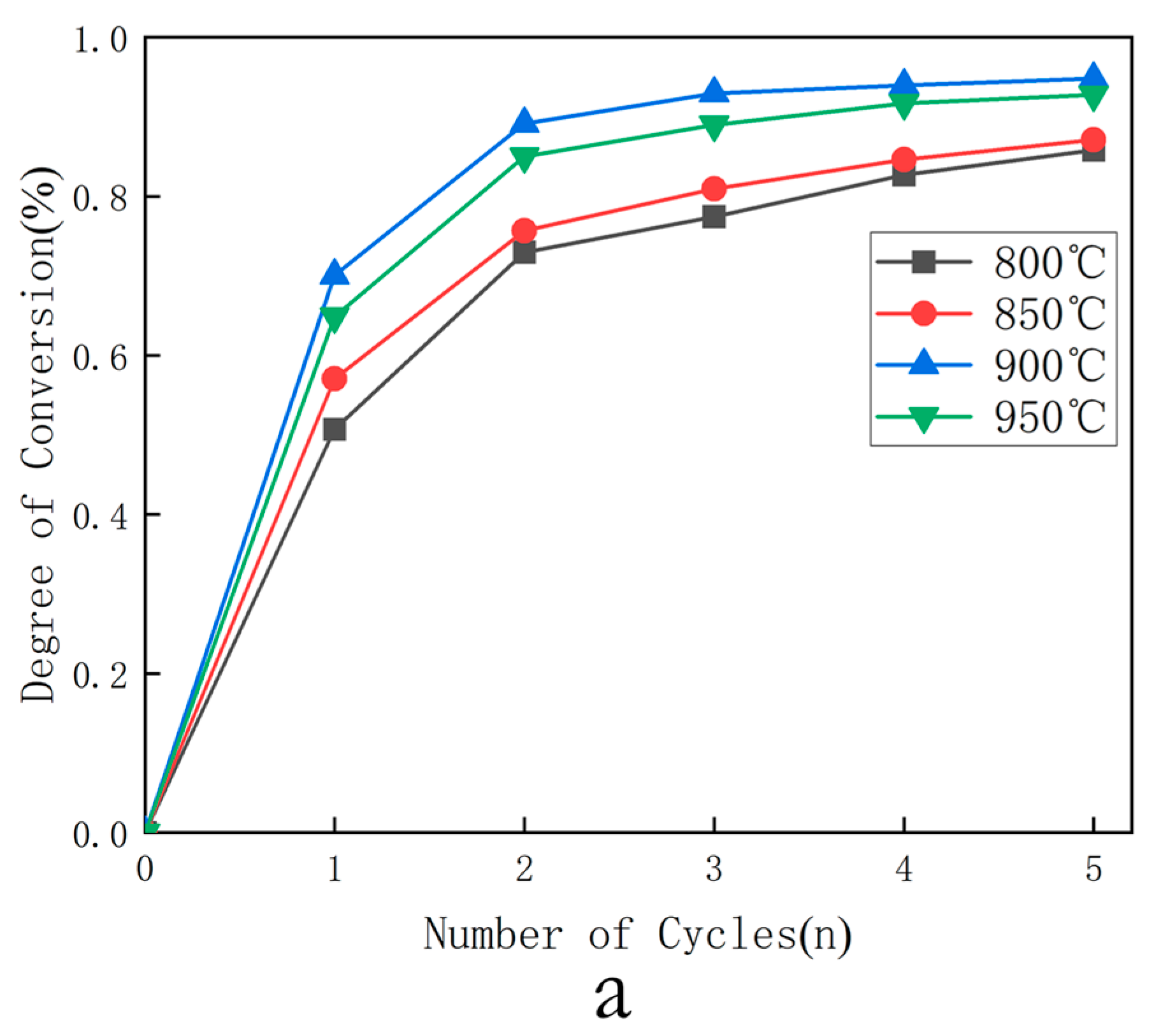
- The AR reaction temperature was set to 800 °C. This optimized temperature ensured stable regeneration of oxygen carriers while maintaining structural integrity throughout cyclic operation [52].
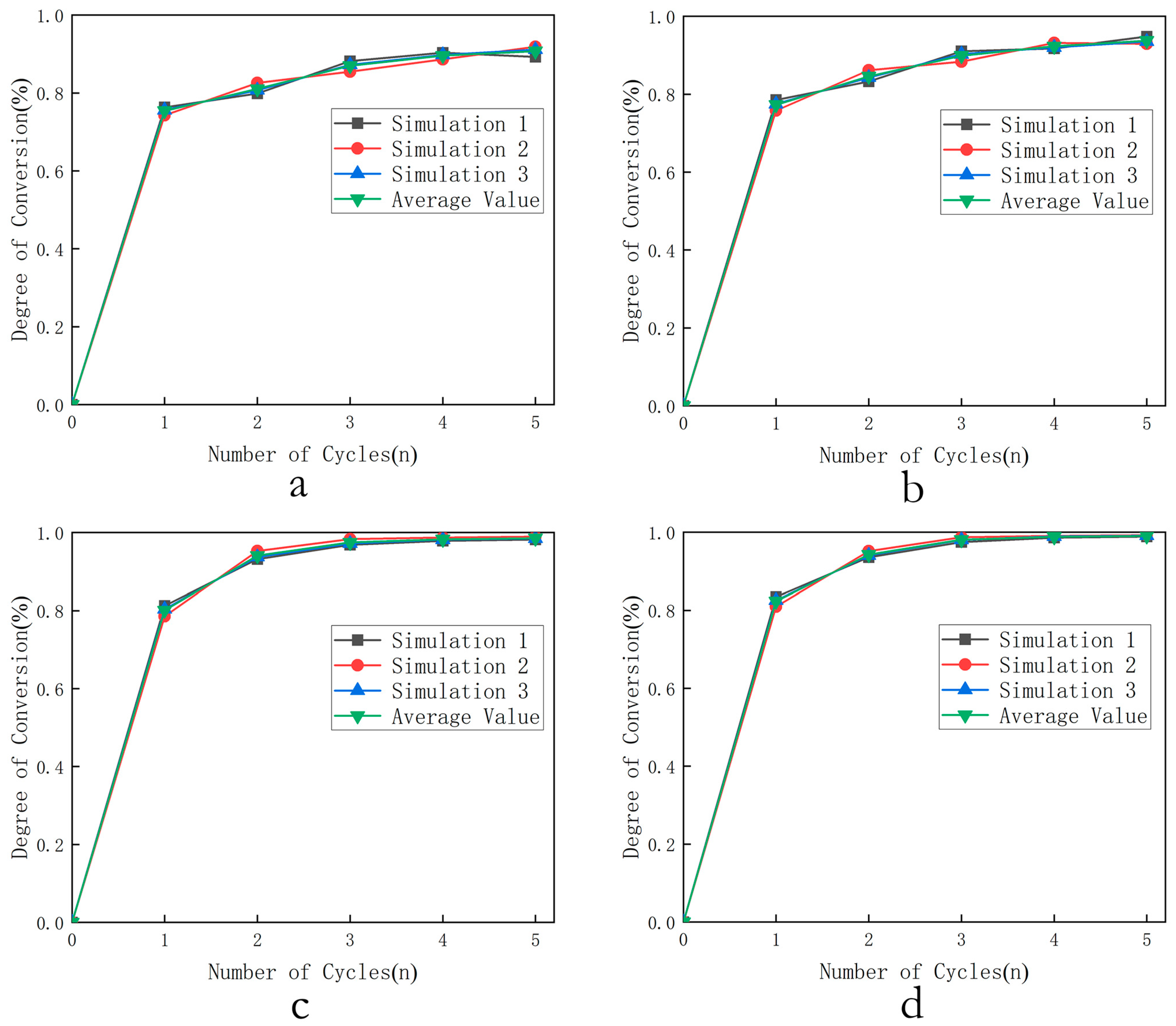
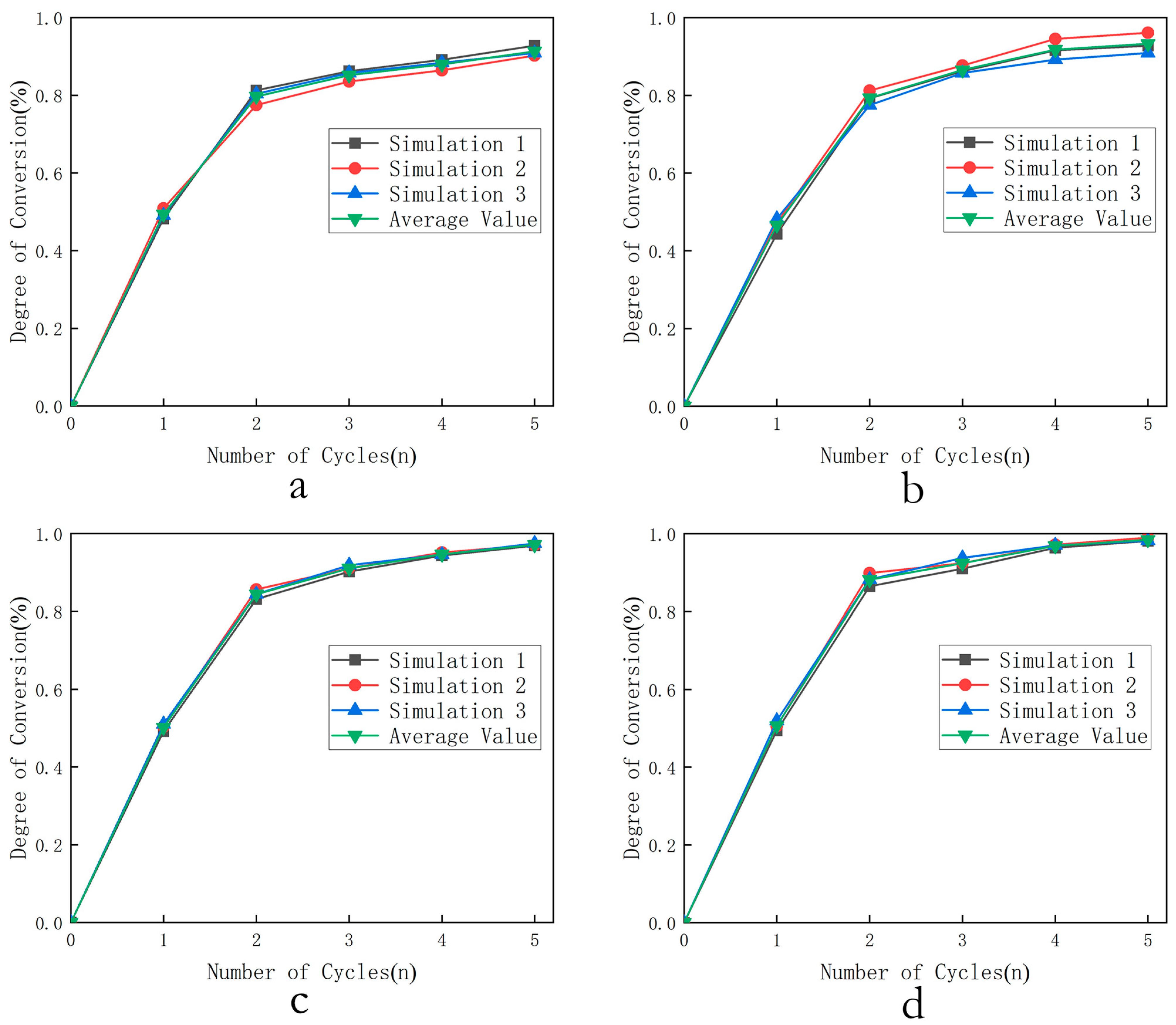
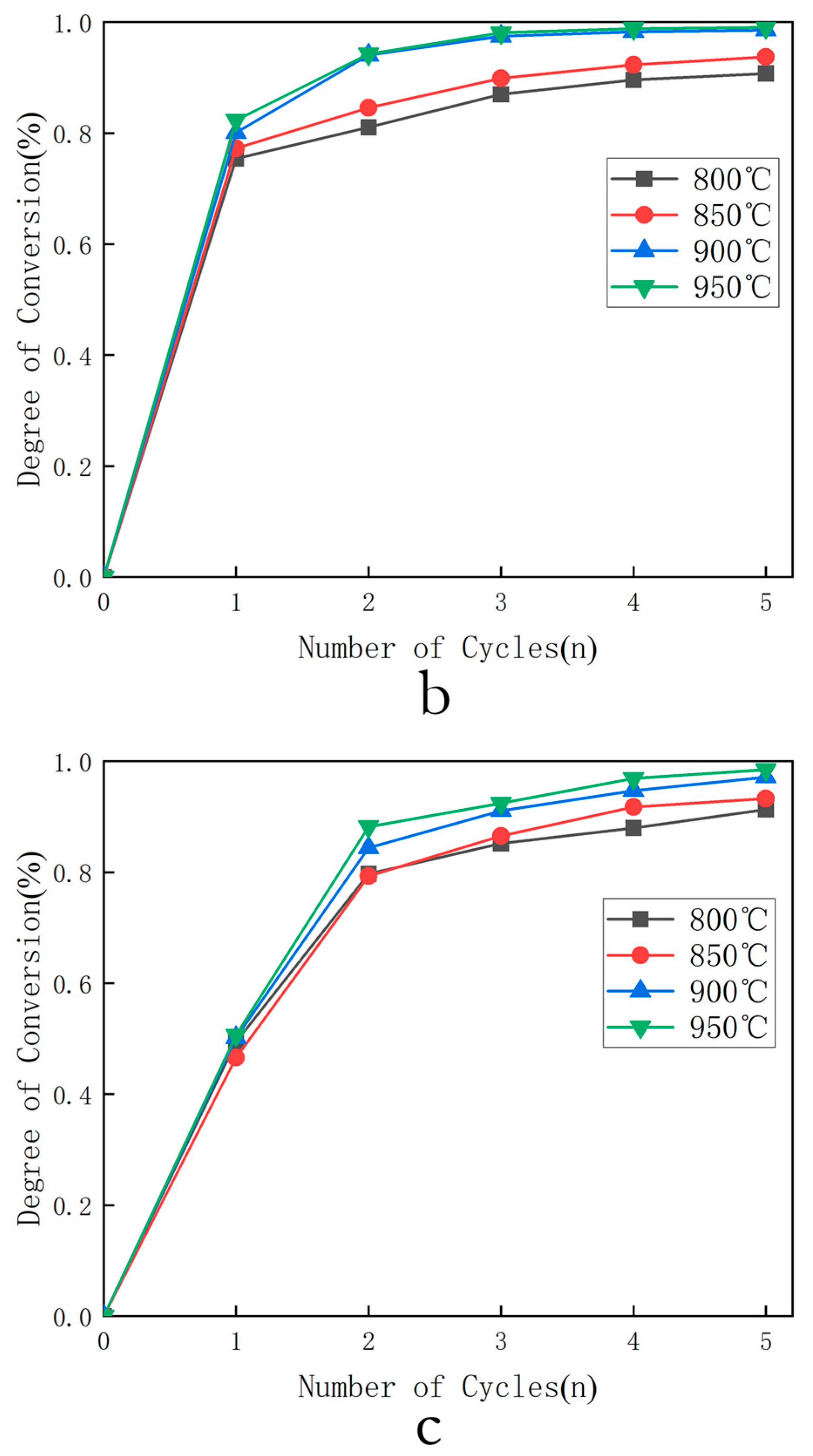
