Membrane-Active Phenolic Compounds from Cephalaria uralensis (Murray) Roem. & Schult.: Isolation, Structural Characterization, and Antioxidant Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction Procedure and Isolation
2.4. NMR Analysis
2.5. LC-MS Analysis
2.6. UV–Vis
2.7. Lipid Monolayer Experiment
2.8. Antioxidant Activity Assays
2.8.1. DPPH● Assay
2.8.2. ABTS●+ Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Structural Characteristics of C. uralensis Phenolics
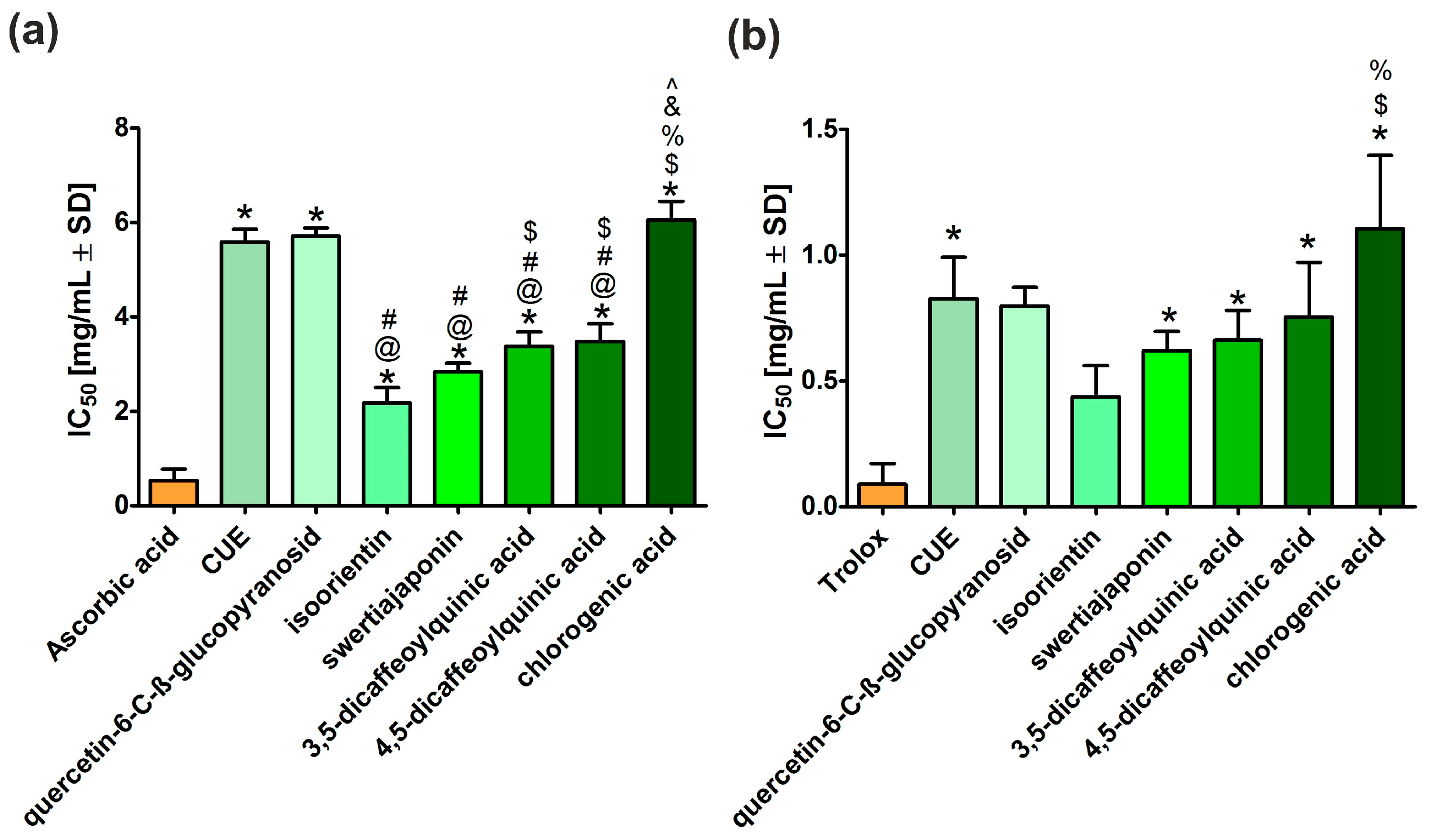
