Abstract
(1) Background: Arteriovenous fistula (AVF) thrombosis represents a major cause of vascular access failure in patients undergoing maintenance hemodialysis. Identifying early biochemical markers associated with thrombosis may facilitate timely intervention and improve vascular outcomes. This study aimed to evaluate the association between baseline biochemical markers and the risk of AVF thrombosis and mortality during long-term follow-up. (2) Methods: We conducted a prospective observational study involving 249 chronic hemodialysis patients with functional AVFs. Baseline data included intact parathyroid hormone (iPTH), hemoglobin, phosphate, potassium, albumin, dialysis adequacy (Kt/V), age, diabetes status, and antivitamin K (AVK) therapy. Patients were followed for five years for the occurrence of AVF thrombosis and mortality. Statistical analysis was performed using one-way ANOVA with Levene’s test and Scheffé post hoc comparisons. (3) Results: iPTH levels were significantly higher in patients who developed AVF thrombosis (mean 494.6 pg/mL) than in those without thrombosis (mean 381.5 pg/mL; p = 0.047). Other variables, including hemoglobin, phosphate, Kt/V, age, diabetes, and AVK therapy, were not significantly associated with thrombosis. Mortality was more frequent among patients with diabetes mellitus and those receiving antivitamin K therapy; however, only the association with diabetes reached statistical significance. (4) Conclusions: Elevated iPTH was associated with AVF thrombosis. Routine monitoring may help identify high-risk patients and guide timely interventions.
1. Introduction
Patients with end-stage renal disease (ESRD) undergoing hemodialysis require functioning vascular access (VA) to ensure effective treatment delivery. Among the different access points, the autologous arteriovenous fistula (AVF) is widely regarded as the first-line option due to its superior patency and lower infection rates when compared to arteriovenous grafts (AVG) and central venous catheters [1,2]. However, maintaining AVF functionality remains clinically challenging, as thrombosis continues to be a leading cause of VA dysfunction [1]. Recent observational studies have reported that the prevalence of AVF thrombosis can reach 16.2% in chronic hemodialysis cohorts, emphasizing its clinical burden and frequency as a complication [3].
VA thrombosis represents a significant problem in the dialysis population, with some estimates suggesting that it accounts for up to 85% of permanent access losses [1]. This complication is not only a technical concern but has also been linked to systemic patient vulnerability [4]. Observational data have shown that patients who experience VA thrombosis may be at increased risk for both early and late mortality [5]. In particular, elevated mortality rates have been reported within the first three months following a thrombotic event, suggesting a potential association between VA failure and overall patient prognosis [6].
Several demographic and clinical factors have been associated with an increased risk of AVF thrombosis. These include advanced age, female sex, distal AVF location, elevated inflammatory markers such as C-reactive protein, hypertension, and the use of arteriovenous grafts (AVGs) [7]. Additional evidence points to both acquired and genetic contributors, with comorbidities like diabetes and variations in vascular access type and location further influencing thrombosis risk [8].
In addition to clinical and anatomical determinants, increasing attention has been directed toward biochemical abnormalities associated with chronic kidney disease–mineral and bone disorder (CKD-MBD), particularly the role of parathyroid hormone (PTH) in vascular pathology. Elevated levels of intact PTH (iPTH) are commonly observed in patients on maintenance hemodialysis and have been implicated in adverse vascular remodeling processes that may compromise AVF integrity. Experimental findings suggest that PTH can actively promote vascular dysfunction through fibrotic and proliferative mechanisms. It has been shown to stimulate the transformation of vascular fibroblasts into contractile myofibroblasts, leading to structural changes in the vessel wall such as neointimal hyperplasia and luminal narrowing, which may predispose to stenosis and thrombosis [9]. These molecular events disrupt normal flow dynamics and impair the adaptive remodeling required for AVF patency.
Clinical guidelines also underscore the impact of mineral metabolism on vascular access outcomes. Persistently elevated iPTH levels are associated with increased vascular calcification and altered vessel compliance, both of which can negatively affect AVF performance [10]. Such calcific remodeling is thought to stiffen the vascular wall and increase susceptibility to occlusion or thrombosis, particularly in the context of hemodynamic stress.
Although the pathophysiological link between iPTH and AVF dysfunction is biologically plausible, the predictive value of iPTH as a single biomarker remains uncertain. It does not always emerge as an independent determinant of AVF outcomes, suggesting that its vascular effects may be modulated by concurrent factors such as blood pressure variability, vascular geometry, and systemic inflammation [11,12].
Taken together, these findings support a nuanced role for iPTH in the maintenance of vascular access in hemodialysis patients. While not deterministic on its own, iPTH may contribute to a broader pathophysiological network that governs AVF stability, warranting its consideration in risk stratification and longitudinal monitoring frameworks [13,14].
Taking into account the emerging evidence linking mineral metabolism abnormalities to vascular access complications, this study set out to examine whether elevated iPTH levels are associated with the development of AVF thrombosis in patients undergoing maintenance hemodialysis. The investigation was designed to clarify whether iPTH is associated with AVF thrombosis in this setting and to contribute to the broader understanding of how biochemical disturbances characteristic of chronic kidney disease may influence long-term vascular access outcomes.
2. Materials and Methods
We conducted a 5-year prospective cohort study across two dialysis centers, involving 249 adult patients with end-stage renal disease (ESRD) undergoing maintenance hemodialysis (HD) and possessing a functional, mature native arteriovenous fistula (AVF). Maturity was defined as the ability to deliver effective hemodialysis with adequate blood flow and without the use of a central venous catheter for at least three months prior to enrollment. All patients had been on HD for at least three months prior to enrollment.
Patients were excluded if they were receiving dialysis through a central venous catheter (CVC), had immature or non-functional AVFs, prior AVF thrombosis, arteriovenous grafts, incomplete baseline data, or were scheduled for kidney transplantation or a change in renal replacement therapy during the follow-up period. Patients lost to follow-up before the 5-year assessment were also excluded.
