Artificial Intelligence in Computed Tomography Radiology: A Systematic Review on Risk Reduction Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection
2.4. Data Management and Collection
3. Results
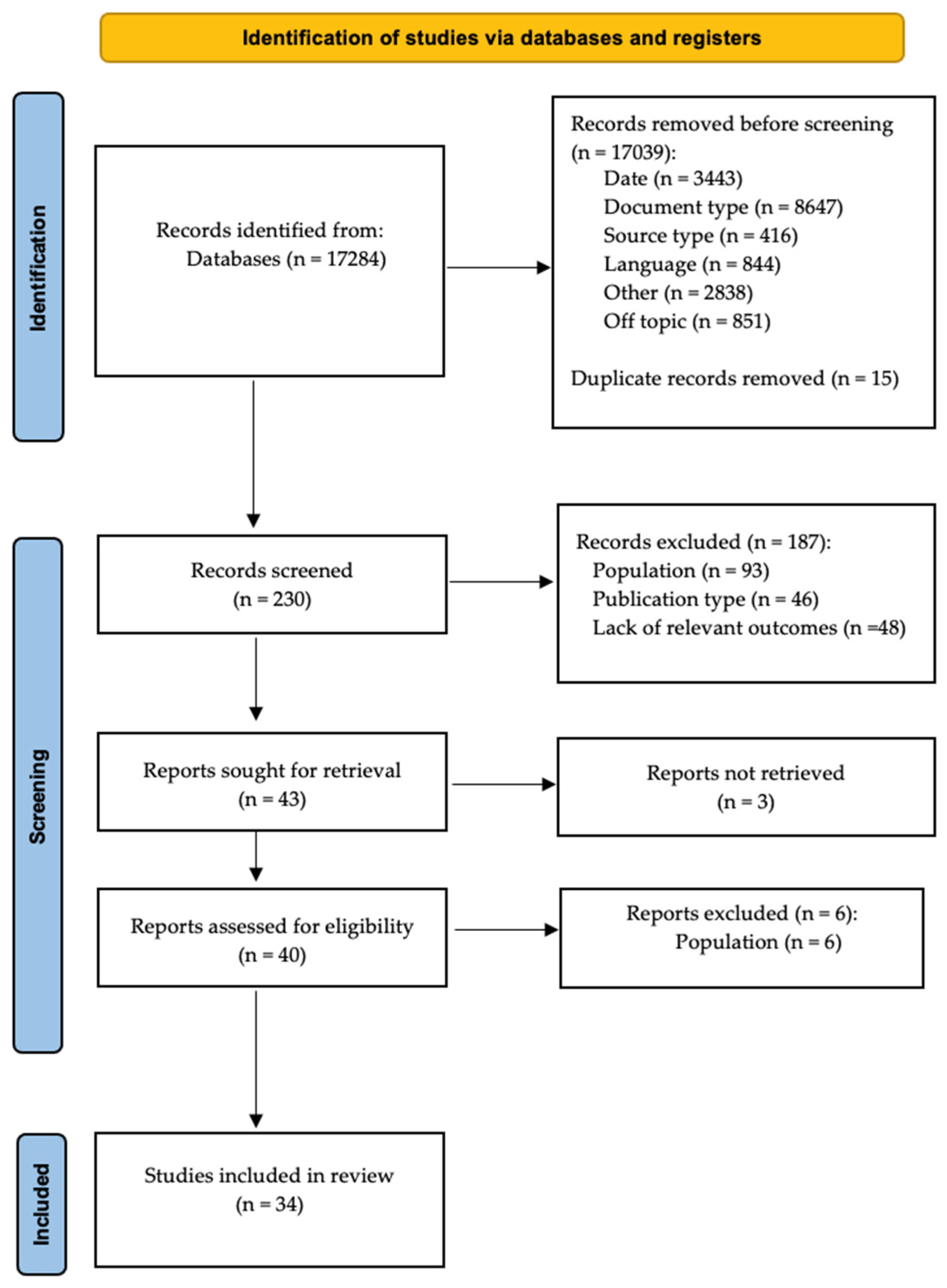
3.1. Selection of Sources of Evidence
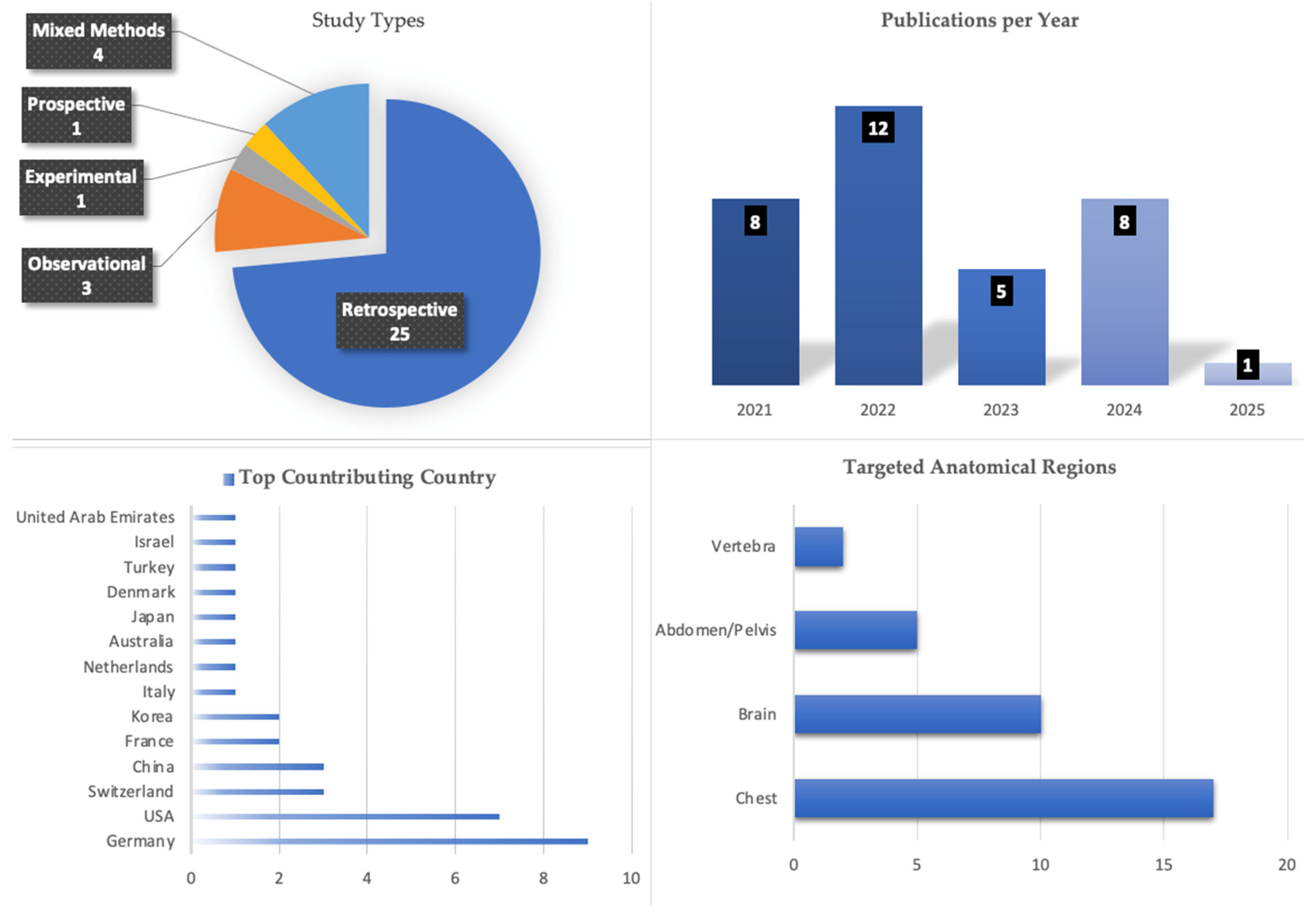
3.2. Study Descriptors
3.3. Results of Individual Studies
3.3.1. Anatomic Segment
3.3.2. Role in Clinical Workflow
3.3.3. Type of Use Cases
3.3.4. Diagnostic Accuracy
4. Discussion
4.1. Limitations
4.2. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial Intelligence in Radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef]
- Bhandari, A. Revolutionizing Radiology with Artificial Intelligence. Cureus 2024, 16, e72646. [Google Scholar] [CrossRef]
- Pierre, K.; Haneberg, A.G.; Kwak, S.; Peters, K.R.; Hochhegger, B.; Sananmuang, T.; Tunlayadechanont, P.; Tighe, P.J.; Mancuso, A.; Forghani, R. Applications of Artificial Intelligence in the Radiology Roundtrip: Process Streamlining, Workflow Optimization, and Beyond. Semin. Roentgenol. 2023, 58, 158–169. [Google Scholar] [CrossRef]
- Buijs, E.; Maggioni, E.; Mazziotta, F.; Lega, F.; Carrafiello, G. Clinical Impact of AI in Radiology Department Management: A Systematic Review. Radiol. Medica 2024, 129, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraj, P.; Ramadass, K.; Bao, S.; Basford, M.; Jones, L.M.; Lee, H.H.; Xu, K.; Schilling, K.G.; Carr, J.J.; Terry, J.G.; et