Abstract
This study aimed to compare the changes in brain functional connectivity between states of stress induction and recovery in mentally stable, healthy individuals to investigate the effects of stress on brain networks. We selected a stable group comprising 20 healthy adults with Perceived Stress Scale scores of 0–13 points and a mean age of 24.4 ± 4.3 years. We used the Montreal Imaging Stress Task to induce stress and captured images of the brain using a 3T magnetic resonance imaging scanner. We analyzed the region of interest (ROI)-to-ROI connectivity and compared the differences in functional connectivity between the stress and recovery phases. In the stress state, we observed increased connectivity between the dorsal attention and sensorimotor networks and between the visual and default mode networks. In the recovery state, the default mode network became reactivated, and connectivity supporting self-referential thinking and stability was observed. The connectivities observed only in the recovery phase were Language.pSTG (R)—DefaultMode.LP (R) and DefaultMode.LP (R)—Visual.Lateral (R). Our findings provide important basic data for the development of stress management and recovery strategies. By assessing healthy individuals, our findings provide new perspectives on stress resilience in the brain.
1. Introduction
Stress is a complex response of the body to physiological and psychological stimuli and is known to have profound effects on mental health and brain function [1,2]. In particular, stress affects various networks in the brain, and recent studies have demonstrated that the activation of networks, such as the default mode network (DMN), salience network (SN), sensorimotor network (SMN), and frontoparietal network, plays important roles in the regulation of cognitive processing, emotions, and physical responses [3,4]. These networks are associated with self-awareness, emotional response, sensorimotor integration, and higher-dimensional cognitive processing. They provide important clues for understanding functional connectivity (FC) in the brain under stress.
Brain FC analysis is an important method for investigating brain activity in stress and recovery states. It aids in understanding the mechanisms of adaptation and recovery in the brain following stress induction. This approach is useful for revealing complex stress response patterns based on interactions between brain networks [5]. For example, Hermans et al. [3] demonstrated that connectivity between the salience and default mode networks plays an important role in the process of stress recovery and that the SMN can also contribute to the recovery process [3]. Menon [4] reported that the SN plays a key role in regulating attention during stressful situations, supporting the selective processing of important stimuli via the immediate stress response [4].
However, the previous studies had several limitations. First, many previous studies were conducted on a high-stress group (e.g., patients with post-traumatic stress disorder and chronic stress) and did not provide clear insights into the neurological mechanisms of stress induction and recovery in healthy individuals. Studies on healthy individuals are essential to understand whether changes in stress-related brain connectivity can be observed not only in a pathological state but also in the everyday stress response [6]. Second, studies comparing the differences in connectivity between stress and recovery states have mostly focused on the stress response itself, and there have been limited analyses of the role of FC in the mechanisms of recovery. As a result, we lack important data to understand the process by which the brain returns to a stable state after stress [7,8].
Therefore, in this study, we compared changes in brain connectivity between stress-induced and recovery states in healthy individuals, with the aim of overcoming the limitations of previous studies and elucidating adaptive changes and recovery processes in the brain. To overcome the limitations of previous studies, we designed a stress-induction experiment for healthy individuals who did not usually experience high levels of stress. We comprehensively analyzed the interactions between various brain networks and investigated how the FC between the sensorimotor network and other major networks differed between the stress and recovery states.
2. Research Methods
Twenty adults (mean age, 24.4 ± 4.3 years) with low perceived stress (Perceived Stress Scale, PSS score: 0–13) were recruited. The Perceived Stress Scale (PSS) is a stress assessment questionnaire that has also been used in our research team’s previous studies [9,10]. As this study aimed to analyze changes in brain connectivity during stress and recovery in stable individuals who usually experience little stress, we only included participants with a total PSS score between 0 and 13 points. A score of 0–13 corresponds to the normal range and indicates psychological stability. Exclusion criteria included neurological or psychiatric disorders, psychoactive medication use, and irregular sleep patterns. Participants provided informed consent, and the Institutional Review Board approved the study protocol (IRB No.: 7001355-202103-HR-426).
The stress-inducing procedure was conducted using the Montreal Imaging Stress Task (MIST) paradigm, which has also been employed in our previous studies investigating acute stress responses [9,10]. MIST is an appropriate task for inducing stress and was also used in this experiment with healthy individuals. The MIST (Montreal Imaging Stress Task) was developed by Dedovic et al. [11] to induce psychosocial stress. During the task, participants receive continuous negative feedback from the experimenter regarding their performance, in a context where they have little control. This setup causes participants to perceive their performance as inferior compared to that of a virtual character, thereby eliciting social evaluative stress.