AVF thrombosis was diagnosed based on the clinical absence of bruit or thrill, supported by physical examination findings by the attending nephrologist. In the majority of cases, diagnosis was further confirmed by Doppler ultrasound performed in a vascular surgery or interventional radiology setting as part of routine clinical care. However, ultrasound confirmation was not part of the study protocol and was not uniformly available for all events.
Information on smoking status, detailed vascular access history, atherosclerotic disease, and cardiac arrhythmias was not systematically collected, as the study protocol focused primarily on biochemical and dialysis-related parameters.
All procedures were performed in accordance with the ethical standards of the institutional research committee and the Declaration of Helsinki. The study protocol received approval from the Ethics Committee of the University of Medicine and Pharmacy “Victor Babes” Timisoara Nr. 34/30.06.2021. Written informed consent was obtained from all participants before inclusion.
2.1. Baseline Clinical Assessment and Laboratory Parameters
All enrolled patients underwent clinical and laboratory evaluation at baseline, during the same month in which AVF ultrasound assessments were performed. Blood samples were drawn from the AVF prior to the dialysis session. The following laboratory parameters were assessed: intact parathyroid hormone (iPTH, pg/mL), hemoglobin (Hb, g/dL), phosphorus (mg/dL), calcium (mg/dL), bicarbonate (mmol/L), ferritin (ng/mL), albumin (g/dL), and potassium (mmol/L). A complete blood count was also obtained.
iPTH levels were measured at baseline using standard chemiluminescence immunoassay techniques. Although iPTH was routinely monitored every six months, only a single baseline measurement was used for this study and for all analyses. Elevated iPTH was defined as a serum level above the cohort median value of 224.5 pg/mL. This threshold was used to stratify patients into low and high iPTH groups for subgroup comparisons. Additionally, the clinical cutoff of >600 pg/mL, consistent with KDIGO guidelines, was also explored for sensitivity analyses.
Clinical variables collected at inclusion included patient age, sex, presence of diabetes mellitus, dialysis adequacy (Kt/V), and treatment with vitamin K antagonists (antivitamin K). Medical records were used to extract dialysis duration, comorbidities, and ongoing medications.
AVF thrombosis events were tracked over a 5-year follow-up period based on clinical records. Mortality data were also recorded for the same period.
2.2. Statistical Analysis
Statistical analysis was conducted using MedCalc Software, version 12.5.0 (MedCalc, Mariakerke, Belgium). Continuous variables were tested for normality using the Kolmogorov–Smirnov test with Lilliefors correction, applied to the residuals of each model. All tested distributions failed the normality assumption (D = 1.000, p < 0.0001). However, given the sample size and the robustness of ANOVA to deviations from normality, parametric methods were retained for group comparisons.
Group comparisons for continuous variables—including iPTH, hemoglobin, serum phosphate, dialysis adequacy (Kt/V), and age—were conducted using one-way analysis of variance (ANOVA). Levene’s test was applied to assess the homogeneity of variances. Post hoc pairwise differences were explored using the Scheffé test. Due to the observed skewness and variance heterogeneity in iPTH values (Levene’s p < 0.001; K–S p < 0.0001), we acknowledge that the use of parametric ANOVA may not fully satisfy the assumptions of the test. Nonetheless, it was chosen for comparability with the prior literature and its robustness in moderate deviations from normality.
Survival analysis was performed using Cox proportional hazards regression to assess the relationship between baseline iPTH levels and time to AVF thrombosis. iPTH was modeled as a continuous predictor. Patients were censored at death or at the end of the 5-year follow-up if thrombosis did not occur.
Categorical variables, including diabetes mellitus status and use of AVK therapy, were analyzed using the Chi-square test of independence to assess associations with both AVF thrombosis and five-year mortality outcomes.
All residual distributions were also visually inspected to confirm analytical assumptions. Statistical significance was established at a two-tailed p-value of <0.05 for all tests.
A post hoc power analysis was performed to assess whether the sample size was sufficient to detect moderate associations with AVF thrombosis. Based on 246 evaluable patients and an event rate of 15% (n = 37), the study had 91.4% power to detect a small-to-moderate effect size (Cohen’s h = 0.3, equivalent to an odds ratio of approximately 1.5) at a two-sided alpha level of 0.05, thus supporting the adequacy of the sample size for evaluating primary associations, though smaller or confounded effects may have been underpowered in multivariable models.
3. Results
3.1. Baseline Characteristics of the Study Population
The study cohort consisted of 249 adult patients with end-stage renal disease (ESRD), all of whom had a mature and functional arteriovenous fistula (AVF) and were undergoing maintenance hemodialysis (HD) for more than three months at baseline. The mean age of the study population was 59.5 ± 13.1 years, with a predominance of male patients (65%). Among the participants, 53 patients (21.3%) had a diagnosis of diabetes mellitus. When stratified by iPTH levels, diabetes mellitus was more common in the low iPTH group (25.8%) compared with the high iPTH group (17.2%). A total of 42 patients (16.9%) were undergoing treatment with vitamin K antagonists (antivitamin K, AVK). The median body mass index (BMI) of the cohort was 28.0 kg/m2, with 34.0% of patients meeting criteria for obesity (BMI ≥ 30 kg/m2). When stratified by iPTH levels, obesity prevalence was similar between the low iPTH group (32.3%) and the high iPTH group (33.3%).
At baseline, hypertension was present in 219 patients (88%), consistent with its known high prevalence in the ESRD population.
The average dialysis adequacy, assessed by Kt/V, was approximately 1.44 ± 0.29 across the sample. All patients had stable AVF access at the time of inclusion, and none were receiving dialysis through central venous catheters (CVCs).
