1. Introduction
Superparamagnetic magnetite (Fe
3O
4) nanoparticles are a key focus of contemporary nanotechnology research due to their superparamagnetic behaviour, biocompatibility, and tunable surface chemistry. These nanoparticles respond strongly to external magnetic fields while exhibiting negligible remanent magnetisation, rendering them highly attractive for biomedical and technological applications, including magnetic resonance imaging (MRI), targeted drug delivery, hyperthermia therapy, and biosensing [
1,
2]. Recent advances have enabled the synthesis of monodisperse iron oxide nanoparticles with well-defined sizes and morphologies, essential for optimised magnetic performance and controlled biological interaction [
3,
4].
Synthesis of superparamagnetic magnetite nanoparticles is typically undertaken via chemical coprecipitation, hydrothermal or solvothermal treatment, and thermal decomposition routes. Each pathway imparts control over properties such as size, crystallinity, magnetic saturation, and shape. For instance, coprecipitation is scalable and simple but may yield polydisperse particles; thermal decomposition, in contrast, yields highly crystalline and uniform nanoparticles though requiring more complex conditions [
5,
6]. The synthesis parameters, such as reaction temperature, pH, precursor ratios, surfactant selection, stirring rate, critically influence particle size [
7]. Nanoparticles below approximately 10 nm generally exhibit superparamagnetism [
8], while larger particles tend towards ferrimagnetic behaviour and increased aggregation [
9].
In order to be used in ferrofluids with biomedical and technological applications, magnetite nanoparticles must be very close to spherical shape and homogeneous in size, and well dispersed in a polar liquid [
10,
11]. Furthermore, the critical diameter of spherical magnetite nanoparticles should define them as magnetic mono-domains and their behaviour will be superparamagnetic [
12,
13].
However, superparamagnetic iron oxide nanoparticles inherently tend to agglomerate due to van der Waals and magnetic dipole–dipole forces, diminishing colloidal stability and functional performance in ferrofluids. Stabilisation techniques thus play an important role, including electrostatic repulsion via ionic surfactants and steric hindrance by polymer (polyethylene glycol, dextran, polyvinyl alcohol) coatings [
3,
6]. An increasingly adopted complementary approach is ultrasonic treatment, which utilises high-frequency acoustic energy to disrupt aggregates via cavitation-induced microstreaming, enhancing dispersion and preventing re-agglomeration. Ultrasonic treatment can be particularly effective when applied alongside surfactants or polymer coatings. Ultrasonication parameters, such as amplitude, duration and pulsing, must be optimized to avoid damage to coatings or alteration of magnetic properties [
14,
15].
Beyond stabilisation, one of the most dynamic applications of magnetite-based ferrofluids lies in their controlled transport through microfluidic channels, driven by external magnetic fields. The unique combination of fluidity and magnetism allows superparamagnetic iron oxide nanoparticle-based ferrofluids to be actuated non-invasively, enabling precise manipulation at the microscale. In such systems, external field gradients generate body forces (magnetophoretic effects) that can guide or pump the ferrofluid through microchannels with complex geometries. This approach enables the development of integrated microfluidic platforms for biosensing, diagnostics, or localised drug delivery [
9,
16]. The efficiency of motion depends strongly on the ferrofluid’s viscosity, particle loading, the intensity and spatial profile of the magnetic field, and the hydrodynamic resistance of the microchannel [
17].
One of the key indicators of the stability of magnetic ferrofluids is the zeta potential of magnetite nanoparticles. This parameter is influenced by a wide range of factors that alter the surface charge of the nanoparticles, with pH being one of the most significant. The introduction of an acid into the suspension can render the particles more positively charged due to proton adsorption, whereas alkalinisation may result in the development of negative surface charges on the magnetic nanoparticles.
The pH value at which the zeta potential reaches zero is referred to as the isoelectric point (IEP). At this point, the electrostatic repulsion between particles is minimised, leading to a pronounced tendency toward agglomeration.
Other factors that can influence the surface charge and, consequently, the zeta potential distribution include synthesis parameters (such as reaction temperature and the molar ratio of precursors) as well as post-synthesis treatments, including washing steps and surface functionalization [
18].
Such capabilities make ferrofluid transport a promising element in soft robotics, self-healing materials, and next-generation lab-on-chip systems, where precise, low-power, remotely controlled actuation is essential. Collectively, the synthesis, stabilisation, and magnetic actuation of superparamagnetic magnetite nanoparticles based ferrofluids across microenvironments highlight a growing multidisciplinary domain at the convergence of nanoscience, biomedical engineering, and microfluidics.
The aim of this work is to obtain and characterize magnetite nanoparticles for subsequent use in an experimental microscopic observation of ferrofluid through a microchannel. The microscopic images captured showed the behaviour of magnetite nanoparticles inside the ferrofluid, but especially in the meniscus area.
This work brings as novelty precisely the choice of the most difficult way to use a ferrofluid in a microchannel: a limited stability time by using magnetite nanoparticles not coated with surfactants, but stabilized only by ultrasonication and controlled alkaline pH, a loading of the ferrofluid with a large amount of magnetic nanoparticles.
2. Materials and Methods
2.1. Experimental Plan
The experimental plan involved several stages. Firstly, nanocrystalline magnetite (MNP1-8) was obtained through chemical coprecipitation at pH = 10 [
19]. Next, each sample was analysed to determine the crystallite size, the average particle size, and the magnetic properties, to establish whether the samples have superparamagnetic characteristics or not. Following the physical characterization, the most representative sample of magnetite nanoparticles (MNPs) was selected to prepare ferrofluids with different magnetite contents (10, 15, 20, 25, 30, and 35 wt.%). The ferrofluids were stabilised via ultrasonication, followed by an evaluation of their colloidal stability through zeta potential measurements.
The dynamic viscosity was measured in both the presence and absence of a magnetic field, and the variations in viscosity relative to MNP concentration were studied. The next step involved experimenting under a digital microscope to observe the behaviour of the ferrofluid with 35 wt.% MNP2 through a microchannel with a diameter of 200 μm in a transparent and hydrophobic polydimethylsiloxane (PDMS) matrix.
The selection of the ferrofluid with the greatest amount of magnetite was based on its rapid response to magnetic fields and, evidently, it is the most difficult ferrofluid to manipulate due to its high viscosity.
2.2. Materials
For this research, eight samples of magnetite nanoparticles (MNP) were obtained by chemical coprecipitation with sodium hydroxide (Merck Millipore, Merck KGaA, Darmstadt, Germany) from iron chlorides solutions (iron (III) chloride from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and iron (II) chloride-tetrahydrate extra pure special grade from Merck Millipore, Merck KGaA, Darmstadt, Germany), under argon protection at the temperature of 75 °C, and pH = 10.
Following the reaction of chemical coprecipitation, the obtained product was separated using a magnetic decantation method, then cleaned multiple times with distilled water, and finally filtered.
To prevent nanoparticle agglomeration during of the obtaining process, the black suspension obtained was stabilized by stirring in argon atmosphere, at the temperature of 60 °C, pH = 10, and ultrasonicated for other 3 h. No surfactant was used for stabilizing the magnetite nanoparticles. After this process, each magnetite sample was slowly dried at room temperature in a desiccator, without any heat treatment, to avoid possible structural changes that could affect the magnetic properties of magnetite nanoparticles.
2.3. Apparatus and Methods
For the analytical investigations, the following devices and methods were used:
The X-ray diffraction (XRD) was performed with the Rigaku Ultima IV X-ray diffractometer (Rigaku, Tokyo, Japan) with Cu-Kα radiation, having wavelength λ = 0.15406 nm, for determining the crystallinity and crystallite size of the magnetic powder obtained; the experimental setup has the following parameters: Bragg–Brentano beam geometry; anode voltage 40 KV and intensity 30 mA; continuous scanning at a speed of 1° × min−1, at a step of 0.01°; angular range (2Ꝋ) = 10–100°. The interpretation was done using the software PDXL 2.2 based on the patterns (ICDD cards–contained in the PDF-5+ database, release 2024) from category S (Star Patterns–Extremely high-quality data), respectively, I (Indexed Patterns–Indexed high quality data). The quantitative analysis was performed using the Rietveld method whole powder pattern fitting (WPPF) based on crystal structure parameters.
The saturation magnetization and the coercive field were determined on Lakeshore Model 7304 vibrating sample magnetometer (VSM) (Lake Shore Cryotronics, Westerville, OH, USA), to find the relation between the magnetic properties and the average magnetite’s crystallite sizes and to establish whether magnetite nanoparticles have (or do not have) superparamagnetic character.
Dynamic viscosity was determined on ferrofluid samples with different magnetite contents using a Viscometer Premium Fungilab (FUNGILAB, Barcelona, Spain) equipped with a circular magnet that generates a magnetic field parallel to the axes of symmetry.