3.2. Antioxidant Activity
3.3. Membrane Activity—Monolayer Insertion Assays
3.4. Comparison to Other Medicinal Plants
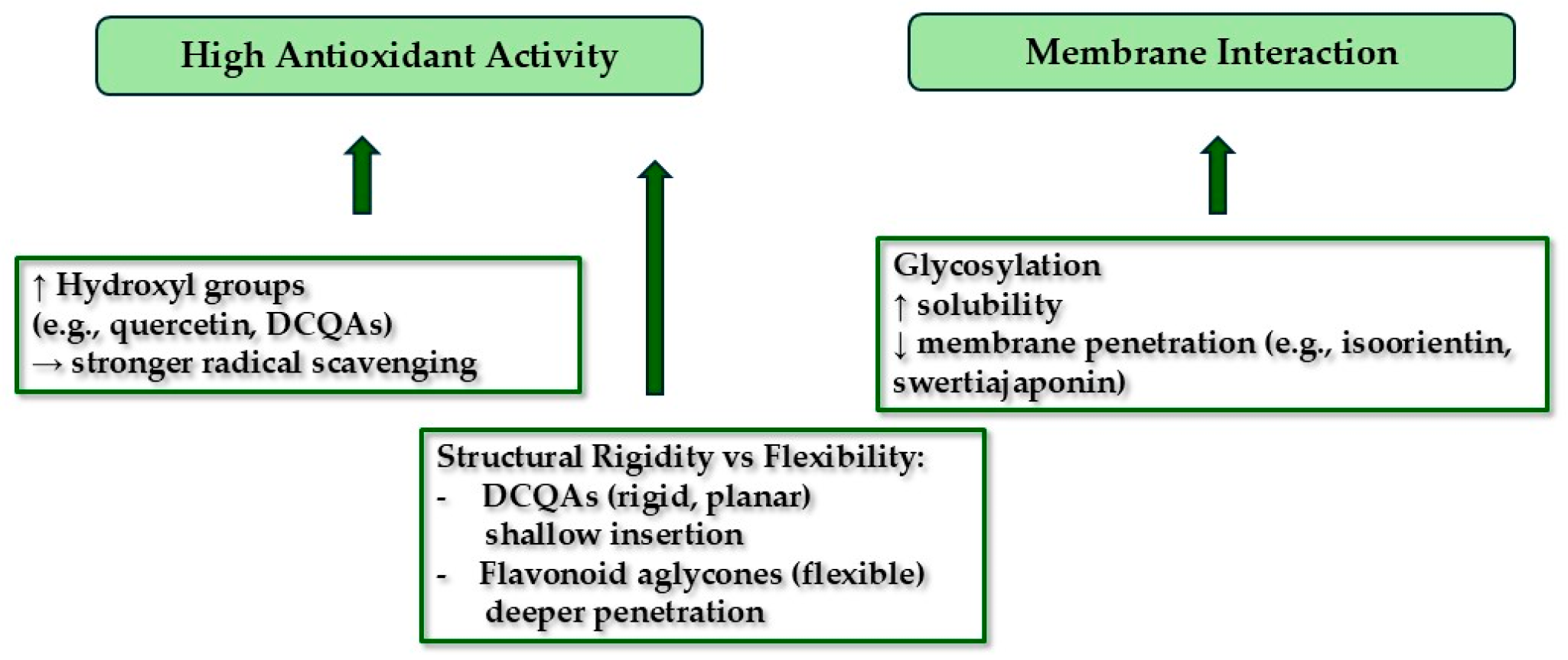
3.5. Mechanistic Implications
3.6. Novelty, Limitations, and Future Work
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farhan, M. The promising role of polyphenols in skin disorders. Molecules 2024, 29, 865. [Google Scholar] [CrossRef]
- WFO Plant List. Cephalaria Schrad. Available online: https://wfoplantlist.org/taxon/wfo-4000007238-2025-06 (accessed on 24 July 2025).
