Recycling Spent Fluorescent Lamp Glass Waste in Calcium Aluminate Cement: Effects on Hydration and Mechanical Performance
Abstract
Featured Application
Abstract
1. Introduction
2. Experimental Process
2.1. Materials, SLFG Waste Preparation, and Characterization
2.2. Preparation of Pastes and Mortars
2.3. Paste and Mortar Characterization
3. Results and Discussion
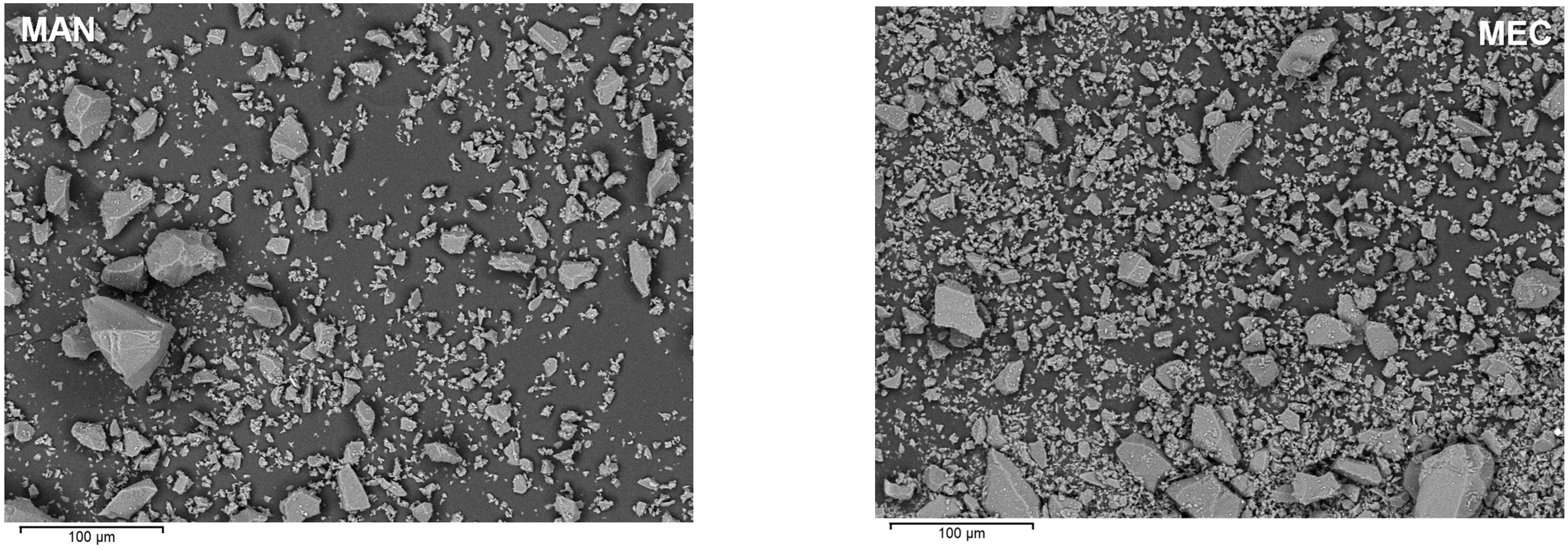
3.1. Raw Material Characterization
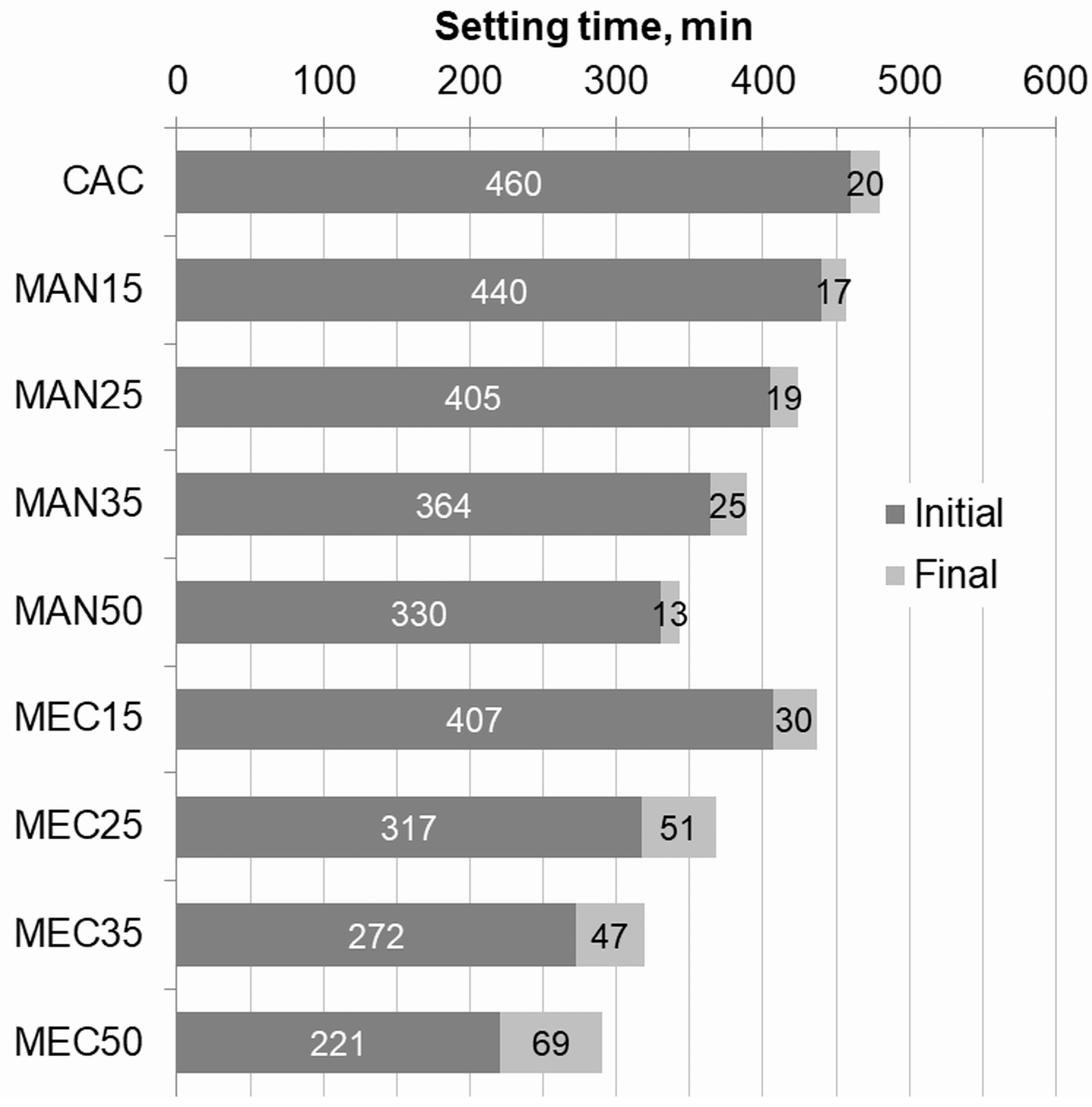
3.2. Setting Time
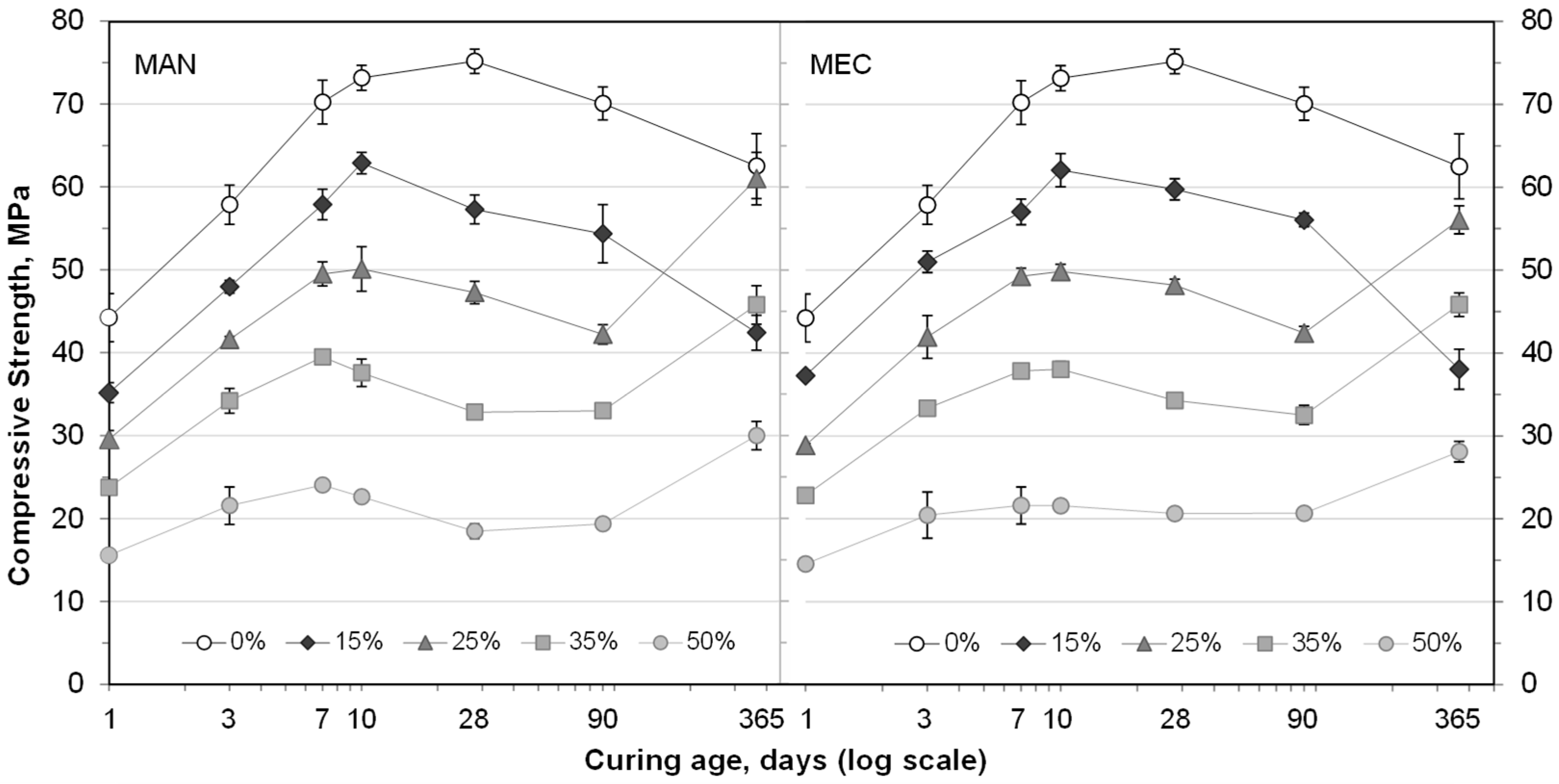
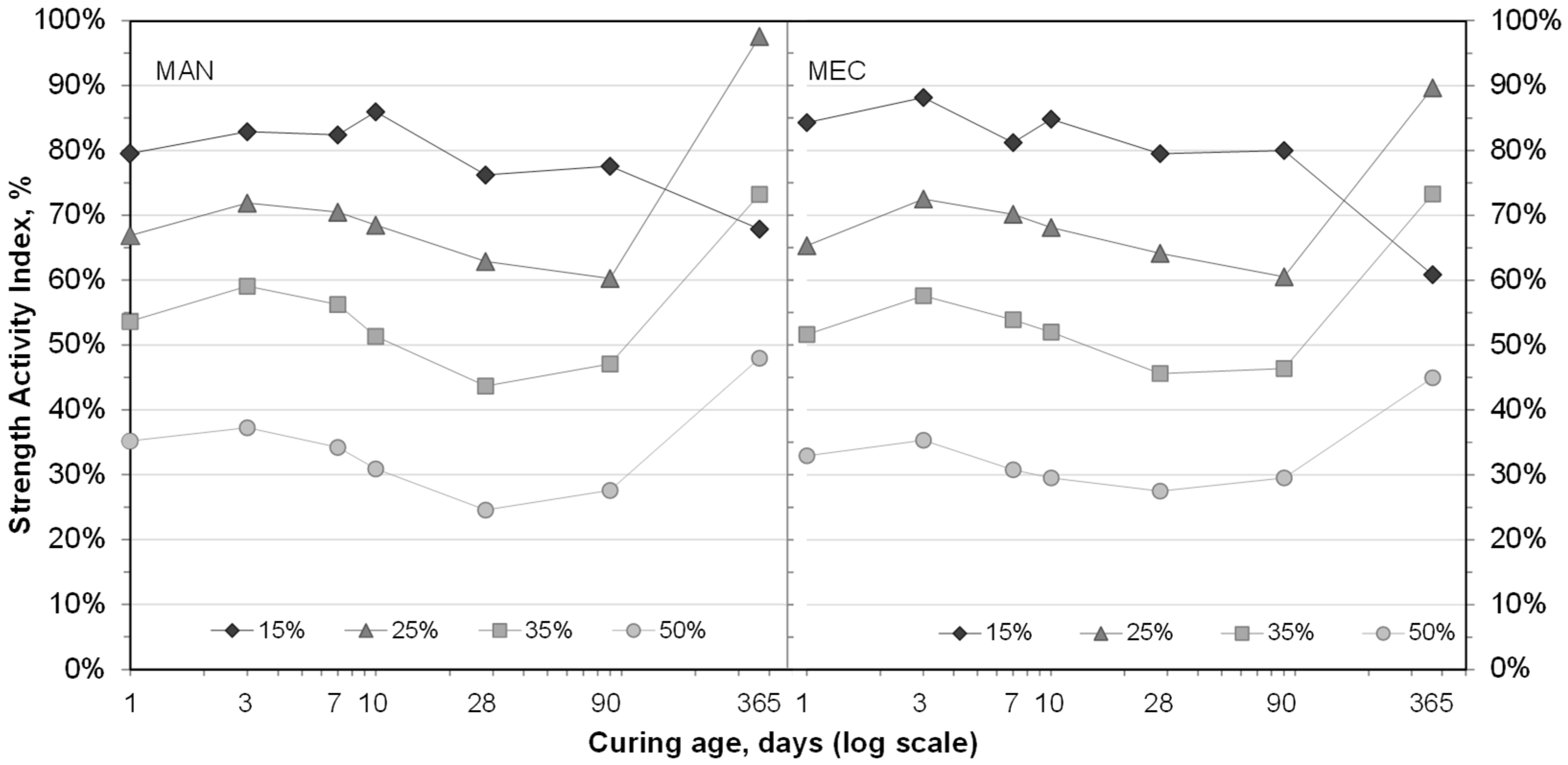
3.3. Compressive Strength of SFLG/CAC Mortars
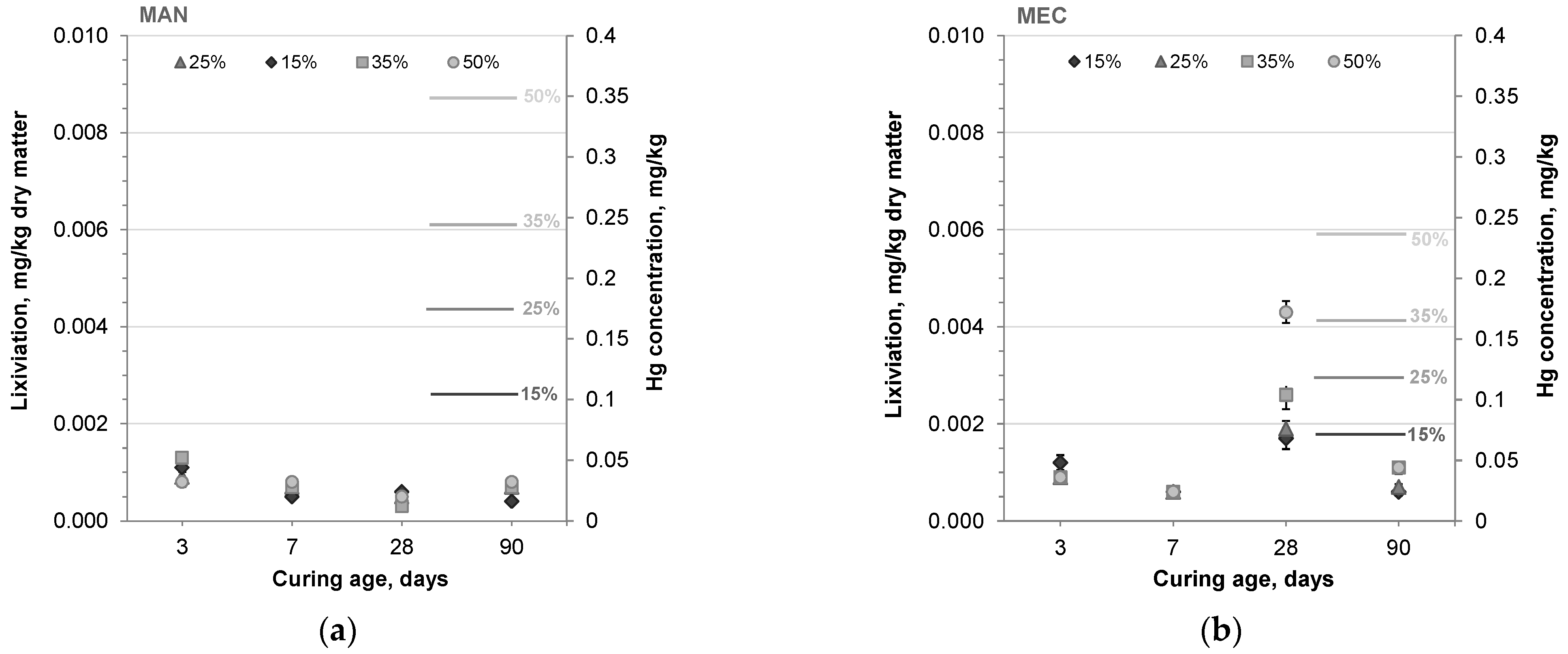
3.4. Lixiviation Results
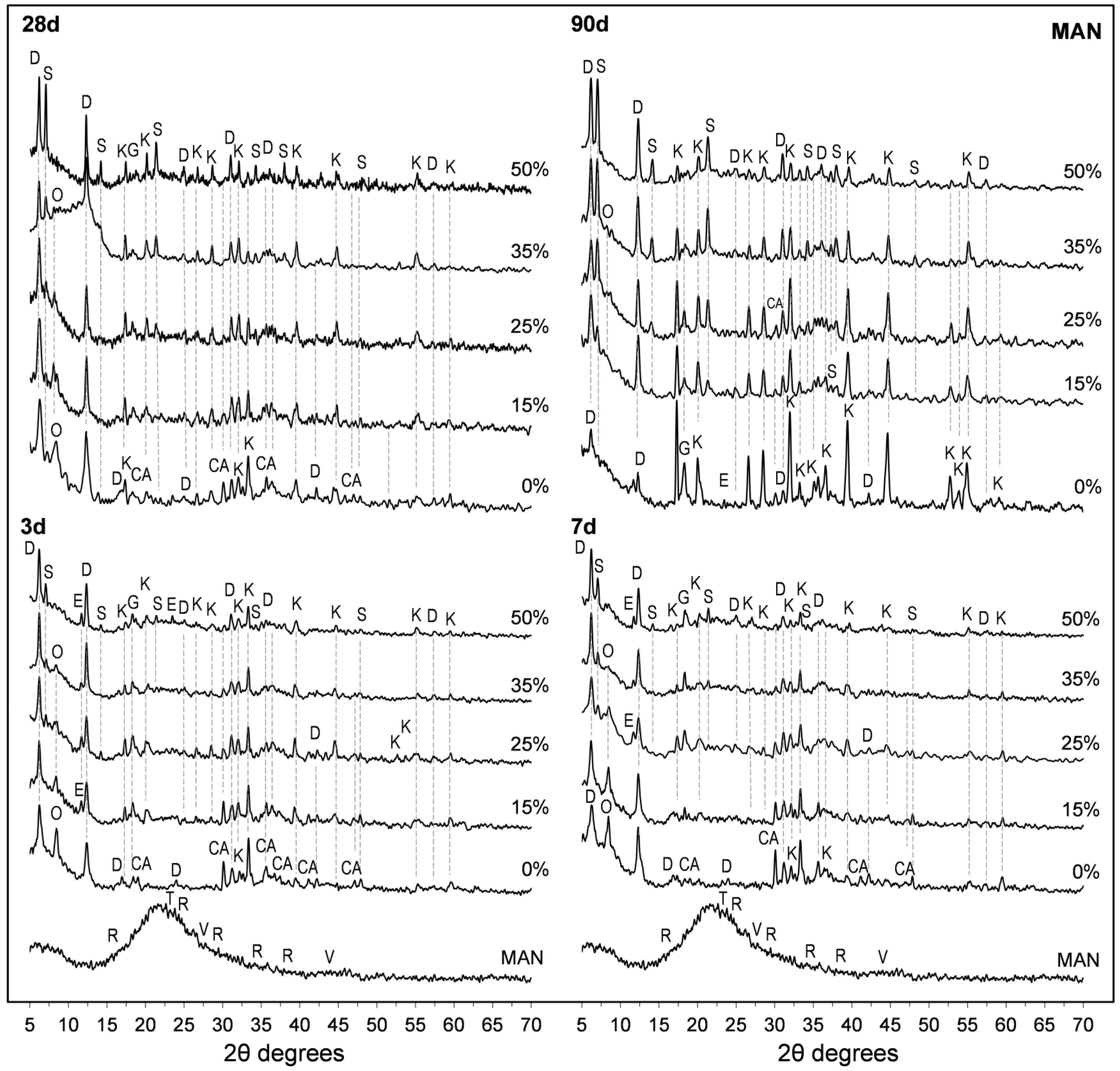
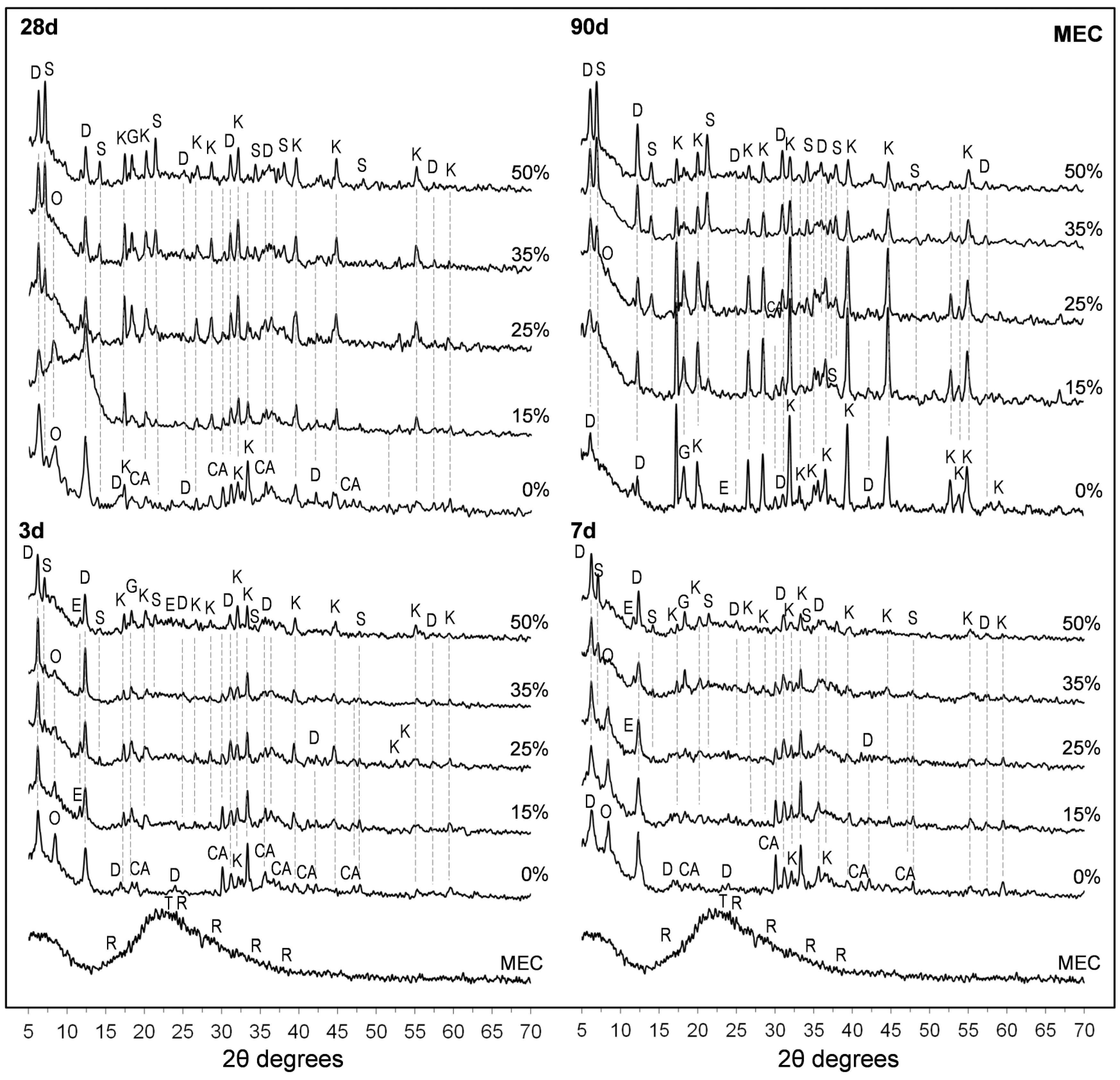
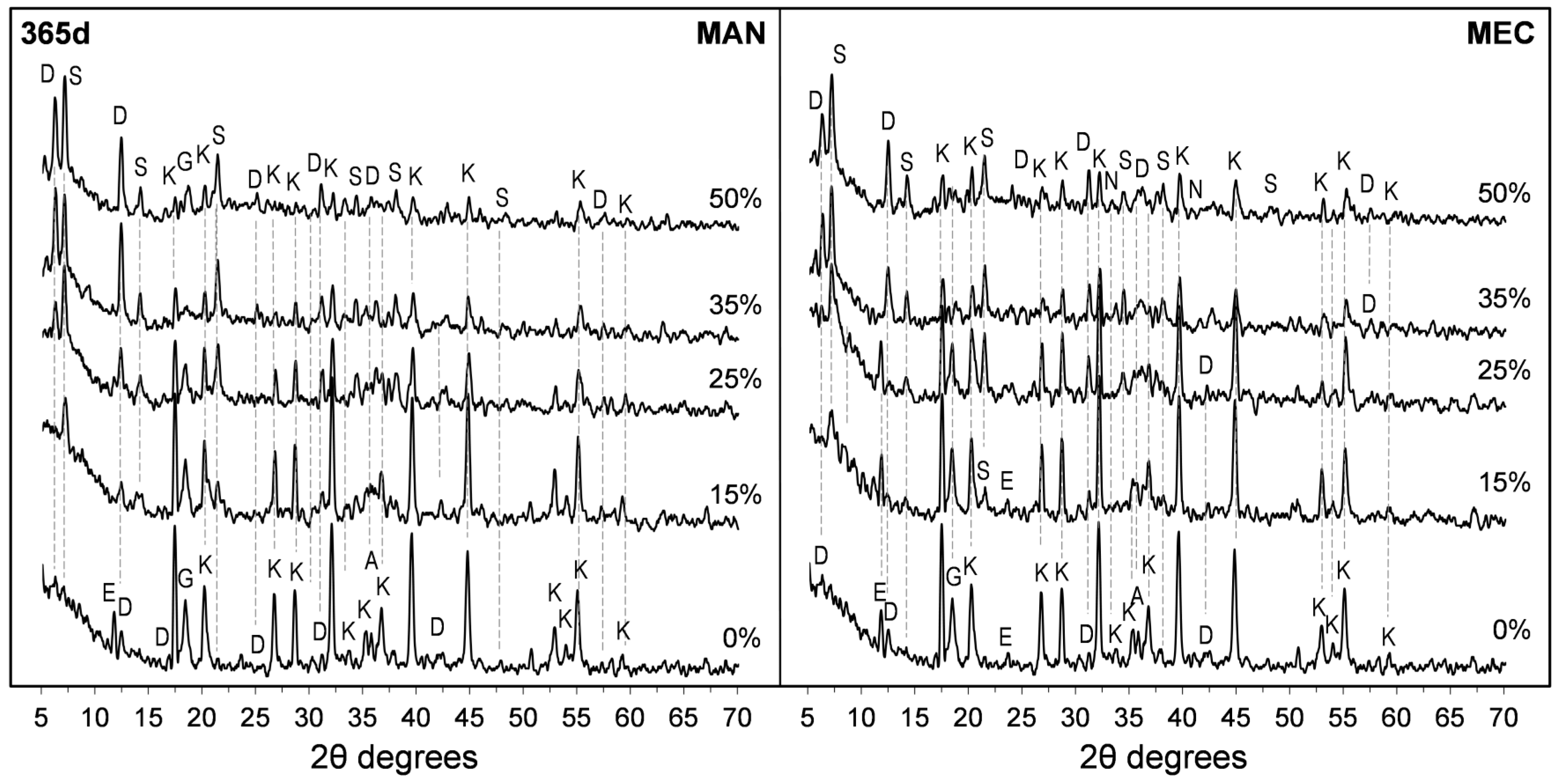
3.5. X-Ray Diffraction (XRD) Studies
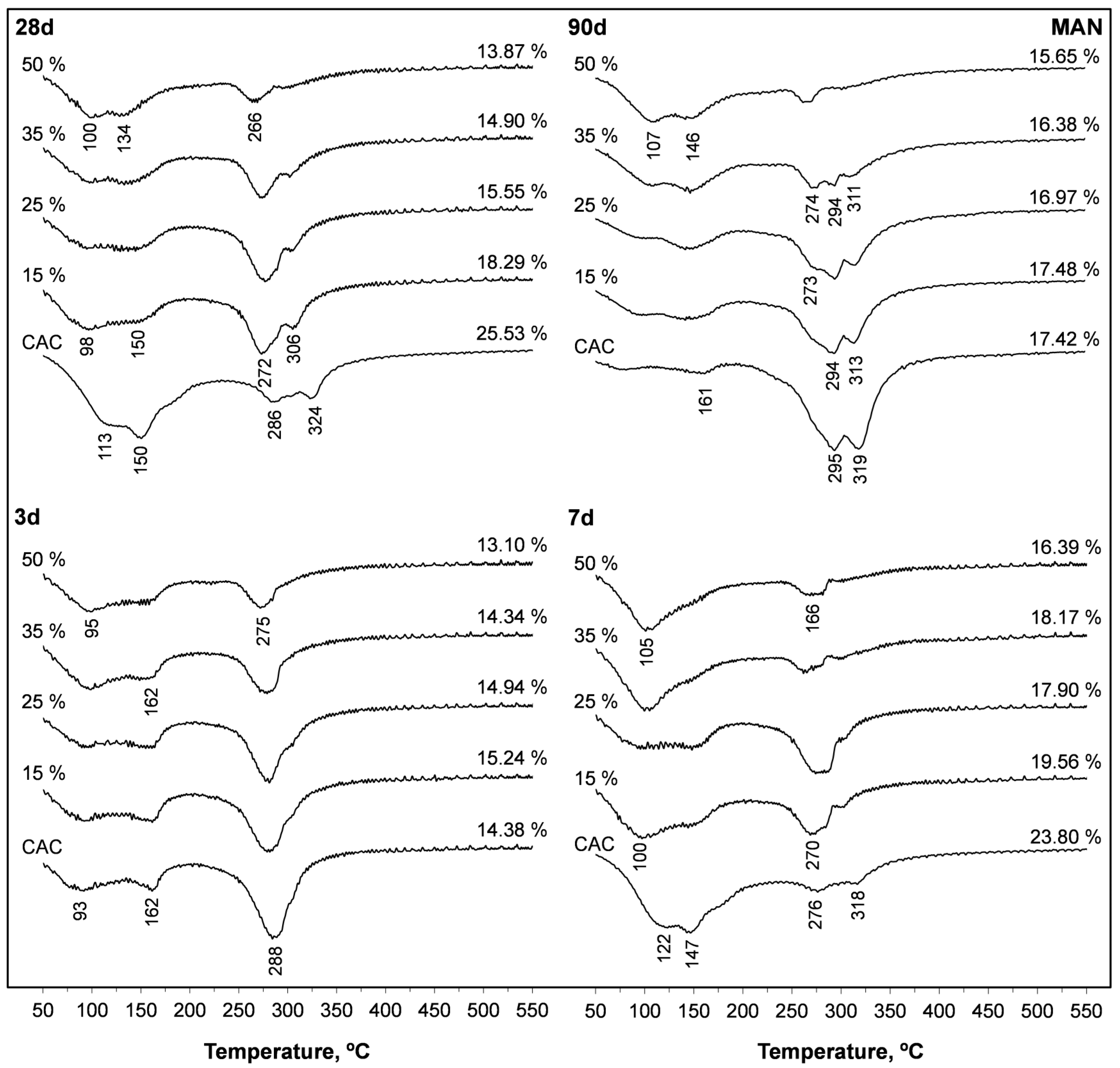
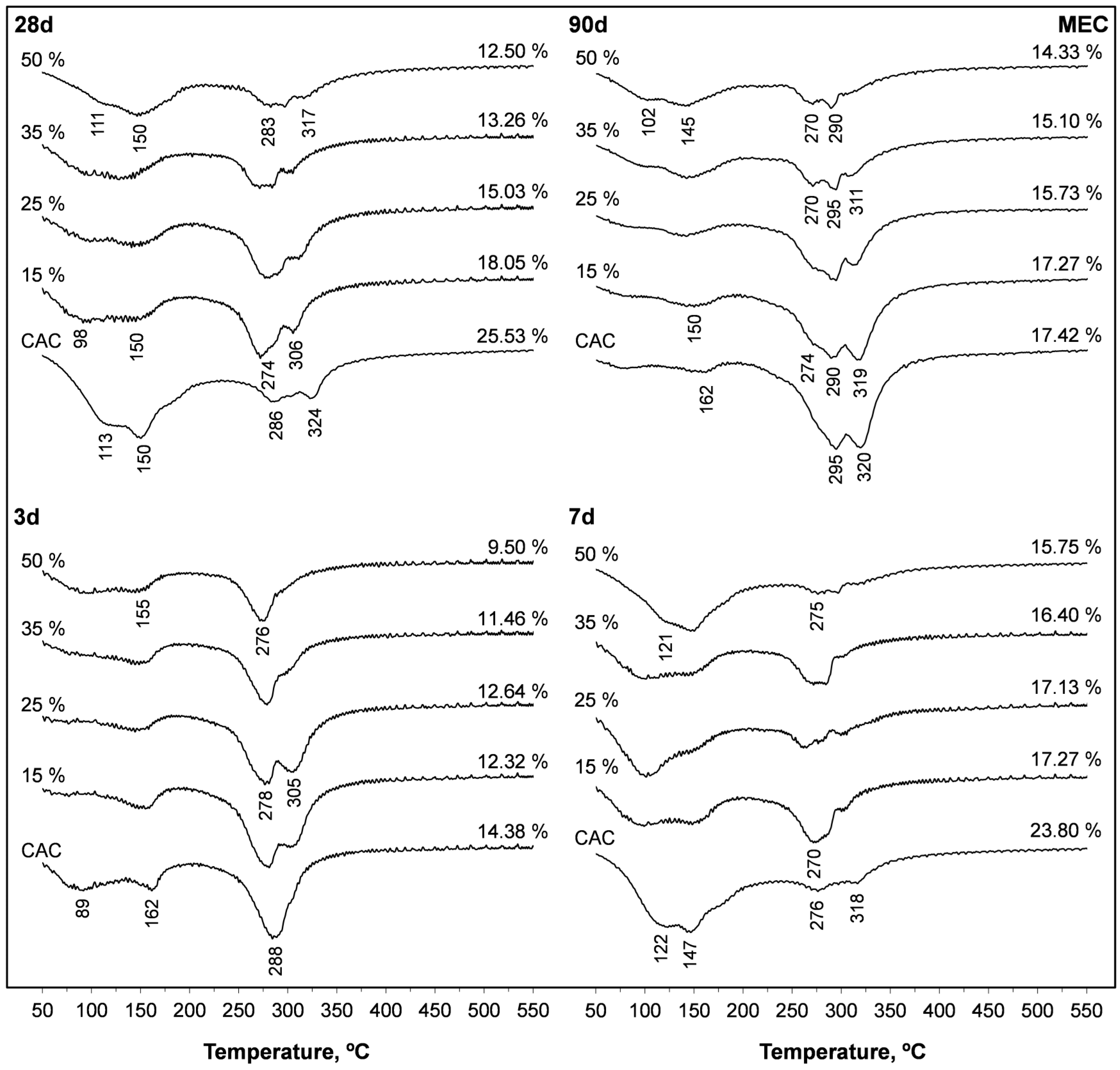
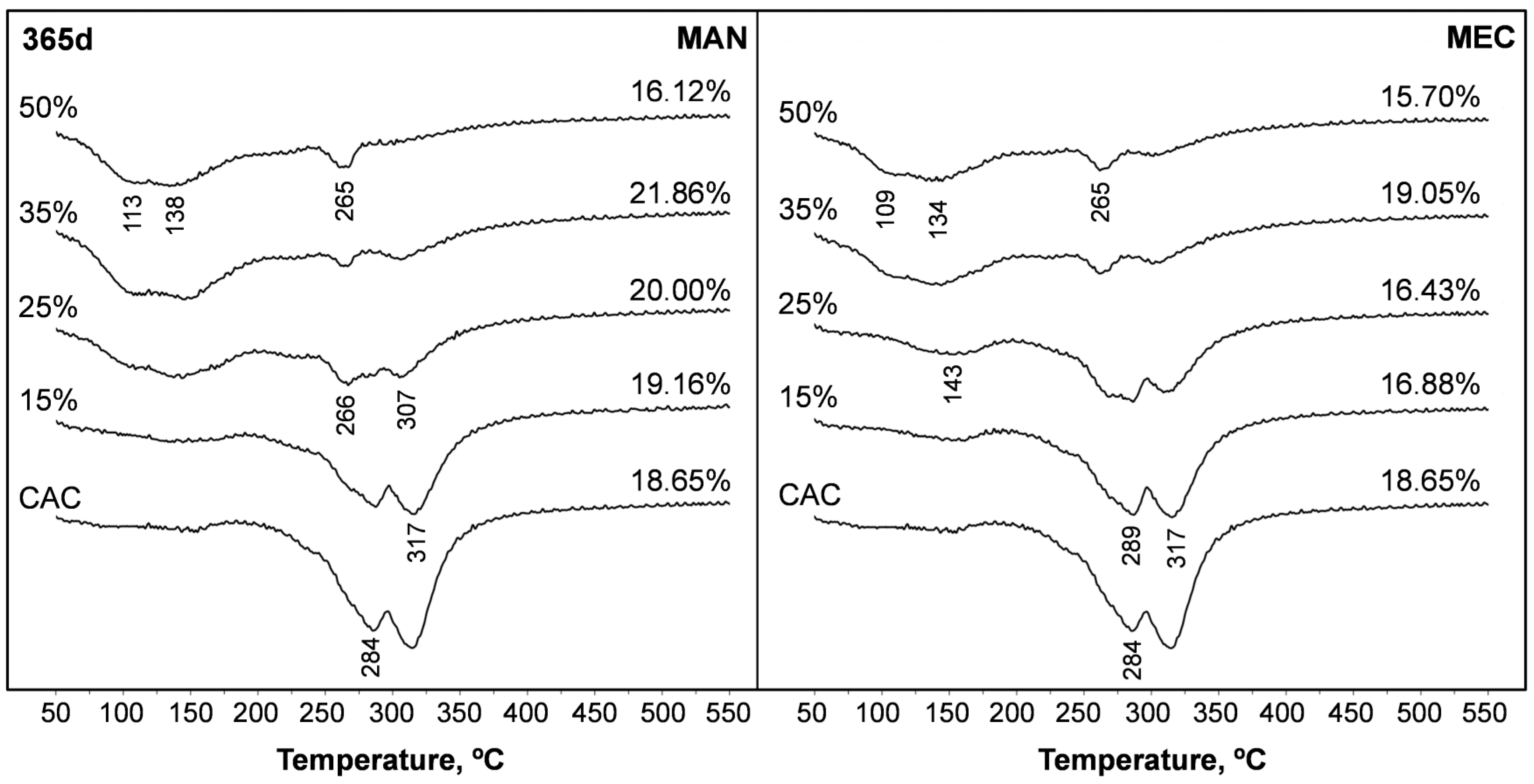
3.6. Thermogravimetric Analyses
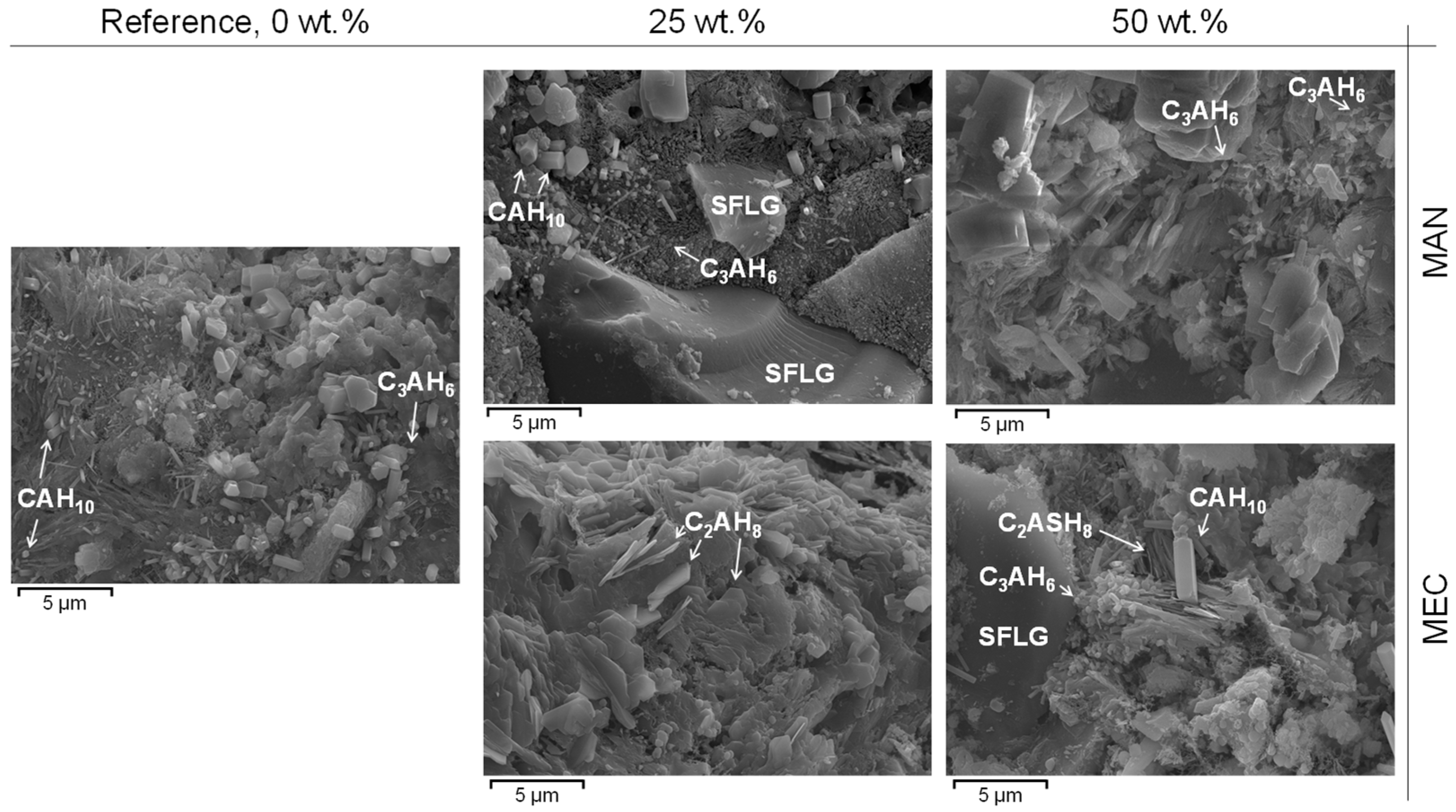
3.7. Scanning Electron Microscopy
4. Conclusions