Transmission electron microscopy (TEM) images and elemental analysis (EDX) were recorded on a Tecnai™ G2 F20 TWIN Cryo-TEM Field Electron (FEI Company, Eindhoven, The Netherlands) operating at an accelerating 200 kV voltage, to determine the shape and the average size of the magnetite nanoparticles before utilization in microchannel movement. The graph of the dimensional distribution of the MNP2 nanoparticle diameters was created by analysing 7 TEM images (62000×) using SigmaPlot 14.5 (Systat Software Inc., San Jose, CA, USA).
Zeta potential measurements were conducted using the Amerigo ZetaView electrophoretic analyser (Cordouan Technologies, Pessac, France) by assessing the mobility of particles under an applied electric field. The MNPs samples were investigated in aqueous suspension at 25 °C, with an applied voltage of 30 V. A dedicated software package (Cordouan Technologies, Pessac, France) monitored particle displacement at 11 distinct positions within the measurement channel, yielding the zeta potential distribution as a function of signal intensity (Total Counts). The reported values represent the mean of three independent measurements for each sample. Experimental parameters, including conductivity, pH, and sample volume, were rigorously controlled to ensure measurement accuracy and reproducibility, using the AmeriQ software (Cordouan Technologies, Pessac, France).
The study of the behaviour in the microchannel was done on an optical microscope, Levenhuk MED 10M (magnification 40–1000× g), with a digital camera Levenhuk M800 Plus (Levenhuk Optics Ltd., Praha, Czech Republic).
2.4. Samples Preparation
For XRD and VSM analyses, it was necessary to crush and grind the magnetite samples into a fine powder. This ensures homogeneity and allows for accurate diffraction measurements.
For VSM study, the samples were prepared as a mixture of 2/3 of MNP powder and 1/3 of polyethylene wax (PEwax) as binder. This mixture was pressed in the form of pellets with dimensions of 3 mm in diameter and 6.35 mm long, to be easily mounted in the sample holder of the VSM. All magnetic measurement was made at room temperature.
For XRD analysis, the MNP powder samples were deposited on glass slides (20 × 20 × 0.5 mm).
For the studies on viscosity, zeta potential measurement (only for ferrofluid sample with 35 wt.% MNP2) and motion through microchannel, ferrofluids were prepared based on distilled water, using only MNP2 powder in weight percentages of 10, 15, 20, 25, 30, and 35 wt.%. Each ferrofluid was ultrasonicated again to ensure stability. Following this, viscosity tests were performed and the behaviour of the ferrofluid was recorded through the microchannel.
To prepare the sample for TEM imaging and elemental analysis (EDX), a few drops of ferrofluid with 35 wt.% MNP2 were placed onto Tedpella copper grids covered with carbon.
3. Results and Discussion
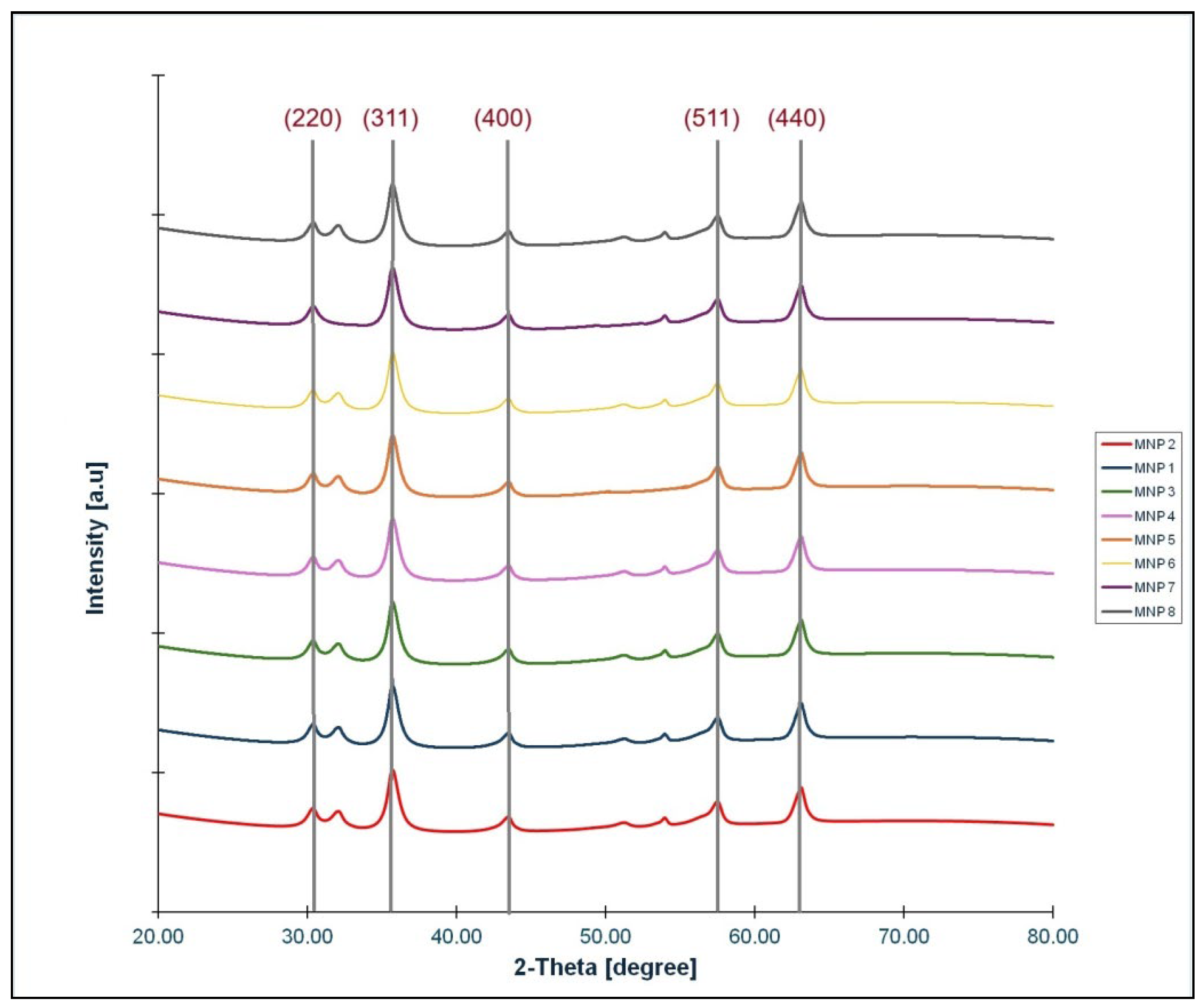
3.1. X-Ray Diffraction Analysis
To determine qualitatively and quantitatively the crystal structure of the magnetic powders that have obtained X-ray diffraction (XRD) analysis was used. All eight samples of magnetite were analysed and the results were very similar (
Figure 1)
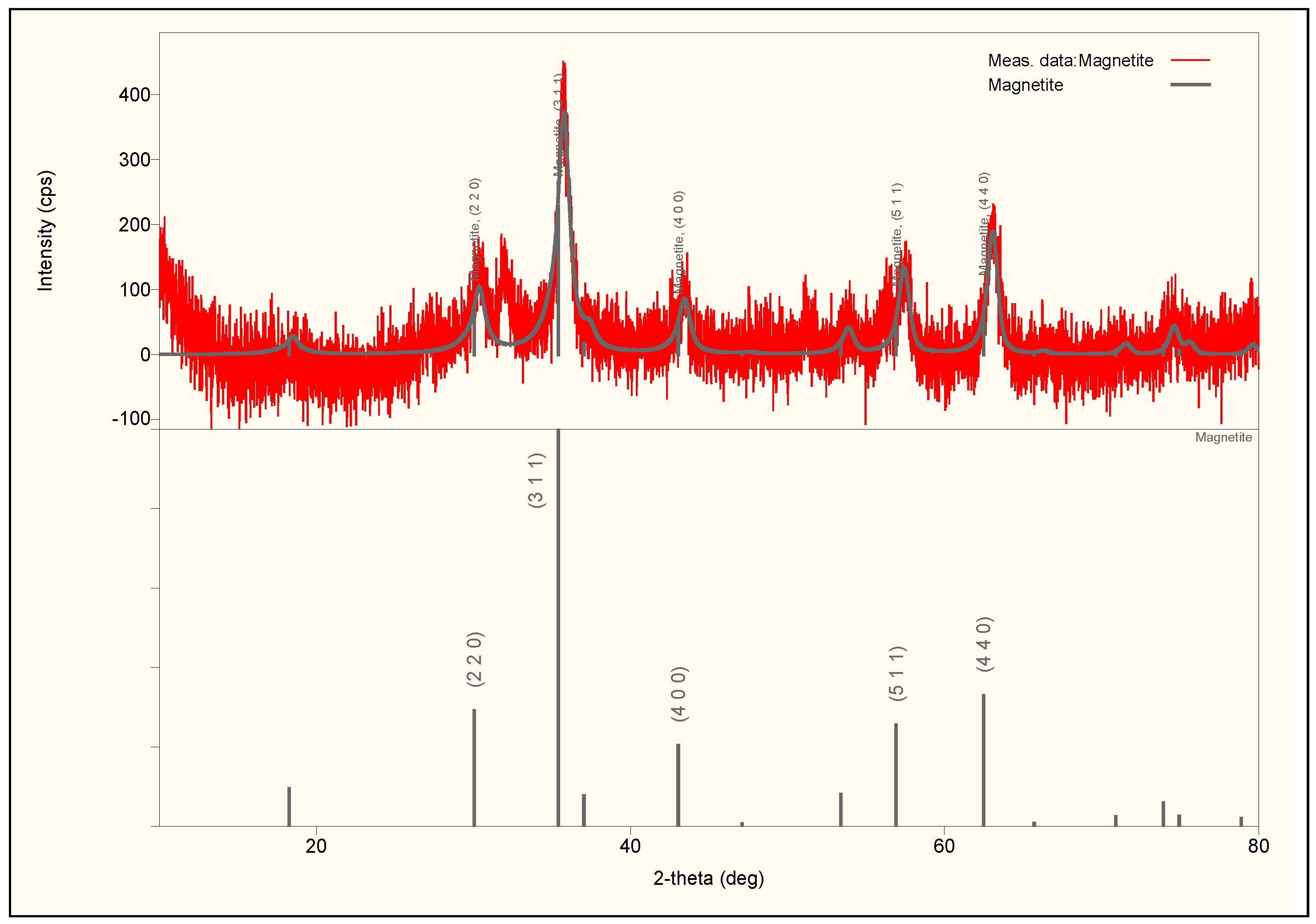
The only difference was in the average crystallite size of the particles. The XRD results show the same characteristic peaks of cubic inverse spinel structure of nanocrystalline magnetite for all samples: (220), (311), (400), (511), and (440). Only the X-ray diffractogram of sample MNP2 together with structure analysis results, the most representative sample from our experiment, are presented in
Figure 2 and the
Table 1.