al. Workflow Integration of Research AI Tools into a Hospital Radiology Rapid Prototyping Environment. J. Digit. Imaging 2022, 35, 1023–1033. [Google Scholar] [CrossRef]
- Mahedi, R.A.; Iqbal, H.; Azmee, R.; Azmee, M.; Jakir, F.; Nishan, M.A.; Uddin, M.B.; Afrin, S. Current Trends and Future Prospects of Artificial Intelligence in Transforming Radiology. J. Curr. Health Sci. 2024, 4, 95–104. [Google Scholar] [CrossRef]
- Fathi, M.; Eshraghi, R.; Behzad, S.; Tavasol, A.; Bahrami, A.; Tafazolimoghadam, A.; Bhatt, V.; Ghadimi, D.; Gholamrezanezhad, A. Potential Strength and Weakness of Artificial Intelligence Integration in Emergency Radiology: A Review of Diagnostic Utilizations and Applications in Patient Care Optimization. Emerg. Radiol. 2024, 31, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Malamateniou, C.; Knapp, K.M.; Pergola, M.; Woznitza, N.; Hardy, M. Artificial Intelligence in Radiography: Where Are We Now and What Does the Future Hold? Radiography 2021, 27, S58–S62. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Mendeley. Com/Reference-Manager. Available online: https://www.mendeley.com/reference-management/reference-manager (accessed on 13 August 2025).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- SCISPACE. Available online: http://www.scispace.com (accessed on 5 June 2025).