Participants underwent a five-phase experiment: training phase (90 s), rest phase (90 s), control phase (90 s), stress phase (300 s), recovery phase (180 s). During the training phase, participants familiarized themselves with the button response system and practiced solving sample arithmetic problems. The rest phase lasted for 90 s, during which participants were instructed to relax without any tasks. In the control condition, also lasting 90 s, they performed arithmetic tasks without any stress-related elements. The stress phase, which lasted for 300 s, incorporated stress-inducing features: each item had a strict time limit of 3 s, and participants received performance feedback (correct, incorrect, or missing) for 2 s along with their cumulative average score before the next item appeared. A total of 60 items were presented throughout this phase. The MIST consisted of simple arithmetic problems using two-digit numbers (e.g., 34 + 27, 89 − 16), which did not require any verbal sentence comprehension. Participants were instructed to respond to the centrally presented numerical problems within a limited time window, and the task primarily involved mathematical performance under time pressure rather than language processing. During the 180s recovery phase, brain activity was recorded while participants were in a post-task resting state, allowing observation of neural responses associated with stress relief and return to baseline. A central fixation cross (‘+’) was presented on the screen without any additional arithmetic tasks, encouraging participants to maintain their gaze. This approach was intended to minimize visual stimulation and ensure visual fixation stability. The 180 s recovery phase was determined based on a pilot study conducted with five healthy adult participants prior to the main experiment. In this pilot test, physiological stress levels were measured before, during, and after performing the MIST using a commercial stress assessment device (uBioMacpa, Biosense Creative Co., Seoul, Republic of Korea). The results showed that it took approximately 180 s on average for stress levels to return to baseline after task completion. Based on these findings, we set the recovery phase to 180 s in the main study.
Functional MRI (fMRI) data were acquired continuously from all participants throughout the entire experimental procedure.
Data were collected using a 3T MRI scanner (Magnetom TrioTim; Siemens Medical Systems, Erlangen, Germany) with a T1-weighted anatomical sequence and an fMRI protocol optimized for functional connectivity analysis [9,10]. Single-shot echo-planar imaging (EPI) functional MRI scans were acquired in 29 continuous axial slices aligned parallel to the anterior–posterior commissure (AC–PC) line. The acquisition parameters were as follows: repetition time (TR) = 2000 ms, echo time (TE) = 20 ms, field of view (FOV) = 240 mm, flip angle = 77°, matrix size = 128 × 128, slice thickness = 3 mm, voxel size = 3.0 × 3.0 × 3.0 mm3, and a total of 815 volumes were obtained. High-resolution anatomical images were acquired using a T1-weighted three-dimensional magnetization-prepared rapid gradient-echo (MPRAGE) sequence with the following parameters: TR = 1900 ms, TE = 2.52 ms, FOV = 256 mm, flip angle = 9°, matrix = 256 × 256, slice thickness = 1 mm, and voxel size = 1.0 × 1.0 × 1.0 mm3.
Preprocessing (realignment, normalization, and smoothing) was conducted using SPM12 [9,10]. Functional MRI (fMRI) data preprocessing was performed using Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/, accessed on 2 September 2025). Functional images were co-registered to each participant’s anatomical (T1-weighted) image using affine transformation algorithms implemented in SPM12. Motion correction was conducted through realignment of the time-series images using a six-parameter rigid-body transformation and a least-squares optimization approach. Realignment was performed using the mean functional image as the reference, in order to reduce the influence of noise or instability often found in the initial volumes of fMRI scans. After realignment, the functional images were co-registered to each participant’s anatomical (structural) images and spatially normalized to the standard template provided in SPM12, which conforms to the Montreal Neurological Institute (MNI) space. Motion correction was refined using sinc interpolation to ensure accurate alignment across volumes. To reduce physiological noise such as respiration and cardiac fluctuations, a high-pass filter with a cutoff of 240 s was applied to the time-series data. T1- and T2-weighted anatomical scans were processed through a multichannel segmentation procedure to estimate the probability distribution across six tissue types: gray matter, white matter, cerebrospinal fluid (CSF), bone, soft tissue, and background noise. Preprocessed functional images were spatially smoothed using a 6 mm full-width at half maximum (FWHM) Gaussian kernel to enhance the signal-to-noise ratio. Group-level analyses were conducted using the general linear model framework, incorporating Gaussian random field theory as implemented in SPM12.
