Machine Learning-Based Predictive Modeling for Solid Oxide Electrolysis Cell (SOEC) Electrochemical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Pre-Processing
2.3. Exploratory Data Analysis
2.4. Model Development
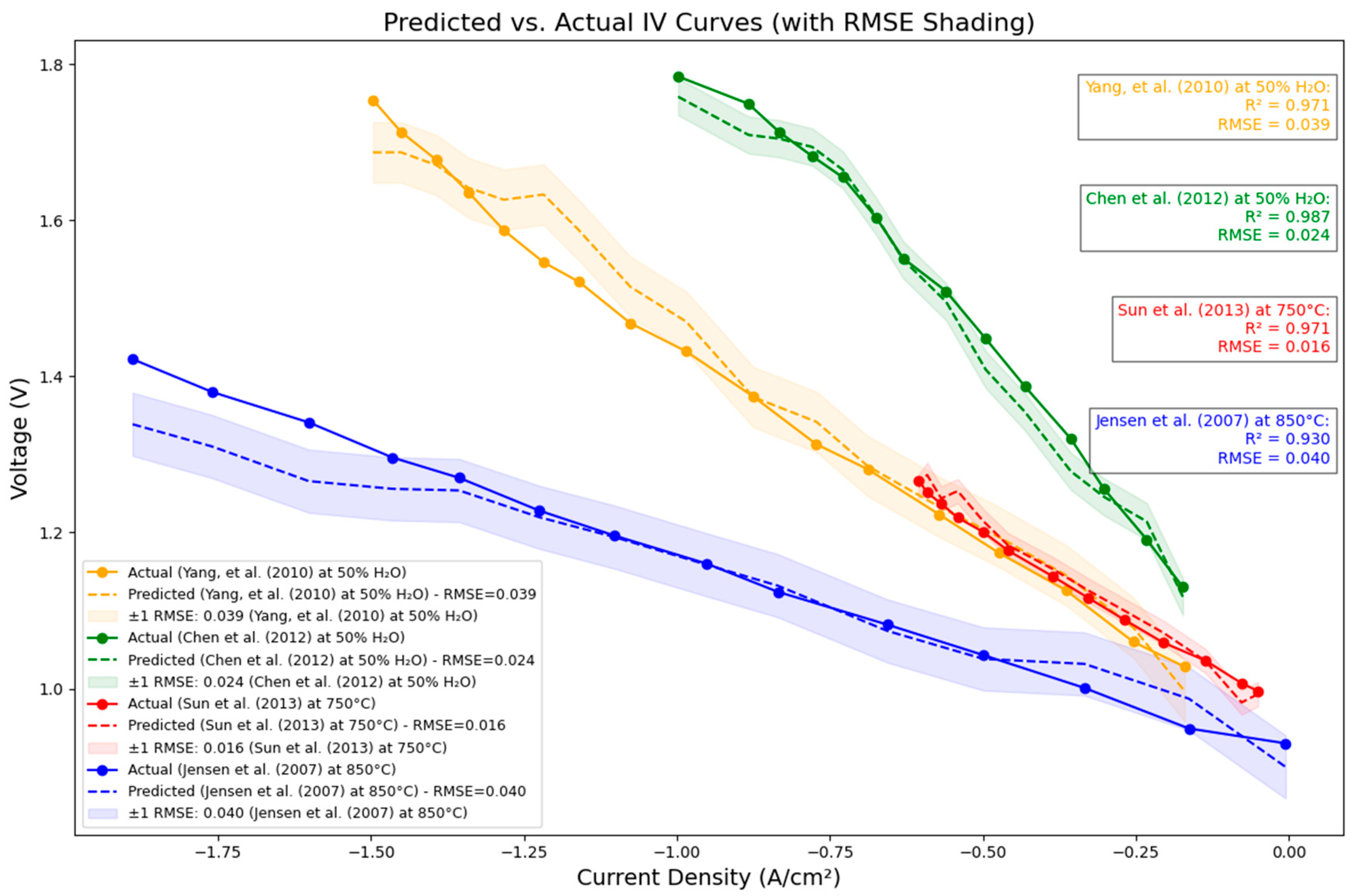
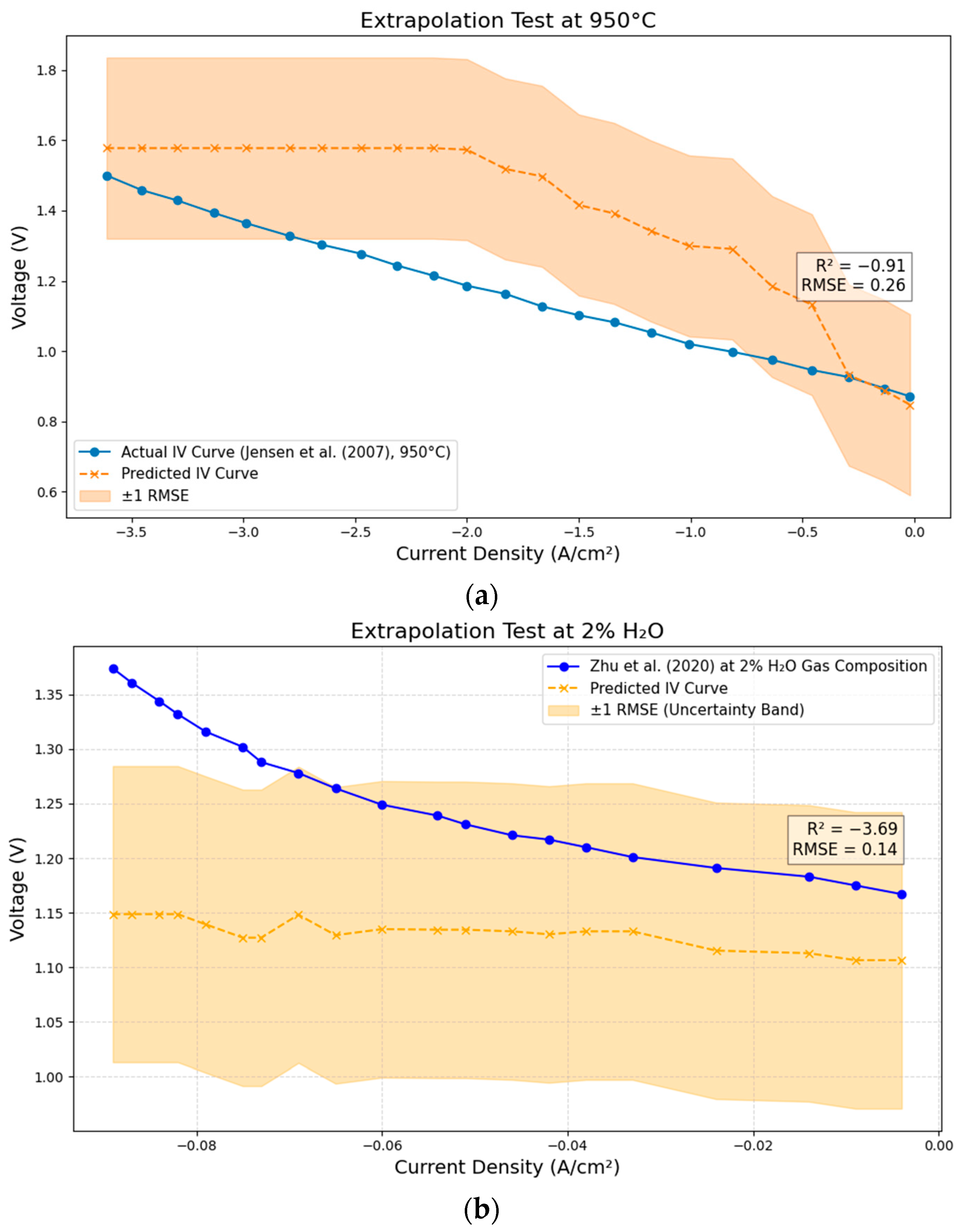
2.5. Curve-Based Validation
2.6. Interpretation
3. Results and Discussion
3.1. Data Profiling
3.2. Exploratory Data Analysis (EDA)
3.3. Hyperparameter Tuning
3.4. Cell Voltage Predictions
3.5. Curve-Based Validation: Interpolation Tests
3.6. Curve-Based Validation: Extrapolation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ML | Machine Learning |
| SOEC | Solid Oxide Electrolysis Cell |
| SOFC | Solid Oxide Fuel Cell |
| PEM | Polymer Exchange Membrane |
| AEM | Anion Exchange |
| IV | Current–Voltage |
| ANN | Artificial Neural Network |
| SVR | Support Vector Regressor |
| RF | Random Forest |
| XGBoost | Extreme Gradient Boosting |
| IEA | International Energy Agency |
| IRENA | International Renewable Energy Association |
| CCS | Carbon Capture and Storage |
| SMR | Steam Methane Reforming |
| LSM | Lanthanum Strontium Manganite |
| YSZ | Yttria-Stabilized Zirconia |
| Ni-YSZ | Nickel Yttria-Stabilized Zirconia |
References
- Herzog, A.; Tatsutani, M. A Hydrogen Future? An Economic and Environmental Assessment of Hydrogen Production Pathways. National Resources Defense Council (NDRC): New York, NY, USA, November 2005; Available online: https://www.nrdc.org/sites/default/files/hydrogen.pdf (accessed on 07 July 2025).
- International Energy Agency. IEA CO2 Emissions in 2023; International Energy Agency: Paris, France, 2023. [Google Scholar]