- Su, B.; Wen, Y.; Liu, Y.; Liao, S.; Fu, J.; Quan, G.; Li, Z. A Deep Learning Method for Eliminating Head Motion Artifacts in Computed Tomography. Med. Phys. 2022, 49, 411–419. [Google Scholar] [CrossRef]
- Barash, Y.; Livne, A.; Klang, E.; Sorin, V.; Cohen, I.; Khaitovich, B.; Raskin, D. Artificial Intelligence for Identification of Images with Active Bleeding in Mesenteric and Celiac Arteries Angiography. Cardiovasc. Intervent Radiol. 2024, 47, 785–792. [Google Scholar] [CrossRef]
- Yuan, H. Anatomic Boundary-Aware Explanation for Convolutional Neural Networks in Diagnostic Radiology. iRadiology 2025, 3, 47–60. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, C.; Kang, S.; Sun, Z.; Wang, Y.; Xiang, M.; Guan, H.; Xia, L.; Wang, S. Application of Computer-Aided Detection (CAD) Software to Automatically Detect Nodules under SDCT and LDCT Scans with Different Parameters. Comput. Biol. Med. 2022, 146, 105538. [Google Scholar] [CrossRef]
- Canayaz, M.; Şehribanoğlu, S.; Özdağ, R.; Demir, M. COVID-19 Diagnosis on CT Images with Bayes Optimization-Based Deep Neural Networks and Machine Learning Algorithms. Neural Comput. Appl. 2022, 34, 5349–5365. [Google Scholar] [CrossRef] [PubMed]
- Gang, Y.; Chen, X.; Li, H.; Wang, H.; Li, J.; Guo, Y.; Zeng, J.; Hu, Q.; Hu, J.; Xu, H. A Comparison between Manual and Artificial Intelligence–Based Automatic Positioning in CT Imaging for COVID-19 Patients. Eur. Radiol. 2021, 31, 6049–6058. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Pathak, R.; Ketireddy, V.; Columbu, M.; Saba, L.; Gupta, S.K.; Faa, G.; Singh, I.M.; Turk, M.; et al. COVLIAS 1.0: Lung Segmentation in COVID-19 Computed Tomography Scans Using Hybrid Deep Learning Artificial Intelligence Models. Diagnostics 2021, 11, 1405. [Google Scholar] [CrossRef]
- Villringer, K.; Sokiranski, R.; Opfer, R.; Spies, L.; Hamann, M.; Bormann, A.; Brehmer, M.; Galinovic, I.; Fiebach, J.B. An Artificial Intelligence Algorithm Integrated into the Clinical Workflow Can Ensure High Quality Acute Intracranial Hemorrhage CT Diagnostic. Clin. Neuroradiol. 2025, 35, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, T.J.; Guillo, E.; Heraud, A.; Rossillon, A.; Bartoli, M.; Herpe, G.; Adam, C.; Fabre, D.; Ardon, R.; Azarine, A.; et al. Multicentric Clinical Evaluation of a Computed Tomography-Based Fully Automated Deep Neural Network for Aortic Maximum Diameter and Volumetric Measurements. J. Vasc. Surg. 2024, 79, 1390–1400.e8. [Google Scholar] [CrossRef] [PubMed]
- Seyam, M.; Weikert, T.; Sauter, A.; Brehm, A.; Psychogios, M.-N.; Blackham, K.A. Utilization of Artificial Intelligence–Based Intracranial Hemorrhage Detection on Emergent Noncontrast CT Images in Clinical Workflow. Radiol. Artif. Intell. 2022, 4, e210168. [Google Scholar] [CrossRef]
- Brejnebøl, M.W.; Nielsen, Y.W.; Taubmann, O.; Eibenberger, E.; Müller, F.C. Artificial Intelligence Based Detection of Pneumoperitoneum on CT Scans in Patients Presenting with Acute Abdominal Pain: A Clinical Diagnostic Test Accuracy Study. Eur. J. Radiol. 2022, 150, 110216. [Google Scholar] [CrossRef]
- Tekin, H.O.; Almisned, F.; Erguzel, T.T.; Abuzaid, M.M.; Elshami, W.; Ene, A.; Issa, S.A.M.; Zakaly, H.M.H. Utilization of Artificial Intelligence Approach for Prediction of DLP Values for Abdominal CT Scans: A High Accuracy Estimation for Risk Assessment. Front. Public. Health 2022, 10, 892789. [Google Scholar] [CrossRef]
- Fink, M.A.; Seibold, C.; Kauczor, H.-U.; Stiefelhagen, R.; Kleesiek, J. Jointly Optimized Deep Neural Networks to Synthesize Monoenergetic Images from Single-Energy CT Angiography for Improving Classification of Pulmonary Embolism. Diagnostics 2022, 12, 1224. [Google Scholar] [CrossRef]
- Artzner, C.; Bongers, M.N.; Kärgel, R.; Faby, S.; Hefferman, G.; Herrmann, J.; Nopper, S.L.; Perl, R.M.; Walter, S.S. Assessing the Accuracy of an Artificial Intelligence-Based Segmentation Algorithm for the Thoracic Aorta in Computed Tomography Applications. Diagnostics 2022, 12, 1790. [Google Scholar] [CrossRef]
- Nadeem, S.A.; Comellas, A.P.; Regan, E.A.; Hoffman, E.A.; Saha, P.K. Chest CT-Based Automated Vertebral Fracture Assessment Using Artificial Intelligence and Morphologic Features. Med. Phys. 2024, 51, 4201–4218. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Wu, D.J.; Wang, Z.; Ma, X.L.; Dong, X.M.; Liu, A.E.; Chen, L. A Fully Automated Rib Fracture Detection System on Chest CT Images and Its Impact on Radiologist Performance. Skeletal Radiol. 2021, 50, 1821–1828. [Google Scholar] [CrossRef]