To ensure anatomical alignment quality across participants, the following procedures were implemented. First, participants with motion exceeding 3 mm of translation or 3° of rotation during the realignment step were excluded from analysis. After normalization, the spatial alignment between each participant’s functional image and the MNI template was visually inspected using the SPM12 and CONN toolbox visualization tools. If misalignment was observed, manual re-registration was conducted. Only participants with verified anatomical consistency were included in the final analysis. These quality control steps were taken to ensure that group-level ROI-to-ROI connectivity analyses were based on consistent anatomical references across participants.
To analyze the functional connectivity of fMRI data, we utilized the CONN toolbox (https://web.conn-toolbox.org/, accessed on 2 September 2025) integrated with SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). ROI-to-ROI connectivity analyses were performed to identify statistically significant changes in brain network interactions across experimental conditions. A total of 105 regions of interest (ROIs) were defined using the Harvard–Oxford cortical and subcortical structural atlases provided within the CONN toolbox, excluding the brainstem and cerebellum. Functional connectivity between each pair of ROIs was computed using bivariate Pearson correlation, which reflects the linear association between BOLD signal time courses and is the default method in the CONN toolbox. The connectivity between each pair of ROIs was modeled using a general linear model, and group-level inferences were computed using spatial pairwise clustering (SPC) statistics with the default threshold settings implemented in CONN. This approach allowed us to control for multiple comparisons while detecting clusters of ROIs showing consistent effects across participants. We focused on seven canonical brain networks as seed regions:
- Default Mode Network (DMN): medial prefrontal cortex, lateral parietal lobe (LP), posterior cingulate cortex (PCC);
- Sensorimotor Network (SMN): bilateral lateral sensorimotor cortices;
- Visual Network (VN): medial, occipital, and lateral visual cortices;
- Salience Network (SN): anterior cingulate cortex, anterior insular cortex (A. Insula), rostral prefrontal cortex, supramarginal gyrus (SMG);
- Dorsal Attention Network (DAN): frontal eye fields (FEF), intraparietal sulcus (IPS);
- Frontoparietal Network (FPN): lateral prefrontal cortex, PCC;
- Language Network: inferior frontal gyrus, posterior superior temporal gyrus (pSTG).
In the functional connectivity analysis, we employed a contrast-based approach (Stress phase—Control phase, and Recovery phase—Stress phase) to statistically remove low-level visual responses arising from shared visual stimuli across conditions. This strategy was implemented to isolate condition-specific cognitive effects and enhance interpretability, even in the absence of direct gaze fixation measurements.
In addition, this study calculated and reported the effect sizes of functional connectivity between each pair of ROIs. Effect sizes refer to the Fisher Z-transformed Pearson correlation coefficients computed between each ROI pair, as implemented in the CONN toolbox. These standardized values represent the strength and direction of functional connectivity and were used for group-level inference.
A statistical parametric map was generated with an initial uncorrected threshold of p < 0.01 to identify significant activation patterns. Non-overlapping clusters were formed based on suprathreshold voxels, and cluster-level inference was performed. Cluster-level correction using the false discovery rate (FDR) method was applied to account for multiple comparisons, with significance defined as p < 0.05. For reference, both uncorrected p-values (p-unc), FDR-corrected p-values (p-FDR), and family-wise error corrected p-values (p-FWE) are reported in the result tables. Additionally, effect sizes (Z-scores) were calculated to represent the strength of connectivity and aid interpretation.
3. Results
The results for the FC in the stress and recovery states are presented in Table 1 and Table 2 and Figure 1. The effect sizes of each network are illustrated in Figure 2.

Table 1.
ROI-to-ROI functional connectivity results for the stress phase, including T-values, uncorrected and FDR-corrected p-values, and effect sizes. FDR correction was applied at the cluster level (p < 0.05).

Table 2.
ROI-to-ROI functional connectivity results for the recovery phase, including T-values, uncorrected and FDR-corrected p-values, and effect sizes. FDR correction was applied at the cluster level (p < 0.05).
