Biochar Control of Water Regime and Adsorption Rate in Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Soils
2.2. Soil Additives
2.3. Preparation of Soil Composites
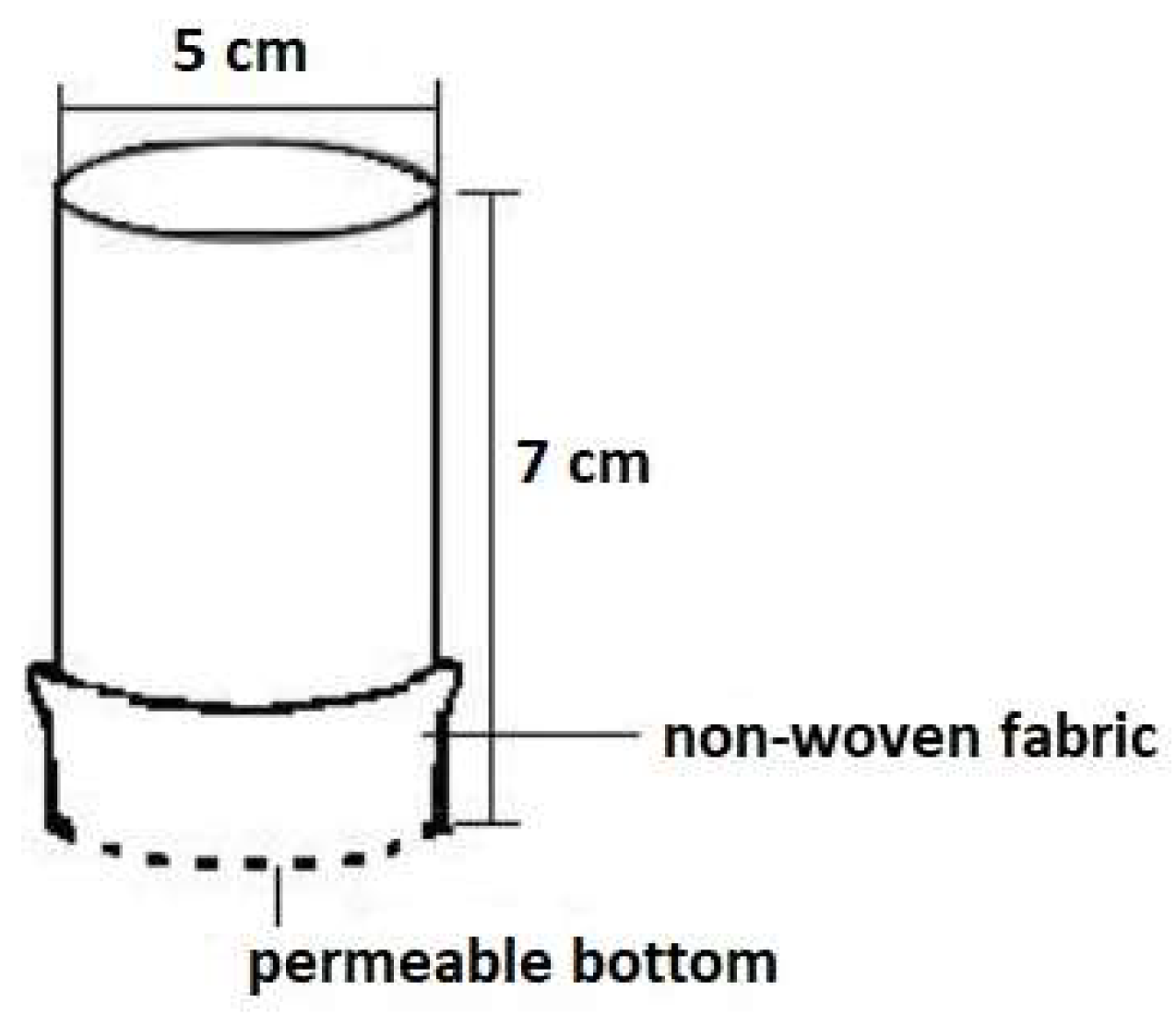
2.4. Water Regime of Soil Composites
2.5. Model Solutions
2.6. Adsorption Experiments
2.7. Analytical Methods
3. Results and Discussion
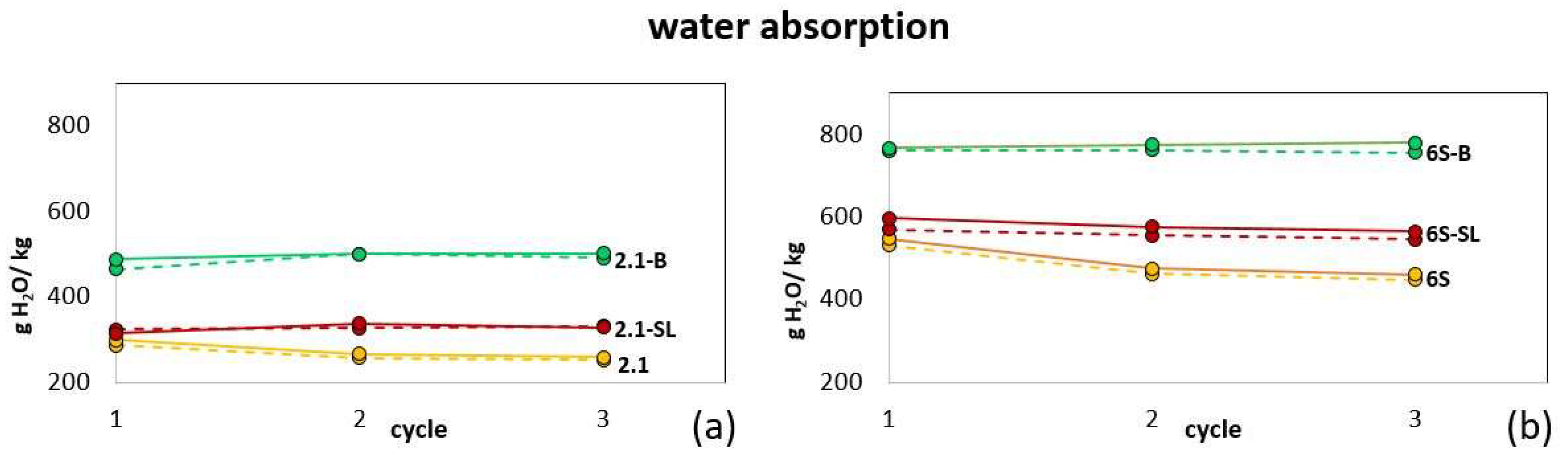
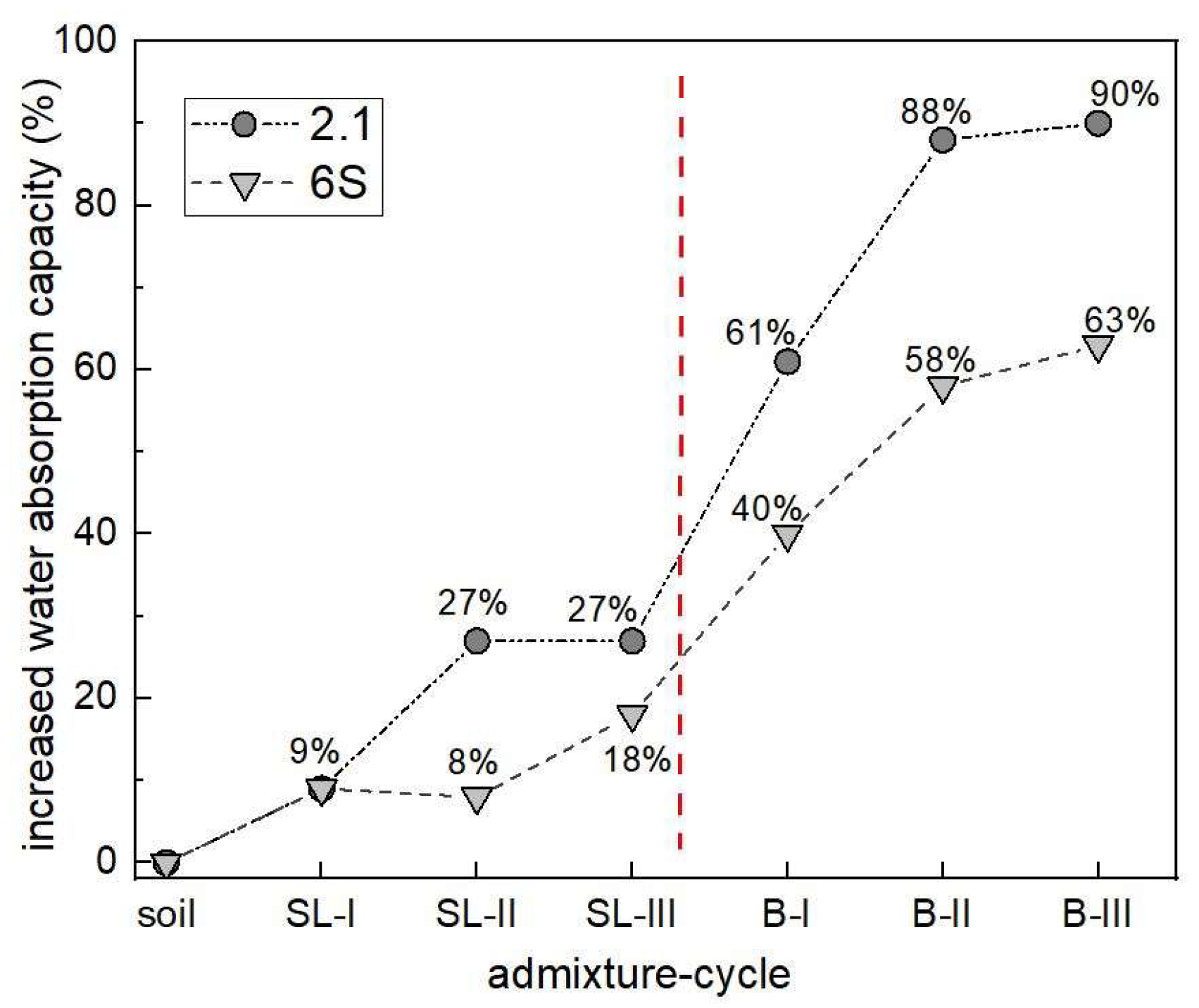
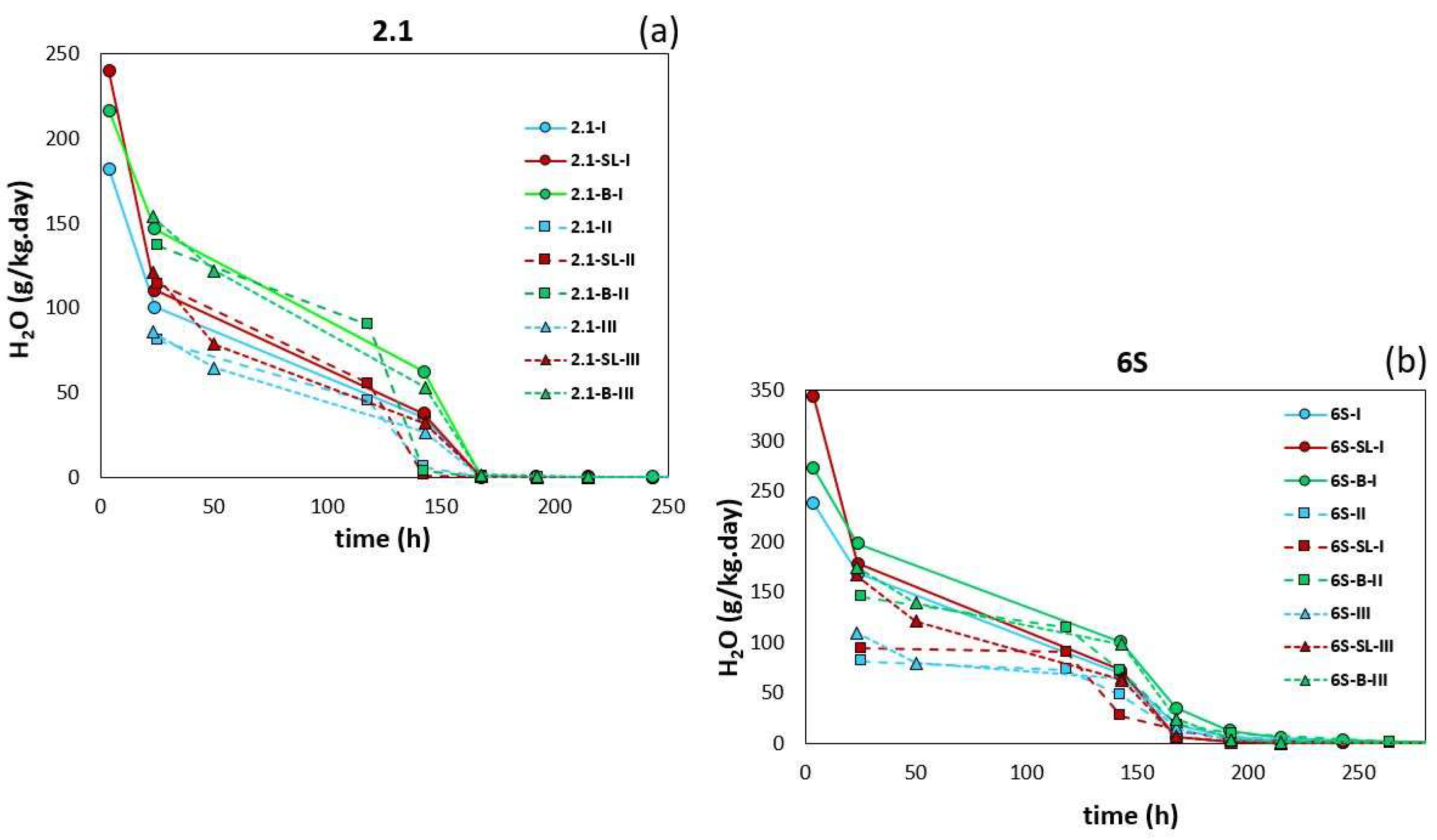
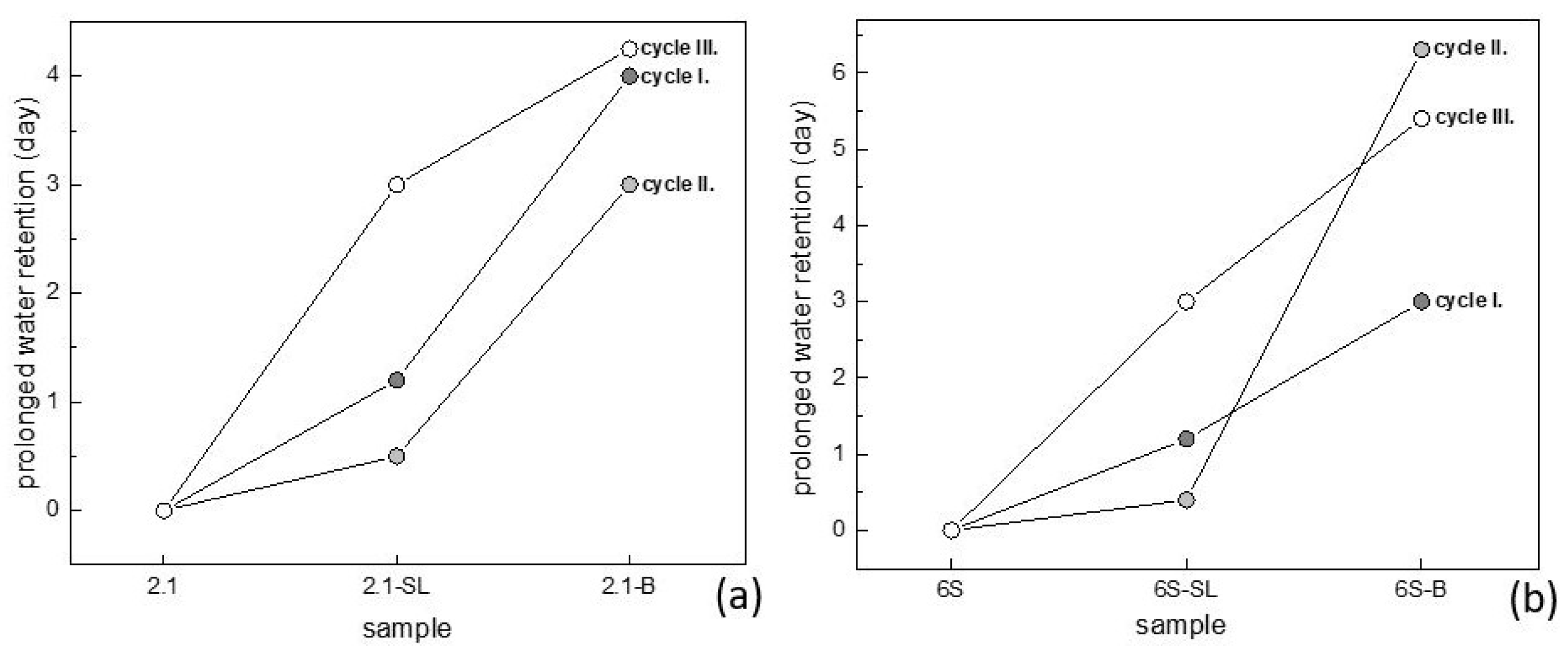
3.1. Water Regime of Standard Soils and Soil Composites
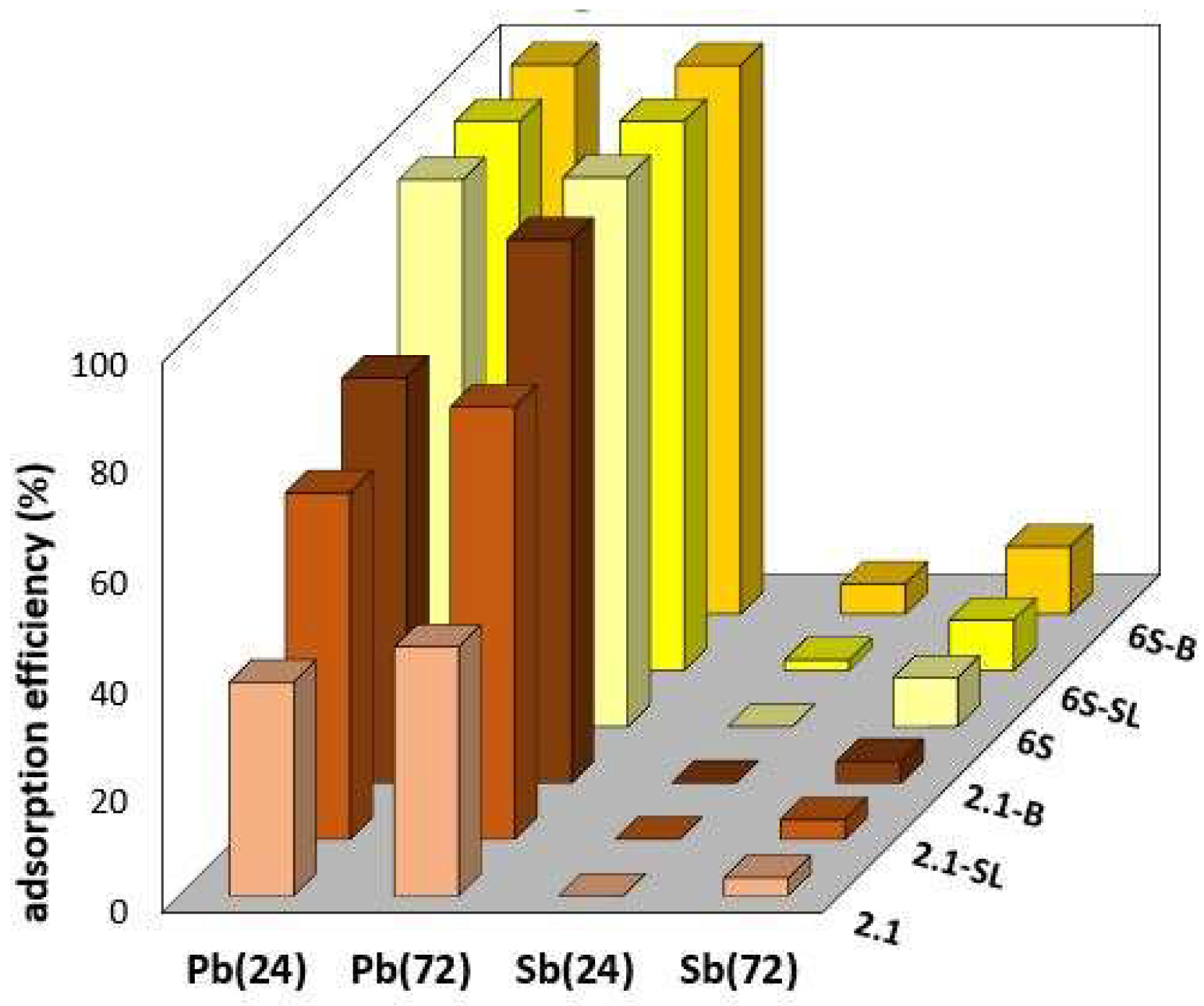
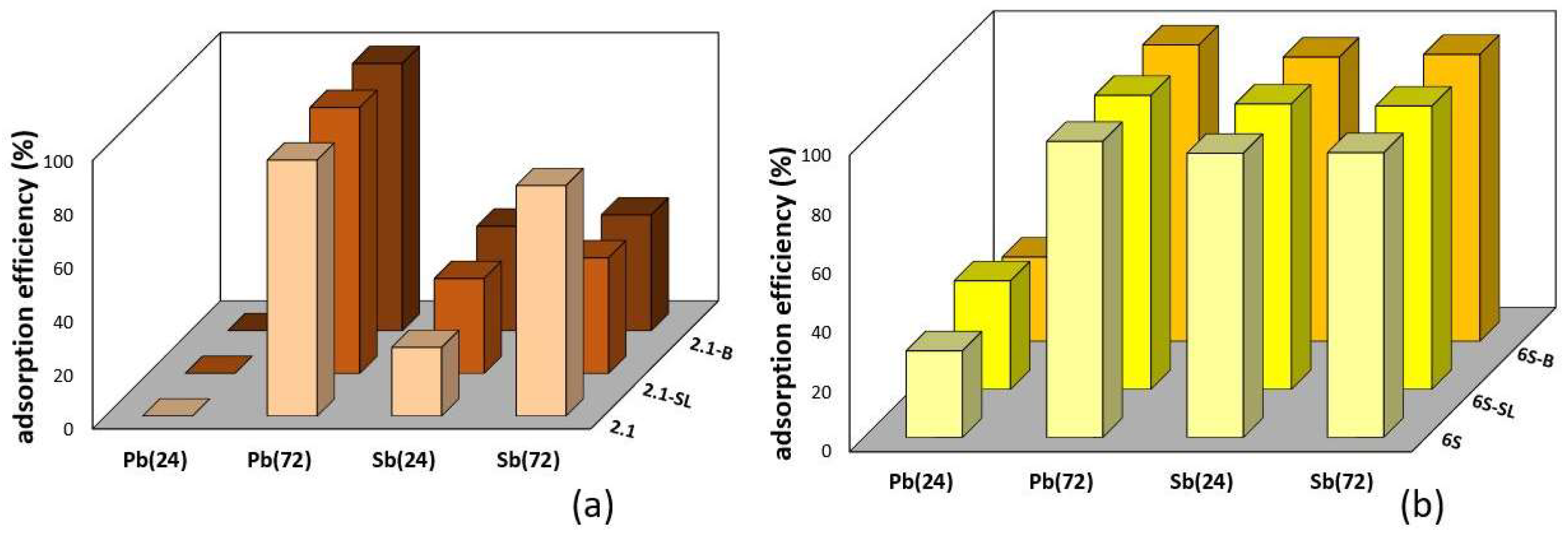
3.2. Adsorption of Pb2+ and Sb(OH)6− on Individual Soils and Soil Composites
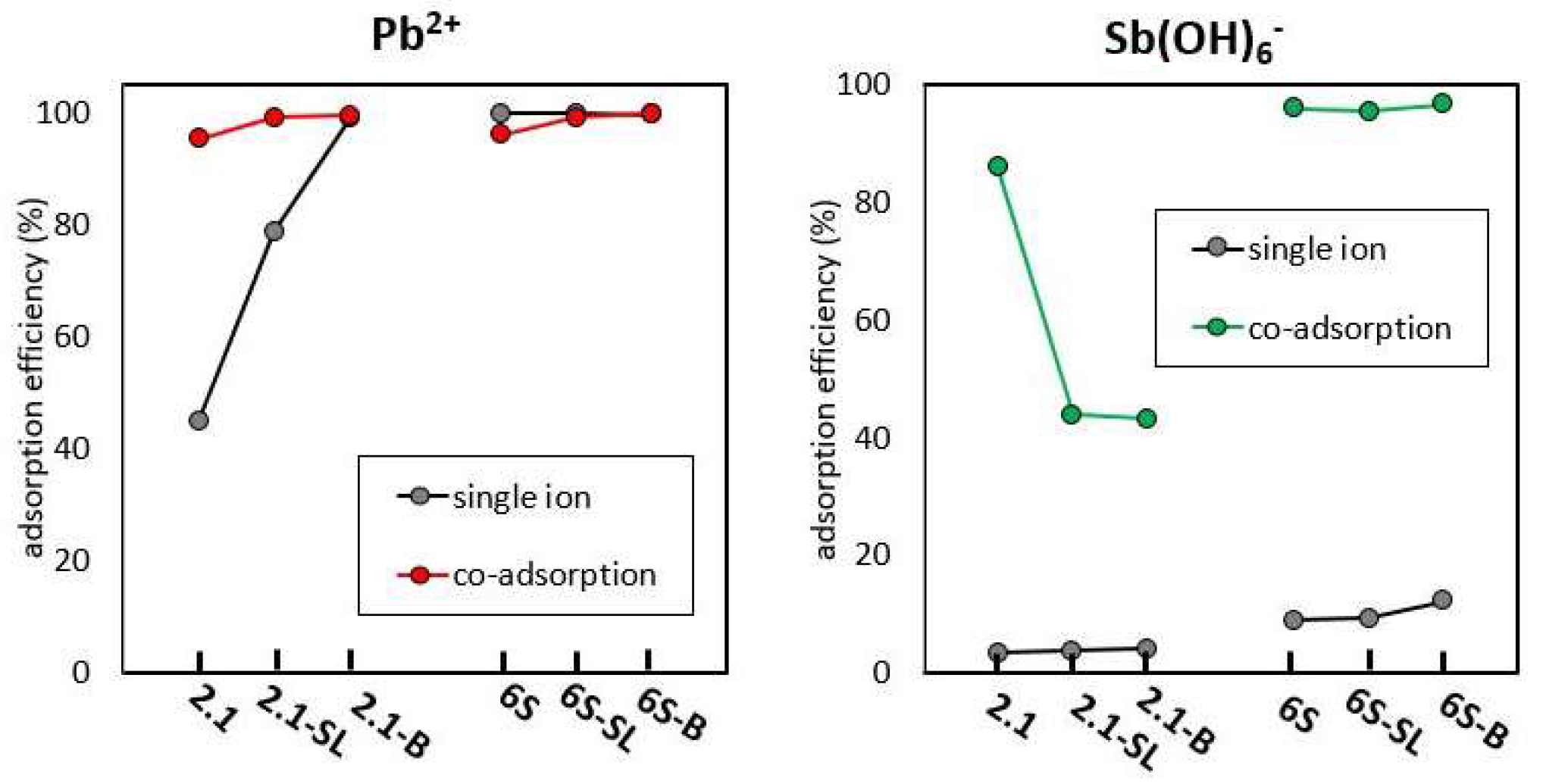
3.3. Co-Adsorption of Pb2+ and Sb(OH)6− on Pure Soils and Soil Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Angin, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Meca, S.; Zhang, S.; Clarens, F.; Hu, X. Understanding the dependence of biochar properties on different types of biomass. Waste Manag. 2024, 182, 142–163. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Li, A.-Y.; Deng, H.; Ye, C.-H.; Wu, Y.-Q.; Linmu, Y.-D.; Hang, H.-L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Lei, O.; Zhang, R. Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties. J. Soils Sediments 2013, 13, 1561–1572. [Google Scholar] [CrossRef]
