Advances in Hydrogel Film Fabrication and Functional Applications Across Biomedical and Environmental Fields
Abstract
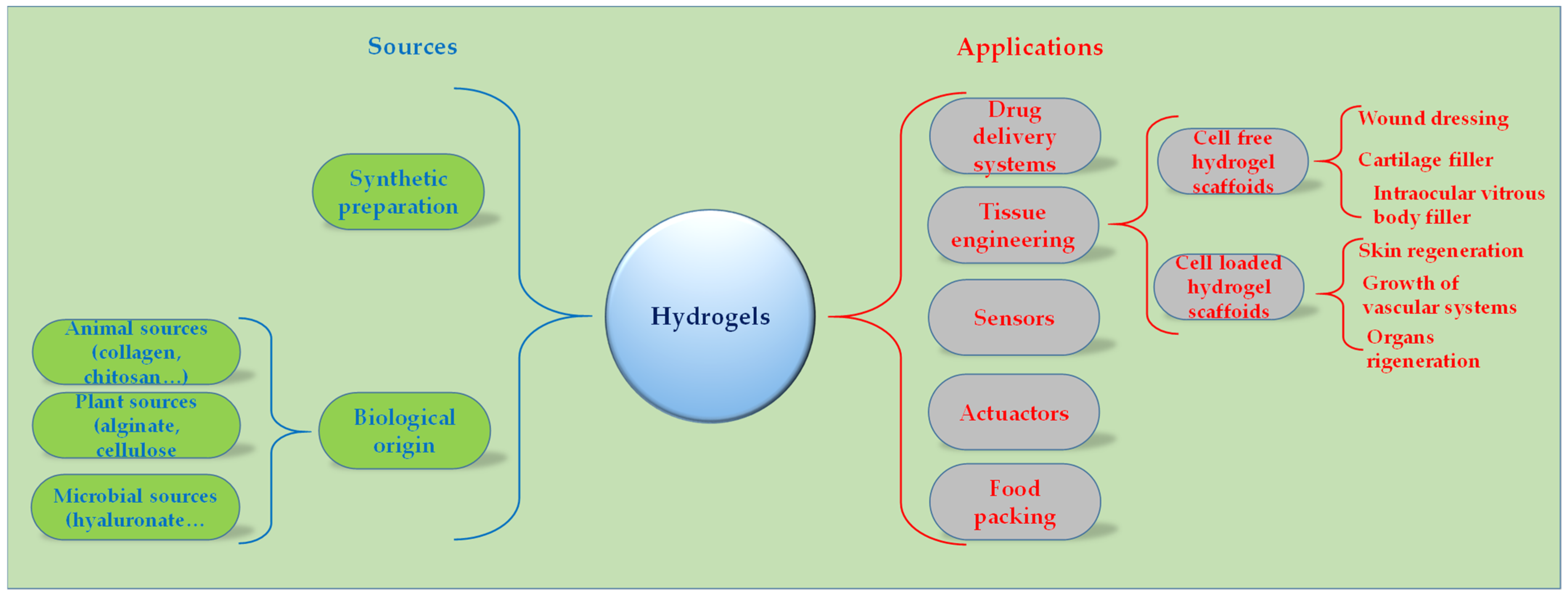
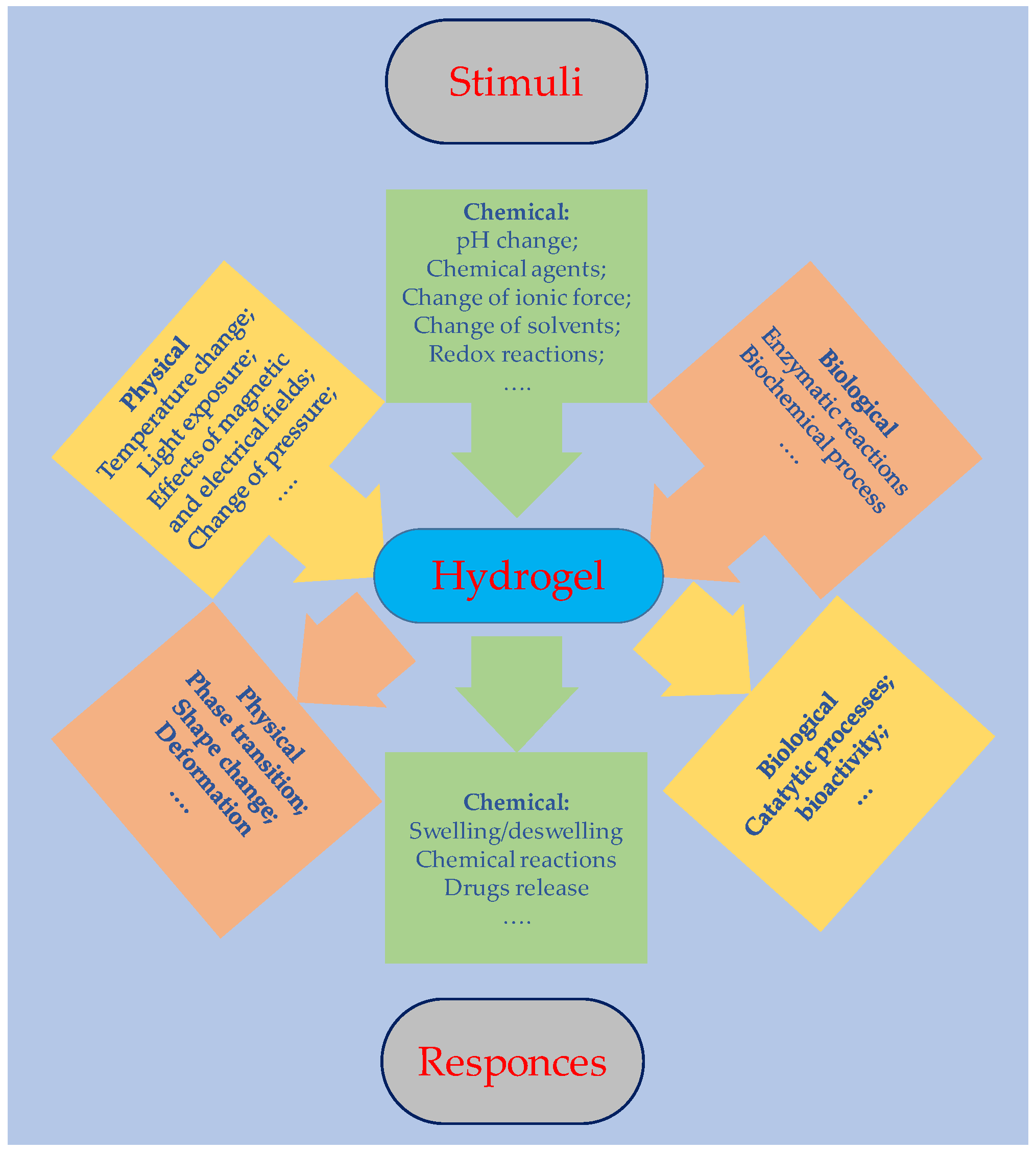
1. Introduction
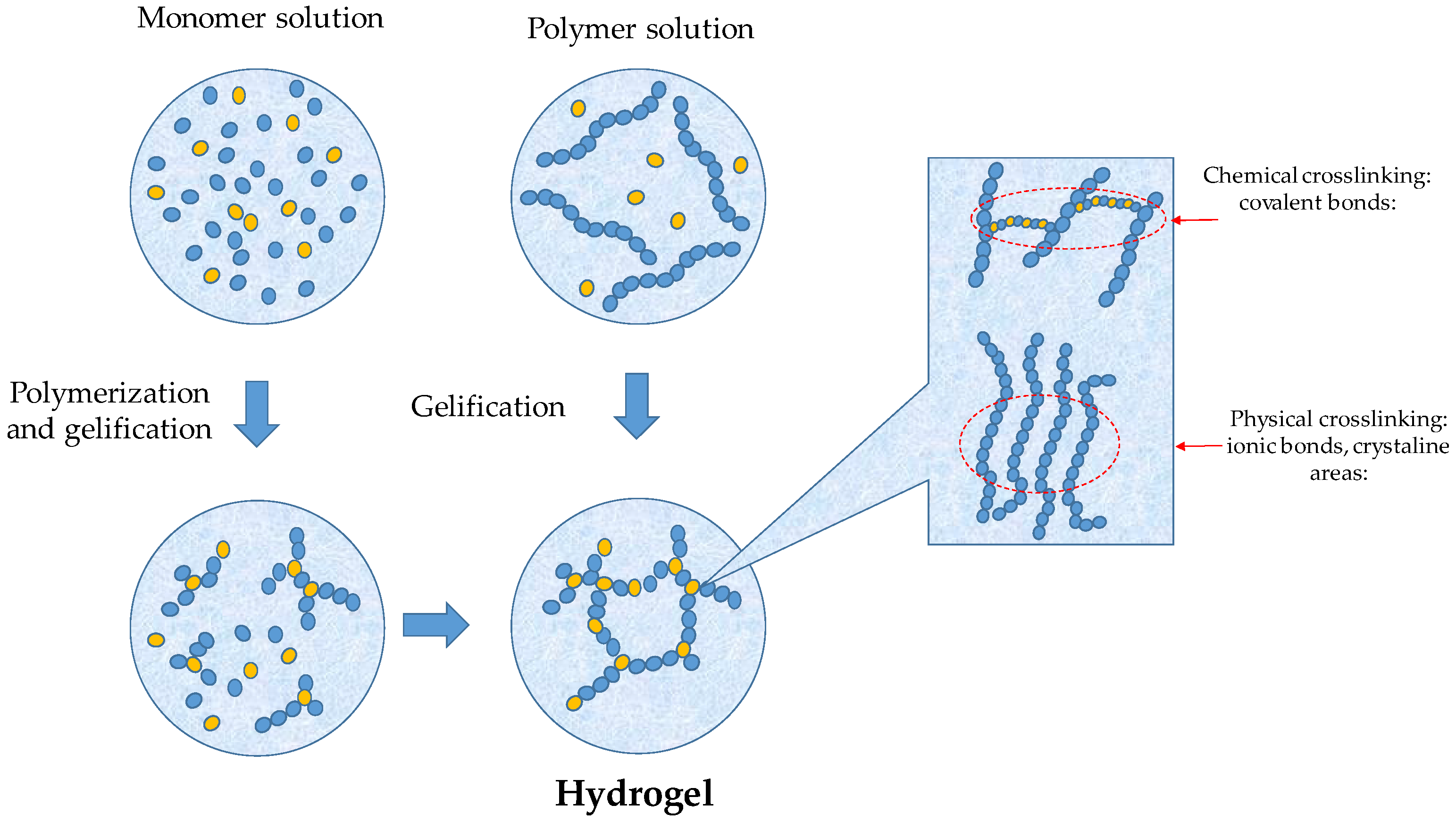
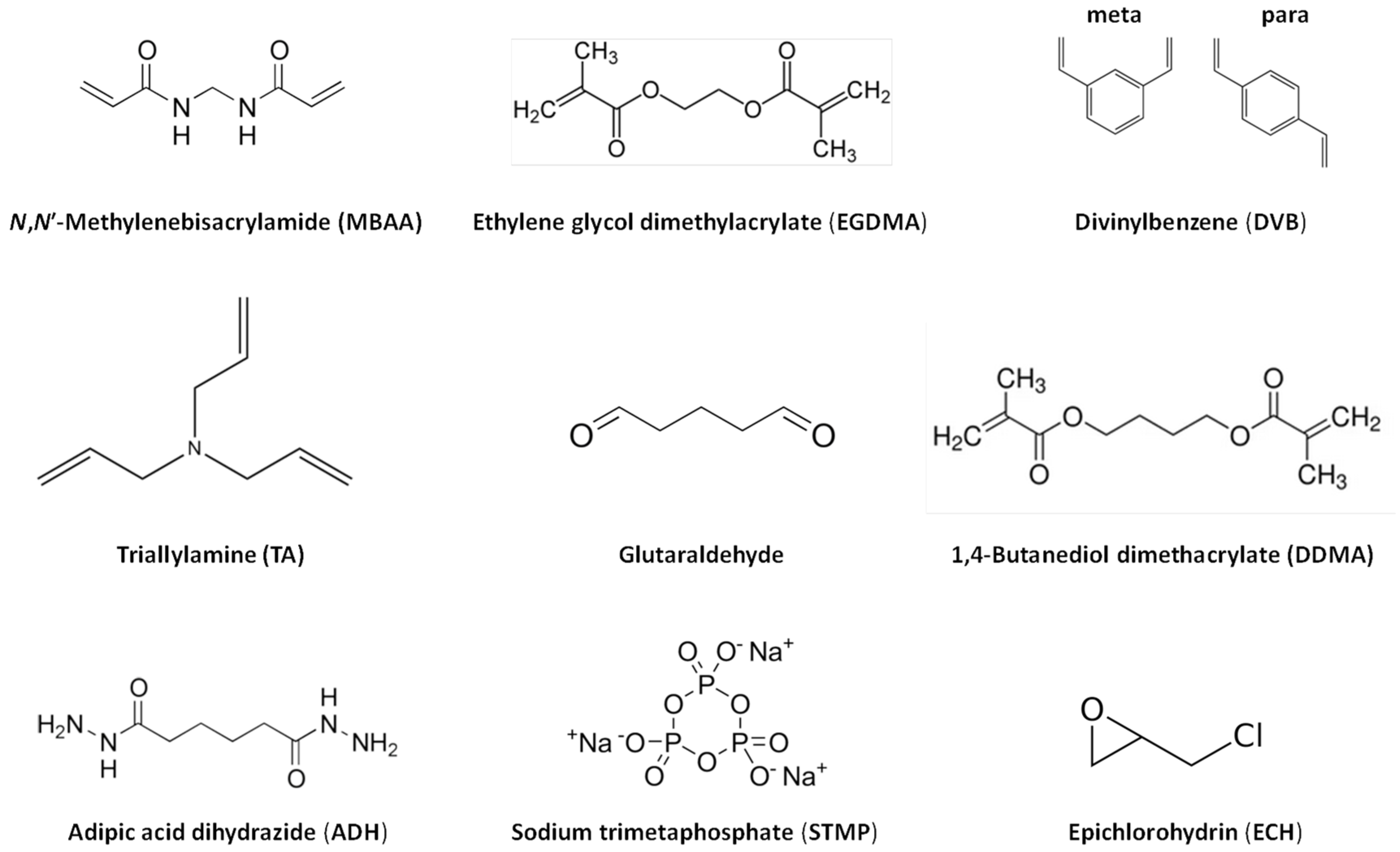
2. Hydrogel Synthesis and Film Preparation
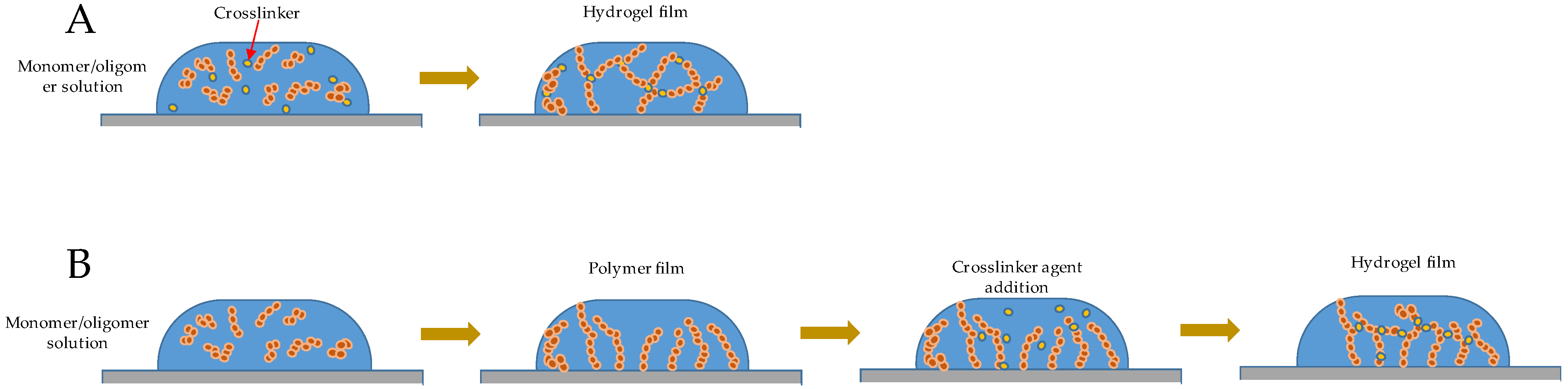
2.1. Film Formation
2.2. Preparation Methods
- Solvent casting [48]: In this case, the film is obtained by dissolving the polymer in a suitable solvent, subsequently casting the solution onto a substrate and then evaporating the solvent, which leaves a thin film.