- IRENA. Making the Breakthrough: Green Hydrogen Policies and Technology Costs; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021. [Google Scholar]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; De Haart, L.G.J.; Guillon, O.; Eichel, R.-A.; Menzler, N.H. Solid Oxide Electrolysis Cells–Current Material Development and Industrial Application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- Flis, G.; Wakim, G. Solid. Oxide Electrolysis: A Technology Status Assessment; Clean Air Task Force: Washington, DC, USA, 2023. [Google Scholar]
- Zong, S.; Zhao, X.; Jewell, L.L.; Zhang, Y.; Liu, X. Advances and Challenges with SOEC High Temperature Co-Electrolysis of CO2/H2O: Materials Development and Technological Design. Carbon. Capture Sci. Technol. 2024, 12, 100234. [Google Scholar] [CrossRef]
- Shirasangi, R.; Lakhanlal; Dasari, H.P.; Saidutta, M.B. Current-Voltage (i-V) Characteristics of Electrolyte-Supported (NiO-YSZ/NiO-SDC/ScSZ/LSCF-GDC/LSCF) Solid Oxide Electrolysis Cell during CO2/H2O Co-Electrolysis. Chem. Phys. Impact 2024, 9, 100670. [Google Scholar] [CrossRef]
- Stempien, J.P. Fundamental Aspects of Solid Oxide Electrolyzer Cell Modelling and the Application for the System Level Analysis. Ph.D. Thesis, Nanyang Technological University, Singapore, 2016. [Google Scholar]
- Kim, S.-D.; Seo, D.-W.; Dorai, A.K.; Woo, S.-K. The Effect of Gas Compositions on the Performance and Durability of Solid Oxide Electrolysis Cells. Int. J. Hydrogen Energy 2013, 38, 6569–6576. [Google Scholar] [CrossRef]