- Matos, T.T.S.; Fornari, M.R.; Mangrich, A.S.; Schultz, J.; Batista, E.M.C.; Ribeiro, R.O.; Romão, L.P.; Yamamoto, C.I.; Grasel, F.S.; Bayer, C.; et al. Low temperature production of biochars from different biomasses: Effect of static and rotary lab reactors and application as soil conditioners. J. Environ. Chem. Eng. 2021, 9, 105472. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Tan, Z.; Zou, J.; Zhang, L.; Huang, Q. Morphology, pore size distribution, and nutrient characteristics in biochars under different pyrolysis temperatures and atmospheres. J. Mater. Cycles Waste Manag. 2018, 20, 1036–1049. [Google Scholar] [CrossRef]
- Das, O.; Mensah, R.A.; George, G.; Jiang, L.; Xu, Q.; Neisiany, R.E.; Umeki, K.; Jose E, T.; Phounglamcheik, A.; Hedenqvist, M.S.; et al. Flammability and mechanical properties of biochars made in different pyrolysis reactors. Biomass Bioenergy 2021, 152, 106197. [Google Scholar] [CrossRef]
- Zhou, M.; Ying, S.; Chen, J.; Jiang, P.; Teng, Y. Effects of Biochar-Based Fertilizer on Nitrogen Use Efficiency and Nitrogen Losses via Leaching and Ammonia Volatilization from an Open Vegetable Field. Environ. Sci. Pollut. Res. Int. 2021, 28, 65188–65199. [Google Scholar] [CrossRef]
- Maziarka, P.; Wurzer, C.; Arauzo, P.J.; Dieguez-Alonso, A.; Mašek, O. Do you BET on routine? The reliability of N2 physisorption for the quantitative assessment of biochar’s surface area. Chem. Eng. J. 2021, 418, 129234. [Google Scholar] [CrossRef]