- Dip coating [49], which consists of the application of a polymer solution to a substrate using a dipping technique.
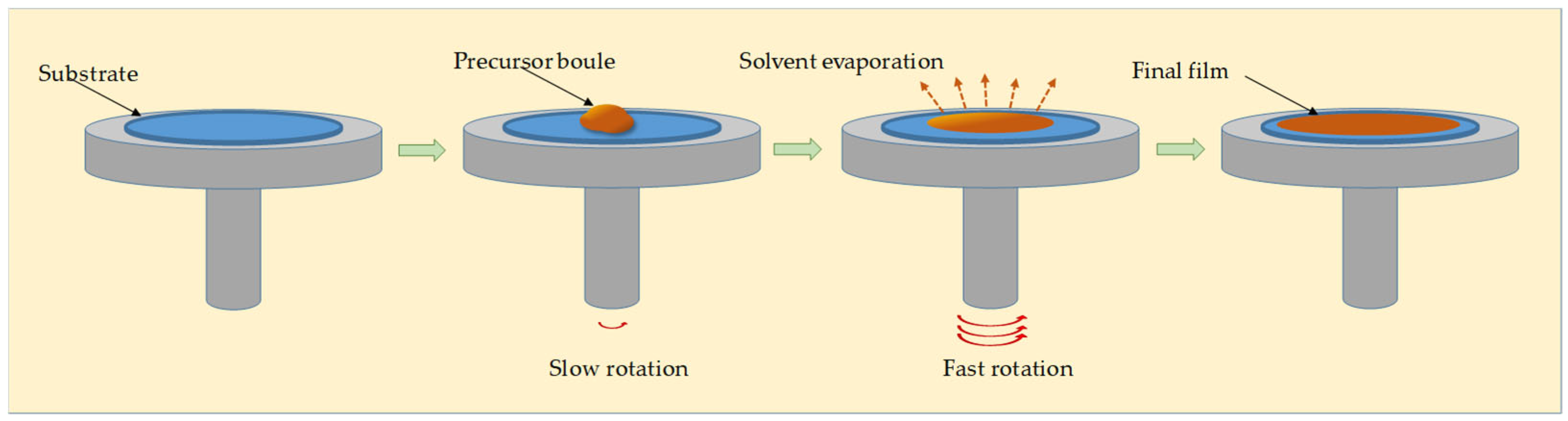
- Spin coating [50], which consists of the application of a polymer solution to a substrate using a spinning technique.
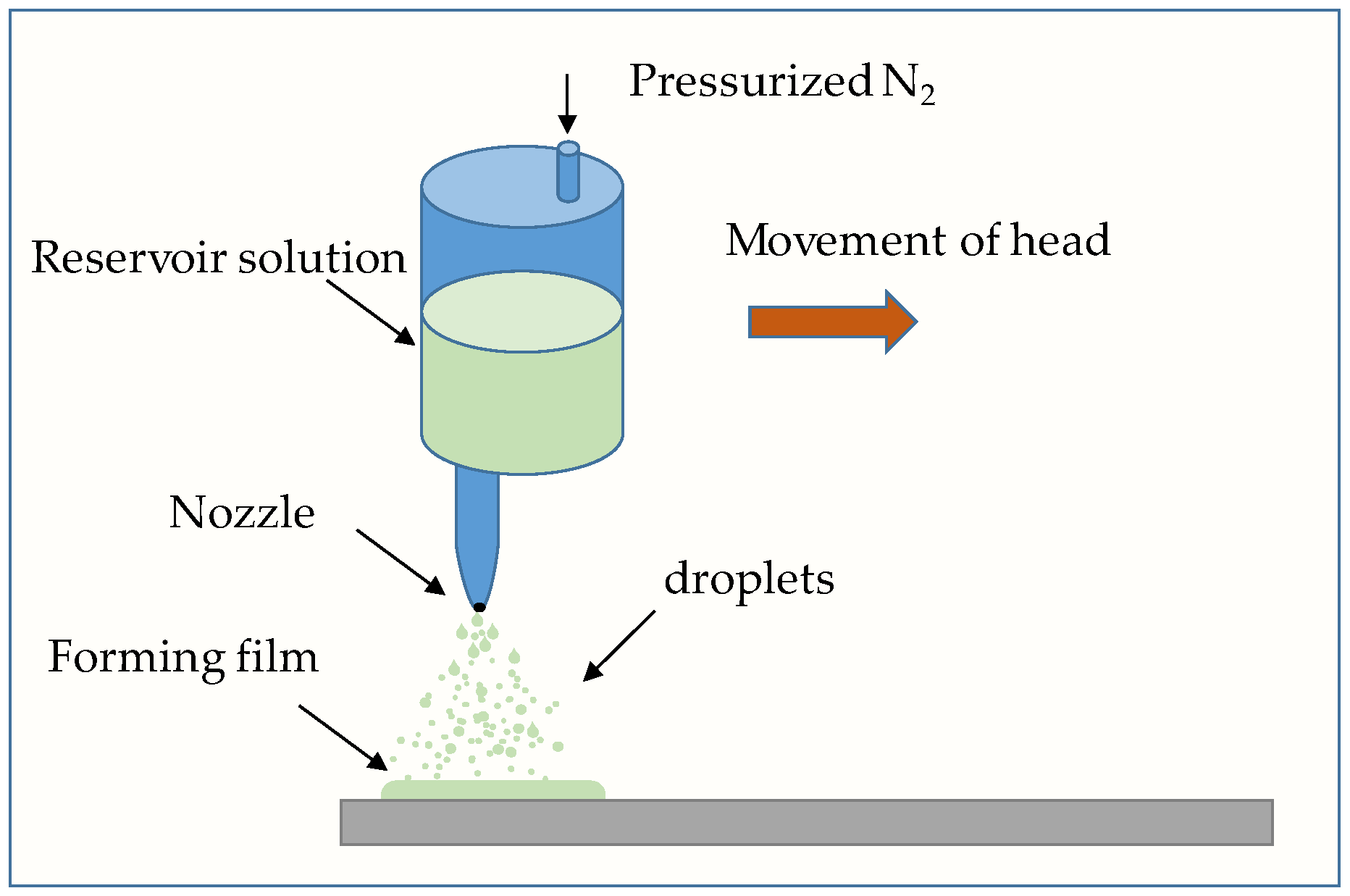
- Spray coating [51], which consists of the application of a polymer solution to a substrate using a spraying technique.
- Blade coating [52]: a method where the film is obtained by spreading the starting solution on the substrate using a blade.
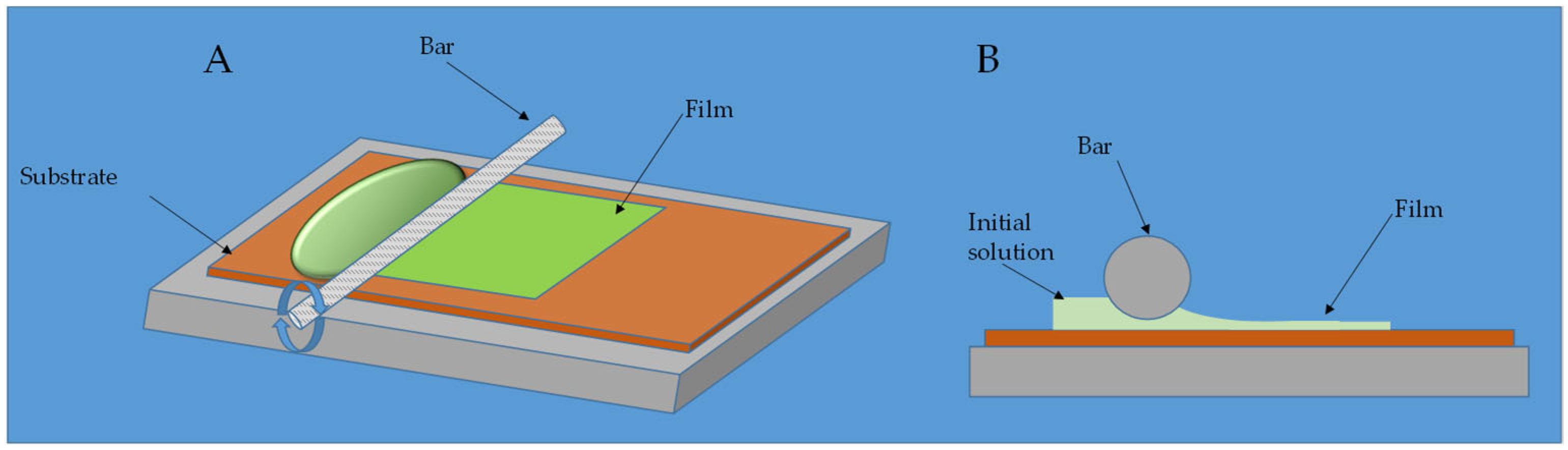
- Bar coating [53]: It is very similar to blade coating; solution is spread across a substrate via a cylindrical bar with wire spiraling around it.
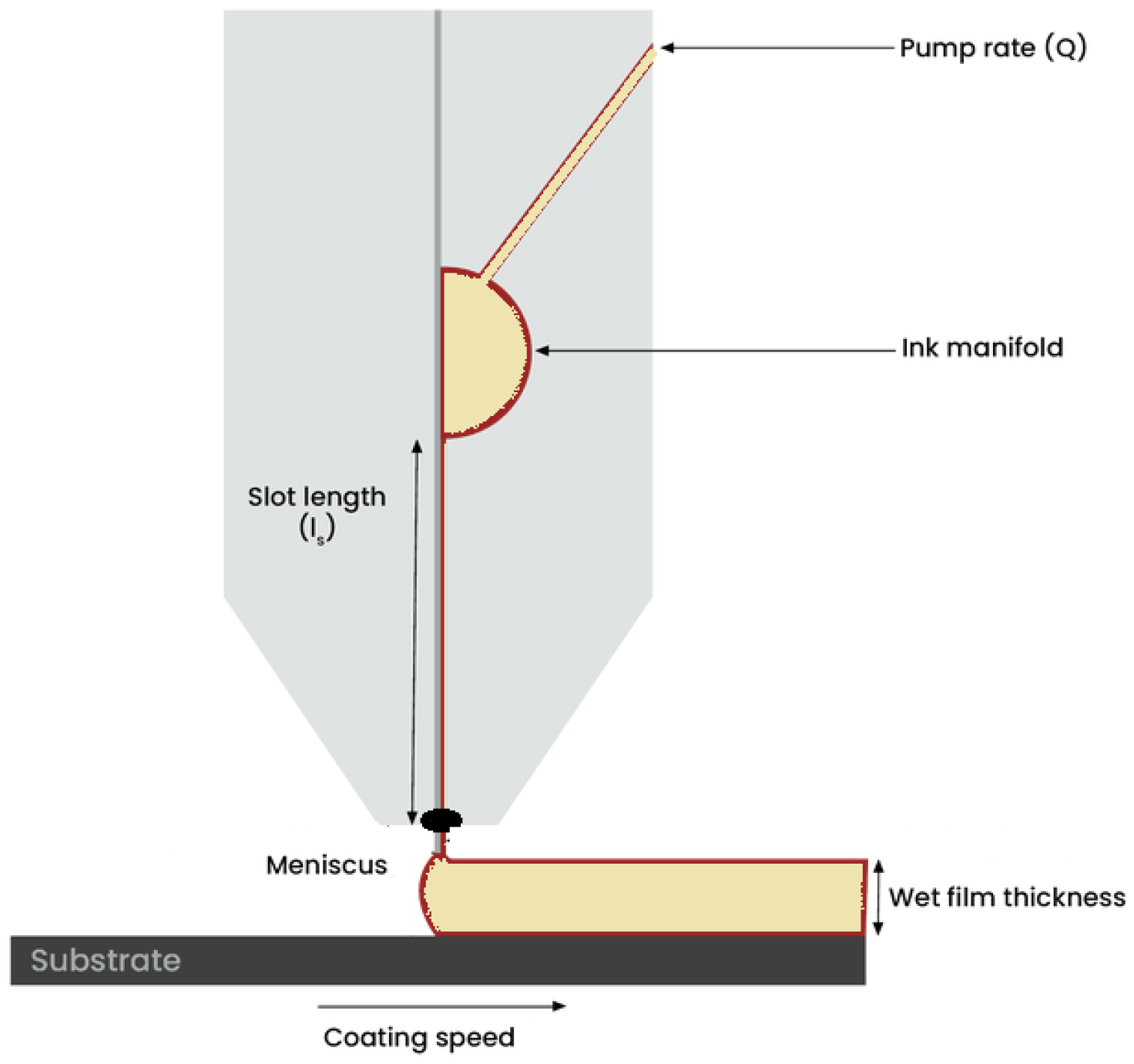
- Slot die coating [54], in which the solution is coated directly onto the substrate. The solution flows through a ‘head’ at a determined rate as the substrate moves relative to the head.
- Photolithography [55], which is a technique used to create films on a substrate by exposing a photosensitive material to light radiation, usually ultraviolet. Often, a mask can be used to create complex structures.
