Abstract
PFASs, compounds to which the C-F bond—the strongest known in nature—bestows high resistance to degradation, have been detected in surface and groundwater worldwide, including drinking water supplies. Current regulations on long-chain PFASs resulted in the shift to short-chain PFASs in industrial uses, with their increasing environmental detection. Currently, suggested BATs for PFAS removal from aqueous solutions include mainly adsorption or membrane filtration; however, different response behavior to even simple treatment was observed concerning long- and short-chain PFAS molecules. In order to permanently destroy (mineralize) PFASs and their precursors, treatment technologies that can deliver sufficiently high energy to crack the C-F bond are needed. This paper discusses current PFAS removal technologies and state of the art advanced methods for PFAS removal and destruction, critically discussing their efficiency, applicability, emerging issues, and future prospects.
1. Introduction
Per- and polyfluoroalkyl substances (PFASs), anthropogenic hydrocarbon (C4–C18) compounds in which all hydrogen atoms in the alkyl chain are replaced by fluorine, have been detected in surface and groundwater worldwide, including drinking water supplies. As an example, Figure 1 reports known PFAS-contaminated sites in Europe, according to the European Environment Agency [1].
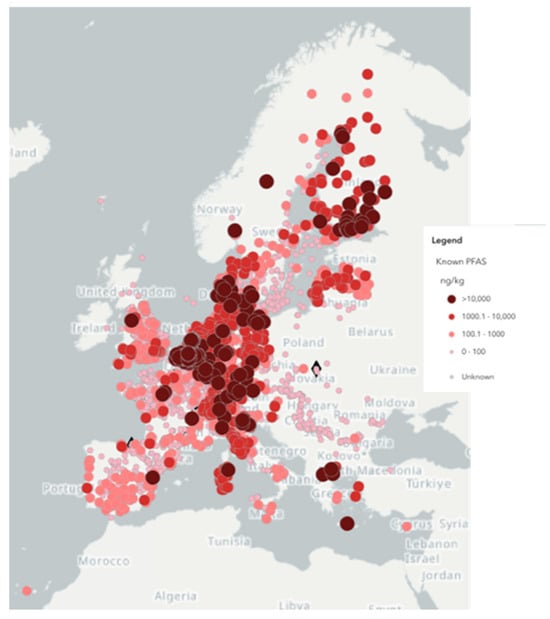
Figure 1.
Map of known PFAS contamination detected in Europe [1].
Curiously, PFASs were originally never intended for large-scale use: Teflon (polytetrafluoroethylene, PTFE), the archetypal PFAS, was developed in 1938 and first used at an industrial scale during the Manhattan Project to produce coatings able to resist the highly corrosive substances used in uranium enrichment [2]. PFASs have since been used in many industrial processes, becoming a common component of a multitude of household products; they can thus enter the environment through improper disposal of manufacturing and household waste and other activities (e.g., firefighting foams, etc.).
Like other persistent man-made pollutants (e.g., microplastics), PFASs tend to be globally transported through atmospheric and/or oceanic diffusion, becoming ubiquitous like other types of emerging pollutants [3,4]. Additionally, PFAS precursors, among which fluorotelomer alcohols and fluorotelomer sulfonates are found in many consumer and industrial products, can transform into stable PFAAs (perfluoroalkyl acids) in natural or engineered, abiotic or biotic conditions, resulting in the formation of harmful compounds that contribute to overall PFAS contamination [5,6].
Industrial and municipal wastewater treatment plants (WWTPs) have been identified as the main recipients of PFASs, as well as significant pathways for their transfer to natural waters though effluents and to soils and groundwater through biological sludge disposal [7,8]. The C-F bond, among the strongest single bonds in nature, gives these molecules extremely long environmental persistence (they are also dubbed “forever contaminants”) and high resistance to degradation, whether natural or process-induced. Further, PFASs bioaccumulate; therefore, even small amounts can build up over the years to damaging levels within living organisms [9].
The presence of some PFAS compounds (e.g., PFOA—perfluorooctanoic acid, PFOS—perfluorooctanesulfonic acid) in water and wastewater has been thoroughly investigated, but information on PFAS precursors is still scarce [8]. Millions of PFAS molecules exist: recent estimates indicate that >7 million PFAS and >21 million fluorinated compounds exist, with ongoing research regularly identifying new ones [10]. This number is several orders of magnitude greater than older established PFAS lists (typically up to a couple tens of thousands of entries) [11]. Among PFASs, long-chain PFASs (LC-PFASs) are characterized by a fully or partially fluorinated carbon chain (≥C6 for perfluoroalkane sulfonic acids, PFSAs; ≥C7 perfluoroalkyl carboxylic acids, PFCAs) and molecular weight (MW) > ≈ 400; short-chain PFASs (SC-PFASs) show a shorter carbon chain length: C4–C6 for PFCAs, C3–C5 for PFSAs, and MW < 400. These two classes have different characteristics that influence their fate, as discussed later.