- Both SFLG wastes, rich in amorphous SiO2, demonstrated reactivity, modifying the CAC hydration process. At substitution levels ≥25 wt.%, the formation of stable strätlingite (C2ASH8) was promoted, reducing the conversion of metastable hexagonal hydrates (CAH10 and C2AH8) into the cubic phase katoite (C3AH6). This stabilization is crucial for enhancing long-term durability in CAC systems.
- SFLG incorporation reduced both initial and final setting times, with MEC waste extending the plastic state duration. Moreover, SFLG waste accelerated hydration, promoting earlier strength development across all substitution levels.
- The partial CAC replacement with SFLG initially reduced compressive strength due to binder dilution. However, mortars containing ≥25 wt.% SFLG exhibited significant strength gains after 365 days, reaching ~60 MPa (25 wt.%) and ~45 MPa (35 wt.%). These long-term improvements are associated with the sustained presence of CAH10 and strätlingite and reduced katoite formation, which enhanced microstructural stability. In contrast, the 100 wt.% CAC and 15 wt.% SFLG mortars experienced typical long-term strength loss due to conversion of hexagonal to cubic hydrates.
- Despite the sodium content in both SFLG types, no evidence of alkaline hydrolysis was observed after 365 days. Key reaction products (CaCO3, Na2CO3, and Al(OH)3) were not significantly detected, and only minor amounts of gibbsite and carboaluminates were found in the highest Na2O pastes (35 wt.% and 50 wt.% MEC). The persistence of CAH10 and strätlingite in blends with ≥25 wt.% SFLG, along with continued strength development, further support the absence of this degradation mechanism under the tested conditions. However, further research under more aggressive environments or with calcareous aggregates is essential, particularly for high-Na2O systems such as MEC35 and MEC50.
- All SFLG/CAC mortars met the regulatory limits for inert waste classification, with mercury leaching well below the 0.01 mg/kg threshold. Mercury was effectively stabilized in the cementitious matrix, regardless of SFLG type or content, demonstrating that untreated SFLG waste can be safely reused in CAC systems without environmental risk.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAC | Calcium aluminate cement |
| CAH10 | Calcium aluminate decahydrate |
| C2AH8 | Calcium aluminate octahydrate |
| C3AH6 | Calcium aluminate hexahydrate or hydrogarnet |
| C2ASH8 | Strätlingite |
| LOI | Loss on ignition |