- Shubh, P.S.Y.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Ghimire, N.; Paudel, P.; Shrestha, J.; Oli, B. Biochar application: A sustainable approach to improve soil health. J. Agric. Food Res. 2023, 11, 100498. [Google Scholar] [CrossRef]
- Acharya, B.S.; Dodla, S.; Wang, J.J.; Pavuluri, K.; Darapuneni, M.; Dattamudi, S.; Maharjan, B.; Kharel, G. Biochar impacts on soil water dynamics: Knowns, unknowns, and research directions. Biochar 2024, 6, 34. [Google Scholar] [CrossRef]
- Xu, D.; Yue, C.; Zhou, T.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Env. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Rangasamy, G.; Prasannamedha, G.; Kanmani, S. A review on the applicability of adsorption techniques for remediation of recalcitrant pesticides. Chemosphere 2023, 313, 137481. [Google Scholar] [CrossRef]
- Parisa, M.; Elham, G.A.; Rohollah, F.; Allah, S.H.; Jalil, K. Nitrate removal from agricultural efuent using sugarcane bagasse active nanosorbent. J. Appl. Water Eng. Res. 2021, 10, 238–249. [Google Scholar] [CrossRef]
- Yapicioglu, P.; Yesilnacar, M.I. Grey water footprint assessment of groundwater resources in southeastern Turkey: Effect of recharge. Water Supply 2022, 22, 615–627. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Hui, H.; Jiayuan, Z.; Tian, W.; Pei, W. Adsorption of toxic metal ion in agricultural wastewater by torrefaction biochar from bamboo shoot shell. J. Clean. Prod. 2022, 338, 130558. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, J.; Jin, Q.; Li, Z.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, H. Sludge-based biochar activation to enhance Pb(II) adsorption. Fuel 2019, 252, 101–108. [Google Scholar] [CrossRef]
- Turunen, M.; Hyväluoma, J.; Keskinen, R.; Kaseva, J.; Nikama, J.; Reunamo, A.; Rasa, K. Pore structure of wastewater sludge chars and their water retention impacts in different soils. Biosyst. Eng. 2021, 206, 6–18. [Google Scholar] [CrossRef]
- He, Y.D.; Zhai, Y.B.; Li, C.T.; Yang, F.; Chen, L.; Fan, X.P.; Peng, W.F.; Fu, Z.M. The fate of Cu, Zn, Pb and Cd during the pyrolysis of sewage sludge at different temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Singer, S.; Tong, Y.; Kimbell, L.; Anderson, E.; Hughes, M.; Zitomer, D.; McNamara, P. Characteristics and applications of biochars derived from wastewater solids. Renew. Sustain. Energy Rev. 2018, 90, 650–664. [Google Scholar] [CrossRef]
- Kroiss, H. What is the potential for utilizing the resources in sludge? Water Sci. Technol. 2004, 49, 1–10. [Google Scholar] [CrossRef]
- Mendez, A.; Gomez, A.; Paz-Ferreiro, J.; Gasco, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and metaanalysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Moško, J.; Jeremiáš, M.; Skoblia, S.; Beňo, Z.; Sikarwar, V.S.; Hušek, M.; Wang, H.; Pohořelý, M. Residual moisture in the sewage sludge feed significantly affects the pyrolysis process: Simulation of continuous process in a batch reactor. J. Anal. Appl. Pyrolysis 2022, 161, 105387. [Google Scholar] [CrossRef]