The EU regulates LC-PFASs under the Persistent Organic Pollutants (POPs) (i.e., PFOS, PFOA, PFHxS—perfluorohexane sulfonic acid) and REACH regulations (i.e., C9–C14 PFCAs, their salts, and related substances). The recast Drinking Water Directive (DWD, EU/2020/2184) limits total PFAS in drinking water to 0.5 µg/L, and 20 individual PFASs to 0.1 µg/L each. Article 21 of the Urban Wastewater Treatment Directive recast (UWWTD, EU/2024/3019) foresees future monitoring obligations of parameters listed in Part B of Annex III of the DWD (including PFASs as “PFAS Total” and/or “Sum of PFAS”) for all WWTPs of agglomerations ≥ 10,000 population equivalents (PE), from which wastewater is discharged into sensitive catchment areas.
The US EPA regulates only five molecules, PFOA, PFOS, PFHxS, PFNA (perfluorononanoic acid), and HFPO-DA (hexafluoropropylene oxide dimer acid), in drinking water as individual contaminants through Maximum Contaminant Levels (MCLs) criteria; mixtures of PFHxS, PFNA, HFPO-DA, and PFBS (perfluorobutane sulfonic acid) are instead regulated according to Hazard Index criteria [12].
The regulations introduced so far resulted in the industrial production shift from LC- to SC-PFASs: Göckener et al. [8] suggested that SC-PFCA levels in water bodies had increased since 2013, following larger industrial use of PFAS precursors based on shorter fluorinated chains. Furthermore, SC-PFASs often result from the partial breakdown of LC-PFASs: two common SC-PFASs, perfluorobutane sulfonic acid (PFBS) and perfluorobutanoic acid (PFBA), have been frequently detected in drinking water, sediments, sewage sludge, and even in polar ice [13]. Despite the toxicity of many PFAS compounds being still undetermined, it was estimated that SC-PFASs can have similar or slightly lower acute toxicity than LC-PFASs and lower bioaccumulation potential [14]. SC-PFASs are highly polar and soluble, poorly retained in separation columns (which makes them challenging to detect), and highly mobile and persistent within the environment [15]. Generally, higher concentrations of SC-PFASs are found at, or near, sites where PFAS-containing products are manufactured or used, and even in WWTP effluents, since they may originate from partial LC-PFAS decomposition [16,17].
Although so far the presence of micro- (MPs) and nanoplastics (NPs) in drinking water has not proven hazardous to human health per se, the adsorption of PFASs onto these particles (especially on the smallest NPs, with size < 1 µm) may be an issue of concern, since NPs can penetrate living organisms’ internal tissue membranes and subsequently potentially release adsorbed substances [18]. To date, the adequacy of treatment technologies has been assessed primarily on the removal of LC-PFASs (i.e., PFOA, PFOS) since these were the earliest detected in drinking waters and regulated.
Due to the strong C-F bond, both LC- and SC-PFASs are highly resistant to conventional degradation processes, requiring innovative approaches. Most conventional water and wastewater treatment methods, including advanced oxidation techniques, struggle to break down these compounds’ perfluorinated tail [19]; for this reason, current technologies focus mainly on their removal rather than destruction. Granular activated carbon (GAC), anion exchange resins (AERs), reverse osmosis (RO), and nanofiltration (NF) are among the suggested best available technologies (BATs) [20]. Even then, removal performances vary: for example, GAC adsorption is less effective on SC-PFASs compared to LC-PFASs due to early breakthrough [21].
Although there are no present regulations specifically targeting SC-PFASs, it is expected that these will emerge in the near future. Consequently, it is also expected that technologies for their efficient destruction will be studied more intensively. In order to permanently eliminate all compounds from contaminated streams without transferring the issue to a different medium (with related disposal problems), complete destruction (mineralization) technologies should be preferred.
This paper discusses the state of the art in PFAS removal and destruction in WTPs/WWTPs, then examines the emerging technologies specifically targeted to PFAS destruction, critically discussing their efficiency and present applicability.
