Abstract
Technological advancements, shifting consumer preferences, and societal changes drive the cosmetics industry to evolve continuously. The cosmetics industry is experiencing a renaissance, with new ingredients that are more environmentally friendly, natural, and transparent in terms of sourcing and manufacturing and, last but not least, which are also multifunctional. The use of technology in cosmetics has been rising, including AI (artificial intelligence) and AR (augmented reality) for virtual try-ons, skin analysis tools, and smart beauty devices that provide at-home skincare treatments. Meanwhile, fermented cosmetic ingredients are becoming increasingly popular in the beauty industry due to their improved efficacy and skin benefits. The benefits include enhanced absorption, improved stability (due to the self-produced preservatives), microbiome-friendliness (supporting the skin’s microbiome), and anti-inflammatory and soothing effects. The most common cosmetic ingredients produced by microorganisms are fermented rice, soy, green tea, fruits, and vegetables. Our laboratory investigates a mineral rock called alginite, which has shown many benefits in other fields, such as agriculture and cosmetics (e.g., as a facemask). It has been proven that alginite combined with LAB (lactic acid-producing bacteria) probiotics is beneficial for health and can increase biomass production. However, cell lysates with alginite have never been investigated for cosmetic purposes. This study aimed to investigate the potential of alginite, a mineral rock, in enhancing the cosmetic properties of LAB lysates, specifically focusing on antioxidant effects, skin-whitening properties, and, in preliminary tests, skin-moisturising effects. LAB strains were cultured with and without alginite, and the resulting cell lysates were analysed for these cosmetic applications. The preliminary results suggested that alginite may boost the hydrating effect of LAB lysate, increasing it tenfold compared to LAB lysate alone. The antioxidant effect was enhanced fivefold in the case of Lactobacillus acidophilus when cultured with alginite. However, no significant effect was observed on mushroom tyrosinase inhibition, suggesting no impact on pigment formation. Further research is needed to fully understand the mechanisms underlying these effects and to explore potential applications in cosmetic formulations. Limitations of this study include the focus on specific LAB strains and the need for in vivo studies to confirm the observed effects on human skin.
1. Introduction
The cosmetics industry is undergoing exciting changes, much like many other sectors worldwide. It is blending age-old traditions, such as fermentation, with modern innovations like artificial intelligence, creating a vibrant new landscape for beauty products. Since prehistoric times, fermentation has been a key part of human culture, and it is now stepping into the spotlight to play a crucial role in how we think about skincare and cosmetics [1,2,3,4,5,6]. Fermented ingredients can be produced by genetically modified or wild-type strains, including fragrance compounds (e.g., terpenoids), organic acids (e.g., lactic acid), and biosurfactants (e.g., alkyl-cyclic oligopeptides).
Fermentation has been shown to produce compounds with positive effects on the skin. These fermented ingredients possess several advantages that enhance the effectiveness of cosmetic products. For example, fermentation improves absorption by breaking larger molecules into smaller, more adaptive ones [7,8,9]. They have increased potency because fermentation amplifies the concentration of beneficial compounds, such as vitamins and antioxidants, enhancing their skin-related benefits [10,11]. They also have enhanced stability due to the production of natural preservatives like organic acids, alcohols, and antibiotics during fermentation [12]. Fermented ingredients can help balance the skin’s microbiome by promoting the growth of beneficial bacteria, which is crucial for maintaining a healthy skin barrier and preventing issues like acne and inflammation [13]. Many fermented ingredients possess natural anti-inflammatory properties, making them suitable for sensitive and irritated skin [14].
Integrating fermented minerals in cosmetics offers notable advantages, primarily through the enhanced bioavailability of essential nutrients. According to Majchrzak et al., the fermentation process significantly increases the effectiveness of raw cosmetic materials by producing beneficial compounds such as amino acids, proteins, and antioxidants [15]. Numerous ions are indispensable, while others exhibit supportive and auxiliary characteristics. They function within the skin, facilitating specific processes pertinent to the organ’s unique circumstances at the environmental interface. Skin bioenergetics, redox equilibrium, epidermal barrier integrity, and dermal remodelling are among the essential processes influenced by or utilising mineral elements [16]. Skin regenerative processes and ageing can be favourably influenced by sufficient accessibility, distribution, and equilibrium of inorganic ions [16]. Wu and colleagues also demonstrated that B. mucilaginosus enhances the breakdown of granite, gneisses, and sandstone by secreting organic acids, amino acids, polysaccharides, and other metabolites [17]. They found that the contents of various organic acids, amino acids, and polysaccharides in the fermentation liquid increased the concentration of several metal oxides in the ferment broth. The background is that the acids can react with mineral structures, leading to a more bioavailable form of these minerals [17]. Thus, the complexity of microbial-mineral interactions during fermentation offers a biotechnological avenue for enhancing mineral recovery and may improve nutrient cycling in natural and engineered ecosystems, including cosmetics, after thorough in vivo investigations on skin.
Alginite, a mineral derived from the decomposition of algae, is emerging as a viable ingredient in the cosmetic industry, thanks to its unique properties and potential health benefits. Alginite has already been proven to have many unique benefits, such as enhancing crop production and efficient probiotic effects when combined with LABs [18,19,20,21,22,23].
Our recent studies have explored the effects of alginite, a mineral-rich substance, on probiotic fermentation and its potential applications in cosmetics, revealing promising results and new avenues for research. Alginite supplementation has consistently significantly impacted probiotic bacterial growth and biomass production across various strains. Multiple measurement techniques, including cell dry weight (CDW), colony-forming units (CFUs), and inline capacitance-based living cell sensors, have confirmed that alginite-supplemented cultures achieve 1.5–2-fold higher cell density compared to alginite-free cultures. Furthermore, specific growth rates and dry matter content during fermentation have shown marked increases, with the Lactobacillus acidophilus study reporting an increase in the specific growth rate from 0.16 to 0.75 1/L and the dry matter content from 5.1 to 8.3 g/L in the presence of alginite [24]. These findings have important implications for the efficient production of probiotic biomass, potentially leading to more cost-effective manufacturing processes. However, the relationship between enhanced growth and cosmetic efficacy presents a complex picture that requires further investigation. In the case of Lactobacillus paracasei, with the hydration levels of 13.9% and 8.1%, respectively, the ferment filtrate without alginite performed better than those with alginite [25]. However, in the case of L. lactis, L. reuteri, and L. rhamnosus, there was no significant difference between the alginite and alginite-free ferment filtrates’ moisturising effect [26]. On the other hand, the presence of alginite decreased the antioxidant impact in all cases except one (L. acidophilus), which was unexpected because alginite has been shown to include fulvic and humic acids, which are known to have strong antioxidant properties [26]. However, because humic acids may only be dissolved in alkaline media, that is why we did not particularly see their impact. While alginite-enriched ferment filtrates of Limosilactobacillus reuteri and Bifidobacterium adolescentis showed promise for use in tanning creams, pending skin toxicity tests [26]. Despite this discrepancy, the potential for alginite-enriched probiotic ferments in cosmetic applications remains significant. These ingredients are noted for being sustainable, environmentally friendly, and highly effective, aligning with growing consumer demand for natural and eco-conscious skincare products. Combining probiotics and alginite offers a novel approach to creating cosmetic ingredients that may provide multiple benefits beyond hydration.
The European Cosmetic Ingredient database (CosIng database) contains 621 entries for “lactobacillus,” 471 for “lactobacillus filtrate,” and 34 for “lactobacillus lysate.” The database contains several ingredients, yet research publications regarding them are scarce in the literature. LABs are now registered as moisturisers, humectants, and conditioners for skin and/or hair; some even possess antioxidant and bleaching (skin whitening) characteristics. Consequently, we initiated a systematic investigation for LAB-based filtrates [26] and lysates alone and in combination with the prospective alginite mineral.