- Kupecki, J.; Niemczyk, A.; Jagielski, S.; Kluczowski, R.; Kosiorek, M.; Machaj, K. Boosting Solid Oxide Electrolyzer Performance by Fine Tuning the Microstructure of Electrodes–Preliminary Stud. Int. J. Hydrogen Energy 2023, 48, 26436–26445. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Jensen, S.H.; Larsen, P.H.; Mogensen, M. Hydrogen and Synthetic Fuel Production from Renewable Energy Sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Liang, M.; Yu, B.; Wen, M.; Chen, J.; Xu, J.; Zhai, Y. Preparation of LSM–YSZ Composite Powder for Anode of Solid Oxide Electrolysis Cell and Its Activation Mechanism. J. Power Sources 2009, 190, 341–345. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and Innovative Solid Oxide Electrolysis Cell Materials: A Short Review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Grondin, D.; Desuere, J.; Brisse, A.; Zahid, M. Ozil Multiphysics Modeling and Simulation of a Solid Oxide Electrolysis Cell. In Proceedings of the COMSOL Conference, Hannover, Germany, 4–6 November 2008. [Google Scholar]
- Mendoza, R.M.; Mora, J.M.; Cervera, R.B.; Chuang, P.-Y.A. Experimental and Analytical Study of an Anode-Supported Solid Oxide Electrolysis Cell. Chem. Eng. Technol. 2020, 43, 2350–2358. [Google Scholar] [CrossRef]
- Menon, V.; Janardhanan, V.M.; Deutschmann, O. A Mathematical Model to Analyze Solid Oxide Electrolyzer Cells (SOECs) for Hydrogen Production. Chem. Eng. Sci. 2014, 110, 83–93. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, J.; Realff, M.J. Machine Learning: Overview of the Recent Progresses and Implications for the Process Systems Engineering Field. Comput. Chem. Eng. 2018, 114, 111–121. [Google Scholar] [CrossRef]