- Schmuelling, L.; Franzeck, F.C.; Nickel, C.H.; Mansella, G.; Bingisser, R.; Schmidt, N.; Stieltjes, B.; Bremerich, J.; Sauter, A.W.; Weikert, T.; et al. Deep Learning-Based Automated Detection of Pulmonary Embolism on CT Pulmonary Angiograms: No Significant Effects on Report Communication Times and Patient Turnaround in the Emergency Department Nine Months after Technical Implementation. Eur. J. Radiol. 2021, 141, 109816. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.; Bagga, B.; Melamud, K.; O’Donnell, T.; Vega, E.; Westerhoff, M.; Dane, B. Quality Assessment of Expedited AI Generated Reformatted Images for ED Acquired CT Abdomen and Pelvis Imaging. Abdom. Radiol. 2025, 50, 1441–1447. [Google Scholar] [CrossRef]
- Wilder-Smith, A.J.; Yang, S.; Weikert, T.; Bremerich, J.; Haaf, P.; Segeroth, M.; Ebert, L.C.; Sauter, A.; Sexauer, R. Automated Detection, Segmentation, and Classification of Pericardial Effusions on Chest CT Using a Deep Convolutional Neural Network. Diagnostics 2022, 12, 1045. [Google Scholar] [CrossRef]
- Wang, D.; Jin, R.; Shieh, C.-C.; Ng, A.Y.; Pham, H.; Dugal, T.; Barnett, M.; Winoto, L.; Wang, C.; Barnett, Y. Real World Validation of an AI-Based CT Hemorrhage Detection Tool. Front. Neurol. 2023, 14, 1177723. [Google Scholar] [CrossRef] [PubMed]
- Palm, V.; Norajitra, T.; von Stackelberg, O.; Heussel, C.P.; Skornitzke, S.; Weinheimer, O.; Kopytova, T.; Klein, A.; Almeida, S.D.; Baumgartner, M.; et al. AI-Supported Comprehensive Detection and Quantification of Biomarkers of Subclinical Widespread Diseases at Chest CT for Preventive Medicine. Healthcare 2022, 10, 2166. [Google Scholar] [CrossRef] [PubMed]
- Zsarnoczay, E.; Rapaka, S.; Schoepf, U.J.; Gnasso, C.; Vecsey-Nagy, M.; Todoran, T.M.; Hagar, M.T.; Kravchenko, D.; Tremamunno, G.; Griffith, J.P.; et al. Accuracy of a Deep Neural Network for Automated Pulmonary Embolism Detection on Dedicated CT Pulmonary Angiograms. Eur. J. Radiol. 2025, 187, 112077. [Google Scholar] [CrossRef]
- Cotena, M.; Ayobi, A.; Zuchowski, C.; Junn, J.C.; Weinberg, B.D.; Chang, P.D.; Chow, D.S.; Soun, J.E.; Roca-Sogorb, M.; Chaibi, Y.; et al. Enhancing Radiologist Efficiency with AI: A Multi-Reader Multi-Case Study on Aortic Dissection Detection and Prioritization. Diagnostics 2024, 14, 2689. [Google Scholar] [CrossRef]
- Zaazoue, K.A.; McCann, M.R.; Ahmed, A.K.; Cortopassi, I.O.; Erben, Y.M.; Little, B.P.; Stowell, J.T.; Toskich, B.B.; Ritchie, C.A. Evaluating the Performance of a Commercially Available Artificial Intelligence Algorithm for Automated Detection of Pulmonary Embolism on Contrast-Enhanced Computed Tomography and Computed Tomography Pulmonary Angiography in Patients With Coronavirus Disease 2019. Mayo Clin. Proc. Innov. Qual. Outcomes 2023, 7, 143–152. [Google Scholar] [CrossRef]
- Kim, P.E.; Yang, H.; Kim, D.; Sunwoo, L.; Kim, C.K.; Kim, B.J.; Kim, J.T.; Ryu, W.S.; Kim, H.S. Automated Prediction of Proximal Middle Cerebral Artery Occlusions in Noncontrast Brain Computed Tomography. Stroke 2024, 55, 1609–1618. [Google Scholar] [CrossRef]
- Colasurdo, M.; Leibushor, N.; Robledo, A.; Vasandani, V.; Luna, Z.A.; Rao, A.S.; Garcia, R.; Srinivasan, V.M.; Sheth, S.A.; Avni, N.; et al. Automated Detection and Analysis of Subdural Hematomas Using a Machine Learning Algorithm. J. Neurosurg. 2023, 138, 1077–1084. [Google Scholar] [CrossRef]
- Dovrat, A.Y.; Saban, M.; Merhav, G.; Lankri, I.; Abergel, E.; Eran, A.; Tanne, D.; Nogueira, R.G.; Hoffmann, R.S. Evaluation of Artificial Intelligence-Powered Identification of Large-Vessel Occlusions in a Comprehensive Stroke Center. Am. J. Neuroradiol. 2021, 42, 247–254. [Google Scholar] [CrossRef]
- Kim, T.M.; Choi, S.J.; Ko, J.Y.; Kim, S.; Jeong, C.W.; Cho, J.Y.; Kim, S.Y.; Kim, Y.G. Fully Automatic Volume Measurement of the Adrenal Gland on CT Using Deep Learning to Classify Adrenal Hyperplasia. Eur. Radiol. 2023, 33, 4292–4302. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Sugimoto, K.; Suzuki, Y.; Wataya, T.; Kita, K.; Nishigaki, D.; Tomiyama, M.; Hiraoka, Y.; Hori, M.; Takeda, T.; et al. Annotation-Free Multi-Organ Anomaly Detection in Abdominal CT Using Free-Text Radiology Reports: A Multi-Centre Retrospective Study. EBioMedicine 2024, 110, 105463. [Google Scholar] [CrossRef]
- Cheikh, A.B.; Gorincour, G.; Nivet, H.; May, J.; Seux, M.; Calame, P.; Thomson, V.; Delabrousse, E.; Crombé, A. How Artificial Intelligence Improves Radiological Interpretation in Suspected Pulmonary Embolism. Eur. Radiol. 2022, 32, 5831–5842. [Google Scholar] [CrossRef]