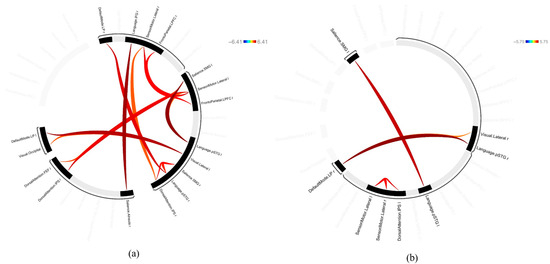
Figure 1.
ROI-to-ROI connectivity map. (a) Stress phase. (b) Recovery phase.
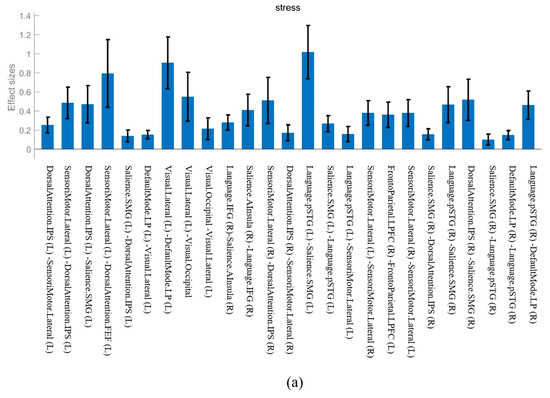
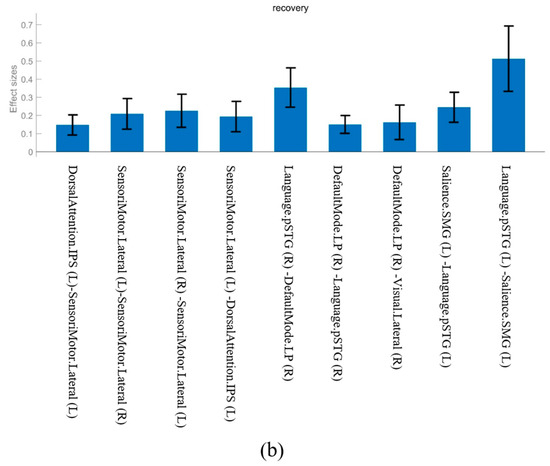
Figure 2.
The effect sizes of common functional connectivity. (a) Stress phase. (b) Recovery phase.
The FC observed in both the stress and recovery conditions was as follows: DorsalAttention.IPS (Left, L)—SensoriMotor.Lateral (L), SensoriMotor.Lateral (L)—DorsalAttention.IPS (L), Language.pSTG (L)—Salience.SMG (L), Salience.SMG (L)—Language.pSTG (L), SensoriMotor.Lateral (L)—SensoriMotor.Lateral (Right, R), SensoriMotor.Lateral (R)—SensoriMotor.Lateral (L), and DefaultMode.LP (R)—Language.pSTG (R) (Figure 1). When we compared the effect sizes of these FCs, most showed a larger effect size after stress induction than during the recovery phase (Figure 2). In particular, the connectivity in Language.pSTG (L)—Salience.SMG (L) showed more than double the effect size in the stress phase compared with that in the recovery phase (Figure 2).
The networks that were only observed in the stress phase were the SensoriMotor.Lateral (L)—DorsalAttention.FEF (L), Visual.Lateral (L)—DefaultMode.LP (L), DorsalAttention.IPS (L)—Salience.SMG (L), Visual.Lateral (L)—Visual.Occipital, and SensoriMotor.Lateral (R)—DorsalAttention.IPS (R) (Figure 1).
The networks observed only in the recovery phase were the Language.pSTG (R)—DefaultMode.LP (R) and DefaultMode.LP (R)—Visual.Lateral (R) (Figure 1).
4. Discussion
In this study, we compared changes in brain FC between the states of stress induction and recovery in psychologically stable, healthy individuals to investigate the effects of stress on brain networks. Although many previous studies have analyzed changes in brain connectivity in high-risk and high-stress groups, our study differs because we analyzed brain FC in stable adults who did not usually experience high stress levels. Thus, we could clearly identify differences in brain connectivity between the states of stress induction and recovery in healthy individuals [6,7].