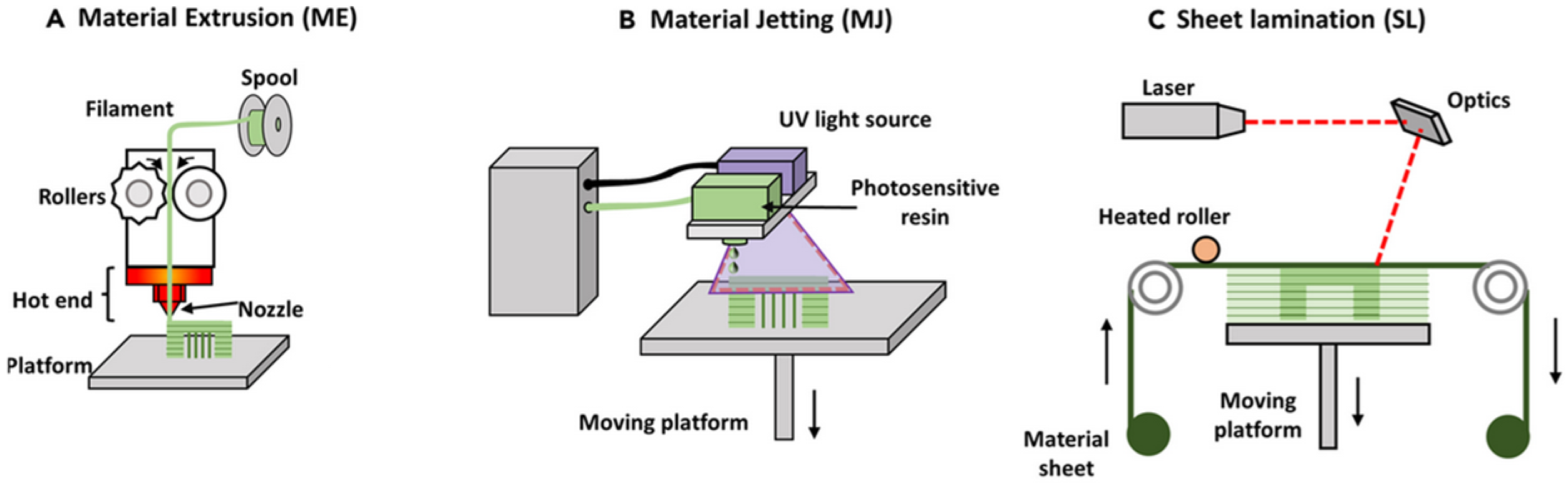
- 3D printing [56], which is a manufacturing technique to create structures, building them up layer by layer, often by extruding a viscous hydrogel ink through a nozzle, although there are other modalities, such as, for instance, stereolithography (SLA), in which a photosensitive resin is polymerized layer by layer by a laser beam or light source, creating objects with high precision and detail.
3. Characterization Techniques
- Fourier-transform infrared spectroscopy (FTIR);
- Raman spectroscopy;
- UV-Vis spectroscopy;
- Nuclear Magnetic Resonance (NMR) spectroscopy;
- Fluorescence spectroscopy.
- Differential Scanning Calorimetry (DSC), which measures heat flow into or out of a sample as temperature changes, allowing for the detection of phase transitions like melting and crystallization.
- Thermogravimetric Analysis (TGA), which measures the weight loss of a sample as it is heated, providing information about thermal stability and decomposition.
- Differential Thermal Analysis (DTA), which is a technique used to study the thermal behavior of materials, including hydrogels, by measuring the temperature difference between a sample and a reference material as they are heated or cooled.
- Elastic modulus, also known as Young’s modulus, which indicates stiffness, often measured using tensile or compression tests. It reflects the hydrogel’s resistance to deformation under stress.
- Tensile strength, which is the maximum stress a hydrogel can withstand before fracturing under tension.