- Kundisch, A.; Hönning, A.; Mutze, S.; Kreissl, L.; Spohn, F.; Lemcke, J.; Sitz, M.; Sparenberg, P.; Goelz, L. Deep Learning Algorithm in Detecting Intracranial Hemorrhages on Emergency Computed Tomographies. PLoS ONE 2021, 16, e0260560. [Google Scholar] [CrossRef]
- Grenier, P.A.; Ayobi, A.; Quenet, S.; Tassy, M.; Marx, M.; Chow, D.S.; Weinberg, B.D.; Chang, P.D.; Chaibi, Y. Deep Learning-Based Algorithm for Automatic Detection of Pulmonary Embolism in Chest CT Angiograms. Diagnostics 2023, 13, 1324. [Google Scholar] [CrossRef]
- Rava, R.A.; Seymour, S.E.; LaQue, M.E.; Peterson, B.A.; Snyder, K.V.; Mokin, M.; Waqas, M.; Hoi, Y.; Davies, J.M.; Levy, E.I.; et al. Assessment of an Artificial Intelligence Algorithm for Detection of Intracranial Hemorrhage. World Neurosurg. 2021, 150, e209–e217. [Google Scholar] [CrossRef]
- Brendel, J.M.; Walterspiel, J.; Hagen, F.; Kübler, J.; Brendlin, A.S.; Afat, S.; Paul, J.-F.; Küstner, T.; Nikolaou, K.; Gawaz, M.; et al. Coronary Artery Disease Detection Using Deep Learning and Ultrahigh-Resolution Photon-Counting Coronary CT Angiography. Diagn. Interv. Imaging 2025, 106, 68–75. [Google Scholar] [CrossRef]
- Ruitenbeek, H.C.; Oei, E.H.G.; Schmahl, B.L.; Bos, E.M.; Verdonschot, R.J.C.G.; Visser, J.J. Towards Clinical Implementation of an AI-Algorithm for Detection of Cervical Spine Fractures on Computed Tomography. Eur. J. Radiol. 2024, 173, 111375. [Google Scholar] [CrossRef]
- Kiefer, J.; Kopp, M.; Ruettinger, T.; Heiss, R.; Wuest, W.; Amarteifio, P.; Stroebel, A.; Uder, M.; May, M.S. Diagnostic Accuracy and Performance Analysis of a Scanner-Integrated Artificial Intelligence Model for the Detection of Intracranial Hemorrhages in a Traumatology Emergency Department. Bioengineering 2023, 10, 1362. [Google Scholar] [CrossRef]
- Seker, F.; Pfaff, J.A.R.; Mokli, Y.; Berberich, A.; Namias, R.; Gerry, S.; Nagel, S.; Bendszus, M.; Herweh, C. Diagnostic Accuracy of Automated Occlusion Detection in CT Angiography Using E-CTA. Int. J. Stroke 2022, 17, 77–82. [Google Scholar] [CrossRef]
| Study | Role in Clinical Workflow | Type of Application | Type of Use Cases | Anatomic Segment | Pathology | Sensitivity | Specificity | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Postiglione T.J. et al. [23] | Addition | Segmentation | Separating normal versus not normal | Chest (aorta segments) | Dissections | 85.5% | _____ | Reliability of a fully automated artificial intelligence-driven solution, augmented radiology for vascular aneurysm, capable of quick aortic segmentation and analysis of diameter and volume for each segment, achieving a median absolute diameter difference of 1.6 mm compared to expert measurements. |
| Seyam M. et al. [24] | Addition | Detection | Computer-aided detection Workflow optimization | Head | Intracranial haemorrhage | 87.2% | 93.9% | AI-based tool for detecting intracranial haemorrhage on non-contrast CT images achieved a diagnostic accuracy of 93.0%, Additionally, the implementation of this tool positively impacted clinical workflow. |
| Brejnebol M.W. et al. [25] | Addition | Detection | Computer-aided detection | Abdominal and pelvis | Pneumoperitoneum | 95.0% | 99.0% | AI algorithm demonstrated high specificity and a sensitivity for pneumoperitoneum detection, achieving an area under the curve of 0.77 in the entire patient cohort. Additionally, when excluding cases with smaller amounts of free air, the area under the curve increased to 0.96. |
| Gang Y. et al. [20] | Replacement | Comparison | Workflow optimization | Chest | Ground glass opacification, consolidation opacification and interstitial thickening | - | - | AI-based automatic positioning method was successful for all patients, reducing total positioning time by 28% and achieving a higher proportion of positioning accuracy (99% vs. 92%) compared to the manual positioning method. Resulted in a 16% reduction in radiation dose and a 9% reduction in image noise in the erector spinae area. |
| Suri J.S. et al. [21] | Replacement | Segmentation | Computer-aided detection | Chest | Ground glass opacities | 96.0% | _____ | Development of the COVID Lung Image Analysis System (COVLIAS 1.0), utilises hybrid deep learning models for effective lung segmentation in CT scans of COVID-19 patients. Demonstrated high performance with an area under the curve of approximately 0.96 to 0.98 across different models. |
| Tekin H.O. et al. [26] | Replacement | Classification | Quality assurance Optimise radiation dose management | Abdominal | NA | 82.2% | NA | Successful utilisation of AI approaches for the prediction of Dose Length Product values in abdominal CT scans, achieving high accuracy for risk assessment. Highlights the importance of various parameters affecting its values. |