| MAN | SFLG manually processed |
| MEC | SFLG mechanically processed |
| PC | Portland cement |
| SEM | Scanning electron microscopy |
| SFLG | Spent fluorescent lamp glass |
| TG | Thermogravimetric analysis |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
| Wt.% | Percentage in weight |
References
- Giner-Juan, F.J. Carbonatación vs. Aluminosis. Cercha Rev. Aparejadores Arquit. Técnicos 2011, 107, 56–64. [Google Scholar]
- Son, H.M.; Park, S.; Kim, H.Y.; Seo, J.H.; Lee, H.K. Effect of CaSO4 on hydration and phase conversion of calcium aluminate cement. Constr. Build. Mater. 2019, 224, 40–47. [Google Scholar] [CrossRef]
- Koňáková, D.; Pommer, V.; Jerman, M.; Keppert, M.; Černý, R.; Vejmelková, E. Utilization of ceramic powder, calcined shale and sintered mullite as partial replacements of calcium aluminate cement. Constr. Build. Mater. 2022, 326, 126824. [Google Scholar] [CrossRef]
- Şengül, K.; Erdoğan, S.T. Influence of ground perlite on the hydration and strength development of calcium aluminate cement mortars. Constr. Build. Mater. 2021, 266, 120943. [Google Scholar] [CrossRef]