In addition to demographic and clinical data, baseline laboratory and dialysis-related characteristics were further analyzed to better define the study cohort. The mean iPTH level at inclusion was 413.5 ± 492.5 pg/mL, with serum calcium averaging 8.97 ± 0.77 mg/dL, phosphate 5.15 ± 1.53 mg/dL, and hemoglobin 11.04 ± 1.47 g/dL. Mean serum albumin was 4.08 ± 0.39 g/dL. When stratified by iPTH levels, mean serum albumin was slightly higher in the high iPTH group (4.14 ± 0.35 g/dL) compared with the low iPTH group (3.99 ± 0.44 g/dL), whereas ferritin levels were similar across groups (median ~500 µg/L).
Patients had been on maintenance hemodialysis for an average of 63.1 ± 58.2 months, and the average AVF age was 51.5 ± 52.0 months at baseline.
Out of the initial 249 patients, 246 had complete 5-year follow-up data, among whom 37 (15.0%) developed AVF thrombosis and 101 patients (41.1%) died.
3.1.1. Relationship Between Dialysis Duration and Fistula Age
Among the 249 patients enrolled, complete data for both dialysis duration and fistula age were available for 246 individuals. A strong positive correlation was observed between dialysis duration and arteriovenous fistula age (Pearson’s r = 0.81), suggesting that fistula age increases proportionally with time on hemodialysis (Figure 1). This finding aligns with the expected parallel between vascular access exposure and cumulative dialysis duration and may reflect time-dependent vascular remodeling.
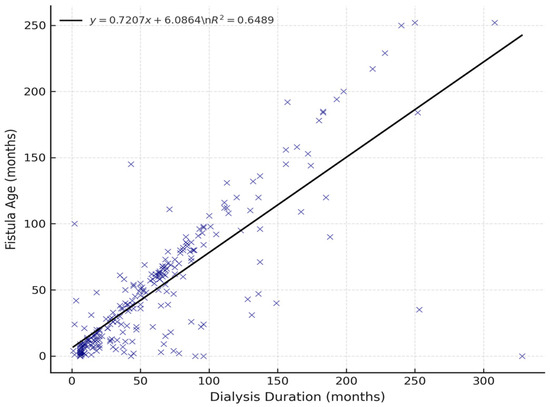
Figure 1.
Correlation between dialysis duration and fistula age (n = 246). A strong positive linear association was observed (r = 0.81). The regression line and corresponding R2 value are shown.
3.1.2. Fistula Location and Thrombosis
Among the 246 patients with complete follow-up, fistula location was documented for all cases. The majority had distal radiocephalic fistulas (n = 70), followed by brachiocephalic (n = 17), and brachiobasilic (n = 16). Thrombosis rates varied by location: twelve events occurred in distal radiocephalic fistulas (17.1%), two events in brachiocephalic fistulas (11.8%), and four events in brachiobasilic fistulas (25.0%). These findings suggest that distal radiocephalic fistulas experienced a moderate risk of thrombosis, while brachiobasilic accesses had the highest relative risk, consistent with their more complex surgical anatomy.
3.2. Biochemical Parameters and AVF Thrombosis Outcomes
Baseline biochemical parameters, including intact parathyroid hormone (iPTH), hemoglobin (Hb), serum phosphate (PO4), dialysis adequacy (Kt/V), and age, were compared between patients who developed arteriovenous fistula (AVF) thrombosis during the five-year follow-up and those who did not. The results of these comparisons are presented in Table 1.

Table 1.
Baseline biochemical and clinical parameters in patients with and without AVF thrombosis.
Among 230 patients with complete iPTH data, patients who developed AVF thrombosis exhibited significantly higher baseline iPTH levels (567.7 ± 740.8 pg/mL) compared to those without thrombosis (383.9 ± 435.4 pg/mL), with this difference reaching statistical significance (p = 0.047). No significant differences were found in hemoglobin levels (11.07 ± 1.36 vs. 11.03 ± 1.50 g/dL; p = 0.880), serum phosphate (5.17 ± 1.53 vs. 5.15 ± 1.54 mg/dL; p = 0.935), Kt/V (1.46 ± 0.26 vs. 1.44 ± 0.30; p = 0.688), or age (59.2 ± 12.2 vs. 59.7 ± 13.4 years; p = 0.830) between the thrombosis and non-thrombosis groups.
These findings suggest that among the analyzed biochemical variables, only elevated iPTH levels were significantly associated with subsequent AVF thrombosis.
3.3. Post Hoc Analysis for iPTH
Out of the 249 patients enrolled, 246 were evaluable at the 5-year follow-up. Among these, 37 patients (15.0%) developed AVF thrombosis, reflecting the prevalence of long-term vascular access complications in this chronic hemodialysis cohort.
To identify baseline predictors of AVF thrombosis, a comparative analysis using one-way ANOVA was performed across clinical and biochemical variables. Among all parameters assessed, only iPTH levels demonstrated a statistically significant association with the occurrence of thrombosis (F = 3.975, p = 0.047). A total of 230 patients had complete iPTH data and were included in the analysis. Patients who developed AVF thrombosis had significantly higher baseline iPTH values (567.71 ± 740.83 pg/mL) compared to those without thrombosis (383.98 ± 435.44 pg/mL) (Table 2). Post hoc analysis using the Scheffé method confirmed the significance of this difference (p < 0.05), supporting a potential association between iPTH levels and AVF dysfunction.

Table 2.
Comparison of baseline iPTH levels between patients with and without AVF thrombosis (n = 230). Values are expressed as mean ± SD.
No statistically significant differences were observed for other baseline variables. The mean hemoglobin level was 11.07 ± 1.36 g/dL in patients with thrombosis and 11.03 ± 1.49 g/dL in those without (p = 0.880). Serum phosphorus levels were 5.17 ± 1.53 mg/dL vs. 5.15 ± 1.54 mg/dL (p = 0.935), and dialysis adequacy, measured by Kt/V, was 1.46 ± 0.26 compared to 1.44 ± 0.30 (p = 0.688). Patient age also showed no difference between groups (59.16 ± 12.22 years vs. 59.67 ± 13.38 years, p = 0.830).