| Fink M.A. et al. [27] | Replacement | Classification | Computer-aided detection | Chest (pulmonary arteries) | Pulmonary embolism | NA | NA | Developed a jointly optimised deep learning framework to generate synthetic monoenergetic images from dual-energy CT pulmonary angiography data, improving automatic pulmonary embolism detection in single-energy scans. The framework achieved high-quality visual SMI predictions with a structural similarity index of 0.984 ± 0.002 and a peak signal-to-noise ratio of 41.706 ± 0.547 dB. |
| Artzner C. et al. [28] | Replacement | Segmentation | Computer-aided detection Workflow optimisation | Chest (thoracic aorta) | Post-TEVAR status, intramural haematoma, mediastinal lymphadenopathy, pericardial effusion and dissections | - | - | The prototypical AI-based algorithm accurately measured thoracic aortic diameters at predefined anatomical locations, demonstrating substantial potential for rapid clinical evaluation of aortic pathology, independent of contrast utilisation or pathology. |
| Nadeem S.A. et al. [29] | Replacement | Classification Segmentation Detection | Computer-aided detection Workflow optimisation | Vertebra | Vertebral fractures | 94.8% | 98.5% | New automated methods for segmentation and labelling of individual vertebrae in chest CT images, detecting vertebral deformity fractures using computed vertebral height features and parametric computational modelling. Achieved high accuracy and sensitivity in vertebral fracture assessment. |
| Meng X.H. et al. [30] | Replacement | Classification Detection | Computer-aided detection | Chest | Rib fractures | 92.2% | - | Proposed a fully automated detection method for rib fractures which effectively filtered false positives and improved detection performance by training three networks sequentially. The deep learning model achieved a recall rate of 0.922 and a classification accuracy of 0.863. |
| Schmuelling L. et al. [31] | Replacement | Detection | Computer aided detection Workflow optimisation | Chest | Pulmonary embolism | 79.6% | 95.0% | Deep learning-assisted detection of pulmonary embolism in CT pulmonary angiograms and the use of an electronic notification system for communication of results to referring physicians technically work, did not lead to significant improvements in clinical performance measures such as report reading times and patient turnaround times. |
| Freedman D. et al. [32] | Replacement | Post-processing | Workflow optimisation | Abdominal and pelvis | NA | - | - | Examinations utilising AI-generated multiplanar reformatted images were completed approximately 50% faster than those generated at the console, with no statistical difference in diagnostic confidence or image quality between the two methods. |
| Hu Q. et al. [18] | Triage | Detection | Computer-aided detection Workflow optimisation | Chest | Pulmonary nodules | 78.0%(SDCT) 70.1%(LDCT) | - | Computer-aided detection system demonstrated a sensitivity of 78.03% for detecting pulmonary nodules in standard-dose CT images and 70.15% in low-dose CT images, indicating its effectiveness in identifying nodules under varying conditions. |
| Canayaz M. et al. [19] | Triage | Classification | Computer-aided detection | Chest | COVID-19 | 99.4% | - | Development of a new method using Bayesian optimisation-based MobilNetv2 and ResNet-50 models, along with machine learning algorithms, achieving hight accuracy in diagnosing COVID-19. |
| Villringer K. et al. [22] | Triage | Classification | Computer-aided detection | Head | Intracranial haemorrhage | 90.0% | 96.0% | AI algorithm successfully detected intracranial haemorrhage in 15% of the analysed cranial CT examinations, with a total of 947 out of 6284 cases identified as having it. The algorithm demonstrated high accuracy when compared to experts. |
| Wilder-Smith, A.J. et al. [33] | Triage | Detection Segmentation Classification | Grading and classification Workflow optimisation | Chest (heart chambers) | Pericardial effusions | 97.0% | 100% | Automatic tool for the detection, segmentation and classification of pericardial effusions on CT, achieving high sensitivity and specificity for diagnosing haemopericardium. |
| Wang D. et al. [34] | Triage | Detection | Computer-aided detection Workflow optimisation | Head | Intracranial haemorrhage | 92.0% | 96.0% | VeriScout™ detected haemorrhage with high sensitivity and specificity, effectively flagging cases for expedited clinical review within 10 min. |