- Mitzia, A.; Hudcová, B.B.; Vítková, M.; Kunteová, B.; Hernandez, D.C.; Moško, J.; Pohořelý, M.; Grasserová, A.; Cajthaml, T.; Komárek, M. Pyrolysed sewage sludge for metal(loid) removal and immobilisation in contrasting soils: Exploring variety of risk elements across contamination levels. Sci. Total Environ. 2024, 918, 170572. [Google Scholar] [CrossRef]
- Zaharioiu, A.M.; Bucura, F.; Ionete, R.E.; Marin, F.; Constantinescu, M.; Oancea, S. Opportunities regarding the use of technologies of energy recovery from sewage sludge. SN Appl. Sci. 2021, 3, 775. [Google Scholar] [CrossRef]
- Doušová, B.; Koloušek, D.; Lhotka, M.; Keppert, M.; Urbanová, M.; Kobera, L.; Brus, J. Waste Brick Dust as Potential Sorbent of Lead and Cesium from Contaminated Water. Materials 2019, 12, 1647–1660. [Google Scholar] [CrossRef]
- Doušová, B.; Bedrnová, E.; Reiterman, P.; Keppert, M.; Koloušek, D.; Lhotka, M.; Mastný, L. Adsorption Properties of Waste Building Sludge for Environmental Protection. Minerals 2021, 11, 309. [Google Scholar] [CrossRef]
- Doušová, B.; Buzek, F.; Machovič, V.; Lhotka, M.; Vojtíšek-Lom, M. Effect of “black carbon” on antimony accumulation in traffic-loaded topsoil. Sci. Total Environ. 2024, 933, 173132. [Google Scholar] [CrossRef]