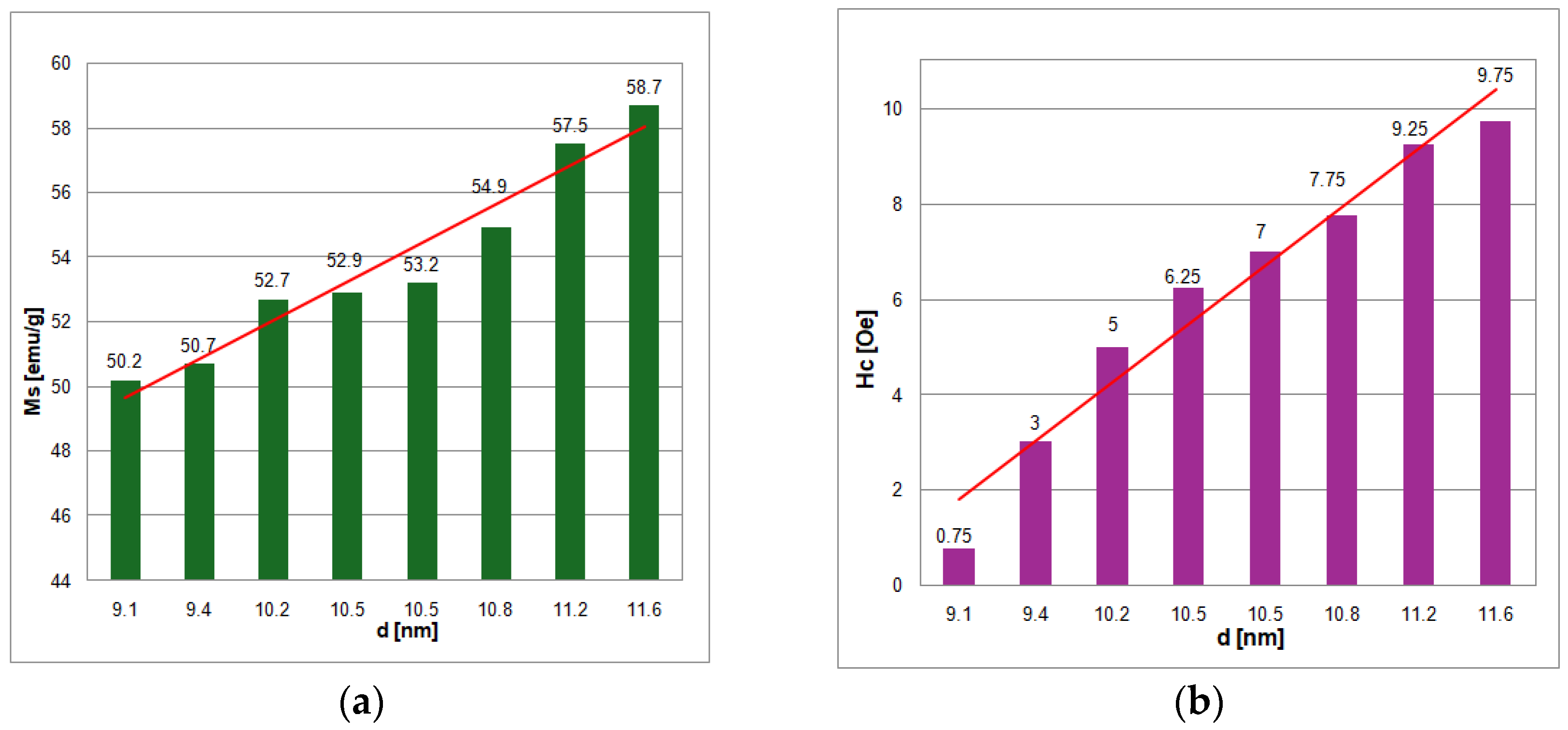
For all samples, the crystallite size was determined using the Williamson–Hall method and found to be in the range 9.1–11.6 nm (
Table 2).
3.2. TEM Imaging
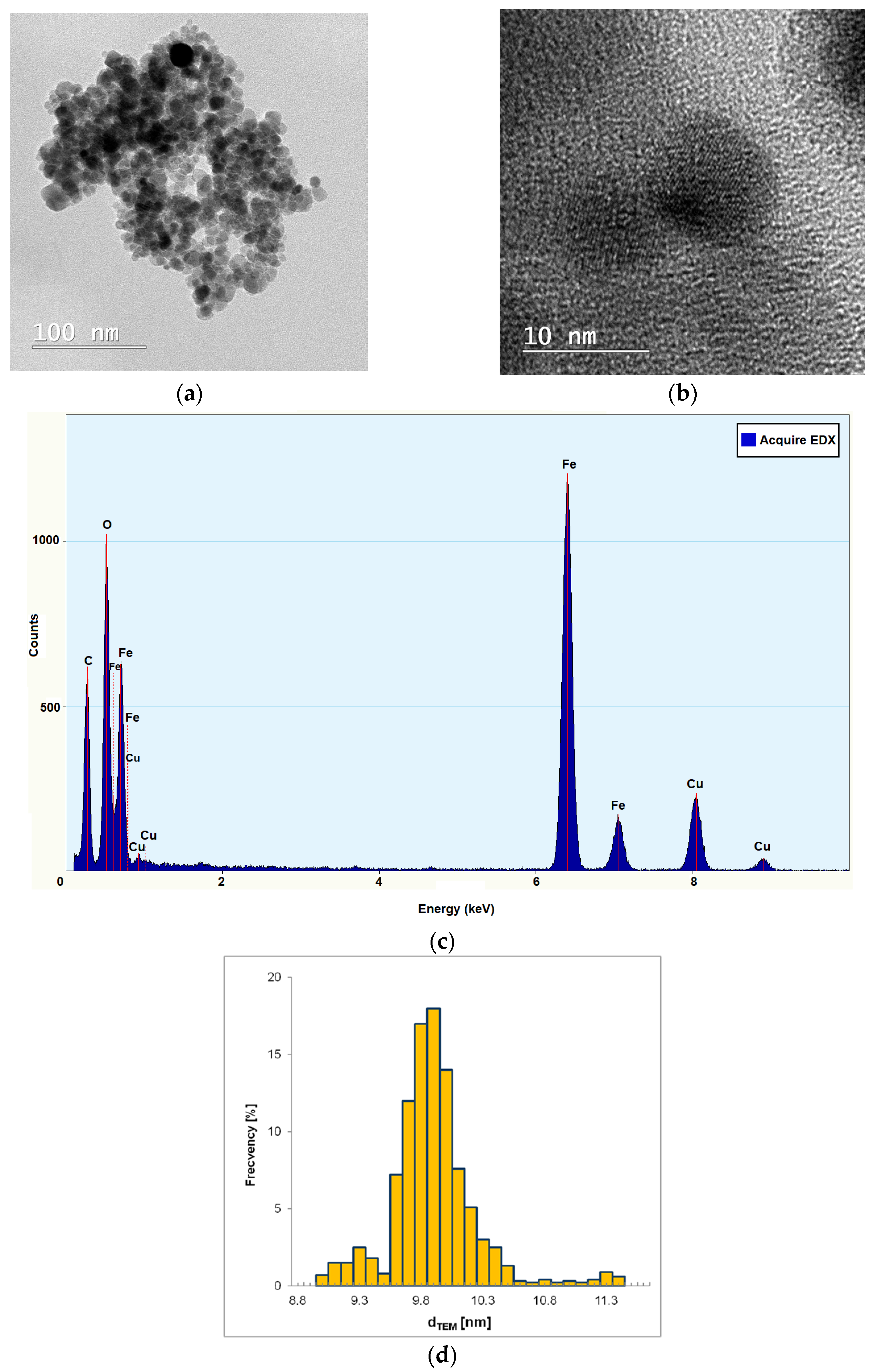
The TEM images (
Figure 3a) of the MNP2 sample indicate that there is a tendency for magnetic agglomeration in clusters of 200–300 nm. This fact requires the ultrasonic treatment of the suspension before using it as ferrofluid for the study of motion in the microchannel.