- Sarıkahya, N.B.; Kırmızıgül, S. Antimicrobially active hederagenin glycosides from Cephalaria elmaliensis. Planta Med. 2012, 78, 828–833. [Google Scholar] [CrossRef]
- Sumer, G.; Sarikahya, N.B.; Kirmizigul, S. Phytochemical and biological investigations on Cephalaria anatolica. Rec. Nat. Prod. 2017, 11, 497–507. [Google Scholar] [CrossRef]
- Kayce, P.; Kırmızıgül, S. Chemical constituents of two endemic Cephalaria species. Rec. Nat. Prod. 2010, 4, 141–148. [Google Scholar]
- Baydoun, S.; Lamis, C.; Dalleh, H.; Nelly, A. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J. Ethnopharmacol. 2015, 173, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Dalar, A.; Mukemre, M.; Unal, M.; Ozgokce, F. Traditional medicinal plants of Ağrı Province, Turkey. J. Ethnopharmacol. 2018, 226, 56–72. [Google Scholar] [CrossRef]
- Alankuş-Çalışkan, Ö.; Anil, H. A bidesmodic triterpene saponin from Cephalaria transsyvanica. Phytochemistry 1995, 38, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Celenk, V.U.; Sarikahya, N.B.; Kirmizigul, S. Isolation and structural studies on saponins from three Cephalaria species from Anatolia. Chem. Nat. Compd. 2020, 56, 180–182. [Google Scholar] [CrossRef]
- Kırmızıgül, S.; Anıl, H.; Uçar, F.; Akdemir, K. Antimicrobial and antifungal activities of three new triterpenoid glycosides. Phytoter. Res. 1996, 10, 274–276. [Google Scholar] [CrossRef]
- Top, H.; Sarıkahya, N.B.; Nalbantsoy, A.; Kırmızıgül, S. Immunomodulatory, hemolytic properties and cytotoxic activity potent of triterpenoid saponins from Cephalaria balansae. Phytochemistry 2017, 137, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mustafayeva, K.; Elias, R.; Balansard, G.; Suleimanov, T.; Mayu-Lede, V.; Kerimov, Y. Iridoid glycosides from Cephalaria kotschyi roots. Chem. Nat. Compd. 2008, 44, 132–133. [Google Scholar] [CrossRef]
- Aliev, A.M.; Movsumov, I.S.; Bagirov, E.K. Alkaloids from certain Cephalaria species. Khim. Prir. Soedin. 1975, 5, 667. [Google Scholar]
- Movsumov, I.S.; Garaev, E.A.; Isaev, M.I. Flavonoids from Cephalaria grossheimii. Chem. Nat. Compd. 2009, 45, 422–423. [Google Scholar] [CrossRef]
- Chrząszcz, M.; Miazga-Karska, M.; Klimek, K.; Granica, S.; Tchórzewska, D.; Ginalska, G.; Szewczyk, K. Extracts from Cephalaria uralensis (Murray) Roem. & Schult. and Cephalaria gigantea (Ledeb.) Bobrov as potential agents for treatment of acne vulgaris: Chemical characterization and in vitro biological evaluation. Antioxidants 2020, 9, 796. [Google Scholar] [CrossRef]
- Kayce, P.; Sarikahya, B.N.; Pekmez, M.; Arda, N.; Kırmızıgül, S. The structure and cytotoxic activity of a new saponin: Cephoside A from Cephalaria elazigensis var. purpurea. Turk. J. Chem. 2017, 41, 345–353. [Google Scholar] [CrossRef]
- Sarıkahya, N.B.; Kırmızıgül, S. Antimicrobial triterpenoid glycosides from Cephalaria scoparia. J. Nat. Prod. 2010, 73, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Kırmızıgül, S.; Anıl, H.; Rose, M.E. Triterpenoid saponins from Cephalaria transsylvanica. J. Nat. Prod. 1996, 59, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, I.I.; Afifi, F.U. Studies on the in vitro and in vivo hypoglycemic activities of some medicinal plants used in treatment of diabetes in Jordanian traditional medicine. J. Ethnopharmacol. 2004, 93, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Wu, W.; Liu, Q.; Liu, X. Effect of isoorientin on intracellular antioxidant defence mechanisms in hepatoma and liver cell lines. Biomed. Pharmacother. 2016, 81, 356–362. [Google Scholar] [CrossRef]