In addition, the influence of AVK therapy and diabetes mellitus on thrombosis risk was evaluated. AVK treatment was recorded in 42 patients, among whom 5 (11.9%) developed AVF thrombosis. In comparison, 32 out of 204 patients not receiving AVK (15.7%) developed thrombosis, indicating no increased risk in the AVK group. Diabetes mellitus was present in 53 patients, with 9 cases of thrombosis (17.0%), compared to 28 events among 193 non-diabetics (14.5%). These differences were not statistically significant, further reinforcing that elevated iPTH was the only factor independently associated with thrombosis risk in this cohort.
3.4. Influence of Antivitamin K Therapy and Diabetes on Mortality
The relationship between five-year all-cause mortality and two key clinical variables—AVK therapy and diabetes mellitus—was examined using the Chi-square test of independence.
Among the 42 patients receiving AVK therapy, 23 deaths were recorded during the follow-up period, corresponding to a five-year mortality rate of 54.8%. In contrast, 78 out of 204 patients not treated with AVK died, resulting in a mortality rate of 38.2%. Although the mortality rate was numerically higher among AVK-treated patients, the difference did not reach statistical significance (χ2 = 3.37, p = 0.067) (Table 3).

Table 3.
Five-year mortality according to antivitamin K therapy and diabetes mellitus status. Comparisons were performed using the Chi-square test of independence.
A total of 53 patients were identified as having diabetes mellitus at baseline. Among these, 30 patients died within five years, yielding a mortality rate of 56.6%. This was substantially higher than the 36.8% mortality observed among the 193 non-diabetic patients, of whom 71 died. The difference was statistically significant according to the Chi-square analysis (χ2 = 6.07, p = 0.014).
These results indicate that diabetes mellitus was significantly associated with increased five-year mortality, while AVK therapy showed a non-significant trend toward higher mortality. Further investigation in larger cohorts may be warranted to clarify the potential effect of AVK treatment on long-term survival in chronic hemodialysis patients.
To assess whether iPTH independently predicts AVF thrombosis when adjusting for potential confounders, we performed a multivariable logistic regression analysis including age, diabetes mellitus, vitamin K antagonist therapy, and Kt/V. In this model, baseline iPTH levels were not statistically significant (p = 0.194). None of the covariates reached significance, possibly due to sample size limitations (Table 4).

Table 4.
Multivariable logistic regression of baseline predictors for 5-year AVF thrombosis (n = 220).
Therefore, while elevated iPTH may contribute to the pathophysiology of AVF thrombosis, it should not be considered an independent clinical predictor without further multivariable validation.
To account for time-dependent risk and censoring, we performed a Cox proportional hazards regression using iPTH as a continuous variable to model time to AVF thrombosis. The model revealed that iPTH was not significantly associated with the hazard of AVF thrombosis over the five-year period (Hazard Ratio [HR] = 1.0003; 95% Confidence Interval [CI]: 0.9999–1.0007; p = 0.158). While elevated iPTH levels were associated with higher overall thrombosis rates in univariate analysis, they did not significantly influence the timing of thrombosis events when evaluated within a time-to-event framework. These findings support the association between high iPTH and AVF thrombosis risk but suggest that its predictive value may not extend to accelerated time to thrombosis in this cohort.
3.5. Summary of Key Findings
This study identified elevated iPTH levels at baseline as the only biochemical parameter significantly associated with the development of AVF thrombosis over a five-year follow-up period. Patients who experienced AVF thrombosis had significantly higher mean iPTH levels compared to those without thrombosis (p = 0.047). No significant associations were found between AVF thrombosis and other baseline parameters, including hemoglobin, serum phosphate, dialysis adequacy (Kt/V), or patient age, as confirmed by one-way ANOVA.
Likewise, the presence of diabetes mellitus and the use of AVK therapy were not significantly associated with AVF thrombosis in this cohort.
In contrast, when evaluating all-cause five-year mortality, diabetes mellitus was found to be a statistically significant predictor of mortality (p = 0.014, Chi-square test), with diabetic patients exhibiting substantially higher mortality rates compared to non-diabetic patients. Although a higher mortality rate was also observed among AVK-treated patients, this difference did not reach statistical significance (p = 0.067).
Taken together, these findings suggest that iPTH is associated with AVF thrombosis, though not predictive in multivariable models, and diabetes mellitus as a significant risk factor for long-term mortality in patients undergoing chronic hemodialysis. Other routine clinical and biochemical parameters, including AVK therapy, were not independently associated with either thrombosis or mortality within this study cohort.
4. Discussion
This study provides evidence that elevated baseline iPTH can be associated with AVF thrombosis in chronic hemodialysis patients, while other common clinical and biochemical parameters showed no such association. Additionally, diabetes mellitus emerged as a strong predictor of five-year mortality. These findings suggest a potential role for iPTH as a vascular risk biomarker and confirm the known mortality burden in diabetic HD patients.
In recent years, iPTH has been increasingly recognized as more than a regulator of calcium-phosphate homeostasis; it plays a broader role in vascular biology, particularly in patients with CKD undergoing hemodialysis. Although the role of iPTH in vascular biology has gained renewed attention in recent years, the association between hyperparathyroidism and vascular access thrombosis was recognized as early as 2003. In a foundational study, Grandaliano et al. [15] reported that hyperparathyroidism was significantly more prevalent in patients with AVF failure, independent of traditional cardiovascular risk factors. They proposed that elevated iPTH may contribute to intimal hyperplasia and vascular injury both directly—through PTH receptor-mediated effects on vascular smooth muscle cells—and indirectly via disruptions in calcium–phosphorus balance. Mechanistically, PTH has been shown to promote vascular calcification by inducing phenotypic switching in vascular smooth muscle cells, leading to osteogenic transformation and matrix deposition in the vessel wall [16,17]. These early insights laid the groundwork for current interest in iPTH as a modifiable risk factor for AVF dysfunction in hemodialysis patients. Lee et al. [18] emphasized that such vascular changes are not only associated with hypertension and diabetes but also result from disrupted calcium-phosphate regulation and oxidative stress, conditions prevalent in CKD patients.