- Fracture toughness: a property related to a hydrogel’s resistance to crack propagation, and that is important for understanding its ability to withstand damage.
- Viscoelasticity, i.e., the time-dependent behavior of hydrogels, including stress relaxation and creep.
4. Materials for Hydrogel Films
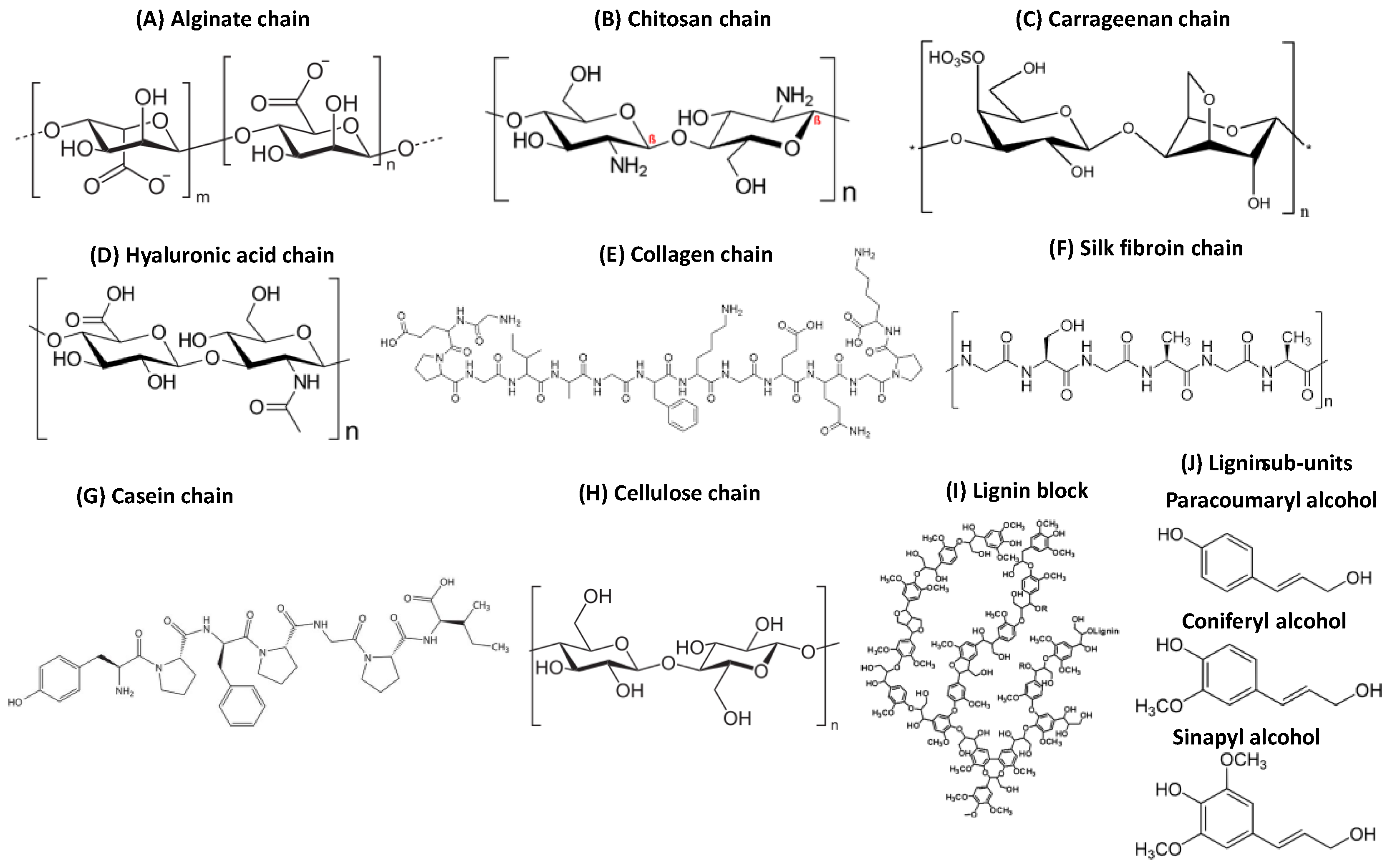
4.1. Materials from Natural Sources
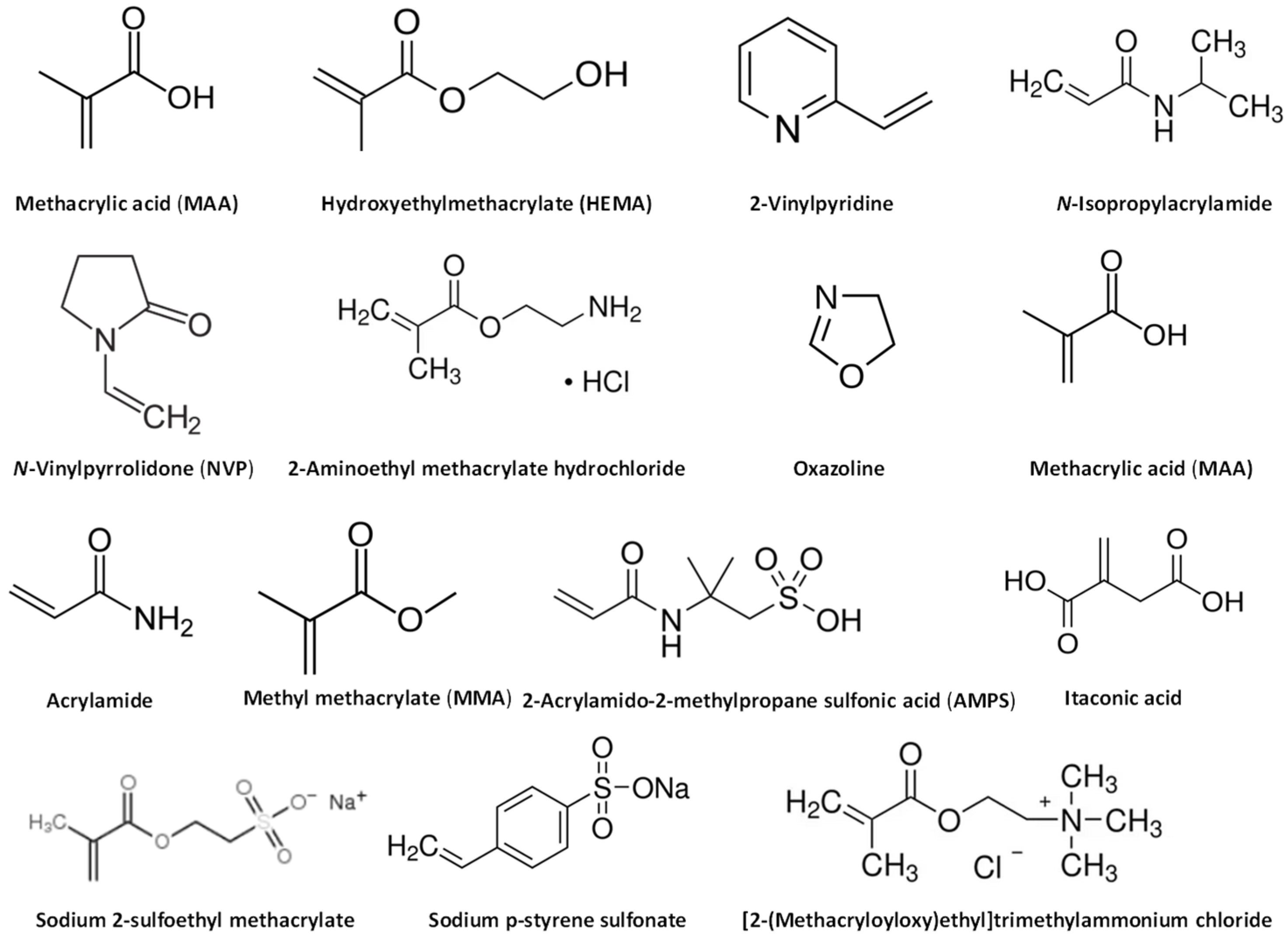
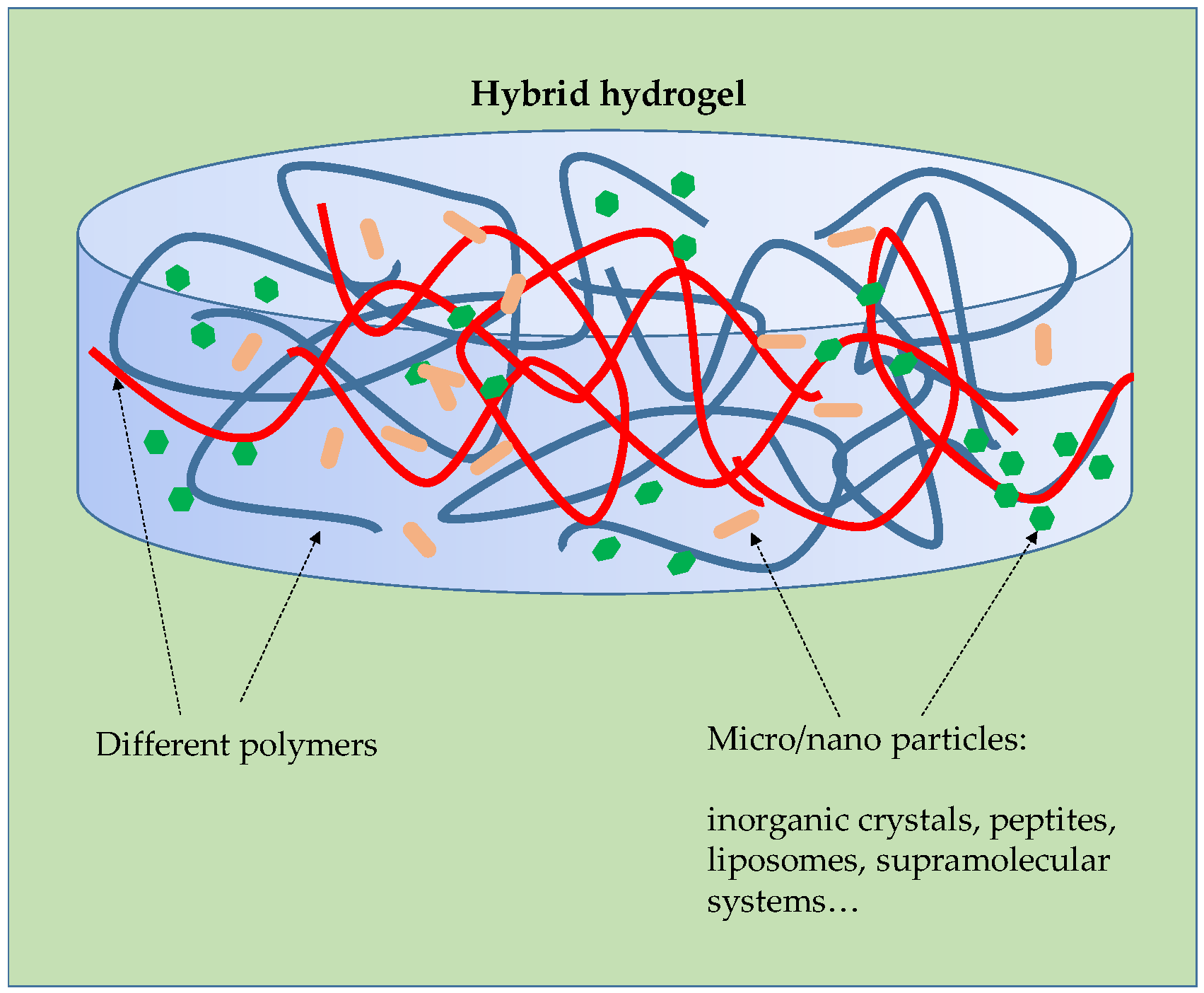
4.2. Synthetic Materials
4.3. Additives for Hydrogels
4.4. Biocompatibility and Cytotoxicity Issues
5. Application of Hydrogel Films
5.1. Biomedical Applications
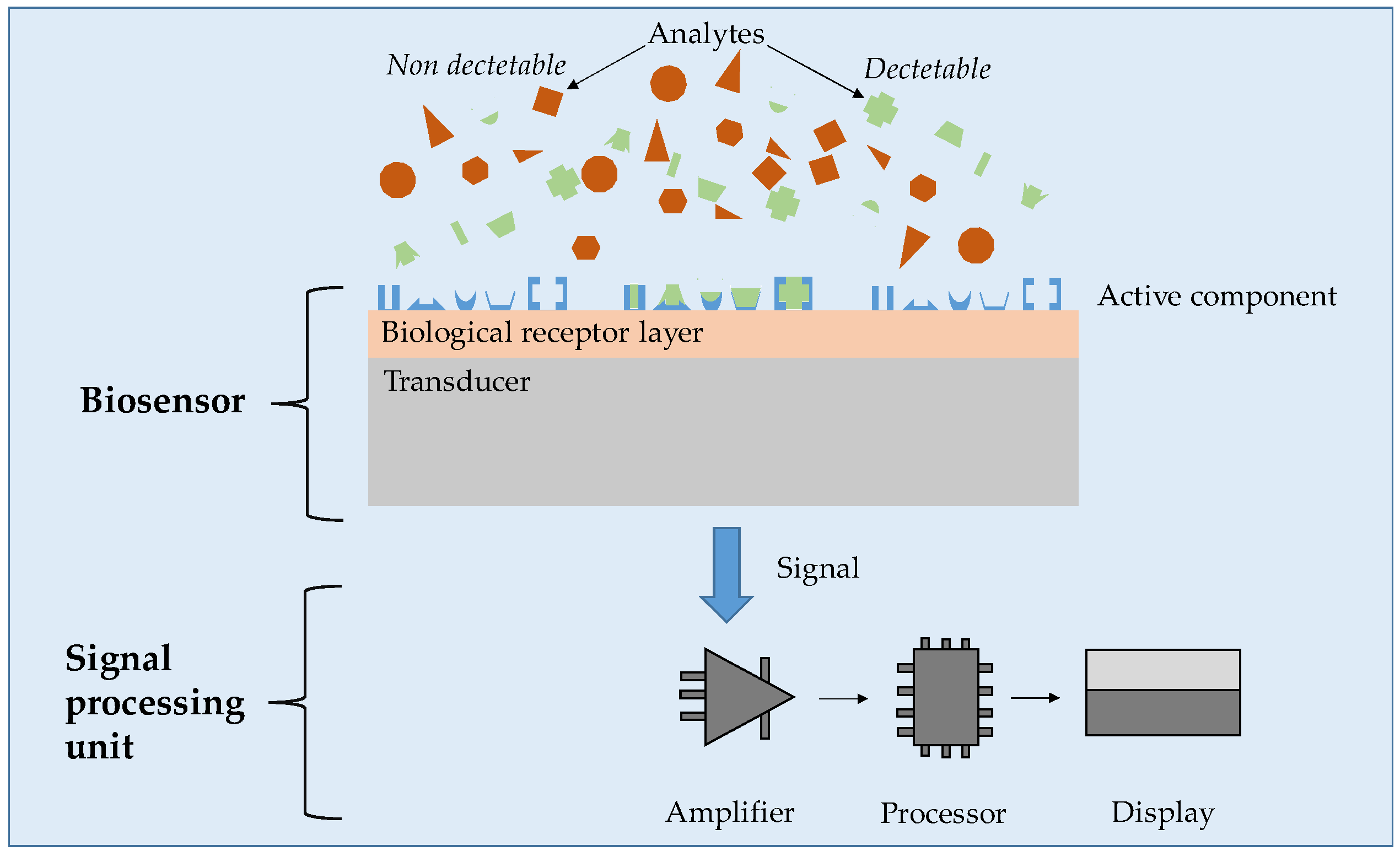
5.2. Biosensing Applications
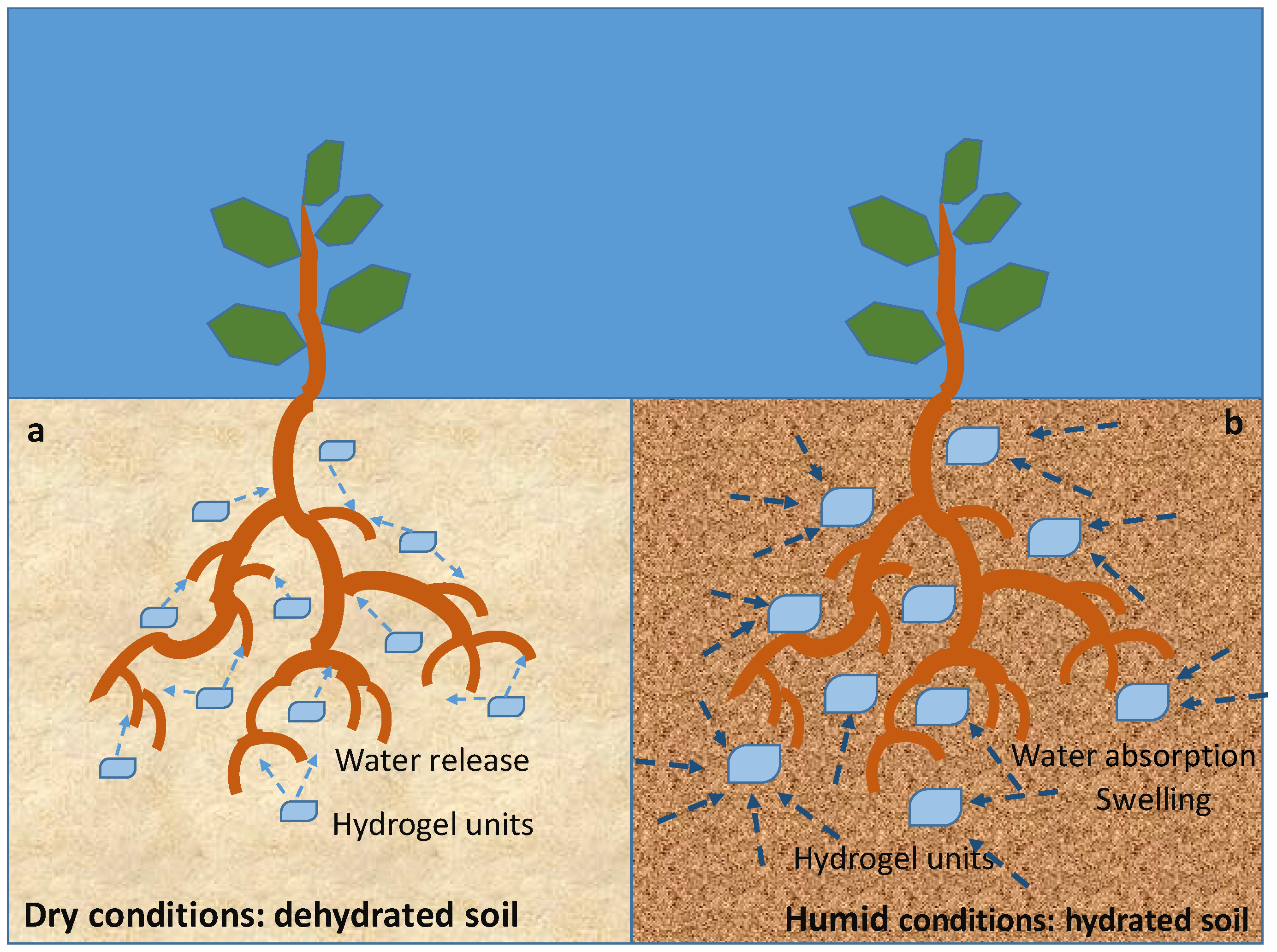
5.3. Environmental and Agricultural Applications
5.4. Other Applications
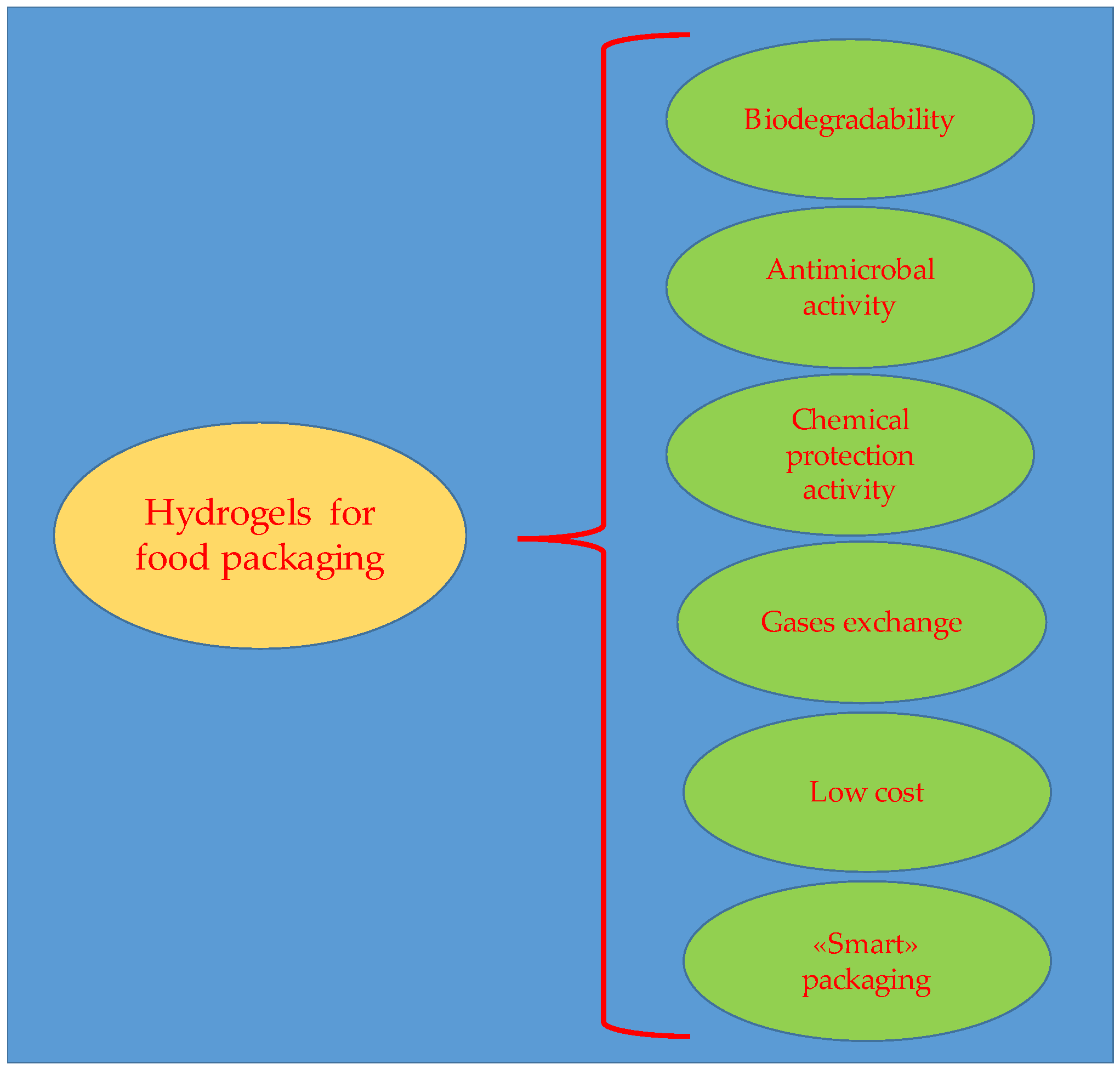
- Food preservation: Hydrogel films for food prevent bacterial growth and the deterioration caused by them, extending the shelf life [90].
- Use in cosmetic industry: Hydrogels can be used in cosmetic products for skin hydration and care [235].
- Use for screen protectors: Hydrogel films are used for the screen of devices such as smartphones and tablets, due to their flexibility and self-healing properties. Additionally, hydrogels can be incorporated into electronic devices, allowing for lighter and more flexible devices [236].
- Applications as sensors: Hydrogels can be used to improve sample collection or act as filters in sensors [237].
- Contact lens preparation: Hydrogels are ideal materials for contact lenses due to their ability to absorb water and oxygenate the eye [238].