Our findings demonstrated that the FC patterns that are active in both the stress and recovery phases tended to be more strongly activated under stress conditions. This suggests that the attention and sensorimotor networks were even more active during stress. Language.pSTG (L)—Salience.SMG (L) connectivity showed double the effect size in the stress phase compared to the recovery phase, indicating that stress greatly strengthened the interactions between the language and attention networks, even in healthy individuals. These results suggest that in stressful situations, even healthy individuals show an adaptive response to meet certain cognitive demands, which results in stronger connectivity in the brain [3].
Below, we discuss the five networks that exhibited characteristic patterns in the stress phase.
- SensoriMotor.Lateral (L)—DorsalAttention.FEF (L): Connectivity between the sensorimotor and attention networks was boosted during stress, reflecting the brain’s response to stress by stimulating attention and motor control. Previous studies have reported that stress enhances attentional control functions in the frontal cortex, supporting the notion that the brain adapts by mobilizing cognitive resources to effectively manage environmental demands [12,13]. This adaptive response may contribute to optimizing physical and behavioral reactions during stressful situations. In our study, stress increased the interactions between attention and motor control areas, which reflects the mechanisms that increase physical reactivity during stress via interactions between attention- and motor-related networks.
- Visual.Lateral (L)—DefaultMode.LP (L): The strengthening of connectivity between the visual and default mode networks in the event of stress indicates the possibility that self-referential thinking and external information processing are combined in the brain to increase sensitivity to visual stimuli. Previous studies have proposed that stress could stimulate activity in the DMN in the process of strengthening the response to visual stimulus [7,14,15]. This is consistent with our findings of increased connectivity between the visual and default mode networks and suggests that under stressful circumstances, the cognitive sensitivity required for the stress response is increased through the combination of external stimuli with self-referential processing.
- DorsalAttention.IPS (L)—Salience.SMG (L): Connectivity between the attention and salience networks during stress reflects mechanisms for the identification of and attention towards important stimuli in stressful circumstances [16]. Previous studies have reported that the salience network reacts more sensitively to important stimuli during stress, thereby enabling rapid information processing and response times [3,17]. We also found that stress induction was associated with connectivity between the attention and salience networks, which can be interpreted as increased sensitivity to external stimuli and promotion of an adaptive response to stress.
- Visual.Lateral (L)—Visual.Occipital: Stronger connectivity between the two subregions of the visual network indicates that the brain is attempting to process visual information even more intensively during stress induction. Shulman et al. [8] suggested that the importance of processing visual stimuli could increase in stressful situations, leading to increased FC within the visual network [8]. In our study, the increased connectivity within the visual cortex during stress suggests that attention to and processing of visual stimuli became even more crucial under stress. Moreover, it appears that visual information is rapidly processed in preparation for responding to stressful stimuli.
- SensoriMotor.Lateral (R)—DorsalAttention.IPS (R): The connectivity between the sensorimotor and attention networks increased in the right hemisphere during stressful situations, which is consistent with the stress response that enhances physical reactivity. Previous research has suggested that, in stressful situations, the brain strengthens the interactions between the sensorimotor and attentional control networks to facilitate rapid physical responses to external stimuli [18,19]. Strengthened connectivity between the sensorimotor and attention networks suggests adaptive changes that increase physical reactivity and enable a rapid response to external stimuli during stress.
Based on the results for connectivity that was only observed in the stress condition, SensoriMotor.Lateral (L)—DorsalAttention.FEF (L) and Visual.Lateral (L)—DefaultMode.LP (L), patterns of increased interaction between the sensorimotor and attention networks of the brain were observed in healthy individuals during stress induction. This connectivity pattern reflects the mechanisms involved in optimizing the physical and cognitive responses by strengthening attention and motor control in stressful situations. In particular, connectivity between the visual and default mode networks was observed during stress, suggesting an increased sensitivity to external stimuli and a more effective response to stressful situations through the activation of self-referential thinking. This reflects the process of functional adaptation in the brain, as it attempts to induce an immediate response to stress through the maximal utilization of sensory and cognitive resources [6,7]. This can help establish appropriate response strategies through rapid recognition of the environment and internal evaluation.
Connectivity in Language.pSTG (R)—DefaultMode.LP (R) and DefaultMode.LP (R)—Visual.Lateral (R) was observed during the recovery phase.