To explore whether a dose–response relationship existed between iPTH levels and AVF thrombosis risk, we stratified patients into quartiles based on baseline iPTH. The incidence of thrombosis increased progressively from the first to the fourth quartile, with the highest rate (17.2%) observed in Q4. Although the trend did not reach statistical significance in the initial analysis, these findings suggest a potential dose–response relationship whereby progressively higher iPTH levels are associated with increased thrombotic risk. Although baseline iPTH values were significantly higher in patients who developed AVF thrombosis (p = 0.047, ANOVA), the distribution of iPTH was highly skewed, and Levene’s test confirmed unequal variances (p < 0.001). These results raise caution regarding the robustness of parametric analysis in this context. While we chose ANOVA to align with the prior literature and maintain clinical interpretability, the findings must be interpreted with care. The association between iPTH and thrombosis was not preserved after log-transformation or in non-parametric analysis. Kadiroglu et al. [19] demonstrated that patients requiring repeated AVF placement had significantly higher iPTH, phosphorus, and Ca × P product levels, highlighting a link between mineral metabolism dysregulation and access failure. Similarly, Morena et al. [20] showed that elevated phosphorus and PTH levels were associated with increased vascular access thrombosis over two years of follow-up, even in the absence of significant inflammatory markers. Consistent with these observations, Gheith and Kamal [21] identified elevated iPTH as an independent predictor of vascular access failure in a cohort of Egyptian hemodialysis patients, reporting a 1.5-fold increased risk of AVF thrombosis associated with higher baseline iPTH levels (p = 0.01).
A strong positive correlation between dialysis duration and AVF age (r = 0.81) in our cohort confirms the expected parallel between time on hemodialysis and vascular access aging. This relationship suggests that longer dialysis exposure is inherently linked to older fistulas, reflecting cumulative vascular remodeling over time. Roozbeh et al. similarly observed that prolonged dialysis duration was associated with increased AVF complications, mediated by thrombogenic and hemodynamic factors [22]. Allon and Robbin emphasized that older fistulas, particularly in high-risk patients, are more prone to dysfunction due to vessel wall changes and repetitive cannulation [23]. While Dember et al. focused on early fistula failure, their findings support the notion that both early and late complications are influenced by the cumulative burden of dialysis-related stress [24].
Hypertension was highly prevalent in our cohort, consistent with its known frequency in the ESRD population. Nevertheless, because of its near-universal presence, hypertension is unlikely to account for the association observed between elevated PTH and AVF thrombosis.
In our study, the presence of diabetes mellitus was significantly associated with increased five-year mortality among chronic hemodialysis patients, with over half (56.6%) of diabetic individuals dying within the follow-up period compared to 36.8% of non-diabetics (p = 0.014). These findings corroborate earlier reports emphasizing diabetes as a key prognostic determinant in dialysis populations. Notably, Soleymanian et al. [25] reported a comparable 2-fold increase in mortality among diabetic HD patients, attributing this excess risk primarily to cardiovascular comorbidities such as coronary artery disease and cerebrovascular events. Similarly, the meta-analysis by Song et al. [26] identified diabetes as an independent predictor of mortality in elderly HD populations, with a pooled odds ratio of 1.19 (95% CI: 1.06–1.33), reinforcing the consistency of this association across age groups and study settings. At a population level, Wierzba et al. [27] also demonstrated that mortality rates in diabetic ESRD patients were more than 15-fold higher than their non-diabetic counterparts, highlighting the profound survival disadvantage associated with diabetes. Furthermore, in a prospective Polish cohort, Grzywacz et al. [28] confirmed the elevated mortality risk among diabetic dialysis patients and identified poor glycemic control and prior cerebrovascular events as additional predictors of death, while regular physical activity appeared protective.
In our cohort, AVK-treated patients exhibited a higher five-year mortality rate (54.8%) compared to those not receiving AVK therapy (38.2%), though the difference did not reach statistical significance (χ2 = 3.37, p = 0.067). This numerical trend aligns with several observational studies suggesting limited survival benefit and increased risk associated with AVK use in hemodialysis patients. A large cohort study by Chan et al. [29] reported a 27% increased mortality in AVK users (HR 1.27; 95% CI: 1.18–1.37) compared to non-users. Similarly, Tsai et al.’s [30] systematic review found no consistent stroke prevention benefit and a frequent association with bleeding complications (HRs for bleeding: 1.41–3.96). Data from the DOPPS cohort further showed that warfarin use may increase stroke risk in elderly HD patients [31]. A meta-analysis by Van Der Meersch et al. [32] involving 17,380 patients found no effect of VKAs on all-cause mortality (HR 1.00), while confirming elevated bleeding risk (HR 1.21). Consistent with these findings, Wong et al. [33] reported no mortality benefit (RR 0.97) from anticoagulation in dialysis patients with atrial fibrillation, while observing increased risks of both hemorrhagic stroke (RR 1.38) and major bleeding (RR 1.31). Nigwekar and Thadhani also emphasize the lack of randomized trials and call for individualized treatment decisions until stronger evidence emerges [34].
No statistically significant differences were found in several key baseline parameters. The mean hemoglobin level was 11.07 ± 1.36 g/dL in the thrombosis group and 11.03 ± 1.50 g/dL in the non-thrombosis group (p = 0.880). Serum phosphorus was 5.17 ± 1.53 mg/dL vs. 5.15 ± 1.54 mg/dL (p = 0.935), dialysis adequacy (Kt/V) was 1.46 ± 0.26 vs. 1.44 ± 0.30 (p = 0.688), and patient age was 59.2 ± 12.2 years vs. 59.7 ± 13.4 years (p = 0.830), respectively. Our findings are consistent with Premuzic et al. [35], who reported no significant association between AVF patency and hemoglobin (106 ± 12 vs. 108 ± 13 g/L; p = 0.275), serum phosphorus (mean levels not significantly different; p = 0.185), Kt/V (1.43 ± 0.28 vs. 1.46 ± 0.26; p = 0.453), or age (59.7 ± 13.2 vs. 58.4 ± 11.9 years; p = 0.428). Similarly, Bodington et al. [36] concluded that neither baseline Kt/V values nor their variation across sessions were predictive of AVF failure using online clearance monitoring. Farber et al. [37] supported our finding on age, noting no significant effect on early AVF thrombosis risk (OR per decade = 0.86, 95% CI: 0.65–1.14; p = 0.28). Jin et al. [38] also reported no significant age difference between AVF thrombosis (56.72 ± 13.10 years) and non-thrombosis (53.58 ± 13.33 years) patients.