5.5. Examples of Studies and Real Applications
5.5.1. Alginate-Based Films
5.5.2. Chitosan-Based Films
5.5.3. Hyaluronic Acid-Based Films
5.5.4. Cellulose-Based Films
5.5.5. Polyacrylic Acid-Based Films
5.5.6. Polyvinyl Alcohol-Based Films
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, H.; Choi, W.-S.; Jeong, J.-O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Pan, H.; Zhang, J.; Guo, Y.; Zhou, Z.; Zheng, J.; Zhang, Z.; Cao, R.; Hou, K.; et al. A Review of Hydrogel Fiber: Design, Synthesis, Applications, and Futures. Chem. Rev. 2025, 125, 5991–6056. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Aslam, M.A.; Abdullah, M.F.B.; Al-Arjan, W.S.; Stojanovic, G.M.; Hasan, A. Hydrogels: Classifications, Fundamental Properties, Applications, and Scopes in Recent Advances in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Arab. J. Chem. 2024, 17, 105968. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef] [PubMed]
- Braido, B.; Rukavina, Z.; Grimstad, Ø.; Franzè, S.; Cilurzo, F.; Vanić, Ž.; Škalko-Basnet, N.; Hemmingsen, L.M. Liposomes-in-hydrogel for topical drug delivery: Mechanical, kinetic, and biological insights. J. Drug Deliv. Sci. Technol. 2025, 113, 107380. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Chronopoulou, L.; Palocci, C. Liposome–Hydrogel Composites for Controlled Drug Delivery Applications. Gels 2024, 10, 284. [Google Scholar] [CrossRef]
- Yoon, M.S.; Lee, J.M.; Jo, M.J.; Kang, S.J.; Yoo, M.K.; Park, S.Y.; Bong, S.; Park, C.-S.; Park, C.-W.; Kim, J.-S.; et al. Dual-Drug Delivery Systems Using Hydrogel–Nanoparticle Composites: Recent Advances and Key Applications. Gels 2025, 11, 520. [Google Scholar] [CrossRef]
- Priya, A.S.; Premanand, R.; Ragupathi, I.; Bhaviripudi, V.R.; Aepuru, R.; Kannan, K.; Shanmugaraj, K. Comprehensive Review of Hydrogel Synthesis, Characterization, and Emerging Applications. J. Compos. Sci. 2024, 8, 457. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators Based on Stimulus Responsive Hydrogels and Their Emerging Biomedical Applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Mateescu, A.; Wang, Y.; Dostalek, J.; Jonas, U. Thin Hydrogel Films for Optical Biosensor Applications. Membranes 2012, 2, 40–69. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Zhang, W.; Qu, G.; Jin, S.; Li, X.; Nie, Z.; Zhou, H. Highly Sensitive Active Powering Pressure Sensor Enabled by Integration of Double Rough Surface Hydrogel and Flexible Batteries. npj Flex. Electron. 2022, 6, 92. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional Hydrogel Coatings. Nat. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Sajkiewicz, P.Ł. Hydrogel-Based Systems as Smart Food Packaging: A Review. Polymers 2025, 17, 1005. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, A.; Vázquez, A.S.; Vázquez, E. Hydrogels in Soft Robotics: Past, Present, and Future. ACS Nano 2024, 18, 20817–20826. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart Materials for Flexible Electronics and Devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Yu, W.; Xu, L. Hydrogels for Lubrication: Synthesis, Properties, Mechanism, and Challenges. Lubricants 2024, 12, 186. [Google Scholar] [CrossRef]
- Samchenko, Y.; Terpilowski, K.; Samchenko, K.; Golovkova, L.; Oranska, O.; Goncharuk, O. Calcium Alginate/Laponite Nanocomposite Hydrogels: Synthesis, Swelling, and Sorption Properties. Coatings 2024, 14, 1519. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Fan, W.; Liu, Y.; Wang, Q.; Weng, L. Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers 2022, 14, 3227. [Google Scholar] [CrossRef]
- Calistri, S.; Ciantelli, C.; Cataldo, S.; Cuzzola, V.; Guzzinati, R.; Busi, S.; Ubaldini, A. Simple Spin-Coating Preparation of Hydrogel and Nanoparticle-Loaded Hydrogel Thin Films. Coatings 2025, 15, 859. [Google Scholar] [CrossRef]
- Dong, M.; Jiao, D.; Zheng, Q.; Wu, Z.L. Recent progress in fabrications and applications of functional hydrogel films. J. Polym. Sci. 2023, 61, 1026–1039. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles Incorporated Hydrogels for Delivery of Antimicrobial Agents: Developments and Trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Djabourov, M. Gelation—A Review. Polym. Int. 1991, 25, 135–143. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and Evaluation of Chitosan/PVA Based Hydrogel Films Loaded with Honey for Wound Healing Application. Gels 2022, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.; Bzainia, A.; Almeida, A.; Martins, C.; Dias, R.C.S.; Costa, M.R.P.F.N. Chemical Routes for the Transformation of Bio-monomers into Polymers. In Plant Biomass Derived Materials: Sources, Extractions, and Applications; Wiley WCH: Weinheim, Germany, 2024. [Google Scholar] [CrossRef]
- Redaelli, F.; Sorbona, M.; Rossi, F. Synthesis and Processing of Hydrogels for Medical Applications. In Bioresorbable Polymers for Biomedical Applications; Perale, G., Hilborn, J., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 205–228. [Google Scholar] [CrossRef]
- López-Santiago, R.F.; Delgado, J.; Castillo, R. Competition Among Physical, Chemical, and Hybrid Gelation Mechanisms in Biopolymers. Soft Matter 2024, 20, 2518–2531. [Google Scholar] [CrossRef]
- Jochi, Y.; Seki, T.; Soejima, T.; Satoh, K.; Kamigaito, M.; Takeoka, Y. Spontaneous Synthesis of a Homogeneous Thermoresponsive Polymer Network Composed of Polymers with a Narrow Molecular Weight Distribution. NPG Asia Mater. 2018, 10, 840–848. [Google Scholar] [CrossRef]
- Blandino, A.; Macias, M.; Cantero, D. Formation of Calcium Alginate Gel Capsules: Influence of Sodium Alginate and CaCl2 Concentration on Gelation Kinetics. J. Biosci. Bioeng. 1999, 88, 686–689. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Abbas, A.; Raza, M.A. Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications. Gels 2025, 11, 47. [Google Scholar] [CrossRef]
- Yammine, P.; El Safadi, A.; Kassab, R.; El-Nakat, H.; Obeid, P.J.; Nasr, Z.; Tannous, T.; Sari-Chmayssem, N.; Mansour, A.; Chmayssem, A. Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes. Chemistry 2025, 7, 61. [Google Scholar] [CrossRef]
- Okihara, M.; Matsuda, A.; Kawamura, A.; Miyata, T. Design of dual stimuli-responsive gels with physical and chemical properties that vary in response to light and temperature and cell behavior on their surfaces. Polym. J. 2024, 56, 193–204. [Google Scholar] [CrossRef]
- Park, K.M.; Park, K.D. In Situ Cross-Linkable Hydrogels as a Dynamic Matrix for Tissue Regenerative Medicine. Tissue Eng. Regen. Med. 2018, 15, 547–557. [Google Scholar] [CrossRef]
- Wolfel, A.; Romero, M.R.; Igarzabal, C.I.A. Post-synthesis modification of hydrogels. Total and partial rupture of crosslinks: Formation of aldehyde groups and re-crosslinking of cleaved hydrogels. Polymer 2017, 116, 251–260. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion mechanism and application progress of hydrogels. Eur. Polym. J. 2022, 173, 111277. [Google Scholar] [CrossRef]
- Cook, J.P.; Goodall, G.W.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Microwave-Assisted Hydrogel Synthesis: A New Method for Crosslinking Polymers in Aqueous Solutions. Macromol. Rapid Commun. 2012, 33, 332–336. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Brady, A.J.; Vicente-Pérez, E.M.; Woolfson, A.D.; Thakur, R.R.S.; Donnelly, R.F. Microwave-Assisted Preparation of Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery Applications. Macromol. Mater. Eng. 2015, 300, 586–595. [Google Scholar] [CrossRef]
- Sun, P.; Li, X.; Kong, B.; Zhu, Y.; Wang, M.; Wang, H.; Liu, Q. Fabrication and characterization of microwave-assisted synthesis of carbon dots crosslinked sodium alginate hydrogel films. Int. J. Biol. Macromol. 2023, 253, 127130. [Google Scholar] [CrossRef]
- Thongsuksaengcharoen, S.; Samosorn, S.; Songsrirote, K. A Facile Synthesis of Self-Catalytic Hydrogel Films and Their Application as a Wound Dressing Material Coupled with Natural Active Compounds. ACS Omega 2020, 5, 25973–25983. [Google Scholar] [CrossRef]
- Mali, K.K.; Ghorpade, V.S.; Dias, R.J.; Dhawale, S.C. Synthesis and characterization of citric acid crosslinked carboxymethyl tamarind gum-polyvinyl alcohol hydrogel films. Int. J. Biol. Macromol. 2023, 236, 123969. [Google Scholar] [CrossRef]
- Brinker, C.J.; Hurd, A.J.; Schunk, P.R.; Frye, G.C.; Ashely, C.S. Review of sol–gel thin film formation. J. Non-Cryst. Solids 1992, 147–148, 424–436. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Tian, Y.; Zhang, X.N.; Du, M.; Song, Y.; Wu, Z.L.; Zheng, Q. Spin-coating-assisted fabrication of ultrathin physical hydrogel films with high toughness and fast response. Soft Matter 2018, 14, 5888–5897. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xue, Y.; Li, S.; Zhang, X.; Miao, Z.; Zeng, Z.; Ruan, D.; Shen, Y.; Yuan, H.; Zhao, Y.; et al. Tough and Functional Hydrogel Coating by Electrostatic Spraying. Small 2025, 21, 2408780. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.J.; Xi, S.; Zhang, Y.; Rao, P.; You, X.; Qu, S. Lignin-Based Ultrathin Hydrogel Coatings with Strong Substrate Adhesion Enabled by Hydrophobic Association. Adv. Funct. Mater. 2024, 35, 2413464. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, J.; Qiu, X.; Liu, H.; Liu, X.; Yao, P.; Huang, J.; Cui, X.; Liang, X.; Huang, C. Towards Large-Scale Fabrication of Self-Healable Functional Hydrogel Coatings for Anti-Fog/Frost Surfaces and Flexible Sensors. Adv. Mater. Technol. 2021, 6, 2001267. [Google Scholar] [CrossRef]
- Pemble, O.J.; Bardosova, M.; Povey, I.M.; Pemble, M.E. A Slot-Die Technique for the Preparation of Continuous, High-Area, Chitosan-Based Thin Films. Polymers 2021, 13, 1566. [Google Scholar] [CrossRef]
- Li, C.Y.; Hao, X.P.; Wu, Z.L.; Zheng, Q. Photolithographically Patterned Hydrogels with Programmed Deformations. Chem. Asian J. 2019, 4, 94–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C. Recent advances in 3D printing hydrogel for topical drug delivery. MedComm—Biomater. Appl. 2022, 1, e211. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Bauer, M.; Duerkop, A.; Baeumner, A.J. Critical review of polymer and hydrogel deposition methods for optical and electrochemical bioanalytical sensors correlated to the sensor’s applicability in real samples. Anal. Bioanal. Chem. 2023, 415, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ni, C.; Cao, R.; Jiang, Y.; Xia, L.; Ren, H.; Chen, Y.; Xie, T.; Zhao, Q. Sprayable porous hydrogel coating for efficient and sustainable evaporative cooling. Matter 2024, 7, 4270–4280. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Jeong, T.-J.; Yu, X.; Harris, T.A.L. Scaled Production of Functionally Gradient Thin Films Using Slot Die Coating on a Roll-to-Roll System. ACS Appl. Mater. Interfaces 2024, 16, 9264–9274. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Ke, L.-Y.; Wei, S.-Y.; Poddar, M.S.; Liu, C.H. Optofluidic thin-film lithography for photocrosslinking hydrogel-based microarchitectures and the assembling of modular cell-embedded microarchitectures. Sens. Actuators B Chem. 2022, 352, 131048. [Google Scholar] [CrossRef]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K. 3D printing of polymer composites: Materials, processes, and applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels…A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Koto, A.; Nishimoto, S.; Sakamoto, H.; Chuang, H.-S.; Uematsu, H.; Tanoue, S.; Takamura, E.; Suye, S. Analysis of Local Viscosity in Alginate Gel via Bead-based Diffusometry. Sens. Mater. 2022, 34, 3123–3131. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; You, T.; Zhao, B.; Dong, L.; Huang, C.; Zhou, X.; Xing, M.; Qian, W.; Luo, G. 3D Printing-Based Hydrogel Dressings for Wound Healing. Adv. Sci. 2024, 11, e2404580. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef]
- Rajendran, A.K.; Jayakumar, R. Preparation of hydrogels for biomedical applications: Biomimetic approach. In Woodhead Publishing Series in Biomaterials, Hydrogel Tissue Analogues; Woodhead Publishing: Sawston, UK, 2025; pp. 15–36. [Google Scholar] [CrossRef]
- Suh, K.Y.; Seong, J.; Khademhosseini, A.; Laibinis, P.E.; Langer, R. A simple soft lithographic route to fabrication of poly(ethylene glycol) microstructures for protein and cell patterning. Biomaterials 2004, 25, 557–563. [Google Scholar] [CrossRef]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, T.; Zheng, W.; Xian, C.; Huang, J.; Chen, Z.; Cai, W.; Zhang, W.; Lai, Y. A robust and transparent hydrogel coating for sustainable antifogging with excellent self-cleaning and self-healing ability. Chem. Eng. J. 2023, 451, 137879. [Google Scholar] [CrossRef]
- Card, M.; Alejandro, R.; Roxbury, D. Decoupling individual optical nanosensor responses using a spin-coated hydrogel platform. ACS Appl. Mater. Interfaces 2023, 15, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Blinova, E.; Korel, A.; Zemlyakova, E.; Pestov, A.; Samokhin, A.; Zelikman, M.; Tkachenko, V.; Bets, V.; Arzhanova, E.; Litvinova, E. Cytotoxicity and Degradation Resistance of Cryo- and Hydrogels Based on Carboxyethylchitosan at Different pH Values. Gels 2024, 10, 272. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Chafran, L.; Carfagno, A.; Altalhi, A.; Bishop, B. Green Hydrogel Synthesis: Emphasis on Proteomics and Polymer Particle-Protein Interaction. Polymers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the application of natural/synthetic hybrid hydrogels in tissue engineering and delivery systems: A comprehensive review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive review of natural based hydrogels as an upcoming trend for food packing. Food Hydrocoll. 2023, 135, 108124. [Google Scholar] [CrossRef]
- Wanniarachchi, P.C.; Paranagama, I.T.; Idangodage, P.A.; Nallaperuma, B.; Samarasinghe, T.T.; Jayathilake, C. Natural polymer-based hydrogels: Types, functionality, food applications, environmental significance and future perspectives: An updated review. Food Macromol. 2025, 2, 84–105. [Google Scholar] [CrossRef]
- Sharma, R.; Malviya, R.; Singh, S.; Prajapati, B. A Critical Review on Classified Excipient Sodium-Alginate-Based Hydrogels: Modification, Characterization, and Application in Soft Tissue Engineering. Gels 2023, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, S.; Nathanael, A.J. A Review on Hydrophobically Associated Alginates: Approaches and Applications. ACS Omega 2024, 9, 4246–4262. [Google Scholar] [CrossRef] [PubMed]
- Roquero, D.M.; Othman, A.; Melman, A.; Katz, E. Iron(iii)-cross-linked alginate hydrogels: A critical review. Mater. Adv. 2022, 3, 1849–1873. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of Alginate-Based Hydrogels in Hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-based hydrogels: From preparation to applications, a review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Hasnain, S.; Aminabhavi, T.M. Drug delivery using interpenetrating polymeric networks of natural polymers: A recent update. J. Drug Deliv. Sci. Technol. 2021, 66, 102915. [Google Scholar] [CrossRef]
- Usov, A.I. Chapter 4—Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 2011; Volume 65, pp. 115–217. [Google Scholar] [CrossRef]
- Yu, H.C.; Zhang, H.; Ren, K.; Ying, Z.; Zhu, F.; Qian, J.; Ji, J.; Wu, Z.L.; Zheng, Q. Ultrathin κ-Carrageenan/Chitosan Hydrogel Films with High Toughness and Antiadhesion Property. ACS Appl. Mater. Interfaces 2018, 10, 9002–9009. [Google Scholar] [CrossRef]
- Moreira, T.D.; Martins, V.B.; da Silva Júnior, A.H.; Sayer, C.; de Araújo, P.H.H.; Immich, A.P.S. New insights into biomaterials for wound dressings and care: Challenges and trends. Prog. Org. Coat. 2024, 187, 108118. [Google Scholar] [CrossRef]
- Valachová, K.; Hassan, M.E.; Šoltés, L. Hyaluronan: Sources, Structure, Features and Applications. Molecules 2024, 29, 739. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef]
- Wu, M.; Cronin, K.; Crane, J.S. Biochemistry, Collagen Synthesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507709/ (accessed on 27 August 2025).