| Palm V. et al. [35] | Triage | Detection Segmentation | Computer-aided detection Workflow optimisation | Chest | Pulmonary nodules | - | - | Holistic imaging diagnostics tool that utilises artificial intelligence to automate the detection, quantification and characterisation of common pulmonary, metabolic, cardiovascular and musculoskeletal comorbidities in chest computed tomography scans. Provides a comprehensive evaluation of patients and improve preventive care. |
| Zsarnoczay E. et al. [36] | Triage | Detection | Computer-aided detection Workflow optimisation | Chest (Pulmonary artery system) | Pulmonary embolism | 84.6% | 95.1% | Demonstrated that a deep neural network-based algorithm effectively detected pulmonary embolism on CT pulmonary angiogram scans, with the majority of false negatives attributed to small chronic pulmonary embolisms in subsegmental arteries. |
| Cotena M. et al. [37] | Triage | Detection Classification | Computer-aided detection Workflow optimisation | Chest (thoracic aorta) | Acute aortic dissection | 94.3% | 100% | Integrating a deep learning-based application for the automated detection and prioritisation of acute aortic dissection on chest CT angiographies significantly reduced the scan-to-assessment time from 15.84 min to 5.07 min, representing a 68% reduction, and the interpretation time from 21.22 s to 14.17 s. |
| Zaazoue K.A. et al. [38] | Triage | Detection | Computer-aided detection | Chest | Pulmonary embolism | 93.2% | 99.6% | AI algorithm demonstrated high sensitivity and specificity for detecting pulmonary embolism in contrast-enhanced CT scans of patients with COVID-19, with optimal accuracy achieved at a pulmonary artery attenuation of more than 362 Hounsfield units. |
| Kim P.E. et al. [39] | Triage | Classification | Clinical outcome prediction | Head | Large vessel occlusion | 80.1% | 88.6% | Machine learning-based algorithm that utilises handcrafted features from non-contrast computed tomography to predict large vessel occlusion in patients with ischemic stroke, demonstrating reliable predictions and the potential to expedite stroke workflow. |
| Colasurdo M. et al. [40] | Triage | Detection Segmentation | Computer-aided detection Workflow optimisation | Head | Subdural haematoma | 91.4% | 96,40% | Potential of AI and deep learning to enhance its detection and analysis, facilitating earlier and more accurate diagnoses. |
| Dovrat A.Y. et al. [41] | Triage | Detection | Computer-aided detection Workflow optimisation | Head and neck | Large vessel occlusion | 81% | 94% | Viz LVO system demonstrated high sensitivity and accuracy in detecting large vessel occlusions in a comprehensive stroke centre, with 61 out of 75 identified by the system. |
| Kim T.M. et al. [42] | Triage | Segmentation Classification | Computer-aided detection | Abdominal (adrenal glands) | Adrenal hyperplasia | 75.0–81.3% | 97.3–100% | Fully automated deep learning model for adrenal segmentation, classification performance for adrenal hyperplasia. |
| Sato J. et al. [43] | Triage | Detection Segmentation | Computer-aided detection Workflow optimisation | Abdominal | Detecting organ abnormalities in abdominal images | - | - | Deep learning-based pipeline for detecting abnormalities in abdominal CT images using information from free-text radiology reports, which allows for accurate anomaly detection without the need for manual annotations. The model achieved an overall area under the curve of 0.886 in external validation. |
| Cheikh A.B. et al. [44] | Triage | Detection | Computer-aided detection Workflow optimisation | Chest (pulmonary arteries) | Pulmonary embolism | 92.60 | 95.8% | The AI algorithm for detecting pulmonary embolism on CT pulmonary angiogram demonstrated high sensitivity, serving as a safety net in emergency radiology practice and enhancing the confidence of radiologists in their diagnoses. |
| Kundisch A. et al. [45] | Triage | Detection | Computer-aided detection | Head | Intracranial haemorrhages | 87.6% | 98.1% | AI algorithm detected an additional 29 instances of intracranial haemorrhages, resulting in a 12.2% increase in the number of detected cases compared to initial radiology reports. AI missed 12.4% of cases, while radiologists missed 10.9%. |
| Grenier P.A. et al. [46] | Triage | Detection Segmentation | Computer-aided detection Workflow optimisation | Chest | Pulmonary embolism | 91.4% | 91.5% | The deep learning-based application, CINA-PE, demonstrated a high degree of diagnostic accuracy. The algorithm correctly identified 170 of 186 exams positive for pulmonary embolism and 184 of 201 exams negative for pulmonary embolism. |