This study evaluated the effectiveness of fermented lysate derived from Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium adolescentis as cosmetic ingredients. In the CosIng database, the indicated Lactobacillus ferment lysates mostly have a moisturising effect. We anticipate favourable moisturisation scores from the lysates, although they are unlikely to surpass those of the filtrates because they also contain lactic acid, providing a well-moisturising effect [26]. Alginite may absorb and retain water, subsequently releasing it to plants, and we anticipate analogous outcomes for the skin. We evaluated the skin moisturising effects, antioxidant activity via DPPH and CUPRAC methodologies, and skin-whitening potential by the mushroom tyrosinase assay, similarly to what was previously conducted with filtrates. This paper details the implementation of the CUPRAC technique. This experiment is generally conducted at a neutral pH, enhancing its relevance to physiological settings. Conversely, the DPPH assay is typically conducted in organic solvents or alcohol-water combinations, which may not consistently represent physiological circumstances. Consequently, it exhibits more selectivity for lipophilic antioxidants due to its solubility in organic solvents; conversely, the CUPRAC method responds to hydrophilic and lipophilic antioxidants.
To summarise, we hypothesised that probiotic bacteria can have a positive effect not only through the enteral mucous membrane but also through skin (based on their structural similarity), and since alginite could boost some probiotic fermentations, resulting in higher cell numbers, we wanted to test whether it could also increase cosmetic benefits of probiotic cell lysates or not. The application of food materials in cosmetics is beneficial for their sustainability, biocompatibility, and user acceptance, but despite some safety advantages (safe foods can be safe for cosmetics), some safety issues may also arise (regarding product perishability), which need to be addressed for better human health in the future.
According to our knowledge, no one has reported research about the cosmetic usage of LABs ferment lysate supplemented with alginite mineral, nor have they reported alginite-boosted cosmetic ingredients.
2. Materials and Methods
The following strains were used: Lactobacillus rhamnosus (NCAIM B.02274) in MRS, Lactobacillus acidophilus (NCAIM B.02085) in MRS, and Bifidobacterium adolescentis (NCAIM B.01822) in Bifidobacterium medium.
De Man, Rogosa, and Sharp (MRS) medium contained the following: peptone 10 g/L; meat extract 10 g/L; yeast extract 5.0 g/L; glucose 20 g/L; K2HPO4 2.0 g/L; sodium acetate 2.0 g/L; ammonium citrate 2.0 g/L; MgSO4 × 7H2O 0.2 g/L; MnSO4 × H2O 0.05 g/L; and Tween-80 1.08 g/L.
Bifidobacterium medium: peptone from casein 10 g/L; yeast extract 5.0 g/L; meat extract 5.0 g/L; soy peptone 5.0 g/L; glucose 10 g/L; K2HPO4 2.0 g/L; MgSO4 × 7 H2O 0.2 g/L; MnSO4 × H2O 0.05 g/L; Tween-80 1.0 mL; NaCl 5.0 g/L; cystein-HCl × H2O 0.5 g/L; resazurin (25 mg/100 mL) 4.0 mL; and trace elements solution 40 mL.
Trace elements solution: 1000 mL distilled water: CaCl2 × 2H2O 0.25 g; MgSO4 × 7H2O 0.50 g; K2HPO4 1.00 g; KH2PO4 1.00 g; NaHCO3 10.00 g; NaCl 2.00 g.
In the case of the alginite-based fermentations, each medium was supplemented with powdered alginite mineral (Gérce, Hungary) 10.0 g/L if necessary. The used alginite mineral had an average 20-micron particle size. According to Kádár et al. [27], the alginite contained 4.4% moisture, 15% CaCO3, and 4.6% organic matter. Total-N was 0.15%, K 63 mg/kg, Al-K2O 386 mg/kg, Al-P2O5 216 mg/kg. The alginite used contained approximately 5% elemental Ca; 3.6% Al; 2.9% Fe; 1.9% Mg; 0.82% K; 0.15% P; 0.12% S. Aqua regia soluble content: Ca 49,942 mg/kg, Al 36,026 mg/kg, Fe 28,501 mg/kg, Mg 19,188 mg/kg, K 8166 mg/kg, P 1501 mg/kg, S 1237 mg/kg, Mn 587 mg/kg, Na 454 mg/kg, Sr 419 mg/kg, Ba 281 mg/kg, Ni 75.0 mg/kg, Zn 65.8 mg/kg, Cr 63.9 mg/kg, B 26.8 mg/kg, Cu 19.2 mg/kg, Co 15.9 mg/kg, Pb 9.75 mg/kg, As 8.84 mg/kg, Sn 2.84 mg/kg, Mo 1.86 mg/kg, Se 1.02 mg/kg, Cd 0.12 mg/kg.
The fermentations were carried out in a 1 L benchtop bioreactor with a working volume of 0.8 L (Biostat Q fermenter, B. Braun Biotech International, Melsungen, Germany) and a 5% v/v inoculum. For production, the temperature was adjusted to 37 °C with an agitation speed of 300 rpm. The pH was controlled by 25% H3PO4 and 25% NaOH. Since the used strains are facultative anaerobic, no oxygen electrode or aeration was applied. The fermentation was monitored by regular manual sampling and CFU determination, cell dry weight determination, and HPLC determination of carbohydrates and lactate. Despite sampling, an at-line living cell sensor (Incyte, Hamilton Inc.) was also applied because of the difficulty of biomass determination, besides partly soluble alginite. Depending on the applied strains, the CFU reached between 109 and 1012 at the harvesting after 30 ± 1 h of inoculation. Harvesting was performed after all sugars were consumed and biomass reached the stationary growth phase. More fermentation details are reported elsewhere [22].
After the fermentation, each broth was centrifuged (6000 rpm, Janetzki K23D centrifuge) to separate the supernatant and cell biomass (also containing alginite content of the broth). The cells (with alginite) were stored in a freezer at −20 °C for at least overnight. The lysates were made by the one-cycle freeze–thaw method: 50 mL Falcon tubes with biomass were placed into a 500 mL beaker filled with warm water (ca. 60 °C). After thawing, a 1 min vortex was applied for better homogenisation, and the obtained lysates were tested for cosmetic effect.
2.1. Skin-Moisturising Measurement
As we previously reported for LAB ferment filtrates [25], the same method was applied now for ferment lysates as preliminary tests to evaluate the short-term/immediate hydration effect. We marked a one-square-centimetre area on the inner forearm three times on ourselves as informed consent participants (having skin of Fitzpatrick type III, age 30–50, 2 males) and determined their initial skin moisture level with the Multi Dermascope MDS 800 (Courage-Khazaka) Corneometer sensor. Then, 0.2 g of biomass paste was applied to the squared skin surfaces for 10 min. After the treatment above, we wiped lysates with a dry hand towel and then measured the hydration of that part of our skin after 5 and 10 min, followed by measurement every 10 min until 50 min with the Corneometer (capacitive). The initial skin moisture values were subtracted from the values at each measurement to determine the relative moisturising effect. Time curves of relative moisturising effects jumped after the start and stabilised at the end of the measurements, following an exponential decay. If time curves decreased below zero, the suggested drying effect was observed; if they remained above zero, a moisturising effect was observed. We have used the average of the last three points (in the stationary phase) as the result of the treatment.