Regarding serum phosphorus, Li et al. [39] found no statistical difference between the patency and dysfunction groups (1.6 ± 0.4 vs. 1.8 ± 0.4 mmol/L; p = 0.004 in univariate but non-significant in multivariate analysis), aligning with our non-significant result (p = 0.935). Hemoglobin similarly showed no significant difference in that cohort (89.7 ± 18.5 vs. 88.2 ± 17.5 g/L; p = 0.726). Contrasting evidence comes from Wen et al. [40], who identified severe anemia as an independent risk factor for AVF dysfunction (p = 0.014).
5. Limitations
While the results of this study are clinically meaningful, several limitations must be acknowledged. The investigation was confined to two centers within a single country, which may limit the broader applicability of the findings. Although the overall sample size was adequate, the number of AVF thrombosis events was relatively modest, potentially reducing the power to detect other relevant risk factors. While Doppler ultrasound was used to confirm AVF thrombosis in most cases, it was performed in external clinical settings rather than within the study protocol. As such, uniform imaging data were not systematically recorded for analysis. No angiographic confirmation was obtained. We acknowledge this as a limitation and suggest that future prospective studies incorporate standardized imaging protocols and quantitative criteria to improve endpoint validity. Inflammatory markers such as CRP, which are known contributors to vascular dysfunction and prothrombotic risk, were not available in our dataset and therefore could not be analyzed. However, surrogate indicators such as ferritin and serum albumin were recorded, providing at least partial information on chronic inflammatory and nutritional status. The absence of CRP limits a more comprehensive assessment of the role of inflammation in AVF outcomes, and future studies should incorporate direct inflammatory markers to better clarify this relationship.
Additionally, our analysis was based on a single baseline iPTH measurement. Given the well-documented biological variability of iPTH and its sensitivity to changes in mineral metabolism, dialysis adequacy, and treatment interventions, a single time point may not fully reflect patients’ long-term iPTH exposure. Serial or time-averaged iPTH values could potentially provide stronger predictive value. Future prospective studies should consider incorporating longitudinal iPTH monitoring and time-varying covariates to better capture dynamic risk profiles.
Several other potential confounders were not available in our dataset and therefore could not be analyzed. Smoking status, prior vascular access history, atherosclerotic disease, and cardiac arrhythmias are all relevant factors that may influence AVF outcomes. Their absence limits our ability to account for their possible effects. Nevertheless, because the study was designed to evaluate biochemical risk factors of AVF thrombosis, vascular access history and cardiovascular comorbidities were not systematically collected. Future studies incorporating these clinical variables will be essential to further clarify their impact.
Future studies should aim to validate these findings in larger and more heterogeneous populations, ideally incorporating longitudinal biochemical monitoring and vascular imaging to capture the dynamic progression of vascular pathology. In addition, interventional trials targeting disordered mineral metabolism, particularly through modulation of iPTH levels, may help determine whether such approaches can mitigate the risk of AVF dysfunction in patients receiving chronic hemodialysis.
6. Conclusions
In this 5-year prospective cohort study, elevated baseline levels of iPTH were associated with a higher risk of AVF thrombosis among patients undergoing chronic hemodialysis. While no other biochemical or clinical parameters—including hemoglobin, phosphate, dialysis adequacy, diabetes, or antivitamin K therapy—were significantly linked to AVF thrombosis, diabetes mellitus was independently associated with increased five-year mortality.
Elevated baseline levels of iPTH were significantly associated with increased risk of AVF thrombosis in univariate analysis. However, multivariable logistic regression did not confirm iPTH as an independent predictor when adjusting for age, diabetes, AVK therapy, and Kt/V. These results suggest a potential role for iPTH in vascular risk stratification, though further studies are needed to clarify its independent prognostic value.