- Dobbelaere, M.R.; Plehiers, P.P.; Van De Vijver, R.; Stevens, C.V.; Van Geem, K.M. Machine Learning in Chemical Engineering: Strengths, Weaknesses, Opportunities, and Threats. Engineering 2021, 7, 1201–1211. [Google Scholar] [CrossRef]
- Jain, A. Machine Learning in Materials Research: Developments over the Last Decade and Challenges for the Future. Curr. Opin. Solid. State Mater. Sci. 2024, 33, 101189. [Google Scholar] [CrossRef]
- Allal, Z.; Noura, H.N.; Salman, O.; Chahine, K. Machine Learning Solutions for Renewable Energy Systems: Applications, Challenges, Limitations, and Future Directions. J. Environ. Manag. 2024, 354, 120392. [Google Scholar] [CrossRef] [PubMed]
- Langner, E.; Dehghani, H.; Hachemi, M.E.; Belouettar–Mathis, E.; Makradi, A.; Wallmersperger, T.; Gouttebroze, S.; Preisig, H.; Andersen, C.W.; Shao, Q.; et al. Physics-Based and Data-Driven Modelling and Simulation of Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2024, 96, 962–983. [Google Scholar] [CrossRef]
- Shomope, I.; Al-Othman, A.; Tawalbeh, M.; Alshraideh, H.; Almomani, F. Machine Learning in PEM Water Electrolysis: A Study of Hydrogen Production and Operating Parameters. Comput. Chem. Eng. 2024, 194, 108954. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, L.; Xiao, J.; Wen, R.; Zhang, F.; Zhang, D. Machine Learning-Assisted Prediction and Optimization of Solid Oxide Electrolysis Cell for Green Hydrogen Production. Green. Chem. Eng. 2024, 6, 154–158. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Q.; Wu, Q.; Zheng, Y.; Zhou, J.; Tu, Z.; Chan, S.H. Modelling of Solid Oxide Electrolyser Cell Using Extreme Learning Machine. Electrochim. Acta 2017, 251, 137–144. [Google Scholar] [CrossRef]