- Selection of optimal temperature: The combustion conversion rates of three oxygen carriers were calculated for the first five cycles at 800 °C, 850 °C, 900 °C, and 950 °C under 0.1 MPa and = 4 (oxygen carrier in large excess relative to fuel). The relationship between cycle number and conversion rate at different temperatures was then output.
- Selection of optimal ratio: The molar fraction of cyclic products of Fe2O3 oxygen carrier was calculated within the range of 0–15 at 0.1 MPa. The relationship between product distribution and ratio was then determined.
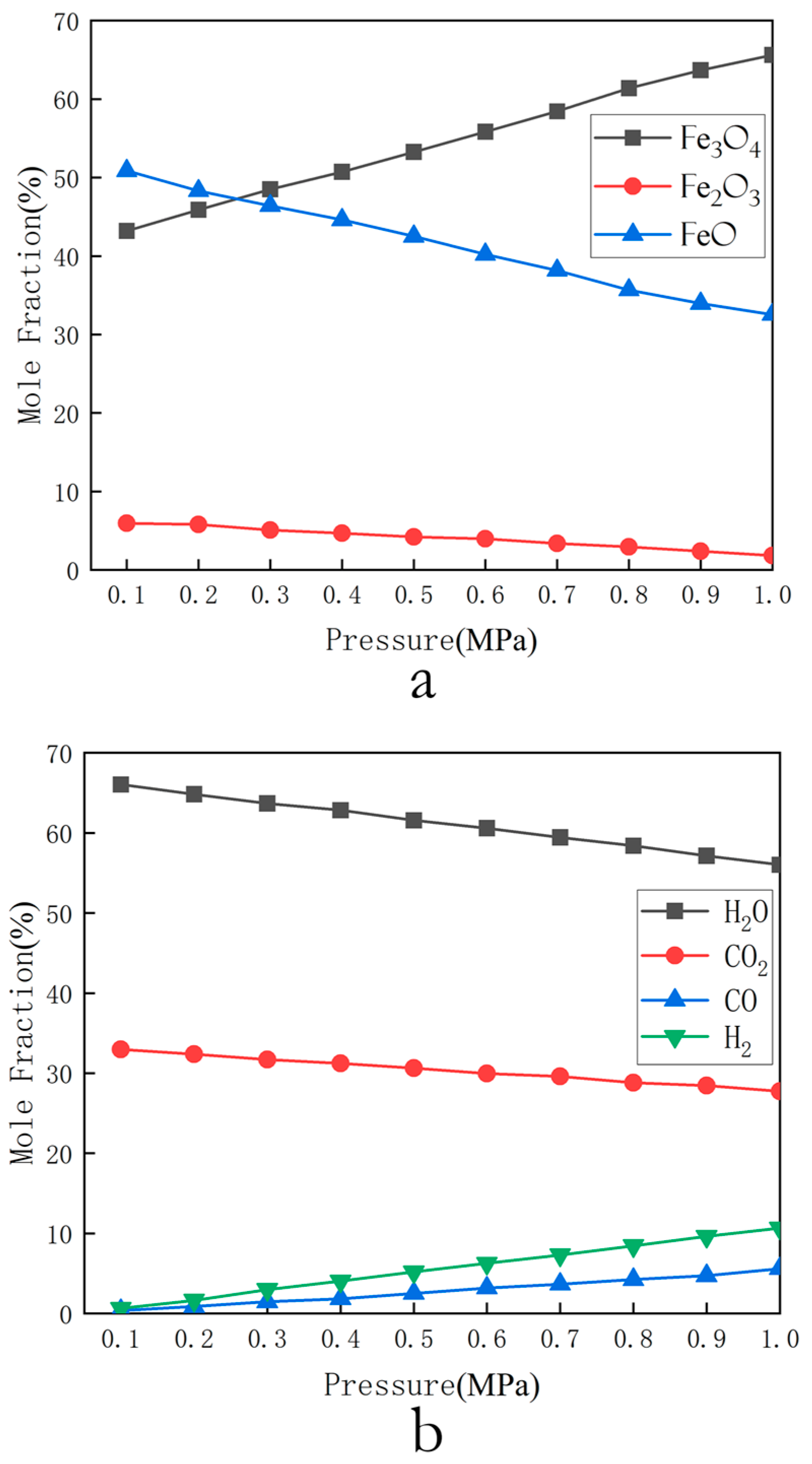
- Selection of optimal pressure: The molar fraction of cyclic products of Fe2O3 oxygen carrier was calculated within the pressure range of 0.1–1.1 MPa at the optimal temperature and ratio. The relationship between product distribution and pressure was output.