- Cognigni, P.; Leonelli, C.; Berrettoni, M. A bibliographic study of biochar and hydrochar: Differences and similarities. J. Anal. Appl. Pyrolysis 2025, 187, 106985. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, S.; Ma, M.; Wang, Z.; Zhao, H.; Wang, Y. Synthesis of novel waste batteries-sawdust-based adsorbent via a two-stage activation method for Pb2+ removal. Environ. Sci. Pollut. Res. 2019, 26, 4730–4745. [Google Scholar] [CrossRef] [PubMed]
- Guilhen, S.N.; Watanabe, T.; Tieko Silva, T.; Rovani, S.; Marumo, J.T.; Tenório, J.A.S.; Mašek, O.; de Araujo, L.G. Role of Point of Zero Charge in the Adsorption of Cationic Textile Dye on Standard Biochars from Aqueous Solutions: Selection Criteria and Performance Assessment. Recent Prog. Mater. 2022, 4, 010. [Google Scholar] [CrossRef]
- Doušová, B.; Pilař, L.; Koloušek, D.; Bedrnová, E.; Lhotka, M.; Maxová, K. Adsorption properties of fly ash–clay composites from Central European localities: Case study. Appl. Clay Sci. 2024, 225, 107395. [Google Scholar] [CrossRef]
- Das, B.; Mondal, N.K.; Bhaumik, R.; Roy, P. Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int. J. Environ. Sci. Technol. 2014, 11, 1101–1114. [Google Scholar] [CrossRef]
- Dousova, B.; Buzek, F.; Herzogova, L.; Machovic, V.; Lhotka, M. Effect of organic matter on arsenic(V) and antimony(V) adsorption in soils. Eur. J. Soil Sci. 2015, 66, 74–82. [Google Scholar] [CrossRef]
- Doušová, B.; Bedrnová, E.; Maxová, K.; Koloušek, D.; Lhotka, M.; Pilař, L.; Angelis, M. Kaolin-fly ash composite for Pb2+ and AsO43− adsorption from aqueous system. Appl. Sci. 2024, 14, 5358. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef] [PubMed]