- Fei, Y.; Li, A.; Zhang, C.; Tu, H.; Zhu, L.; Huang, Z. Performance Optimization of Solid Oxide Electrolysis Cell for Syngas Production by High Temperature Co-Electrolysis via Differential Evolution Algorithm with Practical Constraints. Energy Convers. Manag. 2024, 300, 117911. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Tang, S.; Cheng, M.; Shao, Z. High-Performance Oxygen Electrode Ce0.9Co0.1O2-δ-LSM-YSZ for Hydrogen Production by Solid Oxide Electrolysis Cells. Int. J. Hydrogen Energy 2021, 46, 25332–25340. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Mogensen, M. Electrolysis of Carbon Dioxide in Solid Oxide Electrolysis Cells. J. Power Sources 2009, 193, 349–358. [Google Scholar] [CrossRef]
- Chen, Y.; Bunch, J.; Jin, C.; Yang, C.; Chen, F. Performance Enhancement of Ni-YSZ Electrode by Impregnation of Mo0.1Ce0.9O2+δ. J. Power Sources 2012, 204, 40–45. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Campana, R.; Larrea, A.; Kilner, J.A.; Orera, V.M. Steam Electrolysis Using a Microtubular Solid Oxide Fuel Cell. J. Electrochem. Soc. 2010, 157, B852. [Google Scholar] [CrossRef]
- Kim-Lohsoontorn, P.; Bae, J. Electrochemical Performance of Solid Oxide Electrolysis Cell Electrodes under High-Temperature Coelectrolysis of Steam and Carbon Dioxide. J. Power Sources 2011, 196, 7161–7168. [Google Scholar] [CrossRef]
- Li, Q.; Kuang, K.; Sun, Y.; Zheng, Y.; Liu, Q.; Chan, S.H.; Zhang, H.; Wang, W.; Li, T.; Wang, J. Deficiency of Hydrogen Production in Commercialized Planar Ni-YSZ/YSZ/LSM-YSZ Steam Electrolysis Cells. Int. J. Hydrogen Energy 2022, 47, 23514–23519. [Google Scholar] [CrossRef]