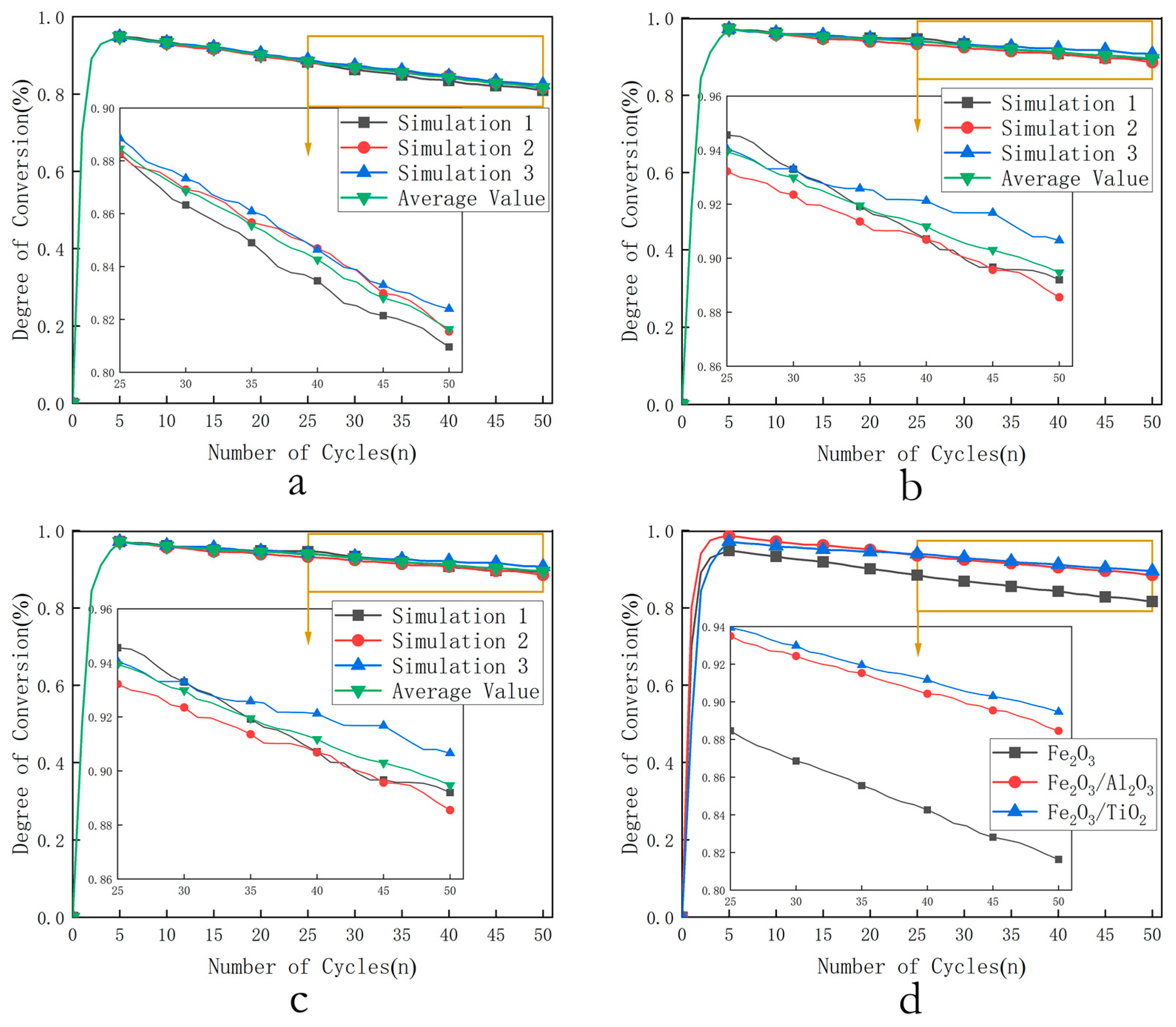
- Degradation simulation: The combustion conversion rates of three oxygen carriers over 50 cycles were calculated under optimal temperature, optimal O/C ratio, optimal pressure, and with degradation considered. The relationship between cycle number and conversion rate at different values was then analyzed.