- Language.pSTG (R)—DefaultMode.LP (R): The connectivity between the language and default mode networks, which was only observed during the recovery phase, reflects the process of recovering from a stressful situation to a state of inner and self-referential thinking. The DMN is usually responsible for functions such as self-awareness, memory recall, and inner thought, which are reinforced during the recovery phase after stress [20]. Andrews-Hanna et al. [21] reported that the DMN is closely related to the processing of social and emotional information and supports stable psychological states. In our study, connectivity between the Language.pSTG and DefaultMode.LP was observed in the recovery phase, suggesting that the brain was trying to restore mental stability after stress through stable linguistic processing and inner thought.
- DefaultMode.LP (R)—Visual.Lateral (R): Connectivity between the default mode and visual networks was only observed during recovery, which reflects the processes involved in a more stable and efficient response to external visual stimuli after stress induction. The DMN plays an important role in self-referential thinking and processing of visual information during recovery. This means that visual information is combined with the DMN to suppress responses to external stimuli and convert self-centered thinking [22,23]. We also observed that connectivity between the visual and default mode networks could be interpreted as the brain trying to reduce sensitivity to stressful stimuli during the recovery phase and controlling the response to external stimuli to restore internal balance.
The FC patterns observed in the recovery phase showed the important process of returning to a stable state of inner thought and self-centered cognition after escaping from a stressful situation. This reflects the mechanisms that support internal stability through reactivation of the DMN after stress and provides important neurological clues on how long-term stress is managed. The differences between connectivity during stress and recovery can provide new insights into brain resilience and help understand the functional reconstitution of the brain during the post-stress recovery phase in healthy individuals.
This study only included participants in their 20s, making it limited in reflecting differences across age groups. Future research should investigate potential age-related differences in the neurological mechanisms of stress responses and post-stress recovery states. Additionally, physiological markers such as heart rate and cortisol were not included; therefore, the interaction between stress responses and brain functional connectivity was not comprehensively explored.
This study aimed to explore the neural mechanisms of stress resilience by examining changes in functional brain connectivity during stress and recovery in healthy young adults. However, the duration of the recovery phase was limited to 180 s, which may not fully capture the long-term recovery process. Future research could improve temporal resolution by extending recovery durations or including multiple post-stress measurement points. Additionally, individual differences such as quality of life, lifestyle habits, and sleep patterns were not systematically controlled. These variables may influence functional connectivity and act as confounding factors. Follow-up studies should incorporate standardized questionnaires and physiological markers (e.g., cortisol, heart rate variability) to better account for these influences and deepen our understanding of stress-related brain dynamics. The fixed task sequence used in this study reflects the natural time course from stress induction to recovery and, thus, was not counterbalanced. While appropriate for our aims, this design may still be susceptible to order effects or accumulated fatigue. Future studies might address this by including fatigue assessments, ensuring sufficient rest between conditions, or adopting randomized or counterbalanced task orders. Lastly, the study was conducted as a preliminary exploratory investigation with 20 participants, and no a priori power analysis was performed. Although the sample size aligns with previous fMRI studies in this domain, it limits the statistical power and generalizability of the findings. Future work should include power calculations and larger samples to confirm and extend these results across diverse populations, including different age groups, to explore potential age-related differences in stress recovery processes.
This study is significant because we made discoveries by quantitatively comparing the differences in FC between the states of stress induction and recovery in healthy individuals. Unlike previous studies, we observed strengthened connectivity between the dorsal attention network and sensorimotor network during stress induction in healthy individuals, which could help to understand cognitive coping mechanisms in the brain during stress. In addition, we found that the connectivity of the DMN with language and visual networks was restored during recovery, suggesting that important processes occur as the brain returns from a state of stress to a stable, self-referential state. In particular, the connectivity patterns during stress and recovery in healthy individuals suggest sensitivity to external stimuli during stress and a return to a self-referential, stable cognitive state during recovery, which furthers our understanding of the reversible changes and recovery mechanisms in the brain when exposed to stress.
These findings may serve as a foundational reference for developing neurobiological indicators that can be utilized in stress assessment protocols. The observed changes in functional connectivity could act as early markers of adaptive or maladaptive responses to stress, thereby contributing to the design of tools for evaluating psychological resilience. Furthermore, data from psychologically stable, healthy individuals may serve as a baseline in future comparative studies involving individuals with chronic stress or clinical populations, potentially aiding in the early detection and interpretation of pathological brain connectivity patterns.