The nanoparticles exhibit a relatively uniform spheroidal shape (
Figure 3a,b) and a dimensional distribution within a narrow range (
Figure 3d). The EDX analysis (
Figure 3c) showed the presence of iron and oxygen as part of the Fe
3O
4 structure, confirming the pure composition of Fe
3O
4 nanoparticles, the carbon and copper content being part of the carbon holey copper grids used for TEM determinations. The average diameter of magnetite nanoparticles (d
TEM ≈ 9.9 nm) graphically determinate from TEM images (
Figure 3d) is very close to the average value of crystallite size of MNP2 determined by XRD (d
W–H = 9.1 nm). This means that almost all MNPs obtained by chemical co-precipitation can be considered mono-crystalline.
3.3. Zeta Potential Measurements
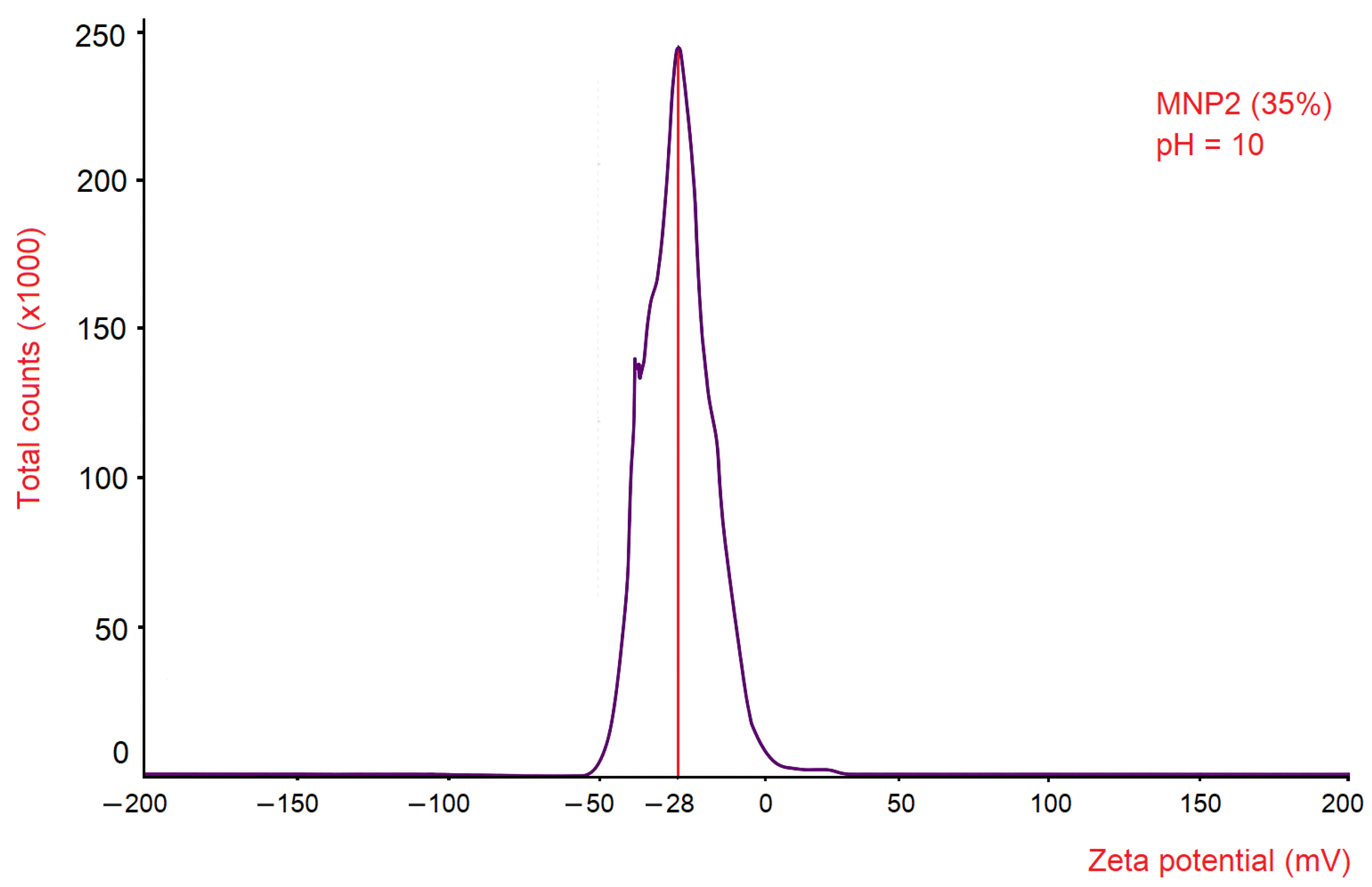
To evaluate the colloidal stability of the ferrofluid containing 35 wt.% MNP2, the zeta potential distribution was determined as a function of signal intensity (Total Counts). The results reveal a Gaussian-shaped distribution curve, characterised by a maximum zeta potential value of −28 mV.
This value indicates that the ferrofluid sample exhibited a negative zeta potential, suggesting that the surfaces of the magnetite nanoparticles carried negative charges, mainly in the form of hydroxyl groups, as a result of the alkaline pH conditions maintained during synthesis via chemical co-precipitation. This negative surface charge promotes electrostatic repulsion between nanoparticles, which in turn contributes to colloidal stability and inhibits agglomeration.
This zeta potential value (−28 mV) is close to the ±30 mV threshold (
Figure 4), which is commonly cited in the literature as an indicator of electrostatic stability in colloidal dispersions [
20].
Therefore, it can be concluded that the magnetite nanoparticles in the ferrofluid used in the experiment through the microchannel exhibit a relatively short-term colloidal stability, being effectively protected against spontaneous agglomeration due to the electrostatic repulsion between the magnetite nanoparticles.