| Item/Soil | 2.1 | 6S | ||||
|---|---|---|---|---|---|---|
| Chem.composition | ||||||
| Ntot (mg·g−1) | 0.5 | 2 | ||||
| Corg (mg·g−1) | 6.5 | 16.4 | ||||
| Fe (mg·g−1) | 11.3 | 106 | ||||
| Sb (µg·g−1) | 2.1 | 3.2 | ||||
| SBET (m2·g−1) | 1.9 | 42.5 | ||||
| pH * | 5.1 | 7.1 | ||||
| pHZPC | 3.7 | 6.5 | ||||
| CEC (meq/100 g) * | 4.3 | 27.2 | ||||
| Hydraulic capacity (g/100 g) * | 31.1 | 40.5 | ||||
| Particle size distribution (mm) according to USDA) (%) * | <0.002 | <0.05 | <2.00 | <0.002 | <0.05 | <2.00 |
| 4.1 | 9.3 | 86.6 | 41.2 | 35.5 | 23.3 | |
| Sample | Chemical Composition (wt%) | Chemical Composition (mg·g−1) | SBET (m2·g−1) | pHZPC | 24 h-WAC * (kg·kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Corg | H | N | Fe | Al | Ca | As | Pb | ||||
| B | 88.8 | 0.7 | 1.0 | 2.6 | 1.4 | 8.0 | 0 | 0.01 | 446.3 | 6.2 | 2.45 |
| SL | 25.1 | 1.0 | 2.4 | 56.2 | 15.1 | 46.9 | 0.01 | 0.06 | 11.0 | 5.4 | 0.96 |
| 2.1 | 2.1-SL | 2.1-B | 6S | 6S-SL | 6S-B | |
|---|---|---|---|---|---|---|
| SBET (m2·g−1) | 1.9 | 2.0 | 23.3 | 42.5 | 32.2 | 57.5 |
| pHZPC | 3.7 | 4.3 | 5.0 | 6.5 | 5.8 | 5.5 |
| Sample | qeq (mmol·g−1) | |||
| Pb2+ | Sb(OH)6− | |||
| 24 h | 72 h | 24 h | 72 h | |
| 2.1 | 0.013 | 0.015 | - | 0.001 |
| 2.1-SL | 0.021 | 0.027 | - | 0.002 |
| 2.1-B | 0.024 | 0.028 | - | 0.004 |
| 6S | 0.090 | 0.022 | - | 0.008 |