- García Alcocel, E.; Garcés, P.; Chinchón, S. General study of alkaline hydrolysis in calcium aluminate cement mortars under a broad range of experimental conditions. Cem. Concr. Res. 2000, 30, 1689–1699. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilińska, I.; Nowacka, M. Studies on the influence of different fly ashes and Portland cement on early hydration of calcium aluminate cement. J. Therm. Anal. Calorim. 2011, 106, 859–868. [Google Scholar] [CrossRef][Green Version]
- Hidalgo, A.; García, J.L.; Alonso, M.C.; Fernández, L.; Andrade, C. Microstructure development in mixes of calcium aluminate cement with silica fume or fly ash. J. Therm. Anal. Calorim. 2009, 96, 335–345. [Google Scholar] [CrossRef]
- Rivas Mercury, J.M.; De Aza, A.H.; Turrillas, X.; Pena, P. Hidratación de los cementos de aluminatos de calcio (Parte I). Bol. Sociedat Esp. Ceram. Vydrio 2003, 42, 269–276. Available online: https://scispace.com/pdf/hidratacion-de-los-cementos-de-aluminatos-de-calcio-parte-i-4nnknv5ys1.pdf (accessed on 1 July 2025). [CrossRef]
- Rivas Mercury, J.M.; De Aza, A.H.; Turrillas, X.; Pena, P. Hidratación de los cementos de aluminatos de calcio. Parte II: Efecto de las adiciones de sílice y alúmina. Bol. Sociedat Esp. Ceram. Vydrio 2003, 42, 361–368. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Astoveza, J.; Trauchessec, R.; Soth, R.; Pontikes, Y. Properties of calcium aluminate blended cement incorporating iron-rich slag: Evolution over a curing period of 1 year. Constr. Build. Mater. 2021, 282, 122569. [Google Scholar] [CrossRef]
- Pacewska, B.; Nowacka, M.; Wilińska, I.; Kubissa, W.; Antonovich, V. Studies on the influence of spent FCC catalyst on hydration of calcium aluminate cements at ambient temperature. J. Therm. Anal. Calorim. 2011, 105, 129–140. [Google Scholar] [CrossRef]
- Wu, K.; Han, H.; Rößler, C.; Xu, L.; Ludwig, H.M. Rice hush ash as supplementary cementitious material for calcium aluminate cement—Effects on strength and hydration. Constr. Build. Mater. 2021, 302, 124198. [Google Scholar] [CrossRef]
- Nowacka, M.; Pacewska, B. Effect of structurally different aluminosilicates on early-age hydration of calcium aluminate cement depending on temperature. Constr. Build. Mater. 2020, 235, 117404. [Google Scholar] [CrossRef]
- European Union. Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment; European Union: Luxembourg, 2011; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011L0065 (accessed on 23 July 2024).