2.2. Antioxidant Capacity Measurement
2.2.1. Procedure of the DPPH Method
The antioxidant capacity of the ferment lysate was determined with the 1,1-diphenyl-2-picrylhydrazyl (DPPH, 97%) scavenging method [26]. For calibration, L-ascorbic acid (AscH2, 99.82%) was used in UV/HPLC grade methanol purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Fresh stock solutions were prepared before each analysis. The spectrophotometric measurements were performed in a Camspec M501 single-beam spectrophotometer with 1 cm glass cuvettes at 517 nm.
For antioxidant activity determination, oxidation of DPPH·-H to DPPH was followed spectrophotometrically, resulting in less oxidised product if antioxidant activity is present, using ascorbic acid (AscH2) as a reference. For that, a 150 µmol/L methanolic DPPH solution was prepared. For DPPH-H, the methanolic solution was prepared with DPPH· and AscH2, both at 150 µmol/L (100% excess of AscH2), protecting the reaction from light for 0.5 h. The DPPH assay was performed by adding constant aliquots of 1.5 mL of an AscH2 methanolic solution (300, 150, 75.0, 37.5, and 18.75 mmol/L) to 1.5 mL of a DPPH solution (150 µmol/L in methanol). The same procedure was applied to the ferment lysate, although there were some modifications. Approximately 1.0 g of the ferment lysate sample was measured, and the liquid volume was adjusted to 4.5 mL (with distilled water), followed by homogenisation using vortexing. The fermentation lysates were diluted twice with methanol and used hereafter in different fold dilutions (2, 4, 8, 16, and 32). The negative control was prepared with 1.5 mL of methanol and 1.5 mL of 150 µmol/L DPPH in methanol. All the reactions were kept in the dark for 30 min (at 25 °C) until measurements. After the reaction, the precipitated substances were centrifuged. All samples and negative controls were measured in triplicate. The following equation calculated the percentage DPPH scavenging activity (AbsNC—average absorbance of negative control, Abssample—average absorbance of sample):
DPPHscav. (%) was plotted versus concentration, and linear regression was used to determine IC50 values.
2.2.2. Procedure of the CUPRAC Method
Preparation of CUPRAC assay solutions: 0.01 M Cu+2 is prepared by dissolving 0.4262 g CuCl2·2H2O in water and diluting to 250 mL (in a volumetric flask at room temperature). Ammonium acetate (NH4Ac) buffer at pH 7.0, 1.0 M, is prepared by dissolving 19.27 g NH4Ac in water and diluting to 250 mL. Neocuproine (Nc) solution, 7.5 × 10−3 M, is prepared daily by dissolving 0.039 g Nc in 96% ethanol and diluting to 25 mL with ethanol.
Approximately 1.0 g of the liquid ferment lysate samples was measured, and the liquid volume was adjusted to 4.5 mL, followed by homogenisation using vortexing. 2.25 mL of the previously prepared solution was measured, followed by the addition of 0.75 mL of 0.01 M CuCl2 solution, 0.75 mL of 1.0 M NH4Ac solution, and 0.75 mL of 7.5 × 10−3 M Neocuprione solution. The resulting 4.5 mL solution was allowed to stand for 30 min at room temperature, followed by centrifugation (5 min at 6500 rpm) to eliminate biomass particles that could interfere with the measurement. The absorbance at 450 nm (A450) was measured relative to a reagent blank. The employed UV-Vis spectrophotometer was a Camspec M501 single-beam spectrophotometer. All samples and negative controls were measured in triplicate. The following equation calculated the percentage CUPRAC activity (AbsNC—average absorbance of negative control, Abssample—average absorbance of sample, Absblanc—average absorbance of blanc):
Cu[II.](%) was plotted versus concentration, and linear regression was used to determine IC50 values.
2.3. Mushroom Tyrosinase Inhibition Method
The ferments’ filtrates’ tyrosinase inhibitory activity was evaluated to determine the filtrates’ skin-whitening potential using mushroom tyrosinase as a model enzyme (human tyrosinase takes part in pigment (melanin) formation; thus, its inhibition results in whiter skin) and L-DOPA (98%). The method Toshiya M. Reference [28] reported was employed with some modifications. Briefly, the cuvettes were designated for A (negative control), B (blank of negative control), C (sample), and D (blank of the sample), which contained the following reaction mixtures: A, 750 µL of a 1/15 M phosphate buffer (pH 6.8) and 200 µL L-DOPA (10 mM in the same buffer with 5% DMSO); B, 950 µL of the same buffer; C, 350 µL of the same buffer, 200 µL L-DOPA (in the same solution), and 400 µL of an appropriate amount of the sample; and D, 550 µL of the same buffer and 400 µL of the same amount of the sample solution. B and C cuvettes for all samples were determined in triplicate. Each was well mixed, and 50 µL of tyrosinase (50 units/mL in the same buffer) was added. After incubation at room temperature (23 °C) for 10 min, each cuvette’s absorbance at 475 nm was measured in a Pharmacia LKB-Ultrospec Plus spectrophotometer. The following equation calculated the percentage inhibition of the tyrosinase activity:
The reference compound used for calibration was kojic acid (50, 25, 12.5, 6.25, 3.125 µg/mL).
2.4. Statistical Evaluation
Statistical significance between alginite-supplemented and alginite-free lysates was observed based on Student’s paired t-test, where probability values were calculated with MS-Excel. When p-values were lower than 0.05, significant differences were described.
Regarding IC50 values, significance was identified by Prism ver. 10 (GraphPad Software LLC.).
3. Results
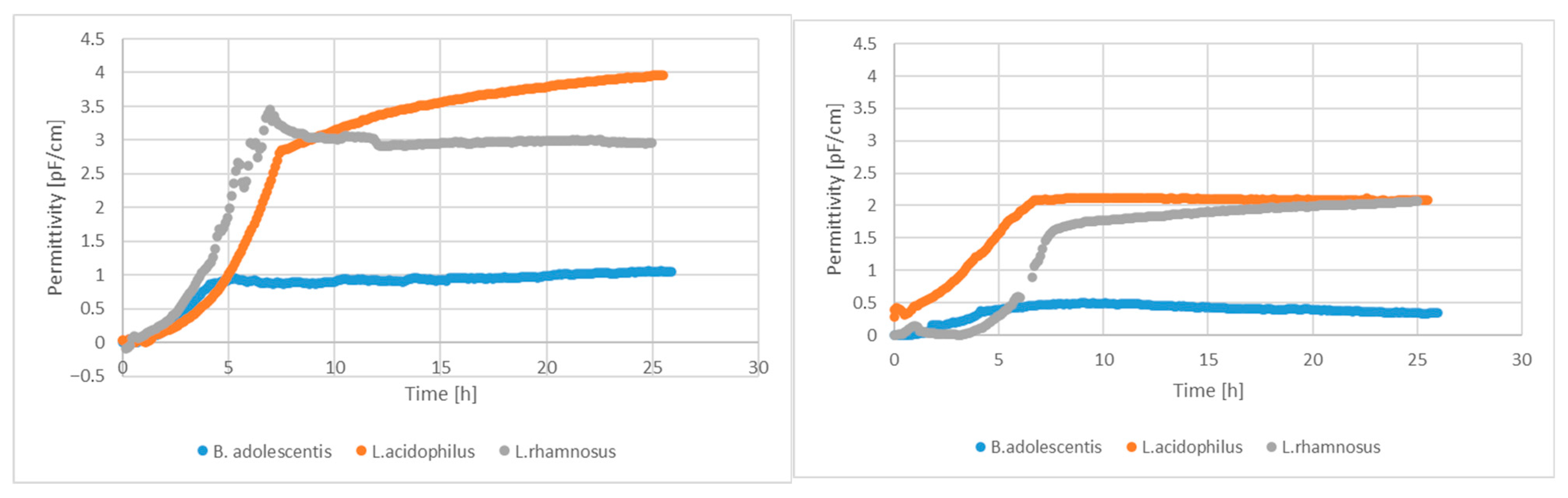
Alginite supplementation in LAB fermentation resulted in higher living cell numbers, as reported elsewhere [24], which is also confirmed by Figure 1.