| 6S-SL | 0.085 | 0.023 | 0.002 | 0.008 |
| 6S-B | 0.092 | 0.035 | 0.001 | 0.010 |
| Sample | qeq (mmol·g−1) | |||
| Pb2+ | Sb(OH)6− | |||
| 24 h | 72 h | 24 h | 72 h | |
| 2.1 | - | 0.010 | 0.001 | 0.007 |
| 2.1-SL | - | 0.011 | 0.002 | 0.004 |
| 2.1-B | - | 0.011 | 0.002 | 0.002 |
| 6S | 0.003 | 0.011 | 0.008 | 0.008 |
| 6S-SL | 0.004 | 0.012 | 0.009 | 0.009 |
| 6S-B | 0.003 | 0.012 | 0.008 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doušová, B.; Bedrnová, E.; Maxová, K.; Lhotka, M.; Pilař, L.; Koloušek, D.; Moško, J.; Pohořelý, M. Biochar Control of Water Regime and Adsorption Rate in Soils. Appl. Sci. 2025, 15, 9392. https://doi.org/10.3390/app15179392
Doušová B, Bedrnová E, Maxová K, Lhotka M, Pilař L, Koloušek D, Moško J, Pohořelý M. Biochar Control of Water Regime and Adsorption Rate in Soils. Applied Sciences. 2025; 15(17):9392. https://doi.org/10.3390/app15179392
Chicago/Turabian StyleDoušová, Barbora, Eva Bedrnová, Kateřina Maxová, Miloslav Lhotka, Lukáš Pilař, David Koloušek, Jaroslav Moško, and Michael Pohořelý. 2025. "Biochar Control of Water Regime and Adsorption Rate in Soils" Applied Sciences 15, no. 17: 9392. https://doi.org/10.3390/app15179392
APA StyleDoušová, B., Bedrnová, E., Maxová, K., Lhotka, M., Pilař, L., Koloušek, D., Moško, J., & Pohořelý, M. (2025). Biochar Control of Water Regime and Adsorption Rate in Soils. Applied Sciences, 15(17), 9392. https://doi.org/10.3390/app15179392