Author Contributions
Conceptualization, M.P. and F.B.; methodology M.P., M.D.T. and F.B.; software, I.D.G.; validation, F.B.; formal analysis, I.D.G. and M.B.; investigation, M.P. and A.S. (Alexandru Sircuta); resources, data curation, writing—original draft preparation, O.S., M.P. and I.D.G.; writing—review and editing, F.B.; visualization, I.D.G.; supervision, F.B. and A.S. (Adalbert Schiller); project administration, F.B.; funding acquisition, L.P. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Medicine and Pharmacy “Victor Babes” Timisoara, Centre for Molecular Research in Nephrology and Vascular Disease, Faculty of Medicine, and “Victor Babes” University of Medicine and Pharmacy, Timișoara, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Medicine and Pharmacy “Victor Babes” Timisoara. Approval nr 34/30.06.2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Stolic, R. Most Important Chronic Complications of Arteriovenous Fistulas for Hemodialysis. Med. Princ. Pract. 2013, 22, 220–228. [Google Scholar] [CrossRef]
- Al-Jaishi, A.A.; Liu, A.R.; Lok, C.E.; Zhang, J.C.; Moist, L.M. Complications of the Arteriovenous Fistula: A Systematic Review. J. Am. Soc. Nephrol. 2016, 28, 1839–1850. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Kang, Y.; Ma, S.; Luo, X.; Fan, Y.; Du, J.; Luo, H.; Wang, X.; Deng, F.; et al. The Positive Association of Homocysteine (Hcy) with Arteriovenous Fistula Thrombosis (AVFT) in Chinese Patients on Hemodialysis: A Retrospective Cohort Study. Ann. Vasc. Surg. 2025, 115, 261–274. [Google Scholar] [CrossRef]
- Sidawy, A.N.; Spergel, L.M.; Besarab, A.; Allon, M.; Jennings, W.C.; Padberg, F.T., Jr.; Murad, M.H.; Montori, V.M.; O’Hare, A.M.; Calligaro, K.D.; et al. The Society for Vascular Surgery: Clinical Practice Guidelines for the Surgical Placement and Maintenance of Arteriovenous Hemodialysis Access. J. Vasc. Surg. 2008, 48 (Suppl. S5), S2–S25. [Google Scholar] [CrossRef]
- Roetker, N.S.; Guo, H.; Ramey, D.R.; McMullan, C.J.; Atkins, G.B.; Wetmore, J.B. Hemodialysis Vascular Access and Risk of Major Bleeding, Thrombosis, and Cardiovascular Events: A Cohort Study. Kidney Med. 2022, 4, 100456. [Google Scholar] [CrossRef]
- Girerd, S.; Han, K.H.; Labreuche, J.; Vaur, L.; Hannedouche, T.; Drueke, T.B.; Massy, Z.A.; Zannad, F. Arteriovenous fistula thrombosis is associated with increased all-cause and cardiovascular mortality in haemodialysis patients from the AURORA trial. Clin. Kidney J. 2020, 13, 116–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, J.; Zhang, X.; Peng, S.; Dong, Y.; Sha, L. Meta-Analysis of Risk Factors for Arteriovenous Fistula Thrombosis in Hemodialysis Patients. Kidney Blood Press. Res. 2022, 47, 643–653. [Google Scholar] [CrossRef]
- Shiri, P.; Rezaeian, S.; Abdi, A.; Jalilian, M.; Khatony, A. Risk Factors for Thrombosis in Dialysis Patients: A Comprehensive Systematic Review and Meta-Analysis. J. Vasc. Nurs. 2024, 42, 165–176. [Google Scholar] [CrossRef]
- Liu, C.-T.; Hsu, S.-C.; Hsieh, H.-L.; Chen, C.-H.; Chen, C.-Y.; Sue, Y.-M.; Lin, F.-Y.; Shih, C.-M.; Shiu, Y.-T.; Huang, P.-H. Parathyroid Hormone Induces Transition of Myofibroblasts in Arteriovenous Fistula and Increases Maturation Failure. Endocrinology 2021, 162, bqab044. [Google Scholar] [CrossRef]
- Chinese Experts Group of the Guideline for the Management of ‘CKD-PeriDialysis’; Chinese Non-Government Medical Institutions Association. Chinese Clinical Practice Guideline for the Management of “CKD-PeriDialysis”—The Periods Prior to and in the Early-Stage of Initial Dialysis. Kidney Int. Rep. 2022, 7 (Suppl. S1), S531–S558. [Google Scholar] [CrossRef]
- Chen, B.; Fang, Q.; Tao, Y.; Peng, S.; Deng, S.; Yuan, Y.; Jiang, N.; Wen, S.; Li, B.; Wu, Q.; et al. Factors associated with dysfunction of autogenous arteriovenous fistula in patients with secondary hyperparathyroidism after parathyroidectomy. Ren. Fail. 2024, 46, 2402515. [Google Scholar] [CrossRef]
- Farber, A.; Imrey, P.B.; Huber, T.S.; Huber, T.M.; Kaufman, J.M.; Kraiss, L.W.; Larive, B.; Li, L.; Feldman, H.I. Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J. Vasc. Surg. 2016, 63, 163–170. [Google Scholar] [CrossRef]
- Long, J.; Chen, H.; Huang, Q.; Chen, X.; Ellis, R.J.; Zanoli, L.; Mussap, M.; Zhu, C. Analysis of risk factors for late arteriovenous fistula failure and patency rates after angioplasty in hemodialysis patients: A retrospective cohort study. Transl. Androl. Urol. 2024, 13, 209–217. [Google Scholar] [CrossRef]
- Kim, J.-K.; Jeong, J.H.; Song, Y.R.; Kim, H.J.; Lee, W.Y.; Kim, K.I.; Kim, S.G. Obesity-related decrease in intraoperative blood flow is associated with maturation failure of radiocephalic arteriovenous fistula. J. Vasc. Surg. 2015, 62, 1010–1017.e1. [Google Scholar] [CrossRef]
- Grandaliano, G.; Teutonico, A.; Allegretti, A.; Losappio, R.; Mancini, A.; Gesualdo, L.; Schena, F.P.; Pertosa, G. The role of hyperparathyroidism, erythropoietin therapy, and CMV infection in the failure of arteriovenous fistula in hemodialysis. Kidney Int. 2003, 64, 715–719. [Google Scholar] [CrossRef]
- Cozzolino, M.; Gallieni, M.; Brancaccio, D. The mechanisms of hyperphosphatemia-induced vascular calcification. Int. J. Artif. Organs 2008, 31, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Ureña Torres, P.A.; De Broe, M. Calcium-sensing receptor, calcimimetics, and cardiovascular calcifications in chronic kidney disease. Kidney Int. 2012, 82, 19–25. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Kadiroglu, A.K.; Sit, D.; Kayabasi, H.; Yilmaz, Z.; Yilmaz, E. The association of demographic, clinical, and thrombophilic factors with the failure of arteriovenous fistula among hemodialysis patients. Saudi Med. J. 2008, 29, 888–891. [Google Scholar] [PubMed]