3.4. Analysis of the Magnetic Behaviour of Magnetite Nanoparticles
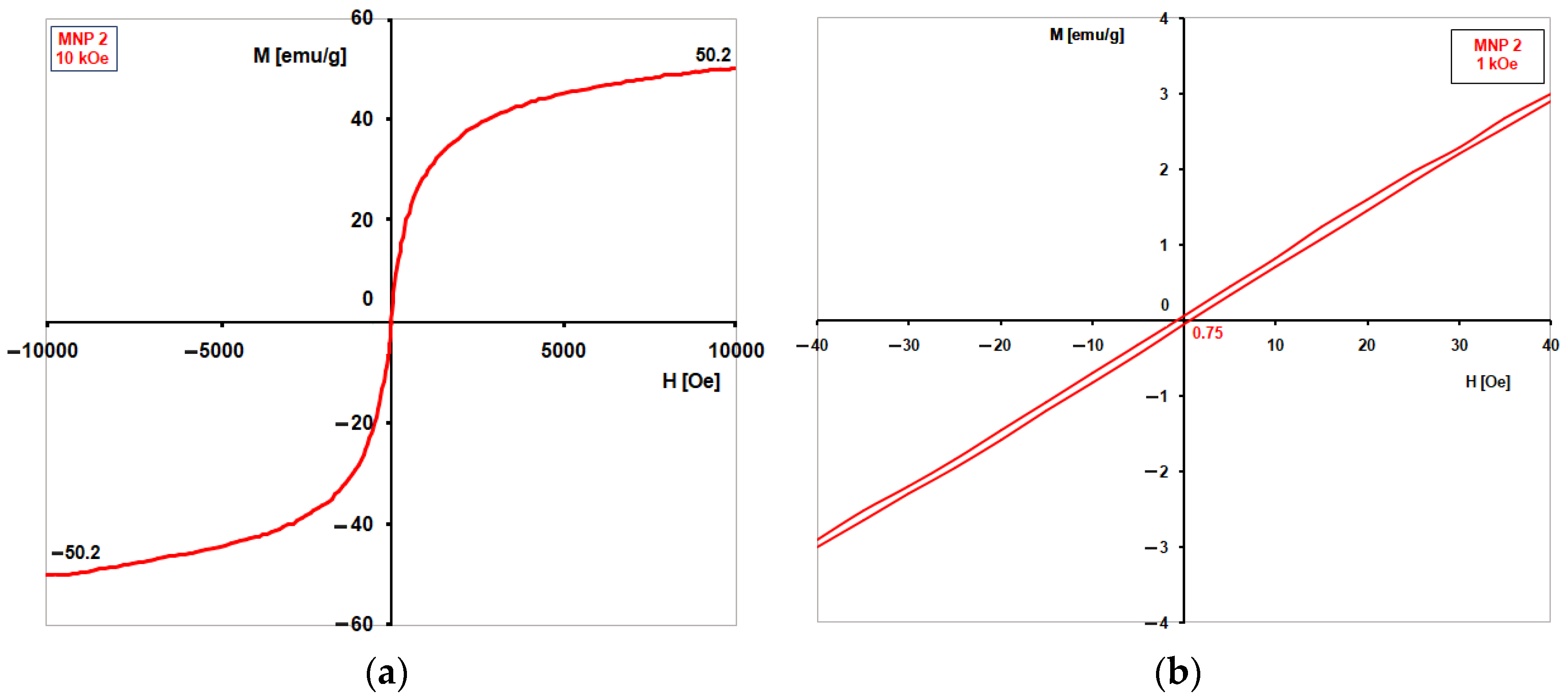
The coercive magnetic field (H
c) and the saturation magnetization (M
s) of MNPs were measured for each magnetite sample using a vibrating sample magnetometer (VSM), and the results are presented in
Table 2 together with average crystallite sizes of MNPs. For each magnetite sample, two types of measurements at room temperature were done: one in high magnetic fields (±10 kOe, increasing by 50 Oe each time, with 1 s for each point) to find the saturation magnetization (M
s), since the samples did not easily reach saturation, even at 10 kOe; and another in a weak magnetic field (±1 kOe) to determine the coercive field (H
c) using small steps (5 Oe) and 1 s per point to get more accurate results. The magnetization curves for the samples MNP1-8 are very similar, exhibiting an extremely small hysteresis, almost absent, which is characteristic of superparamagnetism. However, there are some small differences in the M
s and especially H
c values for each sample. For this reason,
Figure 5 presents only the curves obtained for sample MNP2, where hysteresis is practically absent, with H
c being approximated to zero.
The measured values of saturation magnetization (M
s) are proportional to the crystallite size (d
W–H) and range 50.2–58.7 emu/g, being similar to other reported values [
21]. These values are about half of the saturation magnetization values of bulk magnetite (90–100 emu/g) that are well known.
The H
c values measured for all eight samples of magnetite were very low, up to 10 Oe, with the lowest values of H
c being recorded for samples MNP1 (3 Oe) and MNP2 (0.75 Oe). Superparamagnetism happens when there is no hysteresis, meaning H
c = 0. Because the H
c for sample MNP2 is close to zero, it can be considered superparamagnetic. The magnetization curve of sample MNP2 (
Figure 5) is characteristic of spherical particles with average sizes of 10 nm. This phenomenon may occur during the preparation of VSM samples. Pressing the magnetite powder with a binder that is not magnetic may have created clusters of magnetic nanoparticles.
These nanoparticles then interacted, increasing the anisotropy constant. After comparing all the experimental results obtained, the MNP2 sample was chosen as the most representative because it meets the criteria for superparamagnetism at room temperature for this type of fine particles [
8], as follows: the coercive field H
c tends towards zero, the shape of the magnetization curve is typical for superparamagnetism, and the magnetite nanoparticles can be considered monocrystalline since the average crystalline size determined by XRD (d
W–H = 9.1 nm) is very close to the average particle size determined by TEM (d
TEM ≈ 9.9 nm). Furthermore, the MNP2 sample’s nanoparticles could be regarded as magnetic single domains, according to the literature [
8], and the shape of the magnetite nanoparticles is spherical.
Any magnetic material possesses a specific structure of magnetic domains, which results from the condition of minimizing the total free energy. In the case of completely identical materials, the magnetic behaviour strongly depends on the shape and dimensions of the sample. There is a critical dimension below which the minimum energetic equilibrium corresponds to a magnetic single-domain configuration, meaning that there are no walls between the magnetic domains, and the sample is uniformly magnetized even in the absence of an external magnetic field. This particularity causes the sample to be considered a fine magnetic particle, generally in the nanometric range, depending on the saturation magnetization of the material and the shape of the particle. If the size of the magnetic sample exceeds this range, it is considered to be magnetic grains.
It is very important how the assemblies of dispersed magnetic nanoparticles behave in a fluid. The easy magnetization axes of the particles can be oriented randomly or can be more or less parallel under the external magnetic field, in which case the average dimensions of these particles must be in strictly the nanometric range.
According to classical theories [
22,
23,
24] regarding magnetic single-domain particles, there are two essential goals: the first is to specify when a magnetic single-domain state can exist; the second is to frame the magnetic properties of the particles that make them magnetic single-domains.
A linear relationship between magnetic properties and average crystallite size of magnetite nanoparticles has been found (
Figure 6a,b).
The decrease in saturation magnetization and coercive field with the reduction in the average size of MNPs is the result of surface effects. This phenomenon is explained by a nanoparticle pattern consisting of a nucleus with magnetic properties similar to those of bulk material and an outer shell consisting of a magnetically disordered layer, and the effect is nothing more than an increase in the surface-to-volume ratio of the MNPs. As the average size of the nanoparticles is smaller, the perturbed magnetic order of the surface layer increasingly weakens the magnetic properties of the magnetic nanoparticles. The dead magnetic layer can be attributed to spin inclination, quite high anisotropy, and the loss of long-range order at the surface. Generally, the thickness of the magnetically dead layer can be very close to the value of the lattice constant, a = 0.8394 nm, considering that the ions in the outer layer are the most susceptible to being misaligned in the magnetic field. The decreasing trend of M
s with the size reduction has been reported in many articles [
25,
26,
27,
28,
29].
3.5. Viscosity of Ferrofluids
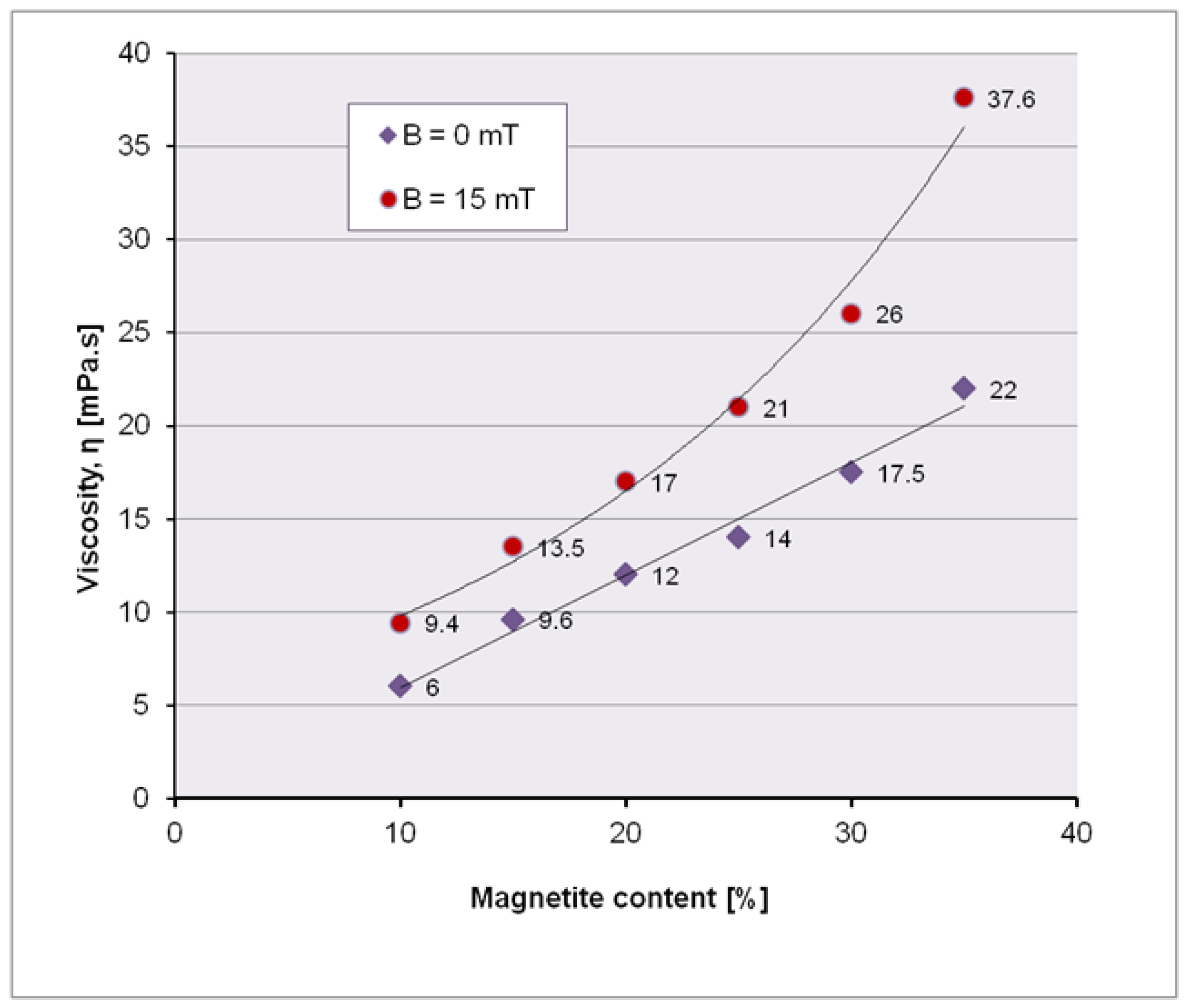
Initially, the dynamic viscosity of the ferrofluid for different charges with magnetite nanoparticles must be determined; in practice, the maximum charge with magnetite nanoparticles of the ferrofluid is determined, which allows an optimum viscosity to move through the microchannel, with or without external magnetic field. Experimentally, dynamic viscosities were measured with (B = 15 mT) and without magnetic field, on ferrofluid samples with 10, 15, 20, 25, 30 and 35 wt.% magnetite MNP2.