- European Union. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE); European Union: Luxembourg, 2012; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012L0019 (accessed on 23 May 2024).
- Ministerio para la Transición Ecológica y el Reto Demográfico, Gobierno de España. Royal Decree 110/2015 of 20 February on Wastes of Electric and Electronic Equipments; Ministerio para la Transición Ecológica y el Reto Demográfico, Gobierno de España: Madrid, Spain, 2015; Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/prevencion-y-gestion-residuos/flujos/aparatos-electr/Royal-Decree-on-wastes-electronic-electric-equipments.aspx (accessed on 23 May 2024).
- Rey-raap, N.; Gallardo, A. Determination of mercury distribution inside spent compact fluorescent lamps by atomic absorption spectrometry. Waste Manag. 2012, 32, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Rey-raap, N.; Gallardo, A. Removal of mercury bonded in residual glass from spent fluorescent lamps. J. Environ. Manag. 2013, 115, 175–178. [Google Scholar] [CrossRef]
- BOE-A-2002-3285; Orden Mam/304/2002, de 8 de Febrero, Por la Que SE Publican Las Operaciones de Valorización Y Eliminación de Residuos Y la Lista Europea de Residuos. Ministerio de Medio Ambiente: Madrid, Spain, 2002. Available online: https://www.boe.es/eli/es/o/2002/02/08/mam304 (accessed on 29 June 2024).
- Ambilamp. Memoria de Sostenibilidad 2023. Available online: https://ambilamp.es/wp-content/uploads/2024/06/Memoria-sostenibilidad-Ambiwaste-2023.pdf (accessed on 15 August 2024).
- Resolución de la Dirección General de Calidad Y Evaluación Ambiental Por la Que SE Publican Los Objetivos Mínimos Estatales Y Autonómicos de Recogida Separada de Residuos de Aparatos Eléctricos Y Electrónicos (Raee) Para El Año 2020. Available online: https://www.miteco.gob.es/content/dam/miteco/es/calidad-y-evaluacion-ambiental/temas/prevencion-y-gestion-residuos/resolucionobjetivosrecogidaraeeestatal-autonomico2020_tcm30-507902.pdf (accessed on 13 August 2024).
- Mehta, A.; Ashish, D.K. Silica fume and waste glass in cement concrete production: A review. J. Build. Eng. 2019, 29, 100888. [Google Scholar] [CrossRef]
- Jiang, D.K.; Ling, T.C.; Mo, K.H.; Shi, C. A critical review of waste glass powder—Multiple roles of utilization in cement-based materials and construction products. J. Environ. Manag. 2019, 242, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Yao, Z.; Ling, T.C.; Sarker, P.K.; Su, W.; Liu, J.; Wu, W.; Tang, J. Recycling difficult-to-treat e-waste cathode-ray-tube glass as construction and building materials: A critical review. Renew. Sustain. Energy Rev. 2018, 81, 595–604. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Tyrer, M.; Richardson, M.; Holmes, N.; Newell, J.; Yio, M.; Wong, H. Predicting the Hydration of Ground Granulated Blast Furnace Slag and Recycled Glass Blended Cements. Appl. Sci. 2025, 15, 6872. [Google Scholar] [CrossRef]
- Alemu, M.Y.; Yehualaw, M.D.; Nebiyu, W.M.; Nebebe, M.D.; Taffese, W.Z. Marble and Glass Waste Powder in Cement Mortar. Appl. Sci. 2025, 15, 3930. [Google Scholar] [CrossRef]
- UNE-EN 14647:2006; Calcium Aluminate Cement—Composition, Specifications and Conformity Criteria. Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain, 2006.
- Pitarch, A.M.; Reig, L.; Gallardo, A.; Soriano, L.; Borrachero, M.V.; Rochina, S. Reutilisation of hazardous spent fluorescent lamps glass waste as supplementary cementitious material. Constr. Build. Mater. 2021, 292, 123424. [Google Scholar] [CrossRef]
- UNE-EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain, 2018.
- UNE-EN 196-3; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain, 2017.
- UNE-EN 12457-4:2003; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/KG for Materials with Particle Size Below 10 MM (Without or with Size Reduction). Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain, 2003.
- Pacewska, B.; Nowacka, M.; Antonovič, V.; Aleknevičius, M. Investigation of early hydration of high aluminate cement-based binder at different ambient temperatures. J. Therm. Anal. Calorim. 2012, 109, 717–726. [Google Scholar] [CrossRef]
- Agencia Estatal, Gobierno de España, Ministerio de la Presidencia. Real Decreto 646/2020, de 7 de Julio, Por El Que SE Regula la Eliminación de Residuos Mediante Depósito en Vertedero; Agencia Estatal, Gobierno de España, Ministerio de la Presidencia: Madrid, Spain, 2020; Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2020-7438 (accessed on 27 June 2024).