- Moon, K.M.; Lee, B.; Cho, W.K.; Lee, B.S.; Kim, C.Y.; Ma, J.Y. Swertiajaponin as an anti-browning and antioxidant flavonoid. Food Chem. 2018, 30, 207–214. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.-D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Perez-Gregorio, R.; Mateus, N.; de Freitas, V. Interactions of dietary polyphenols with epithelial lipids: Advances from membrane and cell models in the study of polyphenol absorption, transport and delivery to the epithelium. Crit. Rev. Food Sci. Nutr. 2021, 61, 3007–3030. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef]
- Czernel, G.; Budziak, I.; Oniszczuk, A.; Karcz, D.; Pustuła, K.; Górecki, A.; Matwijczuk, A.; Gładyszewska, B.; Gagoś, M.; Niewiadomy, A.; et al. ESIPT-related origin of dual fluorescence in the selected model 1,3,4-thiadiazole derivatives. Molecules 2020, 25, 4168. [Google Scholar] [CrossRef] [PubMed]
- Matwijczuk, A.; Budziak-Wieczorek, I.; Czernel, G.; Karcz, D.; Barańska, A.; Jedlińska, A.; Samborska, K. Classification of honey powder composition by FTIR spectroscopy coupled with chemometric analysis. Molecules 2022, 27, 3800. [Google Scholar] [CrossRef]
- Gagoś, M.; Arczewska, M. FTIR spectroscopic study of molecular organization of the antibiotic amphotericin B in aqueous solution and in DPPC lipid monolayers containing the sterols cholesterol and ergosterol. Eur. Biophys. J. 2012, 41, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic composition of the leaves of Pyrola rotundifolia L. and their antioxidant and cytotoxic activity. Molecules 2020, 25, 1749. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Dos Santos Szewczyk, K.; Gawlik-Dziki, U.; Nowak, R. LC-ESI-MS/MS polyphenolic profile and in vitro study of cosmetic potential of Aerva lanata (L.) Juss. herb extracts. Molecules 2022, 27, 1259. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Sharan, K.; Swarnkar, G.; Rawat, P.; Kumar, M.; Manickavasagam, L.; Maurya, R.; Pierroz, D.; Chattopadhyay, N. Quercetin-6-C-β-D-glucopyranoside isolated from Ulmus wallichiana planchon is more potent than quercetin in inhibiting osteoclastogenesis and mitigating ovariectomy-induced bone loss in rats. Menopause 2011, 18, 198–207. [Google Scholar] [CrossRef]
- Kumazawa, T.; Minatogawa, T.; Matsuba, S.; Sato, S.; Onodera, J. An effective synthesis of isoorientin: The regioselective synthesis of a 6-C-glucosylflavone. Carbohydr. Res. 2000, 329, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Koshioka, M.; Kondo, T.; Imizu, K. Flavone C-glucosides responsible for yellow pigmentation induced by low temperature in bracts of Zantedeschia aethiopica. Nat. Prod. Commun. 2015, 10, 425–427. [Google Scholar] [CrossRef]
- Tchoumtchoua, J.; Mathiron, D.; Pontarin, N.; Gagneul, D.; van Bohemen, A.I.; Otogo N’nang, E.; Mesnard, F.; Petit, E.; Fontaine, J.X.; Molinié, R.; et al. Phenolic profiling of flax highlights contrasting patterns in winter and spring varieties. Molecules 2019, 24, 4303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.G.; Yan, Q.Q.; Xue, R.Y.; Zhang, J.; Zhang, Y.Q. Isolation and identification of colourless caffeoyl compounds in purple sweet potato by HPLC-DAD-ESI/MS and their antioxidant activities. Food Chem. 2014, 161, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Hsiao, P.C.; Kuo, T.C.; Chiang, S.T.; Chen, S.L.; Chiou, S.J.; Ling, X.H.; Liang, M.T.; Cheng, W.Y.; Houng, J.Y. Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water, ethanol and supercritical fluid extraction techniques. Ind. Crops Prod. 2016, 89, 543–549. [Google Scholar] [CrossRef]