- Hauch, A.; Jensen, S.H.; Ramousse, S.; Mogensen, M. Performance and Durability of Solid Oxide Electrolysis Cells. J. Electrochem. Soc. 2006, 153, A1741. [Google Scholar] [CrossRef]
- Zhu, Z.; Sugimoto, M.; Pal, U.; Gopalan, S.; Basu, S. Electrochemical Cleaning: An in-Situ Method to Reverse Chromium Poisoning in Solid Oxide Fuel Cell Cathodes. J. Power Sources 2020, 471, 228474. [Google Scholar] [CrossRef]
- Yang, C.; Jin, C.; Coffin, A.; Chen, F. Characterization of Infiltrated (La0.75Sr0.25)0.95MnO3 as Oxygen Electrode for Solid Oxide Electrolysis Cells. Int. J. Hydrogen Energy 2010, 35, 5187–5193. [Google Scholar] [CrossRef]
- Sun, X.; Chen, M.; Liu, Y.-L.; Hjalmarsson, P.; Ebbesen, S.D.; Jensen, S.H.; Mogensen, M.B.; Hendriksen, P.V. Durability of Solid Oxide Electrolysis Cells for Syngas Production. J. Electrochem. Soc. 2013, 160, F1074–F1080. [Google Scholar] [CrossRef]
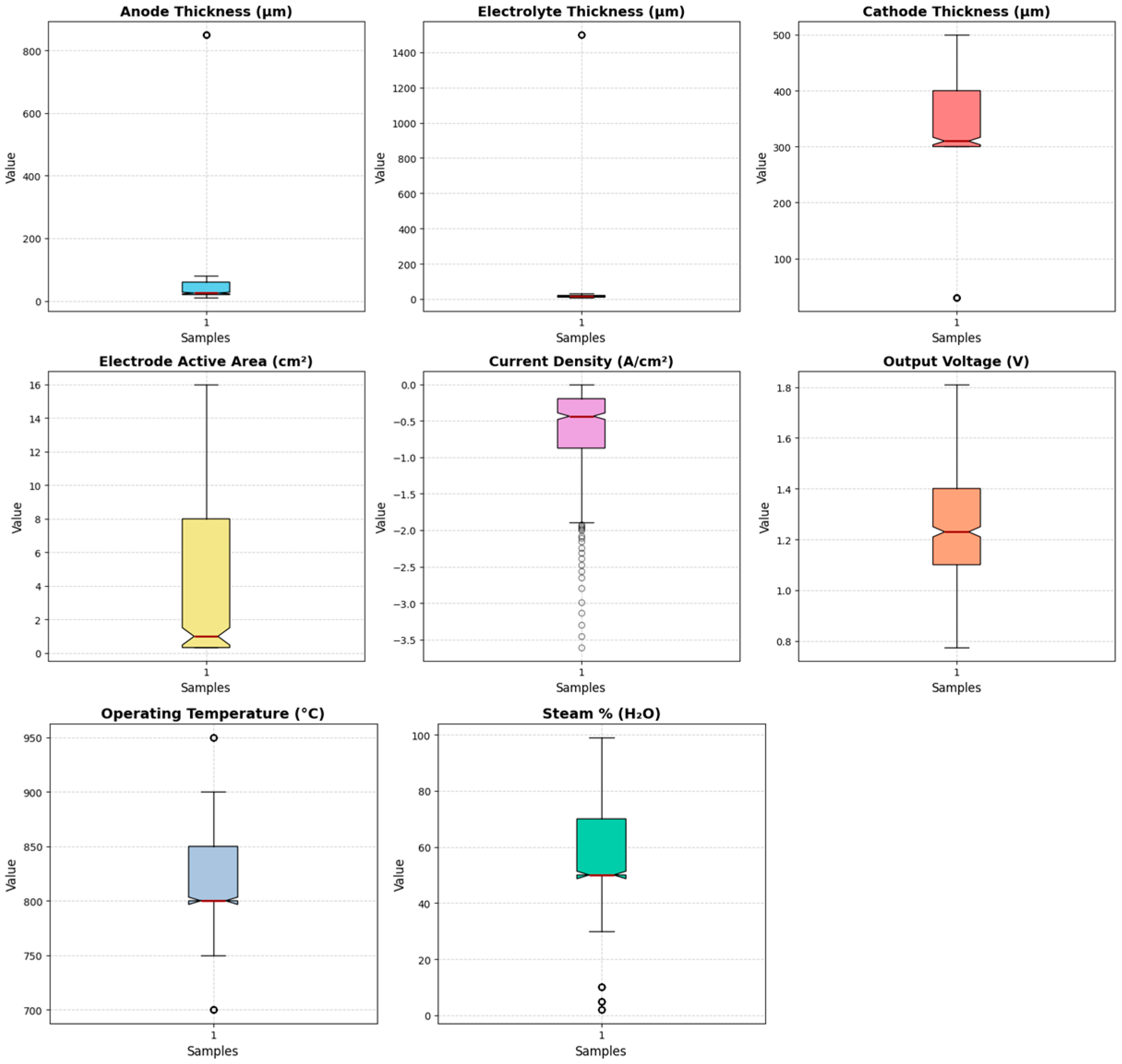
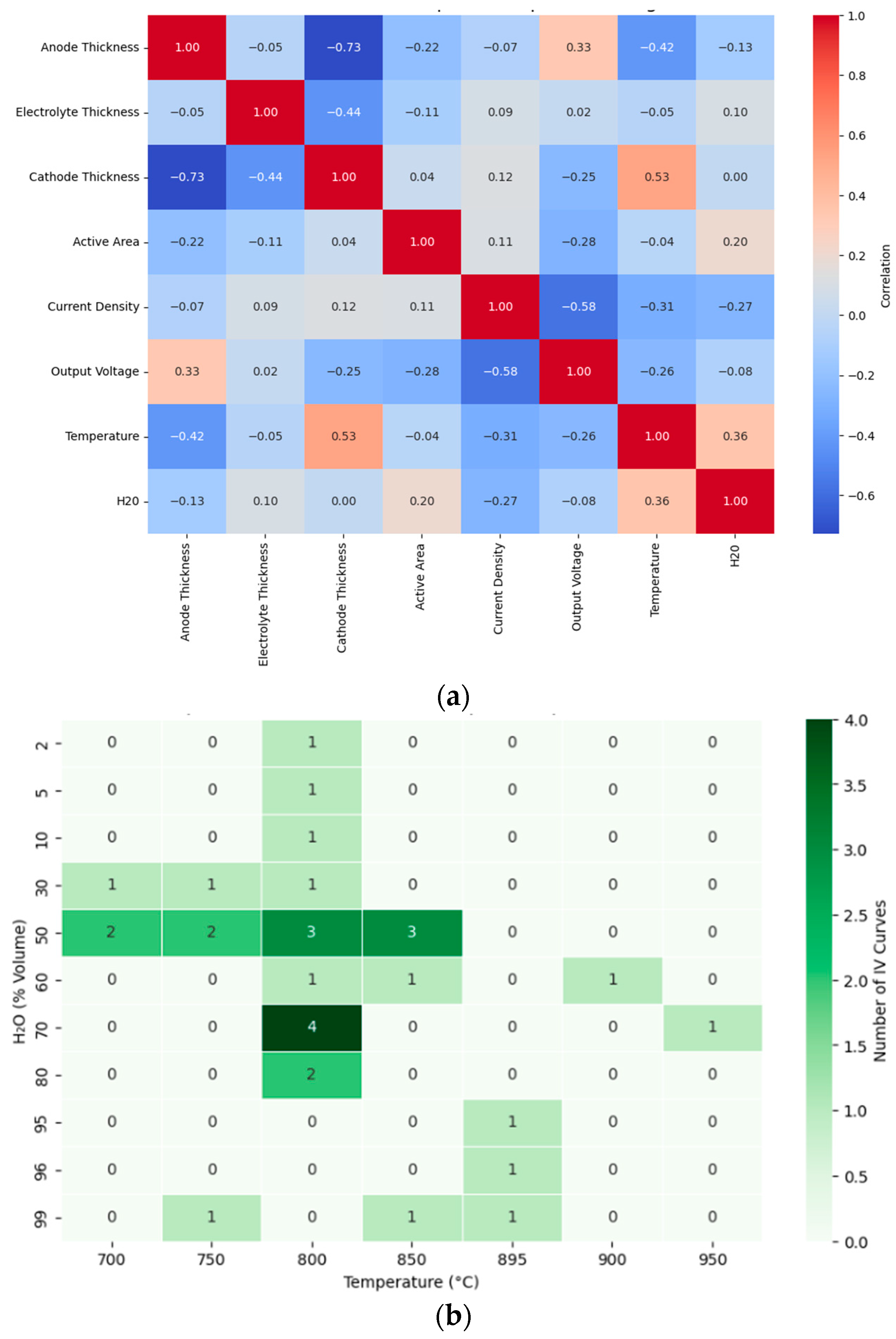
| Feature Category | Feature | Range | Unit/s | %Missing |
|---|---|---|---|---|
| Cell Characteristics | Anode Thickness | 10–850 | µm | 8.43% |
| Cathode Thickness | 30–500 | µm | 8.43% | |
| Electrolyte Thickness | 7–1500 | µm | 8.43% | |
| Active Area | 0.33–63 | cm2 | 0% | |
| Operating Parameters | Temperature | 700–950 | °C | 0% |
| Gas Composition (H2O%) | 2–99% | % | 0% | |
| Electrolytic Parameters | Current Density | −3.611 to −0.002 | A/cm2 | 0% |
| Cell Voltage | 0.774–1.810 | Volts | 0% |
| Model | Hyperparameters | Optimized Parameters |
|---|---|---|