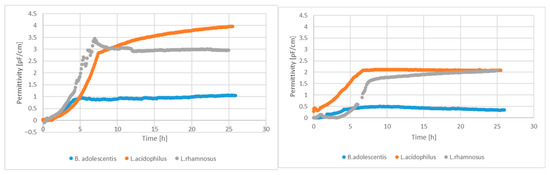
Figure 1.
Permittivity signals of living cell-number sensors with and without alginite supplementation.
We have performed the three primary evaluations of cell lysates employed to assess the potency of individual skincare products. The three measurements include the assessment of the moisturising impact, the analysis of antioxidant activity employing the DPPH and CUPRAC methods, and the evaluation of sun protection efficacy via the mushroom tyrosinase method.
3.1. Skin Moisture Effect Determination
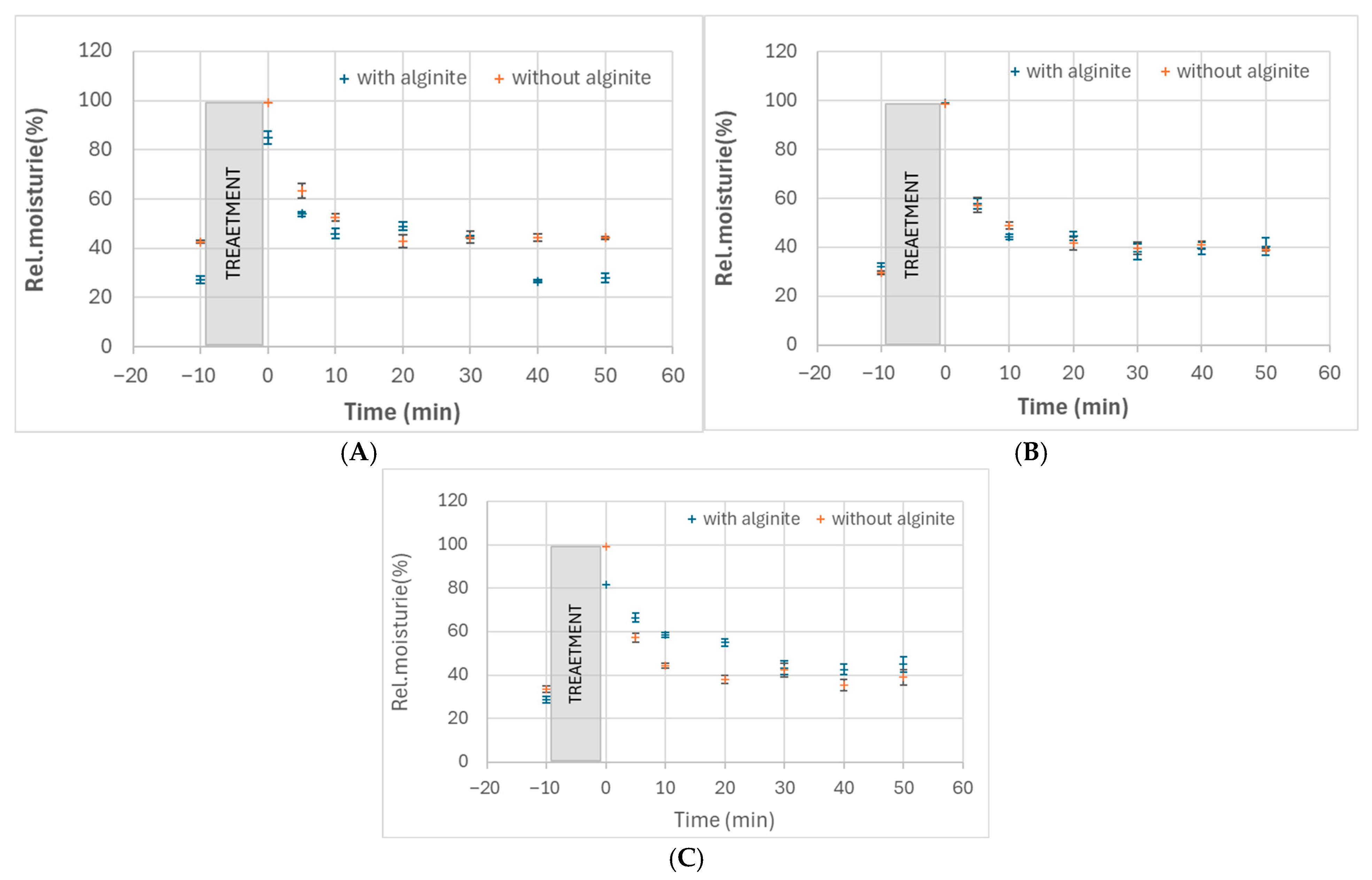
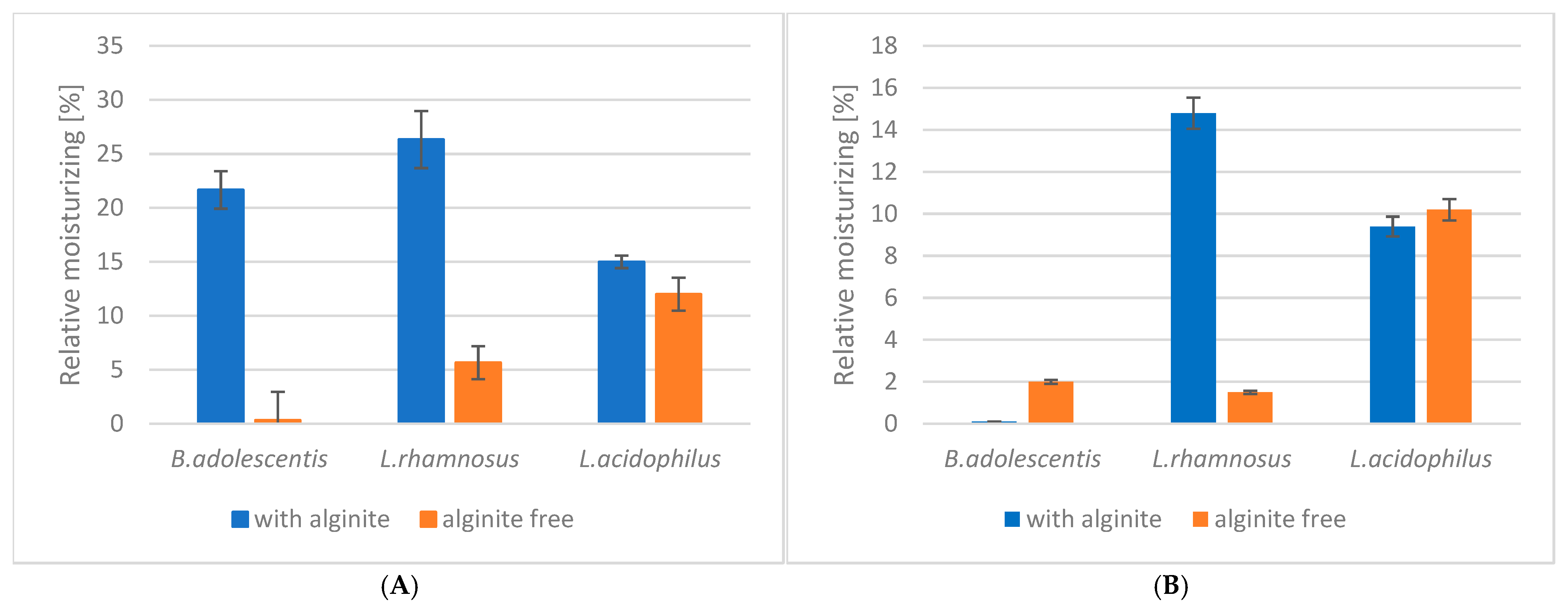
Our study investigated the moisturising effects of three probiotic strains (Bifidobacterium adolescentis, Lactobacillus rhamnosus, and Lactobacillus acidophilus) and their combinations with alginite. The raw data of skin measurements are presented in Figure 2, showing also the initial moisture level as well as stabilisation in time. Hydration potential was observed after subtracting the initial values from the average of the last three (i.e., stabilised) points. The alginite-free probiotic biomasses showed varying degrees of moisturising effects, with L. acidophilus exhibiting the highest effect (10.2%), followed by B. adolescentis (2.0%) and L. rhamnosus (1.5%) (Figure 3). When combined with alginite, the moisturising effects changed notably. In the case of B. adolescentis and L. acidophilus, no significant difference was obtained between alginite-supplemented and alginite-free lysates (p-values of 0.5528 and 0.0889, respectively), while significant differences were observed for L. rhamosus (p = 0.0367). The L. rhamnosus–alginite combination (LR–alginite) demonstrated the most substantial moisturising effect (14.8%), significantly increasing from its alginite-free probiotic form. The L. acidophilus–alginite combination (LA–alginite) maintained a strong moisturising effect (9.4%), similar to its alginite-free version. Interestingly, the B. adolescentis–alginite combination (BA–alginite) showed a marked decrease in moisturising effect (0.1%) compared to its pure form.