- Fernández-Carrasco, L.; Puertas, F.; Blanco-Varela, M.T.; Vázquez, T. Nuevos avances en la carbonatación del cemento aluminoso. Hidrólisis alcalina. Mater. Construcción 1999, 49, 47–55. [Google Scholar] [CrossRef][Green Version]
- Fernández-Carrasco, L.; Blanco-Varela, M.T.; Puertas, F.; Vázquez, T.; Glasser, F.P.; Lachowski, E.E. Hydration of high alumina cement in the presence of alkalis. Adv. Cem. Res. 2000, 12, 143–152. [Google Scholar] [CrossRef]
- Schwarz, N.; Neithalath, N. Influence of a fine glass powder on cement hydration: Comparison to fly ash and modeling the degree of hydration. Cem. Concr. Res. 2008, 38, 429–436. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Saccani, A.; Barbieri, L.; Lancellotti, I. Glass waste as supplementary cementing materials: The effects of glass chemical composition. Cem. Concr. Res. 2015, 55, 45–52. [Google Scholar] [CrossRef]
- Mas, M.A.; Monzó, J.; Payá, J.; Reig, L.; Borrachero, M.V. Ceramic tiles waste as replacement material in Portland cement. Adv. Cem. Res. 2016, 28, 221–232. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Velázquez, S. Evaluation of the pozzolanic activity of fluid catalytic cracking catalyst residue (FC3R). Thermogravimetric analysis studies on FC3R-Portland cement pastes. Cem. Concr. Res. 2003, 33, 603–609. [Google Scholar] [CrossRef]
- Antonovič, V.; Keriene, J.; Boris, R.; Aleknevičius, M. The effect of temperature on the formation of the hydrated calcium aluminate cement structure. Procedia Eng. 2013, 57, 99–106. [Google Scholar] [CrossRef]
- Santacruz, I.; De La Torre, Á.G.; Álvarez-Pinazo, G.; Cabeza, A.; Cuesta, A.; Sanz, J.; Aranda, M.A.G. Structure of stratlingite and effect of hydration methodology on microstructure. Adv. Cem. Res. 2016, 28, 13–22. [Google Scholar] [CrossRef]

| SFLG Waste Type | Designation | Binder/Sand/Water Ratio | CAC Replacement wt.% | Curing T °C | Curing Age Days |
|---|---|---|---|---|---|
| - | CAC | 1:3:0.5 | 0 | 20 | Up to 365 |
| Manual | MAN15 | 15 | |||
| MAN25 | 25 | ||||
| MAN35 | 35 | ||||
| MAN50 | 50 | ||||
| Mechanical | MEC15 | 15 | |||
| MEC25 | 25 | ||||
| MEC35 | 35 | ||||
| MEC50 | 50 |
| SFLG Waste | Setting Time | TG | XRD | SEM | Compressive Strength | Lixiviation |
|---|---|---|---|---|---|---|
| MAN | Performed | 3, 7, 28, 90, 365 | 3, 7, 28, 90, 365 | 28 | 1, 3, 7, 10, 28, 90, 365 | 3, 7, 28, 90 |
| MEC | Performed | 3, 7, 28, 90, 365 | 3, 7, 28, 90, 365 | 28 | 1, 3, 7, 10, 28, 90, 365 | 3, 7, 28, 90 |
| MAN | MEC | |
|---|---|---|
| SiO2 | 75.7 | 74.2 |
| Na2O | 7.6 | 12.2 |
| CaO | 2.9 | 4.4 |
| Al2O3 | 5.2 | 3.1 |
| MgO | 1.0 | 1.9 |
| K2O | 2.6 | 1.8 |
| BaO | 2.4 | 1.3 |
| Other | 1.92 | 0.86 |
| LOI * | 0.65 | 0.36 |
| MAN | MEC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 | 25 | 35 | 50 | 15 | 25 | 35 | 50 | ||
| Hg concentration in blended mortars, mg/kg | 0.1047 | 0.1745 | 0.2443 | 0.3490 | 0.0711 | 0.1185 | 0.1660 | 0.2371 | |
| 3 days | Lixiviation, mg/kg dry matter | 0.0011 | 0.0009 | 0.0013 | 0.0008 | 0.0012 | 0.0009 | 0.0009 | 0.0009 |
| VC, % | 12.86 | 4.71 | 4.99 | 6.56 | 7.25 | 4.61 | 10.15 | 2.34 | |
| 7 days | Lixiviation, mg/kg dry matter | 0.0005 | 0.0007 | 0.0007 | 0.0008 | 0.0006 | 0.0006 | 0.0006 | 0.0006 |
| VC, % | 13.40 | 6.15 | 3.82 | 14.32 | 10.69 | 12.85 | 17.24 | 5.94 | |
| 28 days | Lixiviation, mg/kg dry matter | 0.0006 | 0.0005 | 0.0003 | 0.0005 | 0.0017 | 0.0019 | 0.0026 | 0.0043 |
| VC, % | 1.23 | 21.06 | 6.24 | 18.20 | 18.77 | 9.52 | 7.67 | 10.50 | |
| 90 days | Lixiviation, mg/kg dry matter | 0.0004 | 0.0007 | 0.0007 | 0.0008 | 0.0006 | 0.0007 | 0.0011 | 0.0011 |
| VC, % | 7.07 | 23.93 | 22.43 | 9.18 | 14.89 | 6.64 | 10.57 | 2.26 | |
| SFLG wt.% | 3 d | 7 d | 28 d | 90 d | 365 d | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TWL | 50–220 °C | 220–400 °C | TWL | 50–220 °C | 220–400 °C | TWL | 50–220 °C | 220–400 °C | TWL | 50–220 °C | 220–400 °C | TWL | 50–220 °C | 220–400 °C | ||
| % | % | % | % | % | ||||||||||||
| CAC | 0 | 14.4 | 6.6 | 8.2 | 23.8 | 12.4 | 4.9 | 25.5 | 12.7 | 6.8 | 17.4 | 2.4 | 13.5 | 18.7 | 3.2 | 14.0 |
| MAN | 15 | 15.2 | 7.5 | 7.1 | 19.6 | 8.4 | 5.2 | 18.3 | 7.4 | 7.4 | 17.5 | 5.7 | 10.3 | 19.2 | 3.2 | 10.9 |
| 25 | 14.9 | 7.1 | 6.5 | 17.9 | 7.0 | 4.8 | 15.6 | 6.5 | 6.5 | 17.0 | 4.9 | 8.3 | 20.0 | 7.1 | 7.4 | |
| 35 | 14.3 | 8.0 | 4.6 | 18.2 | 8.7 | 3.7 | 14.9 | 8.8 | 4.1 | 16.4 | 7.1 | 5. 8 | 21.9 | 9.7 | 3.7 | |
| 50 | 13.1 | 6.7 | 3.3 | 16.4 | 7.5 | 2.8 | 13.9 | 7.5 | 3.6 | 15.7 | 7.5 | 2.6 | 16.1 | 7.7 | 3.1 | |
| MEC | 15 | 12.3 | 2.9 | 9.9 | 17.3 | 7.9 | 6.0 | 18.1 | 6.6 | 7.9 | 17.3 | 2.9 | 11.2 | 16.9 | 2.9 | 11.6 |
| 25 | 12.6 | 2.7 | 9.7 | 17.1 | 8.3 | 5.3 | 15.0 | 5.5 | 7.5 | 15.7 | 3.3 | 8.9 | 16.4 | 4.4 | 9.8 | |
| 35 | 11.5 | 4.2 | 5.9 | 16.4 | 6.8 | 4.6 | 13.3 | 5.6 | 6.2 | 15.1 | 5.2 | 5.9 | 19.1 | 7.2 | 5.0 | |
| 50 | 9.5 | 4.9 | 4.4 | 15.8 | 9.7 | 3.6 | 12.5 | 7.0 | 5.0 | 14.3 | 5.3 | 4.6 | 15.7 | 6.7 | 4.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reig, L.; Pitarch, Á.M.; Gallardo, A.; Soriano, L.; Borrachero, M.V.; Payá, J.; Monzó, J.M. Recycling Spent Fluorescent Lamp Glass Waste in Calcium Aluminate Cement: Effects on Hydration and Mechanical Performance. Appl. Sci. 2025, 15, 9629. https://doi.org/10.3390/app15179629
Reig L, Pitarch ÁM, Gallardo A, Soriano L, Borrachero MV, Payá J, Monzó JM. Recycling Spent Fluorescent Lamp Glass Waste in Calcium Aluminate Cement: Effects on Hydration and Mechanical Performance. Applied Sciences. 2025; 15(17):9629. https://doi.org/10.3390/app15179629
Chicago/Turabian StyleReig, Lucía, Ángel M. Pitarch, Antonio Gallardo, Lourdes Soriano, María V. Borrachero, Jordi Payá, and José M. Monzó. 2025. "Recycling Spent Fluorescent Lamp Glass Waste in Calcium Aluminate Cement: Effects on Hydration and Mechanical Performance" Applied Sciences 15, no. 17: 9629. https://doi.org/10.3390/app15179629
APA StyleReig, L., Pitarch, Á. M., Gallardo, A., Soriano, L., Borrachero, M. V., Payá, J., & Monzó, J. M. (2025). Recycling Spent Fluorescent Lamp Glass Waste in Calcium Aluminate Cement: Effects on Hydration and Mechanical Performance. Applied Sciences, 15(17), 9629. https://doi.org/10.3390/app15179629