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Gahlawat, S.; Nanda, V.; Shreiber, D.I. Designing collagens to shed light on the multi-scale structure–function mapping of matrix disorders. Matrix Biol. Plus 2024, 21, 100139. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Yao, L.; Wang, Y.; Wang, L.; Jiang, Z.; Qiu, D. Preparation and Characterization of Nanocomposite Hydrogels Based on Self-Assembling Collagen and Cellulose Nanocrystals. Polymers 2023, 15, 1308. [Google Scholar] [CrossRef]
- Rizo, C.; Espíndola-Serna, L.; Becerra-Rodriguez, J.J.; Cano-Salazar, J.; Flores Guia, L.F.T. Recent Advances in the Synthesis and Applications of Collagen Based Hydrogels: A Review. Mediterr. J. Basic Appl. Sci. 2019, 3, 54–98. [Google Scholar]
- Hu, T.; Lo, A.C.Y. Collagen-Alginate Composite Hydrogel: Application in Tissue Engineering and Biomedical Sciences. Polymers 2021, 13, 1852. [Google Scholar] [CrossRef]
- Noreen, A.; Sultana, S.; Sultana, T.; Tabasum, S.; Zia, K.M.; Muzammil, Z.; Jabeen, M.; Lodhi, A.Z.; Sultana, S. Chapter 3—Natural polymers as constituents of bionanocomposites. In Micro and Nano Technologies, Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 55–85. [Google Scholar] [CrossRef]
- Li, M.; You, J.; Qin, Q.; Liu, M.; Yang, Y.; Jia, K.; Zhang, Y.; Zhou, Y. A Comprehensive Review on Silk Fibroin as a Persuasive Biomaterial for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 2660. [Google Scholar] [CrossRef]
- Carrow, J.K.; Kerativitayanan, P.; Jaiswal, M.K.; Lokhande, G.; Gaharwar, A.K. Chapter 13—Polymers for Bioprinting. In Essentials of 3D Biofabrication and Translation; Academic Press: Cambridge, MA, USA, 2015; pp. 229–248. [Google Scholar] [CrossRef]
- Li, D.; Liang, R.; Wang, Y.; Zhou, Y.; Cai, W. Preparation of silk fibroin-derived hydrogels and applications in skin regeneration. Health Sci. Rep. 2024, 7, e2295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.G.L.; Casanova, F.; Silva, N.F.N.; de Carvalho Teixeira, A.V.N.; de Carvalho, A.F. Casein-based hydrogels: A mini-review. Food Chem. 2020, 314, 126063. [Google Scholar] [CrossRef] [PubMed]
- Guntero, V.A.; Acuña, M.C.; Aon, Y.S.; Gutierrez, L.G.; Ferretti, C.A. Formulation of Casein Hydrogels. Chem. Proc. 2024, 16, 96. [Google Scholar] [CrossRef]
- Anema, S.G. Heat-induced changes in caseins and casein micelles, including interactions with denatured whey proteins. Int. Dairy J. 2021, 122, 105136. [Google Scholar] [CrossRef]
- Zou, P.; Yao, J.; Cui, Y.-N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in Cellulose-Based Hydrogels for Biomedical Engineering: A Review Summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef]
- Raj, K.; Vora, T.; PadmaPriya, G.; Lal, B.; Devi, A.; Sharma, R.S.K.; Chahar, M.; Sudhakar, L.; Suman, R.J.; Nagraik, R. A comprehensive review of sustainable hydrogels from lignin for advanced wastewater solutions. Int. J. Biol. Macromol. 2025, 301, 139963. [Google Scholar] [CrossRef]
- de Freitas, F.A.; de Sá Barros, S.; Saron, C.; Lobo, W.V.; dos Santos, R.I.; Las-Casas, B.; Yupanqui-Mendoza, S.L.; de Souza, L.K.C. Lignocellulose Characterization and Exploitation. In Encyclopedia of Renewable Energy, Sustainability and the Environment, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 565–576. [Google Scholar] [CrossRef]
- Verma, A.; Aljohani, K.; Aljohani, B.S.; Lal, B.; Jadeja, Y.; Ballal, S.; Chahar, M.; Suman, R. Innovations in cellulose-based hydrogels for enhanced wastewater treatment through adsorption. Int. J. Biol. Macromol. 2025, 303, 140660. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, Z.; Lee, H.J. Exploring Applications and Preparation Techniques for Cellulose Hydrogels: A Comprehensive Review. Gels 2024, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Karthäuser, J.; Biziks, V.; Mai, C.; Militz, H. Lignin and Lignin-Derived Compounds for Wood Applications—A Review. Molecules 2021, 26, 2533. [Google Scholar] [CrossRef] [PubMed]
- Erfani Jazi, M.; Narayanan, G.; Aghabozorgi, F.; Farajidizaji, B.; Aghaei, A.; Kamyabi, M.A.; Navarathna, C.M.; Mlsna, T.E. Structure, Chemistry and Physicochemistry of Lignin for Material Functionalization. SN Appl. Sci. 2019, 1, 1094. [Google Scholar] [CrossRef]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.-C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Hamil, S.; Baha, M.; Abdi, A.; Alili, M.; Bilican, B.K.; Yilmaz, B.A.; Cakmak, Y.S.; Bilican, I.; Kaya, M. Use of sea urchin spines with chitosan gel for biodegradable film production. Int. J. Biol. Macromol. 2020, 152, 102–108. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chunshom, N.; Kotcharat, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. The Encapsulation of Green Tea Extract in Cyclodextrin and Loading into Chitosan-Based Composites: Controlled-Release Behavior and Antioxidant Properties. J. Polym. Environ. 2021, 29, 2628–2638. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M. Recent Advances in Poly(vinyl alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Durairaj, V.; Kalpana, R.; Kumar, V. Polyethylene Glycol Cross-Linked Hydrogel for Drug Absorption Properties. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 2), S1201–S1203. [Google Scholar] [CrossRef]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. pHEMA: An Overview for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Tripathi, R.; Yadav, J.P.; Pathak, P.; Almatarneh, M.H.; Verma, A. Chapter 6—Polymer–drug linking through amide bonds: The chemistry and applications in drug delivery. In Polymer-Drug Conjugates; Academic Press: Cambridge, MA, USA, 2023; pp. 147–170. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Nho, Y.-C.; Park, J.-S.; Lim, Y.-M. Preparation of Poly(acrylic acid) Hydrogel by Radiation Crosslinking and Its Application for Mucoadhesives. Polymers 2014, 6, 890–898. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.; Lee, K. Hydrogels as filler materials. In Hydrogels for Tissue Engineering Regenerative Medicine; Oliveira, J.M., Silva-Correia, J., Reis, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 413–432. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Ito, T.; Yamaguchi, S.; Soga, D.; Yoshimoto, T.; Koyama, Y. Preparation of a Bioadhesive Poly(Acrylic Acid)/Polyvinylpyrrolidone Complex Gel and Its Clinical Effect on Dental Hemostasis. Gels 2022, 8, 462. [Google Scholar] [CrossRef]
- Tou, Z.Q.; Koh, T.W.; Chan, C.C. Poly(vinyl alcohol) hydrogel based fiber interferometer sensor for heavy metal cations. Sens. Actuators B Chem. 2014, 202, 185–193. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z. Nanoparticle–Hydrogel Based Sensors: Synthesis and Applications. Catalysts 2022, 12, 1096. [Google Scholar] [CrossRef]
- Lu, D.; Mo, Y.; Sun, S.; Wang, Q.; Wu, Z.; Wang, W.; Zhu, M. Mechanically Reinforced Nanocomposite Hydrogels and Advanced Applications in Biosensing and Bioelectronics. Chem. Mater. 2025, 37, 3871–3902. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.S.; Hu, X.H.; Chu, L.Q. Recent Advances in Antimicrobial Hydrogels Containing Metal Ions and Metals/Metal Oxide Nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal nanoparticle hybrid hydrogels: The state-of-the-art of combining hard and soft materials to promote wound healing. Theranostics 2024, 14, 1534–1560. [Google Scholar] [CrossRef]
- Calistri, S.; Ciantelli, C.; Cuzzola, V.; Strafella, A.; Cellamare, C.M.; Ubaldini, A. Growth of Silver Nanoparticles Embedded in a Polyacrylamide—Alginate Hybrid Hydrogel. Crystals 2025, 15, 211. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Deng, L.; Shen, Z.; Huang, Q.; Shah, N.G.; Chen, W.; Zhang, Y.; Wang, X.; Yu, L.; et al. Antibacterial and antioxidative hydrogel dressings based on tannic acid-gelatin/oxidized sodium alginate loaded with zinc oxide nanoparticles for promoting wound healing. Int. J. Biol. Macromol. 2024, 279, 135177. [Google Scholar] [CrossRef] [PubMed]
- Kaniewska, K.; Karbarz, M.; Katz, E. Nanocomposite hydrogel films and coatings—Features and applications. Appl. Mater. Today 2020, 20, 100776. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Wu, Z.; Wang, Z.; Li, Y.; Jian, K.; He, C.; Zhang, C.; Shi, L.; Dai, J. Enzyme-immobilized nanoclay hydrogel simultaneously reduces inflammation and scar deposition to treat spinal cord injury. Chem. Eng. J. 2024, 484, 149642. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Xu, A.; Li, W.; Qin, Y. Facile preparation of graphene/polyaniline composite hydrogel film by electrodeposition for binder-free all-solid-state supercapacitor. J. Alloys Compd. 2021, 875, 159931. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Z.; Xiang, X. Graphene oxide composite hydrogels for wearable devices. Carbon Lett. 2022, 32, 1395–1410. [Google Scholar] [CrossRef]
- Afruzi, F.H.; Abdouss, M.; Zare, E.N.; Ghomi, E.R.; Mahmoudi, S.; Neisiany, R.E. Metal-organic framework-hydrogel composites as emerging platforms for enhanced wound healing applications: Material design, therapeutic strategies, and future prospects. Coord. Chem. Rev. 2025, 524, 216330. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Tran, D.T.; Leadore, J.L. Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohydr. Polym. 2013, 95, 760–767. [Google Scholar] [CrossRef]
- Sheikh-Oleslami, S.; Tao, B.; D’Souza, J.; Butt, F.; Suntharalingam, H.; Rempel, L.; Amiri, N. A Review of Metal Nanoparticles Embedded in Hydrogel Scaffolds for Wound Healing In Vivo. Gels 2023, 9, 591. [Google Scholar] [CrossRef]
- Yu, Y.-C.; Hu, M.-H.; Zhuang, H.-Z.; Phan, T.H.M.; Jiang, Y.-S.; Jan, J.-S. Antibacterial Gelatin Composite Hydrogels Comprised of In Situ Formed Zinc Oxide Nanoparticles. Polymers 2023, 15, 3978. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.; Torbett-Dougherty, M.; Islam, A.; Soleimani, S.; Bruce-Tagoe, T.A.; Johnson, J.A. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review. Nanomaterials 2025, 15, 285. [Google Scholar] [CrossRef]
- Mascarenhas-Melo, F.; Peixoto, D.; Aleixo, C.; Gonçalves, M.B.S.; Raza, F.; Pawar, K.D.; Veiga, F.; Liu, M.; Paiva-Santos, A.C. Nanoclays for wound management applications. Drug Deliv. Transl. Res. 2022, 13, 924–945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhende, M.; Mulwani, P.; Patil, S. A comprehensive exploration of graphene and graphene oxide based hydrogels—Methods, characteristics, and applications. J. Indian Chem. Soc. 2025, 102, 101782. [Google Scholar] [CrossRef]
- Sharma, S.S.A.; Bashir, S.; Kasi, R.; Subramaniam, R.T. The significance of graphene based composite hydrogels as smart materials: A review on the fabrication, properties, and its applications. FlatChem 2022, 33, 100352. [Google Scholar] [CrossRef]
- Moradi, S.; Hamedi, H.; Tonelli, A.E.; King, M.W. Chitosan/Graphene Oxide Composite Films and Their Biomedical and Drug Delivery Applications: A Review. Appl. Sci. 2021, 11, 7776. [Google Scholar] [CrossRef]
- Vanić, Ž.; Jøraholmen, M.W.; Škalko-Basnet, N. Challenges and considerations in liposomal hydrogels for the treatment of infection. Expert Opin. Drug Deliv. 2025, 22, 255–276. [Google Scholar] [CrossRef]
- Ban, J.; Mo, Z.; Cui, X.; Xu, Y.; Lyu, Z. Comparative study of liposomes and liposomes-in-polymer hydrogel as transdermal carriers for improving the topical delivery of imperatorin. J. Holist. Integr. Pharm. 2021, 2, 32–41. [Google Scholar] [CrossRef]
- Hezari, S.; Olad, A.; Dilmaghani, A. Modified gelatin/iron-based metal-organic framework nanocomposite hydrogel as wound dressing: Synthesis, antibacterial activity, and Camellia sinensis release. Int. J. Biol. Macromol. 2022, 218, 488–505. [Google Scholar] [CrossRef]
- Kush, P.; Singh, R.; Kumar, P. Recent Advances in Metal–Organic Framework-Based Anticancer Hydrogels. Gels 2025, 11, 76. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- He, R.; He, J.; Shen, J.; Fu, H.; Zhang, Y.; Wang, B. Recent advances in multifaceted applications of MOF-based hydrogels. Soft Sci. 2024, 4, 37. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.-M.; Chang, Y.-H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, e50376. [Google Scholar] [CrossRef]
- Andleeb, A.; Mehmood, A.; Tariq, M.; Butt, H.; Ahmed, R.; Andleeb, A.; Ghufran, H.; Ramzan, A.; Ejaz, A.; Malik, K.; et al. Hydrogel patch with pretreated stem cells accelerates wound closure in diabetic rats. Biomater. Adv. 2022, 142, 213150. [Google Scholar] [CrossRef]
- Stewart, C.L.; Hook, A.L.; Zelzer, M.; Marlow, M.; Piccinini, A.M. PLGA-PEG-PLGA hydrogels induce cytotoxicity in conventional in vitro assays. Cell Biochem. Funct. 2024, 42, e4097. [Google Scholar] [CrossRef] [PubMed]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Antibacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Sobczak-Kupiec, A. In vitro cytotoxicity of hydrogels based on chitosan and modified with gold nanoparticles. J. Polym. Res. 2017, 24, 153. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Moratti, S.C. Hydrogels for biomedical applications. Future Med. Chem. 2011, 3, 1877–1888. [Google Scholar] [CrossRef]
- Jose, J.; Athira, V.P.; Michel, H.; Hafeela, A.R.; Bhat, S.G.; Thomas, S.; Maria, L.P. Chapter 1—Hydrogels: An overview of the history, classification, principles, applications, and kinetics. In Sustainable Hydrogels; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–22. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Ravishankar, K.; Venkatesan, M.; Desingh, R.P.; Mahalingam, A.; Sadhasivam, B.; Subramaniyam, R.; Dhamodharan, R. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 447–457. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Fan, Y.; Yu, S.; Liu, M.; Feng, L.; Sun, Q.; Pan, P. Principles and Design of Bionic Hydrogel Adhesives for Skin Wound Treatment. Polymers 2024, 16, 1937. [Google Scholar] [CrossRef]
- Tong, Z.; Jin, L.; Oliveira, J.M.; Reis, R.L.; Zhong, Q.; Mao, Z.; Gao, C. Adaptable hydrogel with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact. Mater. 2021, 6, 1375–1387. [Google Scholar] [CrossRef]
- del Olmo, J.A.; Martínez, V.S.; González, R.P.; Alonso, J.M. Sustained Drug Release from Biopolymer-Based Hydrogels and Hydrogel Coatings; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Xin, H.; Maruf, D.S.A.A.; Akin-Ige, F.; Amin, S. Stimuli-responsive hydrogels for skin wound healing and regeneration. Emergent Mater. 2024, 8, 1339–1356. [Google Scholar] [CrossRef]