| Random Forest (RF) | N Estimators | 200 |
| Max Depth | 16 | |
| Min Samples Split | 2 | |
| Min Samples Leaf | 2 | |
| XGBoost | N Estimators | 500 |
| Max Depth | 3 | |
| Learning Rate | 0.2 | |
| Subsample | 0.8 | |
| Colsample by Tree | 1.0 | |
| ANN | No. of Hidden Layers | 2 |
| Hidden Layer Size | 64 | |
| Activation | ReLU | |
| Optimizer | Adam (default learning rate = 0.001) | |
| Epochs | 100 (early stopped) | |
| Batch Size | 32 | |
| Early Stopping | Yes, patience = 10 | |
| SVR | C | 1 |
| Kernel | Rbf | |
| Gamma | Scale | |
| Epsilon | 0.01 |
| Model | Evaluation Metric | Results |
|---|---|---|
| Random Forest (RF) | Training R2 | 98.41% |
| Training RMSE | 0.0270 | |
| Testing R2 | 95.69% | |
| Testing RMSE | 0.0448 | |
| XGBoost | Training R2 | 99.87% |
| Training RMSE | 0.0077 | |
| Testing R2 | 98.39% | |
| Testing RMSE | 0.0274 | |
| ANN | Training R2 | 98.70% |
| Training RMSE | 0.0244 | |
| Testing R2 | 97.72% | |
| Testing RMSE | 0.0326 | |
| SVR | Training R2 | 93.17% |
| Training RMSE | 0.0059 | |
| Testing R2 | 91.02% | |
| Testing RMSE | 0.0647 |
| Model | Evaluation Metric | Results |
|---|---|---|
| Random Forest (RF) | Training R2 | 98.86% |
| Training RMSE | 0.0223 | |
| Testing R2 | 98.34% | |
| Testing RMSE | 0.0598 | |
| XGBoost | Training R2 | 99.85% |
| Training RMSE | 0.0081 | |
| Testing R2 | 98.10% | |
| Testing RMSE | 0.0332 | |
| ANN | Training R2 | 94.57% |
| Training RMSE | 0.0488 | |
| Testing R2 | 92.21% | |
| Testing RMSE | 0.0673 | |
| SVR | Training R2 | 92.85% |
| Training RMSE | 0.0559 | |
| Testing R2 | 97.11% | |
| Testing RMSE | 0.0647 |