- Nakamura, M.; Ra, J.H.; Jee, Y.; Kim, J.S. Impact of different partitioned solvents on chemical composition and bioavailability of Sasa quelpaertensis Nakai leaf extract. J. Food Drug Anal. 2017, 25, 316–326. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity-a review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Lee, B.; Moon, K.M.; Lee, B.S.; Yang, J.H.; Park, K.I.; Cho, W.K.; Ma, J.Y. Swertiajaponin inhibits skin pigmentation by dual mechanisms to suppress tyrosinase. Oncotarget 2017, 8, 95530–95541. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic acid: A systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Hufnagel, M.; Rademaekers, A.; Weisert, A.; Häberlein, H.; Franken, S. Pharmacological profile of dicaffeoylquinic acids and their role in the treatment of respiratory diseases. Front. Pharmacol. 2024, 15, 1371613. [Google Scholar] [CrossRef]
- Chrząszcz, M.; Szewczyk, K.; Tchórzewska, D. Biotechnological potential of Cephalaria uralensis (Murray) Roem. & Schult. and C. gigantea (Ledeb.) Bobrov—Comparative analysis of plant anatomy and the content of biologically active substances. Plants 2021, 10, 986. [Google Scholar] [CrossRef]
- Luo, J.; He, W.; Li, X.; Ji, X.; Liu, J. Anti-acne vulgaris effects of chlorogenic acid by anti-inflammatory activity and lipogenesis inhibition. Exp. Dermatol. 2021, 30, 865–871. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Kanak, S.; Klimek, K.; Miazga-Karska, M.; Dybowski, M.P.; Typek, R.; Olech, M.; Dos Santos Szewczyk, K. Unveiling the ethnomedicinal potential of Alchemilla speciosa Buser: An underexplored source of bioactive compounds for skin health. J. Ethnopharmacol. 2025, 351, 120068. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant properties of plant-derived phenolic compounds and their effect on skin fibroblast cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef]
- Ghozzi, I.; Fontaine, J.X.; Molinié, R.; Elboutachfaiti, R.; Akkouche, L.; Sebei, K.; Mathiron, D.; Hano, C.; Garros, L.; Choque, E.; et al. Relationship between the structure of the flavone C-glycosides of linseed (Linum usitatissimum L.) and their antioxidant activity. Molecules 2024, 29, 5829. [Google Scholar] [CrossRef]
- Hossain, S.I.; Saha, S.C.; Deplazes, E. Phenolic compounds alter the ion permeability of phospholipid bilayers via specific lipid interactions. Phys. Chem. Chem. Phys. 2021, 23, 22352–22366. [Google Scholar] [CrossRef]
- Wang, R.; Peng, J.; Shi, X.; Cao, S.; Xu, Y.; Xiao, G.; Li, C. Change in membrane fluidity induced by polyphenols is highly dependent on the position and number of galloyl groups. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184015. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Costa, M.; Rebelo, R.; Arques, F.; Ferreira, M.; Gameiro, P.; Barros, T.; Geraldo, D.; Monteiro, L.S.; Paiva-Martins, F. Phytyl phenolipids: Structurally modified antioxidants with superior lipid membrane interaction. Molecules 2025, 30, 2193. [Google Scholar] [CrossRef] [PubMed]
- Tejuca, M.; Serra, M.D.; Ferreras, M.; Lanio, M.E.; Menestrina, G. Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry 1996, 35, 14947–14957. [Google Scholar] [CrossRef] [PubMed]
- Azab, A. Total phenolic content, antioxidant capacity and antifungal activity of extracts of Carthamus tenuis and Cephalaria joppensis. Eur. Chem. Bull. 2018, 7, 156–161. [Google Scholar] [CrossRef]