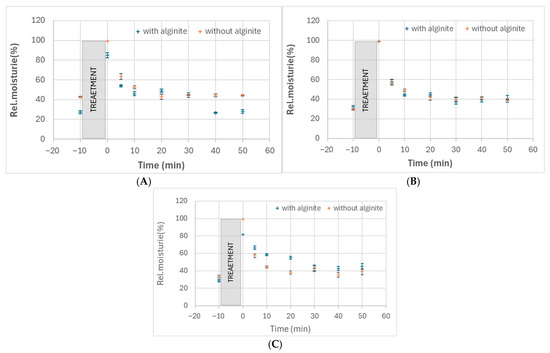
Figure 2.
Relative skin moisture changes in time after treatment with cell lysates of B. adolescentis (A), L. acidophilus (B), and L. rhamnosus (C) fermented either with alginite or without it.
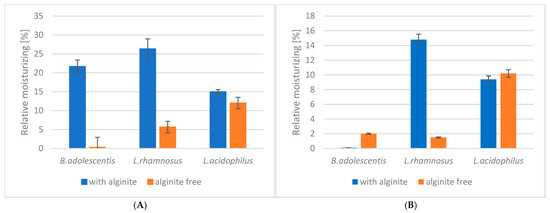
Figure 3.
The ferment lysates’ relative skin moisture in a steady state: (A) at 30 min; (B) at 60 min.
These findings suggest that the combination of probiotics with alginite can significantly alter their moisturising properties, with the effect varying depending on the specific probiotic strain. The LR–alginite combination, in particular, shows promising potential for moisturising applications.
3.2. The Antioxidant Capacity
The antioxidant capacity of three probiotic bacterial strains’ ferment filtrate (Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium adolescentis) was evaluated using both CUPRAC and DPPH methods when either alginite was applied or was not during the fermentation.
CUPRAC method results revealed significant variations in antioxidant capacity. L. acidophilus alginite ferment filtrate showed a marked increase in antioxidant capacity compared to alginite-free filtrates, from 24.9 to 5.2, representing a 79.1% increase (Table 1, the lower IC50 is the better result). B. adolescentis exhibited a moderate increase, from 7.5 to 5.8, a 22.7% improvement. In contrast, L. rhamnosus demonstrated a slight decrease, from 8.3 to 10.3, indicating a 24.1% reduction in antioxidant capacity.

Table 1.
LABs’ biomass antioxidant capacity by the CUPRAC method.
DPPH method results (Table 2) presented a different pattern. L. acidophilus displayed a substantial decrease in antioxidant capacity with alginite, from 4.51 to 2.46, which means a 45.5% reduction. Similarly, B. adolescentis showed a notable decrease, from 3.01 to 1.42, representing a 52.8% decline. L. rhamnosus, however, remained almost unchanged, with values shifting marginally from 6.01 to 6.02, a mere 0.2% increase.

Table 2.
LABs’ biomass antioxidant capacity by the DPPH method.
According to these findings, alginite contributes to the antioxidant effect of ferment lysates, since both methods indicated higher antioxidant activity in alginite-supplemented ferment lysates compared to alginite-free ones, except for L. rhamnosus.
The divergent responses of the three bacterial strains to alginite emphasise the species-specific nature of antioxidant capacity modulation. This variability underscores the importance of strain selection in probiotic formulations, especially when considering potential synergistic effects with additives like alginite.
Furthermore, the discrepancies between CUPRAC and DPPH results highlight the necessity of employing multiple analytical methods when assessing antioxidant capacity, as different assays may capture distinct aspects of the complex antioxidant systems present in probiotic bacteria. These comprehensive findings provide valuable insights into the intricate relationship between alginite and the antioxidant properties of probiotic strains, paving the way for more targeted research and applications in probiotics and functional foods.
The enhanced antioxidant potential of the probiotic-alginite combinations, particularly BA-alginite and LA-alginite, may have important implications for potential applications in various industries, such as cosmetics, pharmaceuticals, and food, where strong antioxidant activity is desirable.
3.3. Mushroom Tyrosinase Inhibition Results
The third important cosmetic parameter that has been studied is mushroom tyrosinase inhibition measurement. This is a standard method used to assess the activity of tyrosinase, an enzyme that plays a crucial role in melanin biosynthesis.