- Rocha-García, D.; Guerra-Contreras, A.; Rosales-Mendoza, S.; Palestino, G. Role of porous silicon/hydrogel composites on drug delivery. Mesoporous Biomater. 2016, 3, 93–101. [Google Scholar] [CrossRef]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef] [PubMed]
- García-García, P.; Reyes, R.; Pérez-Herrero, E.; Arnau, M.R.; Évora, C.; Delgado, A. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111009. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Schaller, E.; Steinmann, P.; Boccaccini, A.R.; Budday, S. Alginate-based hydrogels show the same complex mechanical behavior as brain tissue. J. Mech. Behav. Biomed. Mater. 2020, 111, 103979. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Bîrcă, A.C.; Mogoşanu, G.D.; Rădulescu, M.; Holban, A.M.; Manuc, D.; Alberts, A.; Grumezescu, A.M.; Mogoantă, L. Zinc Alginate Hydrogel-Coated Wound Dressings: Fabrication, Characterization, and Evaluation of Anti-Infective and In Vivo Performance. Gels 2025, 11, 427. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Julin, K.; Ahsan, L.; Basnet, P.; Johannessen, M.; Škalko-Basnet, N. Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Mar. Drugs 2021, 19, 269. [Google Scholar] [CrossRef]
- Ge, G.; Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Muscle-Inspired Self-Healing Hydrogels for Strain and Temperature Sensor. ACS Nano 2020, 14, 218–228. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Fathollahi, A.; Salehi, M.; Gholizadeh, S.; Derakhshankhah, H.; Allahyari, Z.; Jaymand, M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Chan, C.Y.; Rastogi, A.; Grant, A.M.; White, C.M.; Bette, N.; Schaub, N.J.; Corey, J.M. Combining electrospun nanofibers with cell-encapsulating hydrogel fibers for neural tissue engineering. J. Biomater. Sci. Polym. 2018, 29, 1625–1642. [Google Scholar] [CrossRef]
- Obermeyer, J.M.; Tuladhar, A.; Payne, S.L.; Ho, E.; Morshead, C.M.; Shoichet, M.S. Local Delivery of Brain-Derived Neurotrophic Factor Enables Behavioral Recovery and Tissue Repair in Stroke-Injured Rats. Tissue Eng. Part A 2019, 25, 15–16. [Google Scholar] [CrossRef]
- Nam, S.; Stowers, R.; Lou, J.; Xia, Y.; Chaudhuri, O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 2019, 200, 15–24. [Google Scholar] [CrossRef]
- Crocini, C.; Walker, C.J.; Anseth, K.S.; Leinwand, L.A. Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog. Biophys. Mol. Biol. 2020, 154, 71–79. [Google Scholar] [CrossRef]
- Hanak, B.W.; Hsieh, C.Y.; Donaldson, W.; Browd, S.R.; Lau, K.K.S.; Shain, W. Reduced cell attachment to poly(2-hydroxyethyl methacrylate)-coated ventricular catheters in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1268–1279. [Google Scholar] [CrossRef]
- Di, Z.; Shi, Z.; Ullah, M.W.; Li, S.; Yang, G. A transparent wound dressing based on bacterial cellulose whisker and poly(2-hydroxyethyl methacrylate). Int. J. Biol. Macromol. 2017, 105, 638–644. [Google Scholar] [CrossRef]
- Gao, T.; Jiang, M.; Liu, X.; You, G.; Wang, W.; Sun, Z.; Ma, A.; Chen, J. Patterned Polyvinyl Alcohol Hydrogel Dressings with Stem Cells Seeded for Wound Healing. Polymers 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duan, L.; Liu, G.; Sun, J.; Shahbazi, M.A.; Kundu, S.C.; Reis, R.L.; Xiao, B.; Yang, X. Bioinspired Polyacrylic Acid-Based Dressing: Wet Adhesive, Self-Healing, and Multi-Biofunctional Coacervate Hydrogel Accelerates Wound Healing. Adv. Sci. 2023, 10, e2207352. [Google Scholar] [CrossRef]
- Gao, Y.; La, H.; Min, H.; Hou, Z. Study on the mechanical properties of Polyacrylic Acid/Chitosan double network hydrogels in dynamic water content. Mater. Res. Express 2024, 11, 115308. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Environmental and Wastewater Treatment Applications of Stimulus-Responsive Hydrogels. Gels 2025, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Palmese, L.L.; Thapa, R.K.; OSullivan, M.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Choudhary, A.; Sharma, A.; Singh, A.; Han, S.S.; Sood, A. Strategy and Advancement in Hybrid Hydrogel and Their Applications: Recent Progress and Trends. Adv. Eng. Mater. 2024, 26, 2400944. [Google Scholar] [CrossRef]
- Khalilian, A.; Khan, R.R.; Kang, S.-W. Highly sensitive and wide-dynamic-range side-polished fiber-optic taste sensor. Sens. Actuators B Chem. 2017, 249, 700–707. [Google Scholar] [CrossRef]
- Malik, S.; Singha, J.; Goyata, R.; Saharana, Y.; Chaudhrya, V.; Umarb, A.; Ibrahimb, A.A.; Akbarc, S.; Ameend, S.; Baskouta, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Revathi, D.; Panda, S.; Deshmukh, K.; Khotele, N.; Murthy, V.R.K.; Pasha, S.K.K. Smart hydrogels for sensing and biosensing—Preparation, smart behaviours, and emerging applications—A comprehensive review. Polym. Test. 2025, 150, 108912. [Google Scholar] [CrossRef]
- Völlmecke, K.; Afroz, R.; Bierbach, S.; Brenker, L.J.; Frücht, S.; Glass, A.; Giebelhaus, R.; Hoppe, A.; Kanemaru, K.; Lazarek, M.; et al. Hydrogel-Based Biosensors. Gels 2022, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, X.; Tohl, D.; Pham, A.T.T.; Raston, C.; Tang, Y. Hydrogel-Film-Fabricated Fluorescent Biosensors with Aggregation-Induced Emission for Albumin Detection through the Real-Time Modulation of a Vortex Fluidic Device. Molecules 2023, 28, 3244. [Google Scholar] [CrossRef] [PubMed]
- Vedovello, P.; Sanches, L.V.; da Silva Teodoro, G.; Majaron, V.F.; Bortoletto-Santos, R.; Ribeiro, C.; Putti, F.F. An Overview of Polymeric Hydrogel Applications for Sustainable Agriculture. Agriculture 2024, 14, 840. [Google Scholar] [CrossRef]
- Park, J.; Guan, W.; Yu, G. Smart Hydrogels for Sustainable Agriculture. EcoMat 2025, 7, e70011. [Google Scholar] [CrossRef]
- Pattnaik, A.; Ghosh, P.; Poonia, A.K. An overview on advancements in hydrogels for effective wastewater treatment. J. Mol. Liq. 2025, 424, 127120. [Google Scholar] [CrossRef]
- Ali, K.; Asad, Z.; Agbna, G.H.D.; Saud, A.; Khan, A.; Zaidi, S.J. Progress and Innovations in Hydrogels for Sustainable Agriculture. Agronomy 2024, 14, 2815. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Pushparaju, S. Smart Polymer Hydrogels as Matrices for the Controlled Release Applications in Agriculture Sector; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Olad, A.; Gharekhani, H.; Mirmohseni, A.; Bybordi, A. Synthesis, characterization, and fertilizer release study of the salt and pH-sensitive NaAlg-g-poly(AA-co-AAm)/RHA superabsorbent nanocomposite. Polym. Bull. 2017, 74, 3353–3377. [Google Scholar] [CrossRef]
- Helaly, F.M.; Essawy, H.A.; El-Nashar, D.E.; Maziad, N.A. Slow Release of urea as a source of nitrogen from some acrylamide and acrylic acid hydrogels. Polym.-Plast. Technol. Eng. 2005, 44, 253–263. [Google Scholar] [CrossRef]
- Tiwari, R.; Krishnamoorthi, S.; Kumar, K. Synthesis of cross-linker devoid novel hydrogels: Swelling behaviour and controlled urea release studies. J. Environ. Chem. Eng. 2019, 7, 103162. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, M.; Shen, Y. Enhanced swelling ratio and water retention capacity for novel super-absorbent hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123972. [Google Scholar] [CrossRef]
- Chen, M.; Ni, Z.; Shen, Y.; Xiang, G.; Xu, L. Reinforced swelling and water-retention properties of super-absorbent hydrogel fabricated by a dual stretchable single network tactic. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125133. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Sun, X.; Yue, Y.; Tubana, B.; Yang, R.; Cheng, H.N. Novel Alginate-Cellulose Nanofiber-Poly(Vinyl Alcohol) Hydrogels for Carrying and Delivering Nitrogen, Phosphorus and Potassium Chemicals. Int. J. Biol. Macromol. 2021, 172, 330–340. [Google Scholar] [CrossRef]
- Alharbi, K.; Ghoneim, A.; Ebid, A.; El-Hamshary, H.; El-Newehy, M.H. Controlled release of phosphorous fertilizer bound to carboxymethyl starch-gpolyacrylamide and maintaining a hydration level for the plant. Int. J. Biol. Macromol. 2018, 116, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Noppakundilograt, S.; Pheatcharat, N.; Kiatkamjornwong, S. Multilayer-coated NPK compound fertilizer hydrogel with controlled nutrient release and water absorbency. J. Appl. Polym. Sci. 2014, 132, 41249. [Google Scholar] [CrossRef]
- Li, Y.; Hu, C.; Lan, J.; Yan, B.; Zhang, Y.; Shi, L.; Ran, R. Hydrogel-based temperature sensor with water retention, frost resistance and remoldability. Polymers 2020, 186, 122027. [Google Scholar] [CrossRef]
- Inukai, M.; Jin, Y.; Yomota, C.; Yonese, M. Preparation and characterization of hyaluronatehydroxyethyl acrylate blend hydrogel for controlled release device. Chem. Pharm. Bull. 2000, 48, 850–854. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, Y.-H. Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release watersoluble fertilizer. Sci. Total Environ. 2019, 655, 958–967. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; Muñoz-Bonilla, A.; Fernandez-Garcia, M. Amylose modified starches as superabsorbent systems for release of potassium fertilizers. J. Polym. Environ. 2021, 30, 2314–2328. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; De Moura, M.R.; Ribeiro, C.; Longo, E.; Mattoso, L.H.C. Application of polysaccharide hydrogels in adsorption and controlled-extended release of fertilizers processes. J. Appl. Polym. Sci. 2012, 123, 2291–2298. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Mousaa, I.M.; El-Sayyad, G.S. Radiation synthesis of urea/hydrogel core shells coated with three different natural oils via a layer-by-layer approach: An investigation of their slow release and effects on plant growth-promoting rhizobacteria. Progress. Org. Coat. 2021, 151, 106022. [Google Scholar] [CrossRef]
- Bakass, M.; Mokhlisse, A.; Lallemant, M. Absorption and desorption of liquid water by a superabsorbent polymer: Effect of polymer in the drying of the soil and the quality of certain plants. J. Appl. Polym. Sci. 2002, 83, 234–243. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.W.; Zhang, L.; Xie, X.M. Water absorbency of poly (sodium acrylate) superabsorbents crosslinked with modified poly (ethylene glycol). J. Appl. Polym. Sci. 2003, 90, 1851–1856. [Google Scholar] [CrossRef]
- Singh, A.; Sarkar, D.J.; Mittal, S.; Dhaka, R.; Maiti, P.; Singh, A.; Raghav, T.; Solanki, D.; Ahmed, N.; Singh, S.B. Zeolite-reinforced carboxymethyl cellulose-Na⁺-g-cl-poly(acrylamide) hydrogel composites with pH-responsive phosphate release behavior. J. Appl. Polym. Sci. 2019, 136, 47332. [Google Scholar] [CrossRef]
- Liu, M.; Liang, R.; Zhan, F.; Liu, Z.; Niu, A. Synthesis of a slow-release and superabsorbent nitrogen fertilizer and its properties. Polym. Adv. Technol. 2006, 17, 430–438. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Kaewpirom, S. Potassium release kinetics and water retention of controlled-release fertilizers based on chitosan hydrogels. J. Polym. Environ. 2010, 18, 413–421. [Google Scholar] [CrossRef]
- Zhan, F.; Liu, M.; Guo, M.; Wu, L. Preparation of superabsorbent polymer with slow-release phosphate fertilizer. J. Appl. Polym. Sci. 2004, 92, 3417–3421. [Google Scholar] [CrossRef]
- Al Rohily, K.; El-Hamshary, H.; Ghoneim, A.; Modaihsh, A. Controlled Release of Phosphorus from Superabsorbent Phosphate-Bound Alginate-Graft-Polyacrylamide: Resistance to Soil Cations and Release Mechanism. ACS Omega 2021, 5, 32919–32929. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; Ghobashy, M.M. Controlled release fertilizers using superabsorbent hydrogel prepared by gamma radiation. Radiochim. Acta 2017, 105, 865–876. [Google Scholar] [CrossRef]
- Dong, G.; Mu, Z.; Liu, D.; Shang, L.; Zhang, W.; Gao, Y.; Zhao, M.; Zhang, X.; Chen, S.; Wei, M. Starch phosphate carbamate hydrogel based slow-release urea formulation with good water retentivity. Int. J. Biol. Macromol. 2021, 190, 189–197. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Xu, X.; Su, Y.; Yue, Q.; Gao, B. Characterization, swelling and slow-release properties of a new controlled release fertilizer based on wheat straw cellulose hydrogel. J. Taiwan Inst. Chem. Eng. 2016, 60, 564–572. [Google Scholar] [CrossRef]
- Rozo, G.; Bohorques, L.; Santamaría, J. Controlled release fertilizer encapsulated by a κ-carrageenan hydrogel. Polímeros 2019, 29, e2019033. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; Zaidi, S.J. Hydrogel Performance in Boosting Plant Resilience to Water Stress—A Review. Gels 2025, 11, 276. [Google Scholar] [CrossRef]
- Tay, J.-W.; Choe, D.-H.; Mulchandani, A.; Rust, M.K. Hydrogels: From Controlled Release to a New Bait Delivery for Insect Pest Management. J. Econ. Entomol. 2020, 113, 2061–2068. [Google Scholar] [CrossRef]
- Malka, E.; Margel, S. Engineering of PVA/PVP Hydrogels for Agricultural Applications. Gels 2023, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lou, Z.; Zhang, L.; Guo, H.; Wang, Z.; Guo, C.; Fukuda, K.; Ma, S.; Wang, G.; Someya, T.; et al. Ultrathin Hydrogel Films toward Breathable Skin-Integrated Electronics. Adv. Mater. 2023, 35, e2206793. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, G.; Li, S.; Yang, H.; Zheng, J.; Wang, D.; Yang, H.; Wong, L.W.Y.; Fu, J. Breathable Ultrathin Film Sensors Based on Nanomesh Reinforced Anti-Dehydrating Organohydrogels for Motion Monitoring. Adv. Funct. Mater. 2024, 34, 2411725. [Google Scholar] [CrossRef]
- Abdulamier, A.A.; Shaker, L.M.; Al-Amiery, A.A. Advancements in the chemistry of contact Lenses: Innovations and applications. Results Chem. 2024, 12, 101872. [Google Scholar] [CrossRef]
- Panou, A.; Karabagias, I.K. Biodegradable Packaging Materials for Foods Preservation: Sources, Advantages, Limitations, and Future Perspectives. Coatings 2023, 13, 1176. [Google Scholar] [CrossRef]
- Tuesta, T.; Castillo-Barzola, A.; Linares, H.; Ruiz-Pacco, G.; Baena-Moncada, A.M.; Valderrama-Negrón, A.C. Chitosan-based materials for food preservation: Enhancing shelf life and safety through sustainable nanoparticles and films. Food Chem. 2025, 486, 144589. [Google Scholar] [CrossRef]
- Oh, H.; Lee, Y.; Kim, Y.; Seo, Y.; Kang, J.; Park, E.; Yoo, Y.; Sung, M.; Yoon, Y. Development of antimicrobial hydrogel with edible formulations to control foodborne pathogens on food surfaces consumed raw. Innov. Food Sci. Emerg. Technol. 2021, 74, 102845. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Chen, Q.; Zheng, Q.; Chen, H.; Li, X. An antibacterial hydrogel prepared from a licorice residue extract. RSC Sustain. 2024, 2, 646–654. [Google Scholar] [CrossRef]