- Morena, M.; Bosc, J.Y.; Jaussent, I.; Dupuy, A.M.; Terrier, N.; Leray-Moragues, H.; Flavier, J.L.; Maurice, F.; Delcourt, C.; Cristol, J.P.; et al. The role of mineral metabolism and inflammation on dialysis vascular access failure. J. Vasc. Access 2006, 7, 77–82. [Google Scholar] [CrossRef]
- Gheith, O.A.; Kamal, M.M. Risk factors of vascular access failure in patients on hemodialysis. Iran. J. Kidney Dis. 2008, 2, 201–207. [Google Scholar] [PubMed]
- Roozbeh, J.; Serati, A.-R.; Malekhoseini, S.-A. Arteriovenous Fistula Thrombosis in Patients on Regular Hemodialysis: A Report of 171 Patients. Arch. Iran. Med. 2006, 9, 26–32. [Google Scholar] [PubMed]
- Allon, M.; Robbin, M.L. Increasing Arteriovenous Fistulas in Hemodialysis Patients: Problems and Solutions. Kidney Int. 2002, 62, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Dember, L.M.; Beck, G.J.; Allon, M.; Delmez, J.A.; Dixon, B.S.; Greenberg, A.; Himmelfarb, J.; Vazquez, M.A.; Gassman, J.J.; Greene, T.; et al. Effect of Clopidogrel on Early Failure of Arteriovenous Fistulas for Hemodialysis: A Randomized Controlled Trial. JAMA 2008, 299, 2164–2171. [Google Scholar] [CrossRef]
- Soleymanian, T.; Kokabeh, Z.; Ramaghi, R.; Mahjoub, A.; Argani, H. Clinical Outcomes and Quality of Life in Hemodialysis Diabetic Patients versus Non-Diabetics. J. Nephropathol. 2016, 6, 81–89. [Google Scholar] [CrossRef]
- Song, Y.H.; Cai, G.Y.; Xiao, Y.F.; Chen, X.M. Risk Factors for Mortality in Elderly Haemodialysis Patients: A Systematic Review and Meta-Analysis. BMC Nephrol. 2020, 21, 377. [Google Scholar] [CrossRef]
- Wierzba, W.; Śliwczyński, A.; Karnafel, W.; Gujski, M.; Słodki, M.; Lusawa, A.; Pinkas, J. The Association of Diabetes with All-Cause Mortality in Patients with End-Stage Renal Disease Compared to the General Population in Poland—A Comparative Analysis. Arch. Med. Sci. 2022, 18, 314–319. [Google Scholar] [CrossRef]
- Grzywacz, A.; Lubas, A.; Smoszna, J.; Niemczyk, S. Risk Factors Associated with All-Cause Death Among Dialysis Patients with Diabetes. Med. Sci. Monit. 2021, 27, e930152. [Google Scholar] [CrossRef]
- Chan, K.E.; Lazarus, J.M.; Thadhani, R.; Hakim, R.M. Anticoagulant and Antiplatelet Usage Associates with Mortality among Hemodialysis Patients. J. Am. Soc. Nephrol. 2009, 20, 872–881. [Google Scholar] [CrossRef]
- Tsai, C.; Marcus, L.Q.; Patel, P.; Battistella, M. Warfarin Use in Hemodialysis Patients with Atrial Fibrillation: A Systematic Review of Stroke and Bleeding Outcomes. Can. J. Kidney Health Dis. 2017, 4, 2054358117735532. [Google Scholar] [CrossRef]
- Wizemann, V.; Tong, L.; Satayathum, S.; Disney, A.; Akiba, T.; Fissell, R.B.; Kerr, P.G.; Young, E.W.; Robinson, B.M. Atrial Fibrillation in Hemodialysis Patients: Clinical Features and Associations with Anticoagulant Therapy. Kidney Int. 2010, 77, 1098–1106. [Google Scholar] [CrossRef]
- Van Der Meersch, H.; De Bacquer, D.; De Vriese, A.S. Vitamin K Antagonists for Stroke Prevention in Hemodialysis Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Am. Heart J. 2017, 184, 37–46. [Google Scholar] [CrossRef]
- Wong, C.X.; Odutayo, A.; Emdin, C.A.; Kinnear, N.J.; Sun, M.T. Meta-Analysis of Anticoagulation Use, Stroke, Thromboembolism, Bleeding, and Mortality in Patients with Atrial Fibrillation on Dialysis. Am. J. Cardiol. 2016, 117, 1934–1941. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Thadhani, R. Long-Term Anticoagulation for Patients Receiving Dialysis: Tilting the Benefit-to-Risk Ratio? Circulation 2018, 138, e82–e84. [Google Scholar] [CrossRef]
- Premuzic, V.; Hudolin, T.; Pasini, J.; Zimak, Z.; Hauptman, D.; Jelakovic, B.; Kastelan, Z. Hypoproteinemia as a Prognostic Risk Factor for Arteriovenous Fistula Failure. Hemodial. Int. 2018, 22, 37–44. [Google Scholar] [CrossRef]
- Bodington, R.; Hazara, A.M.; Lamplugh, A.; Syed, A.; Bhandari, S. Reassessing the Utility of Access Recirculation and Kt/V for the Prediction of Arteriovenous Fistula Failure Using Online Clearance Monitoring: The SHUNT STUDY. J. Nephrol. 2023, 36, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Imrey, P.B.; Huber, T.S.; Criado, E.; Marston, W.A.; Mendes, R.R.; Bandyk, D.F. Multiple Determinants of Functional Patency of Autogenous Arteriovenous Fistulas: A Multicenter Prospective Study. J. Vasc. Surg. 2016, 63, 163–170.e6. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Fan, Y.; Li, J.; Qi, X.; Li, X.; Li, H. Construction of Risk-Prediction Models for Autogenous Arteriovenous Fistula Thrombosis in Patients on Maintenance Hemodialysis. Blood Purif. 2024, 53, 813–823. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Wang, J.; Zhang, C.; Luo, T. Factors Influencing AVF Dysfunction in Patients with Maintenance Hemodialysis: A Retrospective Study. Ann. Palliat. Med. 2021, 10, 4047–4054. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Li, Z.; Li, J.; Zhou, W.; Liu, Y.; Liu, H.; Chen, G. Risk Factors for Primary Arteriovenous Fistula Dysfunction in Hemodialysis Patients: A Retrospective Survival Analysis in Multiple Medical Centers. Blood Purif. 2019, 48, 276–282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).