2. Energy Considerations in PFAS Removal
The C-F bond is one of the strongest in chemistry: its bond dissociation energy (BDE) is estimated at >130 kcal/mol (460 kJ/mol), much higher than other C-X (halogens) and C-H bonds. For example, the BDE of the C–X bond in a CH3–X molecule, where X = fluorine, hydrogen, chlorine, bromine, iodine, is 115, 104.9, 83.7, 72.1, 57.6 kcal/mol, respectively [22,23]; the O-H BDE in phenol, another generally difficult-to-degrade molecule, is generally reported as 87–90 kcal/mol [24]. The C-F bond size is also shorter (about 1.35 Å) than any other C-X bond and also of single C-N and C-O bonds [25]. Shorter length generally means stronger bond, as atoms are held together more tightly, with greater molecular stability, and command a higher BDE: this increases when more F atoms are attached to a single C atom, as the individual bond lengths diminish [26].
Generally speaking, technologies that can deliver high energy intensity into solutions, e.g., advanced oxidation/reduction processes (AORPs) could be effective for PFAS breakdown [27,28,29]; however, BDE delivery efficiency cannot be immediately determined. AOPs/AORP studies focus primarily on the generation of pollutant-degrading reactive radicals and species, whose efficiency in breaking down the chemical bonds of target molecules is essential for the overall effectiveness of the process. Reactive radicals’ molecule-degrading effects are influenced by various factors (e.g., the presence of scavenger species, presence of electron-donating or -withdrawing groups near the target bond, experimental conditions, etc.) and can be determined solely through case-specific experiments. Hydroxyl radical (•OH), one of the most powerful oxidants, can be produced by various methods, including the administration of O3, H2O2, of UV with catalysis, or by high energy irradiation. The lifetime of these radicals is very brief (few µs for •OH, ms to s for reactive oxygen, O2−) and thus their action must occur immediately after generation, through rapid contact with the target molecules; on the other hand, their short lifetime makes any possible adverse effect negligible. Computational methods, i.e., density functional theory (DFT) or machine learning (ML), could be used to calculate BDE and reaction pathways, theoretically supporting experimental observations to predict the efficiency of bond dissociation in AOPs/AORPs [23,30].
An alternative method for comparing the efficiency of AOP/AORP technologies is the electric energy per order (EEO) figure-of-merit proposed by Bolton [31]. The EEO represents the electric energy required to decrease by 90% (one order of magnitude) the concentration of a target contaminant in a unit volume of solution. Its computational determination is not immediate, as it requires experimental knowledge on target compounds’ molar absorption coefficients, second-order radical reaction rate constants, and other transformation rates, and depends on the original aqueous matrix composition; however, its observation was proven useful for the comparability of the energy efficiency of different AOPs.
Studies on the energy requirements of PFAS destruction report specific energy requirements of various technologies for PFAS destruction [32]. It can be observed that the reported ranges show a high variability, reflecting the uncertainty and nonuniformity of experimental conditions in the respective studies (Table 1).

Table 1.
Summary of reported specific energy use for PFAS destruction using different technologies.
3. PFAS Removal and Destruction in WWTPs: State of the Art
In conventional WWTPs, the removal of organic contaminants occurs by biodegradation and adsorption onto biological flocs; however, since PFASs are poorly susceptible to biodegradation, WWTPs exhibit poor degradation efficiency towards these compounds, with adsorption appearing to be the predominant removal process. Removal depends on the nature of the contaminant: SC-PFASs tend to partition into the aqueous phase, whereas LC-PFASs partition into the solid one. Typically, therefore, WWTPs may remove LC-PFASs as a result of sludge adsorption, while forming recalcitrant SC-PFASs as a result of the former’s possible partial decomposition within biological units [33,34]. Although this may be recorded as removal of (the few) commonly monitored/regulated species, overall higher levels of PFASs have often been observed in WWTP effluents, especially referring to SC-PFASs [35,36], even if the concentration of LC-PFASs (C > 9) and precursors was observed to decrease [37].
Few studies have looked un-depth at PFAS levels in the various units of WWTPs [38]: as a general rule, biological processes operated at longer HRTs and high (meso/thermophylic) temperature are conductive to PFAA formation; operation at higher MLSS (≥5000 mg/L) and longer SRT (≥45 days) improve LC-PFAS adsorptive removal from the aqueous phase. Selected adjustment of the biological processes’ operational parameters (e.g., SRT, temperature, pH) can enhance PFAS partitioning into sludge [39]; while this reduces their effluent concentration, it increases their accumulation in biosolids, requiring further processing of that phase [40]. The fate of PFASs after their partitioning into sludge is relatively unexplored; the load shift from an aqueous to solid phase, often misinterpreted as removal, may induce several adverse environmental effects, requiring special sludge disposal techniques, along with limited biosolid reuse [41].