- Termination conditions: The cycle was terminated when the oxygen carrier was insufficient (i.e., lack of reactant in the Fuel Reactor) or when < (i.e., the reduction reaction in the Fuel Reactor could not proceed spontaneously).
- Definition of key parameters:
2.4.5. Predicted Operating Condition Reactions
3. Results and Discussion
3.1. Selection of Optimal Reaction Conditions
3.1.1. Selection of Optimal Temperature
3.1.2. Selection of Optimal O/C Ratio
3.1.3. Selection of Optimal Pressure
3.2. Degradation Simulation
3.3. Predicted Total Number of Cycles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinmer, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barret, K. Synthesis Report of the IPCC Sixth Assessment Report (AR6); Longer Report; IPCC: Geneva, Switzerland, 2023; Available online: https://mural.maynoothuniversity.ie/id/eprint/17733 (accessed on 25 July 2025).
- Godin, J.; Liu, W.; Ren, S.; Xu, C.C. Advances in recovery and utilization of carbon dioxide: A brief review. J. Environ. Chem. Eng. 2021, 9, 105644. [Google Scholar] [CrossRef]
- Crawford, E. Arrhenius’ 1896 Model of the Greenhouse Effect in Context. Ambio 1997, 26, 6–11. Available online: http://www.jstor.org/stable/4314543 (accessed on 1 July 2025).
- Apfel, U.P.; Weigand, W. Efficient activation of the greenhouse gas CO2. Angew. Chem. Int. 2011, 50, 4262–4264. [Google Scholar] [CrossRef] [PubMed]
- Chilingar, G.V.; Sorokhtin, O.G.; Khilyuk, L.; Gorfunkel, M.V. Greenhouse gases and greenhouse effect. Environ. Geol. 2009, 58, 1207–1213. [Google Scholar] [CrossRef]
- Phillips, E. Crisis in the atmosphere: The greenhouse factor. Natl. Assoc. Biol. Teach. 1990, 54, 59–60. [Google Scholar] [CrossRef]
- Rao, S.; Riahi, K. The Role of Non-CO3 Greenhouse Gases in Climate Change Mitigation: Long-term Scenarios for the 21st Century. Energy J. 2006, 27, 177–200. [Google Scholar] [CrossRef]
- Orr, F.M., Jr. Carbon Capture, Utilization, and Storage: An Update. SPE J. 2018, 23, 2444–2455. [Google Scholar] [CrossRef]
- Endres, D.; Cozen, B.; O’Byrne, M.; Feldpausch-Parker, A.M.; Peterson, T.R. Putting the U in carbon capture and storage: Rhetorical boundary negotiation within the CCS/CCUS scientific community. J. Appl. Commun. Res. 2016, 44, 362–380. [Google Scholar] [CrossRef]
- Palmer, P.I.; O’Doherty, S.; Allen, G.; Bower, K.; Bösch, H.; Chipperfield, M.P.; Connors, S.; Dhomse, S.; Feng, L.; Finch, D.P.; et al. A measurement-based verification framework for UK greenhouse gas emissions: An overview of the Greenhouse gAs Uk and Global Emissions (GAUGE) project. Atmos. Chem. Phys. 2018, 18, 11753–11777. [Google Scholar] [CrossRef]
- Latif, W.M.S.M.; Sabdullah, N.M.; Aenun, S.N.; Bosamah, N.A. A review of global carbon capture and storage (CCS) and carbon capture, utilization, and storage (CCUS). In Proceedings of the E3S Web of Conferences, Dnipro, Ukraine, 15–17 April 2024; p. 01009. [Google Scholar]
- Mikunda, T.; Neele, F.; Akhurst, M.; Pearce, J.; Skagestad, R.; Morgenthaler, S.; Sava, C.; Maver, M. Targeted CCUS R&D activities in industrial clusters. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference, Melbourne, Australia, 21–25 October 2018; pp. 21–26. [Google Scholar]
- Wood, D.A. Carbon dioxide (CO2) handling and carbon capture utilization and sequestration (CCUS) research relevant to natural gas: A collection of published research (2009–2015). J. Nat. Gas Sci. Eng. 2015, 25, A1–A9. [Google Scholar] [CrossRef]
- Tapia, J.F.D.; Lee, J.-Y.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Tontiwachwuthikul, P.; Zeng, F.; Chan, C.W. Special issue on, Carbon Capture, Utilization and Storage (CCUS): Technological developments and future opportunities for petroleum industry. Petroleum 2017, 3, 1–2. [Google Scholar] [CrossRef]
- Kunze, C.; Spliethoff, H. Assessment of oxy-fuel, pre- and post-combustion-based carbon capture for future IGCC plants. Appl. Energy 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Kouri, S.; Tsupari, E.; Kärki, J.; Teir, S.; Sormunen, R.; Arponen, T.; Tuomaala, M. The Potential for CCUS in Selected Industrial Sectors—Summary of Concept Evaluations in Finland. Energy Procedia 2017, 114, 6418–6431. [Google Scholar] [CrossRef]