It is obvious that due to the disorder of magnetite nanoparticles, the resistance to flow increases, thus decreasing the fluidity. If the magnetite nanoparticles are arranged under the influence of the magnetic field along the field lines, then the viscosity decreases (
Table 3,
Figure 7).
Comparing the results obtained without the action of the magnetic field and in the magnetic field, we can graphically conclude that the variation of the viscosity in the magnetic field is parabolic and in the absence of the magnetic field has a linear allure.
Magnetic fields generate important changes in the rheological behaviour of ferrofluids [
8]. The viscosity of pure water is determined by the interaction between water molecules. In ferrofluids, the viscosity is influenced both by the interaction among water molecules and by the interaction between MNPs with hydroxyl groups on the surface. By applying an external magnetic field, the interactions between MNPs are amplified, resulting in an increase in the viscosity of the ferrofluid. The change in viscosity under magnetic field can be explained by the structural modification of the ferrofluids, such as the formation (when magnetization occurs) or the breaking (when demagnetizing) of the MNPs chains. This fact will also be observed in the experiment tracking the behaviour of the ferrofluid through a microchannel, when, under the influence of the magnet, magnetite nanoparticles aggregate into cluster-like structures along the magnetic field lines.
4. The Experimental Observation of the Behaviour of MNP2-Based Ferrofluid Through a Microchannel
4.1. The Selection of the Ferrofluid and Experimental Considerations
According to the experimental plan, out of the eight MNP samples, MNP2 was selected for the preparation of the ferrofluid for the observation experiment of behaviour in a microchannel, considering the fulfilment of criteria for superparamagnetism: the coercive field H
c approaches zero (H
c = 0.75 ≈ 0), which results in a magnetisation curve with no hysteresis (
Figure 5), and the average dimensions of the nanoparticles are approximately 10 nm (d
TEM = 9.9 nm). The superparamagnetic nature of MNP2 is essential for its use in ferrofluids: a rapid and reversible response to magnetic fields which allows for control over the properties of the ferrofluid. MNP2 does not exhibit residual magnetisation, meaning it does not remain magnetised after the removal of the magnetic field, the nanoparticles exhibit a relatively low tendency to aggregate, which provides stability to the ferrofluid, and the ferrofluid can be controlled by external magnetic fields.
The selection of the ferrofluid that exhibited colloidal stability at the highest concentration of magnetite (35 wt.%) was based on its rapid response to the action of the magnetic field. There was also an attempt with a ferrofluid containing 40 wt.% MNP2, but it became blocked in the microchannel. The interesting challenge of this experiment lies in the difficulty of manipulating the ferrofluid with a simple permanent magnet, which generates a non-uniform magnetic field due to its high viscosity and tendency to cluster.
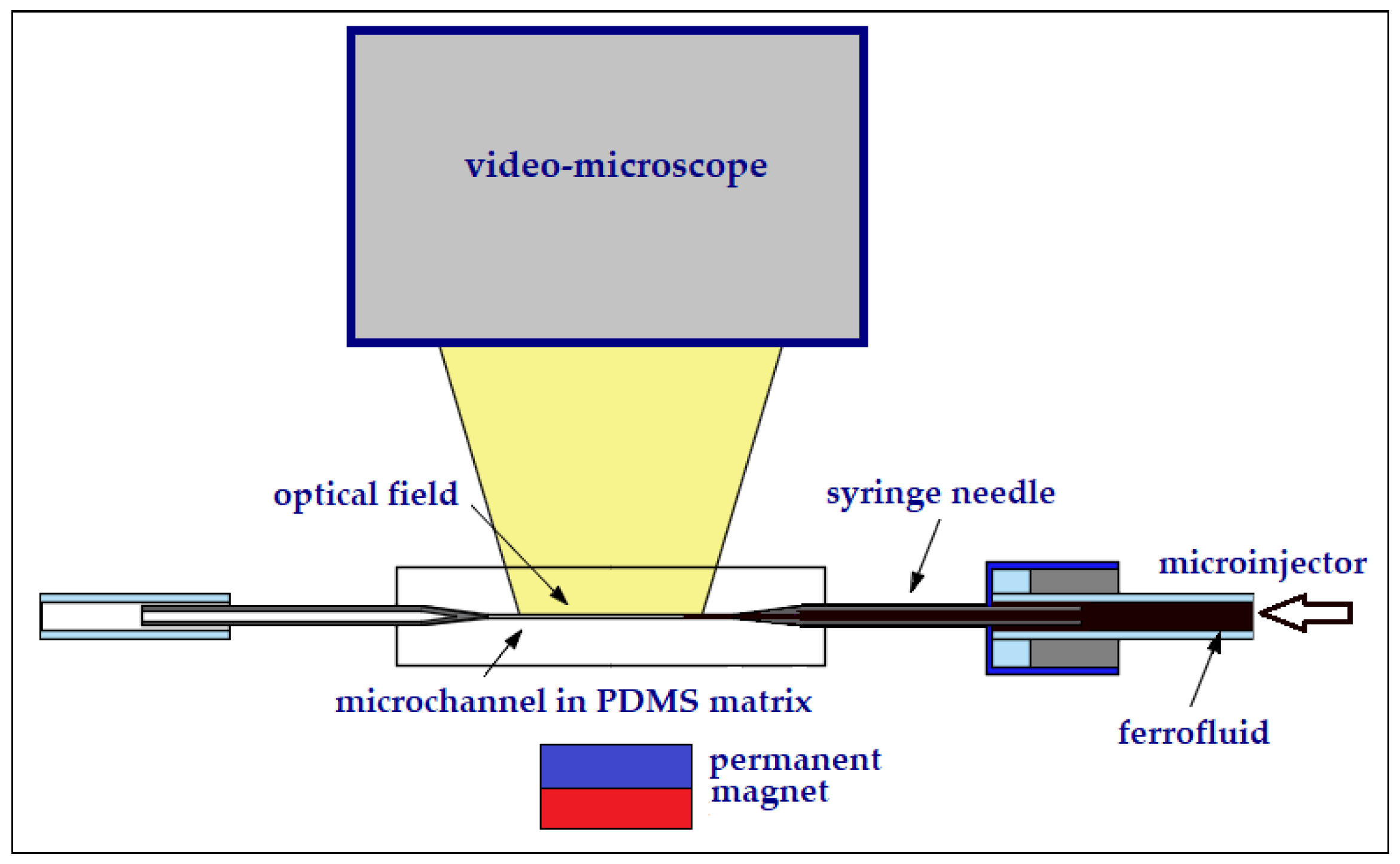
4.2. Experimental Setup
The observation of ferrofluid with 35 wt.% MNP2 through a microchannel was performed through a single microchannel chip from polydimethylsiloxane (PDMS) and the manufacturing technique was reported [
30].
The PDMS chip spatially defines the microchannel with a diameter of 200 μm, mechanically facilitating the connection to an injector and the transparency of the material ensures visualization under the Levenhuk MED 10M microscope. PDMS is a suitable material for making microchannels. PDMS has a repetitive chemical structure consisting of –OSi(CH
3)
2– groups, which confers a hydrophobic surface [
31], which may represent an advantage in their use when moving ferrofluids within them. By applying a constant pressure in the microchannel, the ferrofluid flow will move respecting the internal geometry of the microchannel due to the laws of laminar flow [
32].