| Rava R.A. et al. [47] | Triage Workflow | Detection | Computer-aided detection | Head | Intracranial haemorrhages | 93.0% | 93.0% | Canon’s AUTO Stroke Solution algorithm accurately identified patients with intracranial haemorrhages and those without. Has the potential to significantly improve treatment times for intracranial haemorrhage patients. |
| Brendel J.M. et al. [48] | Triage Workflow | Detection | Computer-aided detection | Chest | Coronary artery disease | 97.2% | 81.7% | Automated deep learning demonstrated remarkable performance in detecting significant coronary artery disease on non-ultra-high-resolution photon-counting coronary CT angiography images. |
| Ruitenbeek H.C. et al. [49] | Triage workflow | Detection | Computer-aided detection Workflow optimisation | Vertebra | Cervical spine fractures | 89.8% | 95.3% | Demonstrated high diagnostic accuracy and a sensitivity for detecting cervical spine fractures on CT scans. Time gain of 16 min to diagnosis for fractured cases after its introduction. |
| Kiefer J. et al. [50] | Triage Workflow | Detection | Computer-aided detection Workflow optimisation | Head | Intracranial haemorrhages | 98.1% | 89.7% | Performance of a scanner-integrated artificial intelligence algorithm for detecting intracranial haemorrhages in a routine clinical setting, achieving high sensitivity and specificity. The algorithm successfully detected brain haemorrhages in 432 out of 435 cases, demonstrating its feasibility and robustness in emergency settings. |
| Seker F. et al. [51] | Workflow | Detection | Computer-aided detection Workflow optimisation | Head | Large vessel occlusions | 84.0% | 96.0% | e-CTA (Brainomix) demonstrates high diagnostic accuracy for the automatic detection of large vessel occlusions in anterior circulation stroke, with sensitivity and specificity values indicating effective performance in identifying proximal occlusions. |
| Anatomical Region | Studies (n) | Main Application |
|---|---|---|
| Chest | 17 | Nodule detection, pulmonary lesion classification, pneumonia triage, pulmonary embolism detection, post-operative aortic evaluation, diameter segmentation |
| Vertebral bodies | 2 | Structural analysis and measurement |
| Brain | 10 | Haemorrhage detection, tumour classification, stroke evaluation, fracture detection, anatomical mapping |
| Abdomen and pelvis | 5 | Organ segmentation, pathology detection |
| Role in Clinical Workflow | Studies (n) | Description |
|---|---|---|
| Triage | 21 | AI pre-analyses imaging data and flags urgent cases for prioritised review, expediting reporting for time-sensitive conditions |
| Replacement | 9 | AI autonomously performs task of image segmentation, anatomical measurements and dose estimation. |
| Addition | 4 | AI supports clinicians with quantitative suggestions and pattern recognition, complementing human judgment. |
| Workflow | 1 | AI autodetection by algorithm training, improving response times |
| Type of Use Case | Studies (n) | Description |
|---|---|---|
| Computer-aided detection | 11 | Systems trained to detect specific conditions |
| Computer-aided detection and workflow optimisation | 17 | Support image interpretation and logistical operations |
| Other use cases | 6 | Radiation dose management, outcome prediction and structured reporting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, S.; Fernandes, A.; Freitas, M.; Fernandes, R.J. Artificial Intelligence in Computed Tomography Radiology: A Systematic Review on Risk Reduction Potential. Appl. Sci. 2025, 15, 9659. https://doi.org/10.3390/app15179659
Coelho S, Fernandes A, Freitas M, Fernandes RJ. Artificial Intelligence in Computed Tomography Radiology: A Systematic Review on Risk Reduction Potential. Applied Sciences. 2025; 15(17):9659. https://doi.org/10.3390/app15179659
Chicago/Turabian StyleCoelho, Sandra, Aléxia Fernandes, Marco Freitas, and Ricardo J. Fernandes. 2025. "Artificial Intelligence in Computed Tomography Radiology: A Systematic Review on Risk Reduction Potential" Applied Sciences 15, no. 17: 9659. https://doi.org/10.3390/app15179659
APA StyleCoelho, S., Fernandes, A., Freitas, M., & Fernandes, R. J. (2025). Artificial Intelligence in Computed Tomography Radiology: A Systematic Review on Risk Reduction Potential. Applied Sciences, 15(17), 9659. https://doi.org/10.3390/app15179659