| Test Case | Mahalanobis Distance | 95% Threshold | 99% Threshold | Multivariate Outlier? | Feature Percentile Ranks 1 |
|---|---|---|---|---|---|
| 1 (Jensen et al. [12]) | 3.3138 | 4.3088 | 5.1154 | No | Anode thickness: 0% |
| Electrolyte thickness: 12.31% | |||||
| Cathode thickness: 36.93% | |||||
| Active area: 63.24% | |||||
| Current density: 4.05% | |||||
| Temperature: 96.12% | |||||
| H2O%: 59.70% | |||||
| 2 (Zhu et al. [34]) | 2.2466 | 4.3088 | 5.1154 | No | Anode thickness: 39.63% |
| Electrolyte thickness: 0% | |||||
| Cathode thickness: 80.10% | |||||
| Active area: 50.93% | |||||
| Current density: 93.42% | |||||
| Temperature: 18.38% | |||||
| H2O%: 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada, N.G.A.; Cervera, R.B.M. Machine Learning-Based Predictive Modeling for Solid Oxide Electrolysis Cell (SOEC) Electrochemical Performance. Appl. Sci. 2025, 15, 9388. https://doi.org/10.3390/app15179388
Estrada NGA, Cervera RBM. Machine Learning-Based Predictive Modeling for Solid Oxide Electrolysis Cell (SOEC) Electrochemical Performance. Applied Sciences. 2025; 15(17):9388. https://doi.org/10.3390/app15179388
Chicago/Turabian StyleEstrada, Nathan Gil A., and Rinlee Butch M. Cervera. 2025. "Machine Learning-Based Predictive Modeling for Solid Oxide Electrolysis Cell (SOEC) Electrochemical Performance" Applied Sciences 15, no. 17: 9388. https://doi.org/10.3390/app15179388
APA StyleEstrada, N. G. A., & Cervera, R. B. M. (2025). Machine Learning-Based Predictive Modeling for Solid Oxide Electrolysis Cell (SOEC) Electrochemical Performance. Applied Sciences, 15(17), 9388. https://doi.org/10.3390/app15179388