- Zheng, L.; Tian, Z.; Ai, B.; Yang, Y.; Zheng, X.; Liu, Y.; Xiao, D.; Sheng, Z.; Qin, J. Double network hydrogel coating improved avocado freshness preservation: Preparation, testing and mechanism. Food Hydrocoll. 2025, 163, 111085. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; de Souza Siqueira Quintans, J.; Freitas, M.M.; Cerqueira, M.A.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Zhang, Y.; Xu, E.; Yan, S.; Xu, H.; Li, M. Recent advances in the fabrication, characterization and application of starch-based materials for active food packaging: Hydrogels and aerogels. Sustain. Food Technol. 2024, 2, 615–634. [Google Scholar] [CrossRef]
- Singh, A.K.; Itkor, P.; Lee, Y.S. State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends. Gels 2023, 9, 433. [Google Scholar] [CrossRef]
- Perera, K.Y.; Mathew, S.S.; Carnaval, L.d.S.C.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Biodegradable curcumin-nanoclay films for extending shrimp shelf-life and freshness. Curr. Res. Food Sci. 2025, 10, 101102. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Hakim, L.; Gaikwad, K.K. Active Packaging Materials. Curr. Food Sci. Technol. Rep. 2023, 1, 123–132. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Int. Food Res. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, Y. Comparison of nano-zinc oxide or calcium chloride incorporated polyvinyl alcohol/chitosan/anthocyanin films for active and intelligent packaging. Colloid Polym. Sci. 2024, 302, 1711–1723. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Jeong, J.-H. Gels/Hydrogels in Different Devices/Instruments—A Review. Gels 2024, 10, 548. [Google Scholar] [CrossRef]
- Tan, J.; Li, X.; Zheng, C.R.; Tan, A.; Li, X.C.; Ni, H.L.; Yu, W.H.; Bai, Y.F.; Hu, P.; Chen, H.M. Development of a shear strengthening conductive hydrogel for impact protection and distress signal emission. Chem. Eng. J. 2025, 511, 162280. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, B.; Li, H.; Li, M.; Song, Y.; Wang, R.; Wang, T.; Zhang, H. Flexible Wearable Sensors in Medical Monitoring. Biosensors 2022, 12, 1069. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, Y.; Zou, H.; Chen, Y.; Wen, Z.; Liang, Q.; Peng, X.; Sui, J.; Chen, J.; He, Y.; et al. Fabrication and Application of Multifunctional Conductive Hydrogel Film for Wearable Sensors via Efficient Freeze-Thaw Cycling and Annealing Process. Chem. Eng. J. 2024, 495, 153487. [Google Scholar] [CrossRef]
- Alhussaini, M.S.; Alyahya, A.R.A.I.; Al-Ghanayem, A.A. Alginate-derived antibacterial and antifungal agents: A review of applications and advancements (2019–2025). Int. J. Biol. Macromol. 2025, 318, 145333. [Google Scholar] [CrossRef] [PubMed]
- Ureña, M.; Carullo, D.; Phùng, T.T.T.; Fournier, P.; Farris, S.; Lagorce, A.; Karbowiak, T. Effect of polymer structure on the functional properties of alginate for film or coating applications. Food Hydrocoll. 2024, 149, 109557. [Google Scholar] [CrossRef]
- Manna, S.; Nath, N.C.; Sarkar, P.; Karmakar, S.; Gupta, P.; Jana, S.; Nandi, G.; Sen, O. Alginate-based target specific bioadhesive drug delivery systems: A review. Int. J. Polym. Mater. Polym. Biomater. 2025, 1–27. [Google Scholar] [CrossRef]
- Yang, B.; Gu, W.; Pan, J.; Zhang, L. Degradable Alginate Hydrogel Intervention Catheters with Gradient Hardness in Length. Adv. Healthc. Mater. 2025, 14, 2405086. [Google Scholar] [CrossRef] [PubMed]
- Dhalsamant, K.; Dalai, A.; Pattnaik, F.; Acharya, B. Biodegradable Carbohydrate-Based Films for Packaging Agricultural Products—A Review. Polymers 2025, 17, 1325. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Panicker, U.G. Sustainable chitosan-based biomaterials for the future: A review. Polym. Bull. 2025, 82, 661–709. [Google Scholar] [CrossRef]
- Hashempur, M.H.; Sabili, A.; Karami, F.; Zomorodian, K.; Shenavari, S.; Vaez, A.; Sahraeian, K.; Zareshahrabadi, Z. Synthesize, antioxidant and antimicrobial properties of a chitosan xerogel film with Nigella Sativa extract. Sci. Rep. 2025, 15, 24635. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Glajc, P.; Chmielniak, D.; Gwizdalska, K.; Swiontek Brzezinska, M.; Dembińska, K.; Shinde, A.H.; Gierszewska, M.; Łukowicz, K.; Basta-Kaim, A.; et al. Development and Characterization of Biocompatible Chitosan-Aloe Vera Films Functionalized with Gluconolactone and Sorbitol for Advanced Wound Healing Applications. ACS Appl. Mater. Interfaces 2025, 17, 15196–15207. [Google Scholar] [CrossRef]
- Nawaz, M.; Saeed, M.; Zahoor, M.; Bibi, S.; Khan, S. Synthesis of chitosan-polyethylene glycol-based superabsorbent for enhanced water retention in agriculture. J. Polym. Res. 2025, 32, 284. [Google Scholar] [CrossRef]
- Madihalli, S.; Masti, S.P.; Eelager, M.P.; Chougale, R.B.; Anilkumar, B.M.; Priyadarshini, A.N. Methylcellulose/Chitosan bioactive films enriched with Achyranthes aspera leaves extract: An innovative approach for sustainable cosmetic face mask applications. Int. J. Biol. Macromol. 2025, 303, 140611. [Google Scholar] [CrossRef]
- Srinivasan, S.; Vijayalekha, A.; Anandasadagopan, S.; Pandurangan, A.K. Hyaluronic Acid: A Comprehensive Review of Its Osteogenic Potential and Diverse Biomedical Applications. Curr. Pharmacol. Rep. 2025, 11, 28. [Google Scholar] [CrossRef]
- Ahmadi, F.; Mojtaba, S.; Fard, H.; Jazeh, M.; Mohammadi, L.; Nilforoushzadeh, M.A. Preparation and Characterization of Hyaluronic Acid-based Composite Films for Wound Dressing Applications. J. Adv. Mater. Eng. 2025, 44, 55–73. [Google Scholar]
- Yu, H.; Li, X.; Xu, M.; Zhao, H.; Cai, Z. Facile fabrication of tear-film-inspired durable hyaluronic acid hydrogel films for persistent anti-fogging. Prog. Org. Coat. 2025, 208, 109537. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Kim, M.; Kwon, Y.; Yun, J.; Choi, J.H.; Youn, H.J. Synergistic moisturizing effect of a cellulose nanofibril/hyaluronic acid/poly-γ-glutamic acid blend system. Cellulose 2025, 32, 4781–4796. [Google Scholar] [CrossRef]
- Lee, T.J.; Lee, H.W.; Kim, H.J. Properties, Preparation, and Applications of Cellulose-Based Hydrogels. J. Korea TAPPI 2025, 57, 5–21. [Google Scholar] [CrossRef]
- Ungureanu, E.; Mikhailidi, A.; Tofanica, B.-M.; Fortună, M.E.; Rotaru, R.; Ungureanu, O.C.; Samuil, C.; Popa, V.I. Sustainable Gels from Polysaccharides in Agriculture. Polysaccharides 2025, 6, 37. [Google Scholar] [CrossRef]
- Oouchi, A.; Ito, T.; Katahira, Y.; Hasegawa, H.; Nakamura, K.; Mizoguchi, I.; Yoshimoto, T.; Koyama, Y. Wound Healing Enhancement and Physical Characterization of Bioadhesive Poly(acrylic acid)/Polyvinylpyrrolidone Complex Gels. Gels 2025, 11, 300. [Google Scholar] [CrossRef]
- Shen, P.; Wu, J.; Han, H.; Bai, Y.; Zhang, X.; Shao, R. Recent progress of hydrogels as sports medical materials: Characteristics, modification strategies and application prospects in sports. J. Biomater. Sci. Polym. Ed. 2025, 1–29. [Google Scholar] [CrossRef]
- Mandal, M.; Singh Lodhi, R.; Chourasia, S.; Das, S.; Das, P. A Review on Sustainable Slow-Release N, P, K Fertilizer Hydrogels for Smart Agriculture. ChemPlusChem 2025, 90, e202400643. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Wang, R.; Ruan, L.; Jiang, G.; Li, P.; Aharodnikau, U.E.; Yunusov, K.E.; Gao, X.; Solomevich, S.O. Fabrication of Curcumin-Loaded Silk Fibroin and Polyvinyl Alcohol Composite Hydrogel Films for Skin Wound Healing. ACS Appl. Bio Mater. 2022, 5, 4400–4412. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Koosha, M.; Li, T.; Geng, X. The favorable role of oxidized pullulan as a multipurpose crosslinker in polyvinyl alcohol (PVA)/chitosan/collagen films for promoting human skin fibroblast viability, antibacterial activity and healing of methicillin-resistant Staphylococcus aureus (MRSA) infected wounds in mice. Int. J. Biol. Macromol. 2025, 311, 143435. [Google Scholar] [CrossRef]
- de Castro, D.P.; dos Santos, C.D.R.; Sant’Ana, J.; Jacques, R.A.; dos Santos Polidoro, A.; da Silva, S.H.F.; Gatto, D.A.; Santana, R.M.C. Exploring the Antifungal and Phytotoxic Activity of Polyvinyl Alcohol Hydrogels Incorporated with Essential Oils. Polym. Adv. Technol. 2025, 36, e70116. [Google Scholar] [CrossRef]







| Characteristics | Solvent Casting | Dip Coating | Spin Coating | Spray Coating | Blade Coating | Bar Coating | Slot Die Coating | Photolithography | 3D Printing |
|---|---|---|---|---|---|---|---|---|---|
| Relative Cost | Low | Low | Medium | High | Medium | Medium | High | High/very high | High |
| Scalability | Limited | No | Possible | Possible | Limited | Possible | Possible | No | |
| Complexity | Low | Low | Low | Medium | Medium | Low | Medium | medium | High |
| Uniformity of Films | Good | High | High | Low | Medium | Medium | High | High | Medium/high |
| Minimum thickness possible | Ten of micrometer | Nanometers | Nanometers | From tens to hundreds of nanometers | Ten of micrometer | Ten of micrometer | Nanometers | From tens to hundreds of nanometers | Micrometers |
| Patterning In Situ | Possible | Possible | No | No | Possible | No | Limited | Possible | Possible |
| Coatable Surfaces | Many types of surfaces they must be smooth and flat | Complex, rigid shapes | Small, flat substrates only | Flexible or rigid substrates, curved or flat surfaces | Flexible or rigid substrates | Flexible or rigid substrates | Flexible or rigid substrates | glass, polymeric, inorganic; pretreatment is often necessary | glass, polymeric, inorganic; pretreatment is often necessary |
| Solution wastage | High | High | High | Moderate | Moderate | Moderate | Low | high | Low |
| Drying Times | High | Slow | Fast | Fast | Slow | Slow | Slow | Slow | medium |
| Coating Speeds | Slow | Slow | Very slow | Fast | Fast | Slow | Fast | Low/medium | Fast |
| Natural Hydrogels | Synthetic Hydrogel | |
|---|---|---|
| Advantages | High biodegradability; High biocompatibility; Low cost | Easily controllable composition; High reproducibility Excellent mechanical properties |
| Disadvantages | Worst mechanical properties; Batch-related variability; Low long-term stability; Possible low reproducibility | Possible cytotoxicity; Possible low biocompatibility |
| Type of Gel | Biocompatibility | Cellular Activity/Proliferation | Wound Dressing | Exudates Absorption | Mechanical Properties | Reference |
|---|---|---|---|---|---|---|
| Alginate | Yes | Yes | Yes | Low | [178,179] | |
| Cellulose | Yes | Yes | Yes | Low | [180] | |
| Chitosan | Yes | Yes | Yes | Low | [177,181] | |
| Collagen | Yes | Yes | Yes | Low | [182,183] | |
| Hyaluronic acid | Yes | Yes | Yes | Low | [184,185] | |
| Poly(ethylen oxide) (PEG) | Yes | Yes | Yes | Good | [186,187] | |
| Poly(hydroxyethyl meta acrylate) (PHEMA) | Yes | Yes | Yes | Yes | Good | [188,189] |
| Poly(vinly alcool) (PVA) | Yes | Yes | Yes | Good | [190] | |
| Polyacrylic acid (PAA) | Yes | Yes | Yes | Good | [191,192] |
| Type of Gel | Main Function | References |
|---|---|---|
| Acrylamide and acrylic acid-based hydrogels | Soil conditioner and water retention material | [208] |
| Acrylamide and N-hydroxymethyl acrylamide hydrogel | Soil conditioner and water retention material | [209] |
| Acrylic acid and acrylamide copolymers | Soil conditioner and water retention material | [210] |
| Acrylic acid-co-acrylic amide based hydrogel | Soil conditioner and water retention material | [211] |
| Alginate-cellulose nanofibers–poly(vinyl alcohol) hydrogel | Controlled release of phosphate fertilizer | [212] |
| Carboxymethyl starch-g-polyacrylamideCarboxymethyl starch-g-polyacrylamide | Controlled release of phosphate fertilizer | [213] |
| Glutaraldehyde crosslinked chitosan-poly(vinylalcohol) hydrogel | Controlled release of nitrogen fertilizer | [214] |
| Glycerol and poly(vinyl alcohol) hydrogel | Soil conditioner and water retention material | [215] |
| Hyaluronate-Hydroxyethyl acrylate blend | Soil conditioner and water retention material | [216] |
| Methylcellulose and hydroxypropyl methylcellulose-based hydrogel | Controlled release of potassium | [217] |
| N,N′-MBA crosslinked starch hydrogel | Controlled release of potassium | [218] |
| Poly(acrylamide) and methylcellulose-based hydrogels | Controlled release of nitrogen fertilizer | [219] |
| Poly(acrylonitril)-based poly acrylic acid hydrogels | Controlled release of nitrogen fertilizer | [220] |
| Poly(ethylene glycol) and Poly(acrylate) copolymer | Soil conditioner and water retention material | [221] |
| Poly(ethylene glycol) and poly(sodium acrylate) | Soil conditioner and water retention material | [222] |
| Poly(lactic acid)/cellulose-based hydrogel composite | Controlled release of potassium | [223] |
| Poly(maleic anhydride-co-acrylic acid) hydrogel | Controlled release of nitrogen fertilizer | [224] |
| Poly(vinyl alcohol)/chitosan crosslinked with glutaraldehyde | Controlled release of potassium | [225] |
| Poly(vinylalcohol)-phosphate gels | Controlled release of phosphate fertilizer | [226] |
| Polyacrylamide hydrogel | Controlled release of phosphate fertilizer | [227] |
| Polyvinylpyrrolidone (PVP)/carboxylmethyl cellulose | Controlled release of nitrogen fertilizer | [228] |
| Starch phosphate carbamate hydrogel | Controlled release of nitrogen fertilizer | [229] |
| Wheat straw cellulose hydrogel | Soil conditioner and water retention material | [230] |
| κ-carrageenan-based hydrogel | Controlled release of potassium | [231] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubaldini, A.; Calistri, S. Advances in Hydrogel Film Fabrication and Functional Applications Across Biomedical and Environmental Fields. Appl. Sci. 2025, 15, 9579. https://doi.org/10.3390/app15179579
Ubaldini A, Calistri S. Advances in Hydrogel Film Fabrication and Functional Applications Across Biomedical and Environmental Fields. Applied Sciences. 2025; 15(17):9579. https://doi.org/10.3390/app15179579
Chicago/Turabian StyleUbaldini, Alberto, and Sara Calistri. 2025. "Advances in Hydrogel Film Fabrication and Functional Applications Across Biomedical and Environmental Fields" Applied Sciences 15, no. 17: 9579. https://doi.org/10.3390/app15179579
APA StyleUbaldini, A., & Calistri, S. (2025). Advances in Hydrogel Film Fabrication and Functional Applications Across Biomedical and Environmental Fields. Applied Sciences, 15(17), 9579. https://doi.org/10.3390/app15179579