- Lacy, R.; Molina, M.; Vaca, M.; Serralde, C.; Hernandez, G.; Rios, G.; Guzman, E.; Hernandez, R.; Perez, R. Life-cycle GHG assessment of carbon capture, use and geological storage (CCUS) for linked primary energy and electricity production. Int. J. Greenh. Gas Control 2015, 42, 165–174. [Google Scholar] [CrossRef]
- Leonzio, G. Special Issue on “CCUS: Paving the Way to Net Zero Emissions Technologies”. Appl. Sci. 2025, 15, 3285. [Google Scholar] [CrossRef]
- Sethi, V.K.; Vyas, S.; Dutta, P.S. A pilot study of post combustion carbon capture & sequestration pilot plant aimed at feasibility analysis of installation of a carbon capture, utilization and sequestration (CCUS) plant on a large thermal unit in India. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference, Melbourne, Australia, 21–25 October 2018; pp. 21–26. [Google Scholar]
- Chandra, A.; Singh, A.A.; Prasad, S.; Andersson, M.R.; Gedefaw, D. Crosslinking Approaches for Polyethylene Imine (PEI) and Its Uses in Adsorption of Heavy Metals, Dyes, and Carbon Dioxide. Appl. Sci. 2025, 15, 4767. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Singh, A.; Singh, A.; Kalauni, K.; Wdowin, M. High-Performance Carbon Dioxide Adsorption with Zeolitic Imidazolate Framework-8-Based Cellulose Air Filters. Appl. Sci. 2024, 14, 11019. [Google Scholar] [CrossRef]
- Ho, Q.D.; Rauls, E. Ab initio study: Investigating the adsorption behaviors of polarized greenhouse gas molecules on pillar[5]arenes. Mater. Today Commun. 2023, 36, 106875. [Google Scholar] [CrossRef]
- Chu, X.; Fu, L.; Liu, Q.; Yu, S. Optimal allocation method of oxygen enriched combustion-carbon capture low-carbon integrated energy system considering uncertainty of carbon-source-load. Int. J. Electr. Power Energy Syst. 2024, 162, 110220. [Google Scholar] [CrossRef]
- Châtel-Pélage, F.; Varagani, R.K.; Pranda, P.; Perrin, N.; Farzan, H.; Vecci, J.; Yongqi, L.; Chen, S.; Rostam-Abadi, M.; Bose, C. Applications of oxygen for NOx control and CO2 capture in coal-fired power plants. Therm. Sci. 2006, 10, 119–142. [Google Scholar] [CrossRef]
- Weiliao, K.; Fang, L.I.U.; Li, Y.; Caifu, L.I.; Chen, S. Study on attrition characteristics and mechanism of chemical looping iron-based oxygen. Coal Sci. Technol. 2022, 50, 159–168. Available online: https://www.mtkxjs.com.cn/en/article/id/fd710326-ab2f-44ed-ad38-61932488875f (accessed on 2 July 2025).
- Zhao, H.; Tian, X.; Ma, J.; Chen, X.; Su, M.; Zheng, C.; Wang, Y. Chemical Looping Combustion of Coal in China: Comprehensive Progress, Remaining Challenges, and Potential Opportunities. Energy Fuels 2020, 34, 6696–6734. [Google Scholar] [CrossRef]
- Imtiaz, Q.; Hosseini, D.; Müller, C.R. Review of oxygen carriers for chemical looping with oxygen uncoupling (CLOU): Thermodynamics, material development, and synthesis. Energy Technol. 2013, 1, 633–647. [Google Scholar] [CrossRef]
- Fang, H.; Haibin, L.; Zengli, Z. Advancements in development of chemical-looping combustion: A review. Int. J. Chem. Eng. 2009, 2009, 710515. [Google Scholar] [CrossRef]
- Dueso, C.; Ortiz, M.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. Reduction and oxidation kinetics of nickel-based oxygen-carriers for chemical-looping combustion and chemical-looping reforming. Chem. Eng. J. 2012, 188, 142–154. [Google Scholar] [CrossRef]
- Shen, L.; Wu, J.; Gao, Z.; Xiao, J. Characterization of chemical looping combustion of coal in a 1kWth reactor with a nickel-based oxygen carrier. Combust. Flame 2010, 157, 934–942. [Google Scholar] [CrossRef]
- Wolf, J.; Anheden, M.; Yan, J. Comparison of nickel- and iron-based oxygen carriers in chemical looping combustion for CO2 capture in power generation. Fuel 2005, 84, 993–1006. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-based oxygen carriers in chemical looping conversions: A review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Dansie, J.K.; Sahir, A.H.; Hamilton, M.A.; Lighty, J.S. An investigation of steam production in chemical-looping combustion (CLC) and chemical-looping with oxygen uncoupling (CLOU) for solid fuels. Chem. Eng. Res. Des. 2015, 94, 12–17. [Google Scholar] [CrossRef]
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J.M. Selection of Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y. Syngas Generation from Methane Using a Chemical-Looping Concept: A Review of Oxygen Carriers. J. Chem. 2013, 2013, 294817. [Google Scholar] [CrossRef]