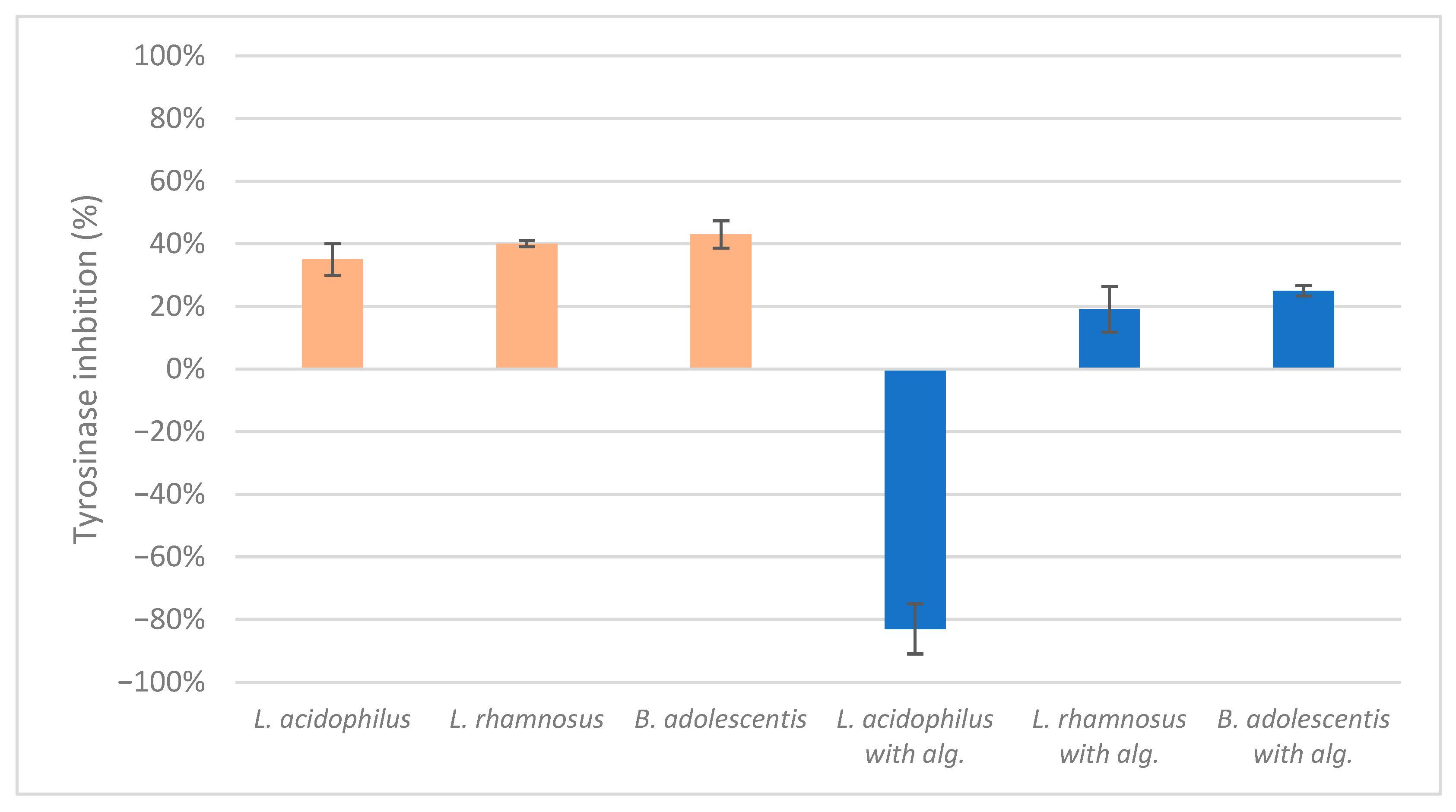
The results of the mushroom tyrosinase inhibition measurements indicated a lack of significant positive or negative effects on inhibition, with the exception of the lysate of L. acidophilus combined with alginite, which enhanced enzyme activity (Figure 4). This finding aligns with our previous study, where the ferment filtrates of L. reuteri, B. adolescentis, and L. acidophilus with alginite also demonstrated a tyrosinase-activating effect, which could be beneficial for self-tanning ingredients but needs further in vivo testing [26].
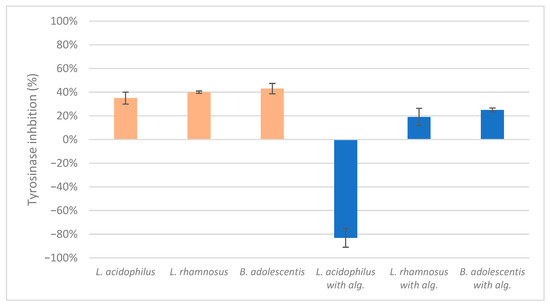
Figure 4.
The ferment lysates’ highest mushroom tyrosinase inhibition score.
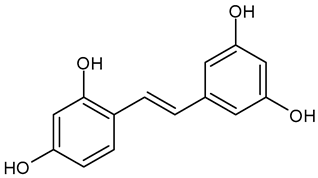
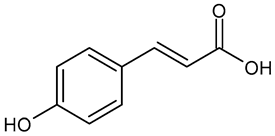
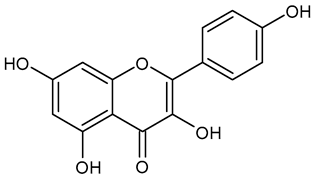
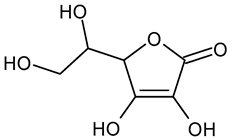
In the mushroom tyrosinase inhibition measurement, none of the samples reached the 50% inhibition level of the tyrosinase enzyme. The known tyrosinase inhibitors commonly have phenolic compounds with more than one heteroatom, which are usually oxygen in the form of oxo, hydroxyl, and ether. In Table 3, we listed some known tyrosinase inhibitors, for example.

Table 3.
Mushroom tyrosinase enzyme inhibitors.
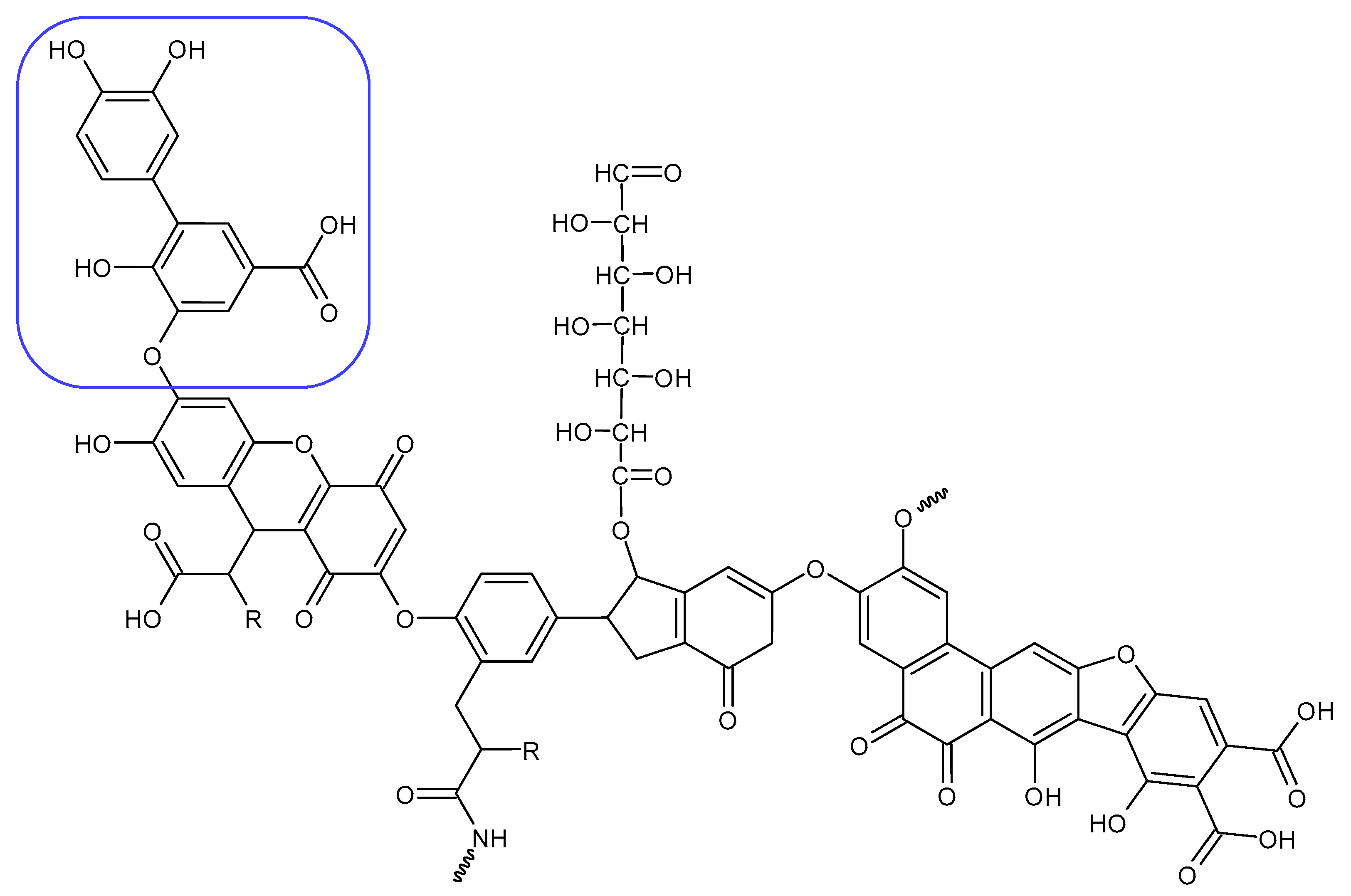
Alginite contains humic and fulvic acids. In the humic acids (Figure 5), we can find a group whose chemical structure is similar to that of the mushroom tyrosinase inhibitors. In the blue bracket, a catechol is linked to a PHBA (para-hydroxybenzoic acid). So we could assume some tyrosinase enzyme inhibitor activity of alginite. However, the inhibitors and the smaller humic substances (which are able to bind to the enzyme) are polar due to the many hydroxyl and oxo groups; thus, they are instead distributed into the aqueous phase (i.e., in ferment filtrate), and none are in the ferment lysate. Unfortunately, no further tyrosinase inhibitors were found.