- Karalija, E.; Ćavar Zeljković, S.; Tarkowski, P.; Muratović, E.; Parić, A. The effect of cytokinins on growth, phenolics, antioxidants and antimicrobial potential in liquid agitated shoot cultures of Knautia sarajevensis. Plant Cell Tissue Organ Cult. 2017, 131, 347–357. [Google Scholar] [CrossRef]
- Kılınc, H. Phytochemical profiles, antimicrobial and antioxidant activity of Knautia integrifolia (L.) Bertol. subsp. integrifolia. Plants 2025, 14, 466. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Orrego, R.; Leiva, E.; Cheel, J. Inhibitory effect of three C-glycosylflavonoids from Cymbopogon citratus (Lemongrass) on human low density lipoprotein oxidation. Molecules 2009, 14, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2017, 27, 169–176. [Google Scholar] [CrossRef] [PubMed]
| Technique | Information Obtained |
|---|---|
| High-Resolution LC–MS | Exact masses and molecular formulas; fragmentation patterns (e.g., loss of sugar moieties) to infer glycosylation. Essential for assigning molecular weight and initial formula hypotheses. |
| NMR | Detailed proton and carbon connectivity, confirming flavonoid vs. caffeoyl structure, position of sugar attachment, and ring substitution. Enabled unambiguous structural elucidation of each glycoside (e.g., identifying C-glycosidic linkages) |
| No. | Experimental Mass [M-H]− (Da) | Theoretical Mass [M-H]− (Da) | Δ mDa | Δ ppm | Elemental Composition |
|---|---|---|---|---|---|
| 1 | 463.08831 | 463.08820 | 0.11 | 0.24 | C21H19O12 |
| 2 | 447.09336 | 447.09329 | 0.07 | 0.16 | C21H19O11 |
| 3 | 461.10885 | 461.10894 | −0.09 | 0.20 | C22H21O11 |
| 4 | 515.11985 | 515.11950 | 0.35 | 0.68 | C25H23O12 |
| 5 | 515.11964 | 515.11950 | 0.14 | 0.27 | C25H23O12 |
| 6 | 353.08760 | 353.08781 | −0.21 | 0.59 | C16H17O9 |
| Compound | Increase in Surface Pressure (SP) [mN/m] |
|---|---|
| ISO | ~2 |
| 3,5-diCQA | ~3.5 |
| 4,5-diCQA | 6.5–7 |
| CUE | ~5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berecka-Rycerz, A.; Chrząszcz-Wróbel, M.; Matwijczuk, A.P.; Hołowiński, P.; Granica, S.; Dos Santos Szewczyk, K. Membrane-Active Phenolic Compounds from Cephalaria uralensis (Murray) Roem. & Schult.: Isolation, Structural Characterization, and Antioxidant Potential. Appl. Sci. 2025, 15, 9585. https://doi.org/10.3390/app15179585
Berecka-Rycerz A, Chrząszcz-Wróbel M, Matwijczuk AP, Hołowiński P, Granica S, Dos Santos Szewczyk K. Membrane-Active Phenolic Compounds from Cephalaria uralensis (Murray) Roem. & Schult.: Isolation, Structural Characterization, and Antioxidant Potential. Applied Sciences. 2025; 15(17):9585. https://doi.org/10.3390/app15179585
Chicago/Turabian StyleBerecka-Rycerz, Anna, Małgorzata Chrząszcz-Wróbel, Arkadiusz Paweł Matwijczuk, Piotr Hołowiński, Sebastian Granica, and Katarzyna Dos Santos Szewczyk. 2025. "Membrane-Active Phenolic Compounds from Cephalaria uralensis (Murray) Roem. & Schult.: Isolation, Structural Characterization, and Antioxidant Potential" Applied Sciences 15, no. 17: 9585. https://doi.org/10.3390/app15179585
APA StyleBerecka-Rycerz, A., Chrząszcz-Wróbel, M., Matwijczuk, A. P., Hołowiński, P., Granica, S., & Dos Santos Szewczyk, K. (2025). Membrane-Active Phenolic Compounds from Cephalaria uralensis (Murray) Roem. & Schult.: Isolation, Structural Characterization, and Antioxidant Potential. Applied Sciences, 15(17), 9585. https://doi.org/10.3390/app15179585