- Tijani, M.M.; Aqsha, A.; Mahinpey, N. Synthesis and study of metal-based oxygen carriers (Cu, Co, Fe, Ni) and their interaction with supported metal oxides (Al2O3, CeO2, TiO2, ZrO2) in a chemical looping combustion system. Energy 2017, 138, 873–882. [Google Scholar] [CrossRef]
- Karimi, E.; Forutan, H.R.; Saidi, M.; Rahimpour, M.R.; Shariati, A. Experimental Study of Chemical-Looping Reforming in a Fixed-Bed Reactor: Performance Investigation of Different Oxygen Carriers on Al2O3 and TiO2 Support. Energy Fuels 2014, 28, 2811–2820. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes; Zenodo: Geneva, Switzerland, 2021. [Google Scholar]
- Saccone, G.; Breda, P.; Natale, P.; Battista, F. Reduced kinetic mechanism for methane/oxygen rocket engine applications: A reliable and numerically efficient methodology. Combust. Theory Model. 2023, 27, 391–417. [Google Scholar] [CrossRef]
- Gunawardena, D.A.; Fernando, S.D. Thermodynamic equilibrium analysis of methanol conversion to hydrocarbons using Cantera methodology. J. Thermodyn. 2012, 2012, 125460. [Google Scholar] [CrossRef]
- Gao, Z.-p.; Shen, L.-h.; Xiao, J.; Zheng, M.; Wu, J.-h. Analysis of reactivity of Fe-based oxygen carrier with coal during chemical-looping combustion. J. Fuel Chem. Technol. 2009, 37, 513–520. [Google Scholar] [CrossRef]
- Ortiz, M.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Gayán, P.; Adánez, J. Hydrogen production by auto-thermal chemical-looping reforming in a pressurized fluidized bed reactor using Ni-based oxygen carriers. Int. J. Hydrogen Energy 2010, 35, 151–160. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Cho, P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 1953–1962. [Google Scholar] [CrossRef]
- Jerndal, E.; Mattisson, T.; Lyngfelt, A. Thermal Analysis of Chemical-Looping Combustion. Chem. Eng. Res. Des. 2006, 84, 795–806. [Google Scholar] [CrossRef]
- Sreejith, C.C.; Arun, P.; Muraleedharan, C. Thermochemical Analysis of Biomass Gasification by Gibbs Free Energy Minimization Model—Part: I (Optimization of Pressure and Temperature). Int. J. Green Energy 2013, 10, 231–256. [Google Scholar] [CrossRef]
- Burcat, A.; Ruscic, B. Third Millenium Ideal Gas and Condensed Phase Thermochemical Database for Combustion (with Update from Active Thermochemical Tables); Argonne National Lab.(ANL): Argonne, IL, USA, 2005. [Google Scholar]
- Wang, R.; Balciunaite, U.; Chen, J.; Yuan, C.; Owens, A.; Tennyson, J. NASA Polynomial representation of molecular specific heats. J. Quant. Spectrosc. Radiat. Transf. 2023, 306, 108617. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Ma, Z.; Xiao, R.; Chen, L. Kinetics of sintering induced surface area decay of iron oxide in the reduction process of chemical looping combustion. Fuel Process. Technol. 2017, 168, 20–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Reduction Kinetics of Fe2O3/Al2O3 by Ultralow Concentration Methane under Conditions Pertinent to Chemical Looping Combustion. Energy Fuels 2015, 29, 337–345. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Li, Z.; Larring, Y.; Cai, N. Heterogeneous reaction kinetics of a perovskite oxygen carrier for chemical looping combustion coupled with oxygen uncoupling. Chem. Eng. J. 2021, 417, 128054. [Google Scholar] [CrossRef]
- Lyngfelt, A. Chemical Looping Combustion: Status and Development Challenges. Energy Fuels 2020, 34, 9077–9093. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of Magnetite (Fe3O4) Oxidation to Hematite (Fe2O3) in Air for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2014, 53, 13320–13328. [Google Scholar] [CrossRef]
- Jerndal, E.; Leion, H.; Axelsson, L.; Ekvall, T.; Hedberg, M.; Johansson, K.; Källen, M.; Svensson, R.; Mattisson, T.; Lyngfelt, A. Using low-cost iron-based materials as oxygen carriers for chemical looping combustion. Oil Gas Sci. Technol. 2011, 66, 235–248. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, S.; Lu, Y. Activation Mechanism of Fe2O3-Al2O3 Oxygen Carrier in Chemical Looping Combustion. Energy Fuels 2020, 34, 16350–16355. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, D.Y.; Symonds, R.T.; Hughes, R.W. Chemical looping reforming of CH4 in the presence of CO2 using ilmenite ore and NiO-modified ilmenite ore oxygen carriers. Chem. Eng. J. 2020, 401, 123481. [Google Scholar] [CrossRef]