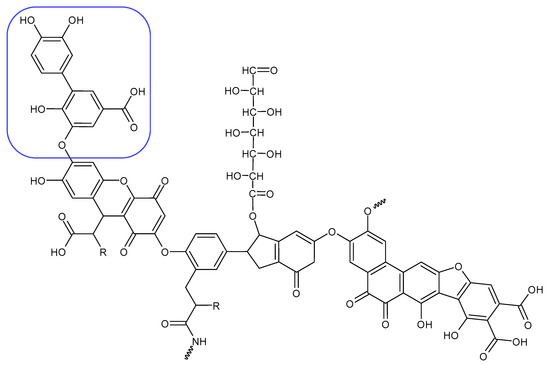
Figure 5.
Humic substance chemical structure.
These findings are consistent with our previous study, where we investigated the LABs’ ferment filtrates. The following results were obtained: the highest achieved inhibition of tyrosinase L. reuteri and B. adolescentis alginite-free filtrates had a value of 88% and 69%, respectively. However, the alginite-based ferment filtrates for the same strains reached values of −94% and −135% at the same dilution [26].
To summarise, the alginite based ferment filtrates enhanced the activity of the mushroom tyrosinase, which leads to the tanning effect on the skin, as indicated by negative inhibition values. In contrast, alginite ferment lysate did not have any skin-whitening effect, i.e., tyrosinase inhibition.
4. Discussion
Our study on the effects of probiotic strains and their combinations with alginite has yielded valuable insights into their potential applications in skincare and functional foods. The research focused on three key aspects: moisturising effects, antioxidant activity, and mushroom tyrosinase inhibition.
In terms of moisturising properties, we observed significant variations among the probiotic strains and their alginite combinations. Alginite by itself has a skin-drying effect, but the combination of Lactobacillus rhamnosus’s ferment filtrate with alginite in fermentation showed the most significant moisturising effect, even outperforming the strong moisturising properties of pure Lactobacillus acidophilus. This finding suggests a synergistic interaction between L. rhamnosus and alginite, which could be particularly beneficial for skincare applications.
The investigation of antioxidant activity demonstrated intricate interactions between the probiotic strains and alginite. The IC50 of antioxidant capacity values were in different ranges depending on the employed methods (CUPRAC or DPPH), but the ratios of tested samples are similar: the disparity between the L. acidophilus lysate with and without alginite was much greater in the CUPRAC assessment than in the DPPH measurement. In both instances, the alginite supplement exhibited superior antioxidant capacity compared to the alginite-free. The lysates of L. rhamnosus alginite and alginite-free produced comparable outcomes in the CUPRAC assay, while alginite-supplemented and alginite-free lysates showed identical results, also with the DPPH assay. The B. adolescentis lysate combined with alginite exhibited the best antioxidant result in the DPPH assay, and also the alginite one reached better antioxidant capacity in the measurement of CUPRAC.
Contrary to our initial expectations, the mushroom tyrosinase tests yielded no significant tyrosinase inhibition across all samples. L. acidophilus with alginite was an exception because it went negative and reached −83% in mushroom tyrosinase inhibition, i.e., activation. We have previously experienced similar results, but only then did alginite filtrates of probiotic strains (L. reuteri, B. adolescentis, and L. acidophilus) show similar skin-tanning properties. Returning to the rest, we hypothesise that this lack of effect may be due to the separation of potential inhibitors during the centrifugation, with active compounds likely remaining in the filtrates rather than the biomass based on their structure and water solubility.
These findings collectively emphasise the strain-specific nature of probiotic-alginite interactions and their effects on various skincare-related properties. The study underscores the potential of certain probiotic-alginite combinations, particularly for moisturising and antioxidant applications. However, it also highlights the need for careful consideration of strain selection and processing methods when developing probiotic-based products.
Moving forward, further research is warranted to optimise probiotic-alginite combinations for specific applications and to understand the mechanisms underlying their biological activities fully. This study lays a foundation for future investigations into the use of probiotics and alginite in the skincare and functional food industries, opening up new possibilities for innovative product development.
5. Conclusions
In summary, we examined the cosmetic impacts of three probiotic lactic acid-producing bacteria (L. acidophilus, L. rhamnosus, and B. adolescentis) fermented lysates, both with and without alginite. The results suggested that alginite may enhance the potential of probiotic ferment lysates as cosmetic additives. Preliminary results of L. acidophilus samples’ skin-hydrating effect suggested that it could increase skin moisture level both with and without alginite; furthermore, the alginite-based samples exhibited superior antioxidant activity, particularly in the CUPRAC assay. Additionally, it behaved as a mushroom tyrosinase activator. The L. rhamnosus alginite-supplemented ferment lysate showed the highest skin hydration potential with a 14.8% relative moisture increment, whereas the alginite-free variant yielded 1.5%. B. adolescentis exhibits strong antioxidant capacity (alginite-based was better in all cases); however, it seemed to be ineffective in skin moisturisation and mushroom tyrosinase inhibition.
According to our findings, L. acidophilus alginite-supplemented ferment lysate can be applicable as a moisturiser, antioxidant, and probably skin tanner after a detailed study, while. L. rhamnosus lysate with alginite might be applied as a moisturiser.
Author Contributions
P.T. methodology, investigation, formal analysis, visualisation, writing—original draft; Á.N. conceptualisation, writing—review and editing, supervision, validation, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by MÉL Biotech K+F Kft (Budapest, Hungary).
Institutional Review Board Statement
The reported experimental materials in this study were tested solely by the authors in a preliminary, exploratory context. As such, no formal ethics committee approval was required, in accordance with the Declaration of Helsinki. The authors further confirm that Informed Consent for Participation was obtained.
Data Availability Statement
The authors confirm that all data supporting the findings of this study are included in the manuscript. Additional data are available upon request from the corresponding author.
Acknowledgments
The alginite sample used in this study was kindly provided by Alginit Kft., Hungary.
Conflicts of Interest
The authors declare no competing interests.