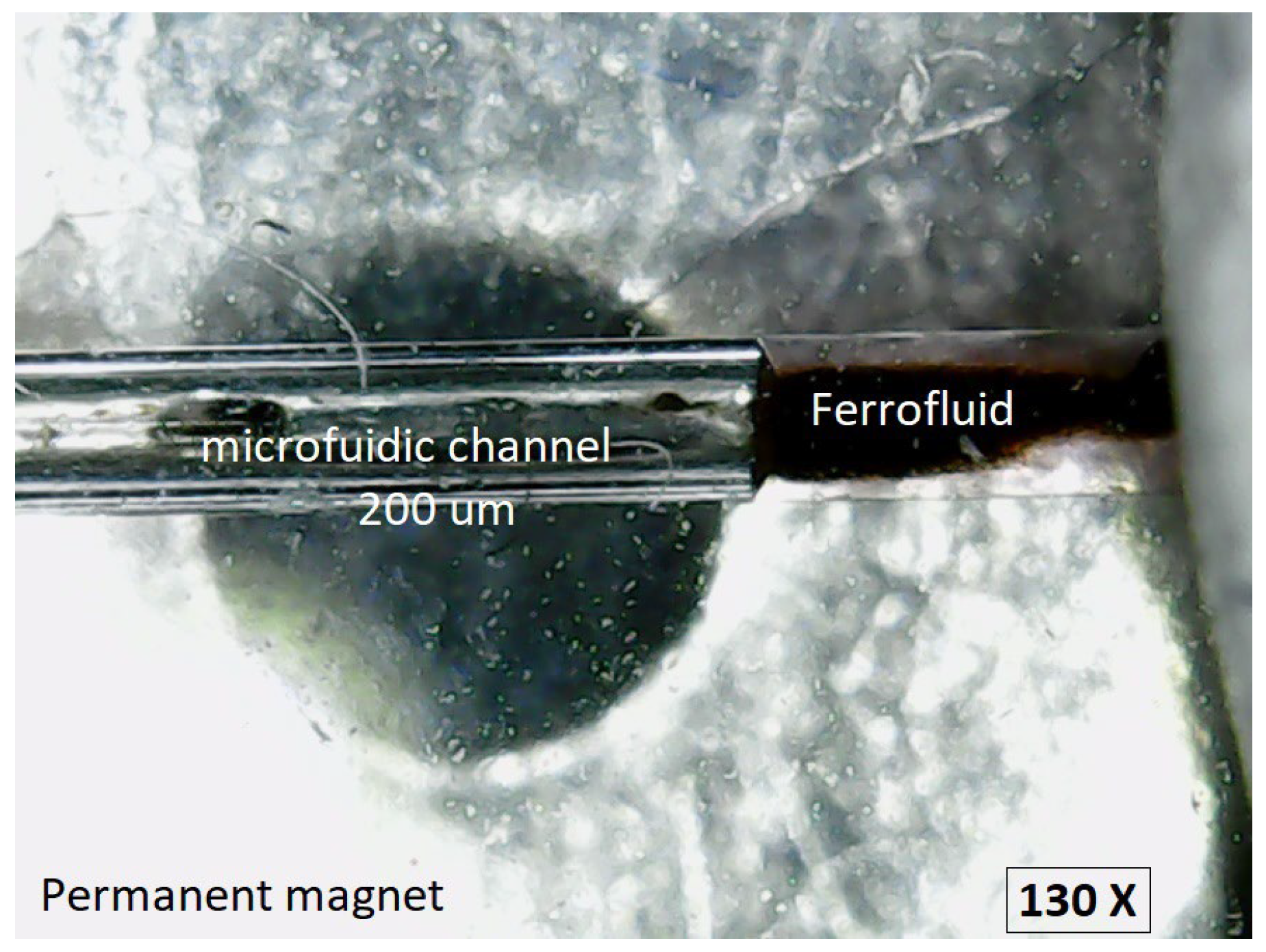
Figure 8 shows the experimental setup with the PDMS chip with the microchannel fixed on a support. Below the chip is the permanent magnet with which the ferrofluid is manipulated. Visualization is done using the microscope mounted above.
In the microscopic image (130×) shown in
Figure 9, the experimental setup can be observed comprising the circular microchannel with the diameter of 200 μm, the ferrofluid, and the permanent magnet positioned as in
Figure 8.
4.3. The Behaviour of Ferrofluid Under the Action of a Non-Uniform Magnetic Field
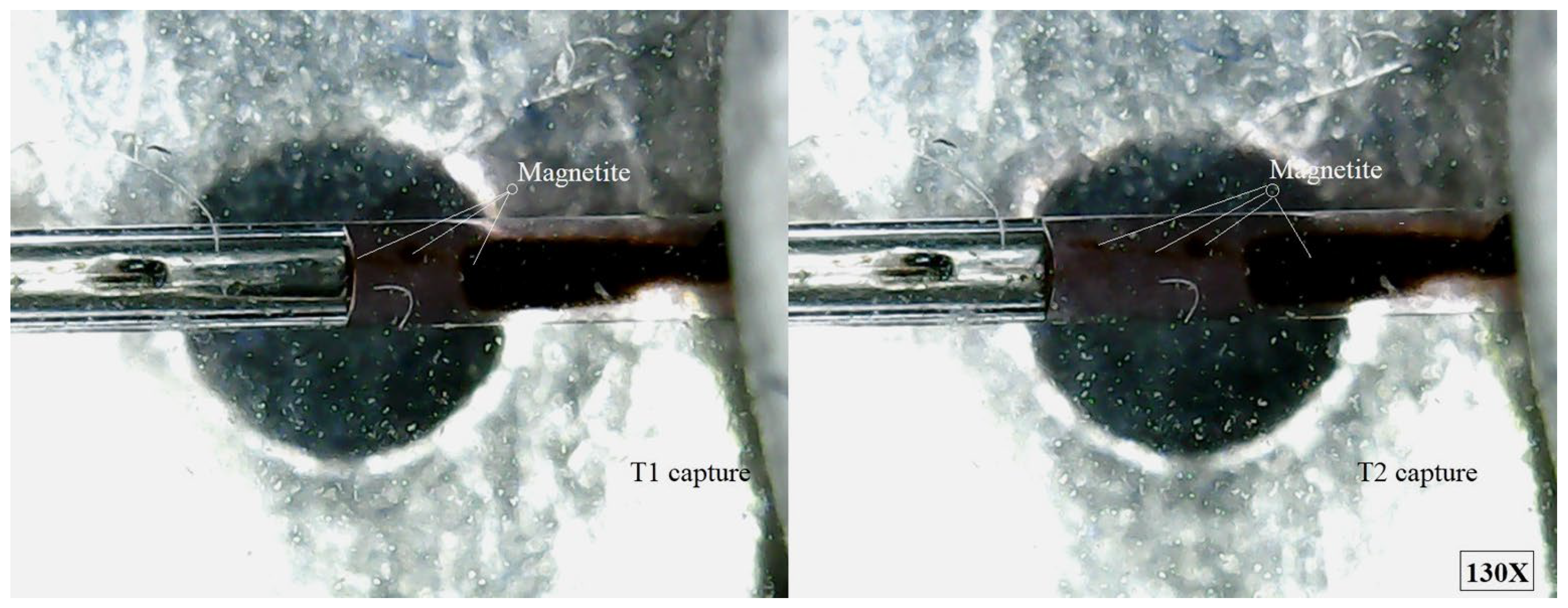
The purpose of this experimental part was to demonstrate that magnetite can be retained from ferrofluid in motion, in a particular area, with the help of a permanent magnet which generates a non-uniform magnetic field.
Figure 10 shows several magnetite micro-clusters formed in ferrofluid, but most of the magnetite nanoparticles are blocked in the magnetic field; the rest of the magnetite clusters are driven by the mechanical movement of the carrier fluid drawn from the syringe.
When the ferrofluid with 35 wt.% MNP2 flows through microchannel under the influence of a simple permanent magnet, the non-uniform magnetic field exerts several pronounced effects. Firstly, magnetic volume forces act on the entire ferrofluid, attracting it to regions where the magnetic field intensity is higher. At the level of individual magnetite nanoparticles, magnetophoretic forces drive their migration towards zones of stronger magnetic field. Due to the high concentration of 35 wt.% MNP2 in the ferrofluid, the effect is accentuated because MNP2 respond strongly to the field gradient and tend to move collectively, forming clusters. This migration is counterbalanced by random Brownian motion, but at such a high loading, diffusion plays a secondary role to magnetic attraction.