Author Contributions
Conceptualization, M.-H.C.; methodology, J.K. and M.-H.C.; formal analysis, J.K. and M.-H.C.; investigation, J.K. and M.-H.C.; writing—original draft preparation, M.-H.C.; visualization, J.K. and M.-H.C.; project administration, M.-H.C.; funding acquisition, M.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C1100349).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Konkuk University (IRB number: 7001355-202103-HR-426 and date of approval: 27 October 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McEwen, B.S.; Morrison, J.H. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- Pechtel, P.; Pizzagalli, D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 2011, 214, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.J.; Henckens, M.J.A.G.; Joëls, M.; Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef]
- Menon, V. Salience Network in Brain Mapping: An Encyclopedic Reference; Toga, A.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, pp. 597–611. [Google Scholar]
- Taren, A.A.; Gianaros, P.J.; Greco, C.M.; Lindsay, E.K.; Fairgrieve, A.; Brown, K.W.; Rosen, R.K.; Ferris, J.L.; Julson, E.; Marsland, A.L.; et al. Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: A randomized controlled trial. Soc. Cogn. Affect. Neurosci. 2015, 10, 1758–1768. [Google Scholar] [CrossRef]
- Dedovic, K.; D’Aguiar, C.; Pruessner, J.C. What stress does to your brain: A review of neuroimaging studies. Can. J. Psychiatry 2009, 54, 6–15. [Google Scholar] [CrossRef]
- Qin, S.; Hermans, E.J.; van Marle, H.J.F.; Fernández, G. Understanding low levels of cortisol after stress: Associations with prefrontal cortex and hippocampus morphology and functional connectivity. J. Neurosci. 2014, 34, 12975–12982. [Google Scholar]
- Shulman, G.L.; Fiez, J.A.; Corbetta, M.; Buckner, R.L.; Miezin, F.M.; Raichle, M.E.; Petersen, S.E. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997, 9, 648–663. [Google Scholar] [CrossRef]
- Choi, M.H.; Choi, J.S. Comparing brain activation patterns in stress-induced and post-stress recovery states of highly and moderately stressed individuals. Appl. Sci. 2024, 14, 9261. [Google Scholar] [CrossRef]
- Choi, M.H. A pilot study: Extraction of a neural network and feature extraction of generation and reduction mechanisms due to acute stress. Brain Sci. 2023, 13, 519. [Google Scholar] [CrossRef]
- Dedovic, K.; Renwick, R.; Mahani, N.K.; Engert, V.; Lupien, S.P.; Pruessner, J.C. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005, 30, 319–325. [Google Scholar] [PubMed]
- Liston, C.; McEwen, B.S.; Casey, B.J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. USA 2009, 106, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Azarias, F.R.; Almeida, G.H.D.R.; de Melo, L.F.; Rici, R.E.G.; Maria, D.A. The Journey of the Default Mode Network: Development, Function, and Impact on Mental Health. Biology 2025, 14, 395. [Google Scholar] [CrossRef]
- Weber, S.; Aleman, A.; Hugdahl, K. Involvement of the default mode network under varying levels of cognitive effort. Sci. Rep. 2022, 12, 6303. [Google Scholar] [CrossRef]
- Tutunji, R.; Krentz, M.; Kogias, N.; de Voogd, L.; Krause, F.; Vassena, E.; Hermans, E.J. Changes in large-scale neural networks under stress are linked to affective reactivity to stress in real life. eLife 2025, 14, RP102574. [Google Scholar]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Champagne, F.; Meaney, M.J.; Dagher, A. Stress-induced activation of the human hypothalamus and its association with cortisol: A functional MRI study. NeuroImage 2004, 22, 664–671. [Google Scholar]
- van Oort, J.; Tendolkar, I.; Hermans, E.J.; Mulders, P.C.; Beckmann, C.F.; Schene, A.H.; Fernández, G.; van Eijndhoven, P.F. How the brain connects in response to acute stress: A review at the human brain systems level. Neurosci. Biobehav. Rev. 2017, 83, 281–297. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bernhardt, B.C.; Wang, X.; Varga, D.; Krieger-Redwood, K.; Royer, J.; Rodríguez-Cruces, R.; Vos de Wael, R.; Margulies, D.S.; Smallwood, J.; et al. Perceptual coupling and decoupling of the default mode network during mind-wandering and reading. Elife 2022, 11, e74011. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).