References
- Dailin, D.J.; Rithwan, F.; Azelee, N.I.W.; Zainan, N.; Low, L.Z.M.I.; Zaidel, D.N.A.; El Enshasy, H. Trends in Bio-Based Cosmetic Ingredients. In Biomass-Based Cosmetics; Arung, E.T., Fatriasari, W., Kusuma, I.W., Shimizu, H.K.K., Kim, Y.-U., Azelee, N.I.W., Edis, Z., Eds.; Springer: Singapore, 2024; pp. 27–47. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Sasounian, R.; Martinez, R.M.; Lopes, A.M.; Giarolla, J.; Rosado, C.; Magalhães, W.V.; Velasco, M.V.R.; Baby, A.R. Innovative Approaches to an Eco-Friendly Cosmetic Industry: A Review of Sustainable Ingredients. Clean Technol. 2024, 6, 176–198. [Google Scholar] [CrossRef]
- Kucharska, E.; Wachura, D.; Elchiev, I.; Bilewicz, P.; Gąsiorowski, M.; Pełech, R. Co-Fermentation of Dandelion Leaves (Taraxaci folium) as a Strategy for Increasing the Antioxidant Activity of Fermented Cosmetic Raw Materials—Current Progress and Prospects. Appl. Sci. 2025, 15, 9021. [Google Scholar] [CrossRef]
- Cavalcanti, A.P.B.; de Araújo, G.P.; Bezerra, K.G.d.O.; de Almeida, F.C.G.; da Silva, M.d.G.C.; Sarubbo, A.; da Silva Júnior, C.J.G.; Soares da Silva, R.d.C.F.; Sarubbo, L.A. Production of a Biosurfactant for Application in the Cosmetics Industry. Fermentation 2025, 11, 451. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nowak, A.; Muzykiewicz-Szymańska, A.; Wójciak, M.; Sowa, I.; Szczepanek, D.; Nizioł-Łukaszewska, Z. Enhancing the Cosmetic Potential of Aloe Vera Gel by Kombucha-Mediated Fermentation: Phytochemical Analysis and Evaluation of Antioxidant, Anti-Aging and Moisturizing Properties. Molecules 2025, 30, 3192. [Google Scholar] [CrossRef]
- Juliano, C.C.A.; Magrini, G.A. A Radish Root Ferment Filtrate for Cosmetic Preservation: A Study of Efficacy of Kopraphinol. Appl. Sci. 2024, 14, 7761. [Google Scholar] [CrossRef]
- Chan, C.-F.; Huang, C.-C.; Lee, M.-Y.; Lin, Y.-S. Fermented Broth in Tyrosinase- and Melanogenesis Inhibition. Molecules 2014, 19, 13122–13135. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Jeong, K. Engineering of leuconostoc citreum for efficient bioconversion of soy isoflavone glycosides to their aglycone forms. Int. J. Mol. Sci. 2022, 23, 9568. [Google Scholar] [CrossRef]
- Hati, S.; Patel, M.; Mishra, B.K.; Das, S. Short-chain fatty acid and vitamin production potentials of Lactobacillus isolated from fermented foods of Khasi Tribes, Meghalaya, India. Ann. Microbiol. 2019, 69, 1191–1199. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2019, 24, 51. [Google Scholar] [CrossRef]
- Duhan, J.S.; Nehra, K.; Gahlawat, S.K.; Saharan, P.; Surekha, D. Bacteriocins from Lactic Acid Bacteria. In Biotechnology: Prospects and Applications; Salar, R., Gahlawat, S., Siwach, P., Duhan, J., Eds.; Springer: New Delhi, India, 2013; pp. 127–141. [Google Scholar] [CrossRef]
- Yu, J.; Ma, X.; Wang, X.; Cui, X.; Ding, K.; Wang, S.; Han, C. Application and mechanism of probiotics in skin care: A review. J. Cosmet. Dermatol. 2022, 21, 886–894. [Google Scholar] [CrossRef]
- Zheng, X.-Q.; Zhang, X.-H.; Gao, H.-Q.; Huang, L.-Y.; Ye, J.-J.; Ye, J.-H.; Lu, J.-L.; Ma, S.-C.; Liang, Y.-R. Green Tea Catechins and Skin Health. Antioxidants 2024, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and cosmetical importance of fermented raw materials: An overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef]
- Haftek, M.; Abdayem, R.; Guyonnet-Debersac, P. Skin minerals: Key roles of inorganic elements in skin physiological functions. Int. J. Mol. Sci. 2022, 23, 6267. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q. Preliminary study on the rock weathering effect of bacillus mucilaginosus. Appl. Ecol. Environ. Res. 2019, 17, 13297–13309. [Google Scholar] [CrossRef]
- Solti, G. Az Alginit; Magyar Állami Földtani Intézet (Alkalmi Kiadványa): Budapest, Hungary, 1987; ISBN 963 671 073 2. [Google Scholar]
- Jan, C.; Lukáš, L.; Zdeněk, V.; Martin, B.; Rostislav, L. The effects of alginite fertilisation on selected tree species seedlings performance on afforested agricultural lands. Cent. Eur. For. J. 2017, 63, 48–56. [Google Scholar] [CrossRef]
- Tužinský, M.; Kupka, I.; Podrázský, V.; Prknová, H. Influence of the mineral rock alginite on survival rate and re-growth of selected tree species on agricultural land. J. For. Sci. 2015, 61, 399–405. [Google Scholar] [CrossRef]
- Hlubeňová, K.; Mudroňová, D.; Nemcová, R.; Gancarčíková, S.; Maďar, M.; Sciranková, Ľ. The Effect of Probiotic Lactobacilli and alginite on the Cellular Immune Response in Salmonella Infected Mice. Folia Vet. 2017, 61, 61–66. [Google Scholar] [CrossRef]
- Viola, S.; Ivana, K.; Jana, F.; Aladár, M.; Soňa, G.; Dagmar, M.; Andrea, L. Evaluation of Probiotic Lactobacillus fermentum CCM 7421 administration with alginite in Dogs. Probiotics Antimicrob. Proteins 2017, 10, 577–588. [Google Scholar] [CrossRef]
- Gancarčíková, S.; Nemcová, R.; Popper, M.; Hrčková, G.; Sciranková, Ľ.; Maďar, M.; Mudroňová, D.; Vilček, Š.; Žitňan, R. The Influence of Feed-Supplementation with Probiotic Strain Lactobacillus reuteri CCM 8617 and alginite on Intestinal Microenvironment of SPF Mice Infected with Salmonella Typhimurium CCM 7205. Probiotics Antimicrob. Proteins 2018, 11, 493–508. [Google Scholar] [CrossRef]
- Tóth, P.; Németh, Á. Exploring the Impact of Alginite Mineral on Lactic Acid Bacteria. Fermentation 2025, 11, 482. [Google Scholar] [CrossRef]
- Tóth, P.; Németh, Á. Investigations Into the usage of the mineral alginite fermented with Lactobacillus paracasei for cosmetic purposes. Hung. J. Ind. Chem. 2022, 50, 16–20. [Google Scholar] [CrossRef]
- Tóth, P.; Németh, Á. Investigation and Characterisation of New Eco-Friendly Cosmetic Ingredients Based on Probiotic Bacteria Ferment Filtrates in Combination with Alginite Mineral. Processes 2022, 10, 2672. [Google Scholar] [CrossRef]
- Kádár, I.; Ragályi, P.; Murányi, A.; Radinszky, L.; Gajdó, A. Effect of Gérce alginit on the fertility of an acid sandy soil. Agrokémia Talajt. 2015, 64, 437–452. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef]
- Hassan, M.; Ashraf, Z.; Abbas, Q.; Raza, H.; Seo, S.-Y. Exploration of novel human tyrosinase inhibitors by molecular modeling, docking and simulation studies. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Seo, S.-Y.; Babar, M.M.; Zaidi, N.-S.S. Design, synthesis and bioevaluation of novel umbelliferone analogues as potential mushroom tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stäb, F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 19–23. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Nokinsee, D.; Shank, L.; Lee, V.S.; Nimmanpipug, P. Estimation of inhibitory effect against tyrosinase activity through homology modeling and molecular docking. Enzym. Res. 2015, 2015, 262364. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).