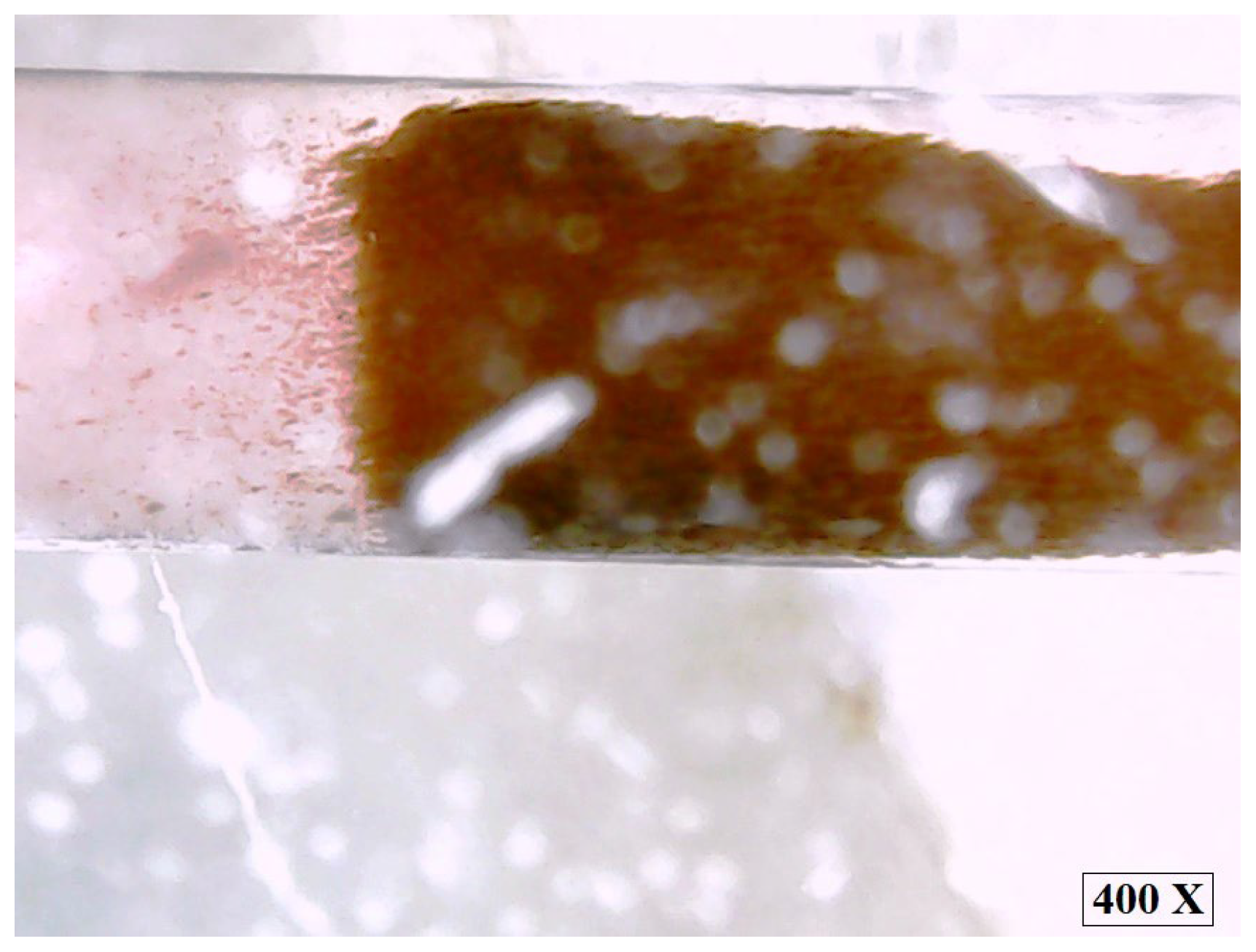
In addition, the applied non-uniform magnetic field induces a torque on the MNP2, which causes them to align along the field lines. This orientation is not only an individual phenomenon, but also favours the emergence of dipole–dipole interactions between MNPs, which lead to the formation of temporary chain-like structures aligned in the direction of the field (
Figure 11). Due to the high content of MNP2 in ferrofluid (35 wt.%), these structures become more stable and extensive, as the distance between the nanoparticles is small and the magnetic interactions are stronger than thermal agitation.
The presence of agglomerated MNP2 formations results in a significant increase in the effective viscosity of the ferrofluid, a phenomenon known as the magnetoviscous effect. As a result, the overall mobility of the ferrofluid decreases, making flow through the microchannel more difficult. The effect is the emergence of areas of non-uniform flow: in some areas, the MNP2 chains act as microscopic obstacles that slow down the ferrofluid, leading to slow or even nearly stagnant flow, as was the case in the failed experiment with the 40 wt.% MNP2 ferrofluid that became blocked in the microchannel.
In contrast, in other areas, where the field is weaker or the MNP2 structures disintegrate, the ferrofluid flows more freely. This heterogeneous behaviour explains why the ferrofluid subjected to a non-uniform magnetic field can develop local instabilities and varying flow velocities, phenomena that are significant for microfluidic applications and flow control based on magnetic fields.
The consequence is the emergence of a non-homogeneous distribution of MNP2 and the velocity of the ferrofluid: magnetite nanoparticles concentrate towards the walls of the microchannel located near the permanent magnet, causing the flow to become less uniform. These effects are directly dependent on the high concentration of MNP2, which amplifies both the magnetic response and the phenomena of temporary agglomeration.
The movement of the ferrofluid containing 35 wt.% MNP2 in a non-uniform magnetic field generated by a permanent magnet was significantly influenced by the properties of its nanoparticles. The small average size (10 nm) of the superparamagnetic MNP2 enabled fast and easy movement within the ferrofluid, facilitating the prompt response of the ferrofluid to the magnetic field. The high saturation magnetization of MNP2 (Ms = 50.2 emu/g) indicates that these nanoparticles could be strongly magnetized, directly influencing the magnetophoretic forces, which allowed a prompt response of the ferrofluid to the action of the magnetic field. The zeta potential of −28 mV indicates that MNP2 are moderately negatively charged, which results in relatively good colloidal stability, preventing agglomeration and sedimentation in the absence of magnetic field action.
The negative charge present on the surface of MNP2 influences the interactions between the ferrofluid and the inner surface of the microchannel, altering the contact angle with the inner surface of the microchannel and affecting the shape and curvature of the meniscus. In the
Figure 9 and
Figure 10, the specific concave meniscus of water-based ferrofluids is observed. The concave meniscus occurs when the attraction between the ferrofluid and the inner surface of the microchannel (adhesion) is greater than half of the attraction between the molecules and nanoparticles of the ferrofluid to each other (cohesion), causing the ferrofluid to rise along the inner surface of the microchannel. Surface tension acts at the interface between the ferrofluid and air, causing the meniscus to curve and minimize the contact area. Additionally, the ferrofluid is also subject to gravitational attraction. The presence of an external non-uniform magnetic field leads to the magnetization of MNP2, generating magnetic forces that can influence the shape and dynamics of the meniscus.
Figure 11 illustrates the final moment of the experiment in which the stability of the ferrofluid ceases, and MNP2 sediments at the base of the microchannel. Subsequently, under the action of the magnetic field, chain-like nanoparticle structures have detached from the sedimented magnetite through the carrier liquid (water), orienting themselves along the lines of the magnetic field, while MNP2 are also grouped within the sediment along the field lines.
5. Conclusions
The crystal structure, average size of nanoparticles, magnetic properties, zeta potential, viscosity and behaviour in microchannel of some ferrofluids prepared in the laboratory by a chemical co-precipitation method, were studied. By precisely controlling reaction parameters according to previous literature and experience, superparamagnetic magnetite nanoparticles can be obtained.
The ferrofluids were prepared with different magnetite contents and the carrier fluid was distilled water. The viscosity is strongly influenced by the magnetic field due to structural modifications of the ferrofluid by the formation of magnetite nanoparticles chains.
The observation of the behaviour through the microchannel was performed using a ferrofluid sample with 35 wt.% magnetite nanoparticles. The retention of magnetite nanoparticles from ferrofluid in motion, in an imposed area, with the help of a permanent magnet, was observed. The non-uniform magnetic field has induced non-linear behaviour in the ferrofluid, leading to complex phenomena such as the formation of magnetite nanoparticle clusters within the ferrofluid. This explains the loss of colloidal stability shortly after the action of the magnetic field. The movement of the ferrofluid in the non-uniform magnetic field generated by the permanent magnet had the following effects: the magnetite nanoparticles concentrated in regions with a stronger magnetic field, which influenced the properties of the ferrofluid; and the ferrofluid was deformed by the magnetic field, resulting in changes in homogeneity and behaviour.
By properly orienting the magnet to the microchannel, a series of studies can be generated regarding the forces at the interface between two liquids or at the interface between a liquid and air at the microfluidic level. The interface meniscus provides precise information about the complex of forces acting on it. Using ferrofluid based on magnetite nanoparticles, we applied magnetic force on the meniscus. The curvature of this meniscus provides information that allows the calculation of the elasticity of the liquid.
The magnetite nanoparticles tended to agglomerate soon after their use as a ferrofluid in the microchannel. To further mitigate this agglomeration tendency, in addition to stabilization through ultrasonication, the use of a surfactant is also recommended [
33,
34,
35]. However, the aim of this work was the strict use of ultrapure magnetite, without any other chemical treatment, to observe how it acts when moving through the microchannel.
Furthermore, experimental data obtained are completing the research realized in this field. All of these provide further development perspectives.