- Condori, O.; García-Labiano, F.; de Diego, L.F.; Izquierdo, M.T.; Abad, A.; Adánez, J. Biomass chemical looping gasification for syngas production using ilmenite as oxygen carrier in a 1.5 kWth unit. Chem. Eng. J. 2021, 405, 126679. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Liu, Y.; Cheng, Y. Preparation and Characterization of Fe2O3/Al2O3 Using the Solution Combustion Approach for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2012, 51, 12773–12781. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Deplazes, S.; Veksha, A.; Wirz-Töndury, C.; Giannis, A.; Lim, T.T.; Lisak, G. Ba–Al-decorated iron ore as bifunctional oxygen carrier and HCl sorbent for chemical looping combustion of syngas. Combust. Flame 2021, 223, 230–242. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; Cuadrat, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 689–702. [Google Scholar] [CrossRef]
- Liu, L.; Zachariah, M.R. Enhanced performance of alkali metal doped Fe2O3 and Fe2O3/Al2O3 composites as oxygen carrier material in chemical looping combustion. Energy Fuels 2013, 27, 4977–4983. [Google Scholar] [CrossRef]
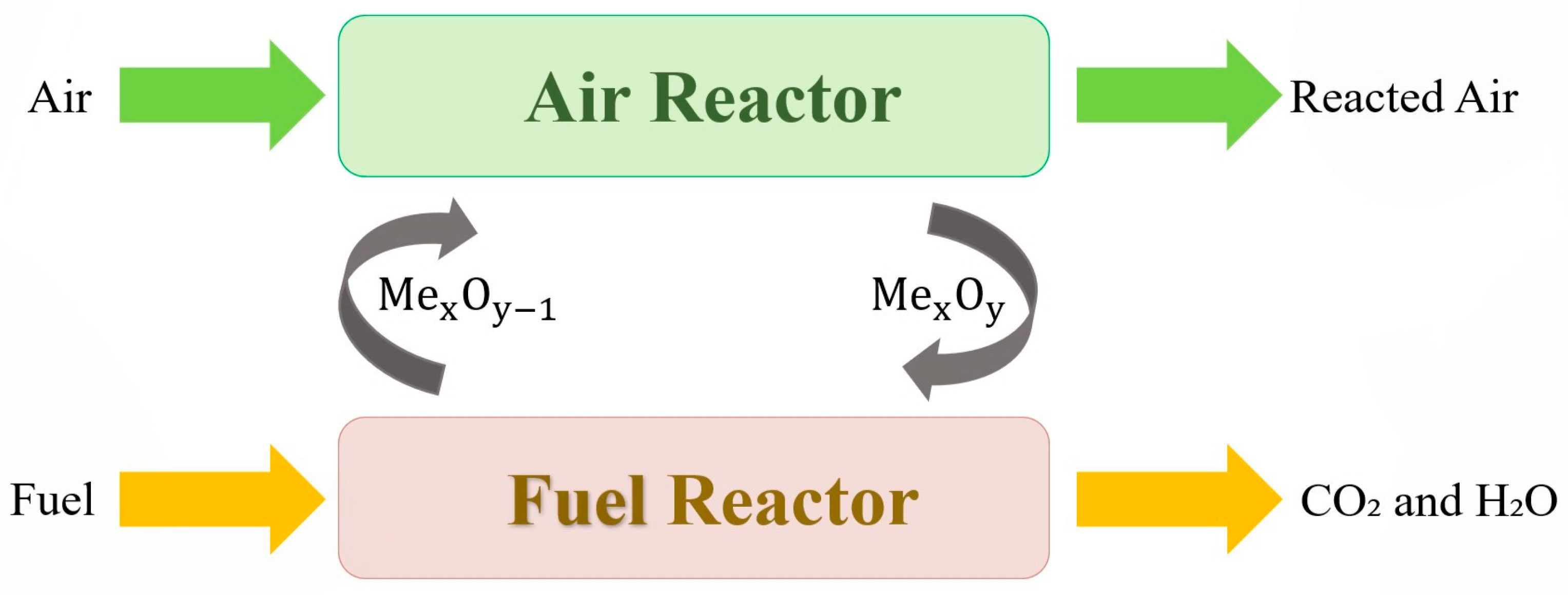


| FR Products | Reaction | ΔH (kJ/mol) | |
|---|---|---|---|
| C | C + 6Fe2O3 → CO2 + 4Fe3O4 | 95.98 | (R4) |
| C + 2Fe2O3 → CO2 + 4FeO | 164.88 | (R5) | |
| H2 | H2 + 3Fe2O3 → H2O + 2Fe3O4 | −2.01 | (R6) |
| H2 + Fe2O3 → H2O + 2FeO | 32.44 | (R7) | |
| CO | CO + 3Fe2O3 → CO2 + 2Fe3O4 | −37.67 | (R8) |
| CO + Fe2O3 → CO2 + 2FeO | −3.22 | (R9) | |
| CO + Fe2O3 + 2Al2O3 → 2FeO·Al2O3 + CO2 | 122.52 | (R10) | |
| CO + Fe2O3·TiO2 + TiO2 → 2FeO·TiO2 + CO2 | −1.57 | (R11) | |
| CH4 | CH4 + 12Fe2O3 → 2H2O + CO2 + 8Fe3O4 | 221.76 | (R12) |
| CH4 + 4Fe3O4 → 12FeO + 2H2O + CO2 | 365.93 | (R13) | |
| CH4 + 4Fe2O3 → 2H2O + CO2 + 8FeO | 317.87 | (R14) | |
| CH4 + 4Fe2O3 + 8Al2O3 → 8FeO·Al2O3 + 2H2O + CO2 | 233.93 | (R15) | |
| CH4 + 12Fe2O3·TiO2 → 8Fe3O4 + 12TiO2 + CO2 + 4H2O | −347.13 | (R16) | |
| CH4 + 4Fe2O3·TiO2 + 4TiO2 → 8FeO·TiO2 + CO2 + 2H2O | 323.35 | (R17) | |
| CH4 + 4Fe3O4 + 12TiO2 → 12FeO·TiO2 + 2CO2 + 2H2O | 23.23 | (R18) |
| Reaction | ΔH (kJ/mol) | |
|---|---|---|
| C + H2O → CO + H2 | 131.46 | (R19) |
| CO + H2 → C + H2O | −131.31 | (R20) |
| CO + H2O → H2 + CO2 | −41.14 | (R21) |
| C + CO2 → 2CO | 172.67 | (R22) |
| 2CO → C + CO2 | −172.46 | (R23) |
| CH4 + H2O → CO + 3H2 | 223.70 | (R24) |
| CH4 + CO2 → 2H2 + 2CO | 247.29 | (R25) |
| 2CO + 2H2 → CH4 + CO2 | −247.29 | (R26) |
| CO2 + 4H2 → CH4 + 2H2O | −165.00 | (R27) |
| C + 2H2 → CH4 | −74.87 | (R28) |
| CH4 → C + 2H2 | 75.00 | (R29) |
| Reaction | ΔH (kJ/mol) | |
|---|---|---|
| 4Fe3O4 + O2 → 6Fe2O3 | −483.07 | (R30) |
| 4FeO + O2 → 2Fe2O3 | −554.68 | (R31) |
| 4FeO·TiO2 + O2 → 2Fe2O3 + 4TiO2 | −562.82 | (R32) |
| 4FeO·Al2O3 + O2 → 2Fe2O3 + 4Al2O3 | −562.82 | (R33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Yin, X.; Liu, H.; Huang, M.; Wang, H. Enhanced Cyclic Stability of Composite-Modified Iron-Based Oxygen Carriers in Methane Chemical Looping Combustion: Mechanistic Insights from Chemical Calculations. Appl. Sci. 2025, 15, 9733. https://doi.org/10.3390/app15179733
Liang D, Yin X, Liu H, Huang M, Wang H. Enhanced Cyclic Stability of Composite-Modified Iron-Based Oxygen Carriers in Methane Chemical Looping Combustion: Mechanistic Insights from Chemical Calculations. Applied Sciences. 2025; 15(17):9733. https://doi.org/10.3390/app15179733
Chicago/Turabian StyleLiang, Dongxu, Xuefeng Yin, Hao Liu, Minjie Huang, and Hao Wang. 2025. "Enhanced Cyclic Stability of Composite-Modified Iron-Based Oxygen Carriers in Methane Chemical Looping Combustion: Mechanistic Insights from Chemical Calculations" Applied Sciences 15, no. 17: 9733. https://doi.org/10.3390/app15179733
APA StyleLiang, D., Yin, X., Liu, H., Huang, M., & Wang, H. (2025). Enhanced Cyclic Stability of Composite-Modified Iron-Based Oxygen Carriers in Methane Chemical Looping Combustion: Mechanistic Insights from Chemical Calculations. Applied Sciences, 15(17), 9733. https://doi.org/10.3390/app15179733