In cleaner streams (e.g., drinking water or treated effluents), common technologies for PFAS removal include mainly GAC adsorption, high-pressure membrane filtration (nanofiltration—NF, and reverse osmosis—RO), and ion exchange resins.
3.1. Adsorption for PFAS Removal
GAC works via surface adsorption of solute molecules onto the medium’s granules due to its high specific surface area of ≈1000 m2/g, effectively removing PFASs from water; the process is influenced by factors like solution chemistry, type of PFAS, and by the presence of other contaminants competing for adsorption sites [42].
GAC is most effective for LC-PFASs, less so for SC-PFASs [43]; its adsorption capacity is surpassed by up to two orders of magnitude by super-fine powder activated carbon (PAC) for LC- and SC-PFASs alike, although with lower efficiency against the latter [44].
Ion exchange is an efficient technology for the removal of anionic LC-PFASs at low concentrations (ng/L), with adsorption capacity higher than GAC and faster adsorption kinetics; however, it is less efficient for solutions containing also organic and inorganic matter. The resin adsorption capacity of LC-PFASs is strongly dependent on the sorbent’s functional group: the more hydrophobic, the higher the resin’s removal efficiency. Two different mechanisms were observed: the adsorption of LC-PFASs is influenced by the formation of micelles/hemimicelles on the positively charged sorbent surface; SC-PFASs are primarily immobilized via electrostatic interactions [45]. The adsorbent medium is typically used once, after which it needs to be disposed of (usually through thermal destruction), or regenerated, producing a concentrated PFAS stream that needs careful subsequent handling [46].
Various studies employed polymeric materials as sorbents, achieving near complete removal of both LC- and SC-PFASs from groundwater [47]. Molecularly imprinted polymer (MIP) beads used as bio-sorbents showed good (up to 83%) removal efficiency of LC-PFASs, but much lower (14–52%) adsorption of SC-PFASs, indicating that compounds’ uptake is strongly correlated to the hydrophobicity of their carbon chain length [48,49].
Covalent organic framework (COF) adsorption has emerged as a very effective technology for PFAS removal from various matrices, showing high affinity for both SC- and LC-PFASs, with up to 90% and 98% efficiency, respectively. COFs are porous materials with a regular porous structure, strong stability, and possibility of varied structural configurations, with outstanding selectivity for target solutes, fast mass transfer, and better adsorption capacity than most common adsorbents. Functionalized COFs (ionic and fluorine-functionalized) are used for PFAS adsorption with observed efficiencies >90% for PFOS and >99% for PFOA [50].
Natural soil and biological sludge (e.g., activated sludge) have been studied in relationship to their PFAS sorbent properties. Sorption is driven by hydrophobic interactions, responsible for PFAS adsorption to the mineral surface, and by weak electrostatic interactions and hydrogen bonding. Studies investigating the PFAS adsorption performance of activated sludge observed increased sorption capacity with the increase in the C–F chain length: up to 90% for LC-PFASs (perfluoroundecanoic acid, PFUnDA) but only 33% for PFOA [51].
So-called “green” sorption media, made from natural recycled materials (mainly sand, with small amounts of clay, iron, and aluminum), have been tested for PFOS and PFOA removal, with limited efficiency (up to 46% for PFOS) [52]. Many other engineered materials have been experimented with as sorbents, including mesoporous cetyltrimethylammonium bromide (CTAB)-functionalized microspheres (mesoporous Fe3O4@SiO2@CTAB–SiO2) capable of adsorbing PFAS at ng/L levels; and poly(ethylenimine)-functionalized cellulose microcrystals (PEI-f-CMCs) with high adsorption capacity for LC-PFASs (98.8% for PFOS, 87.6% for PFOA) but disappointing performance on SC-PFASs (˂2% for PFBA and PFPeA, 7% for PFBS, 12.9% for PFHxA, 26.2%, PFPeS, 38.9% for PFHpA) [53].
Despite the large number of sorbents available and studied, there is still a clear knowledge gap on the removal of SC-PFASs: many studies in fact focus exclusively on LC-PFASs (usually PFOS and PFOA). Work is needed to explore SC-PFAS adsorption mechanisms to develop sorbents targeted for these substances, which, being of an hydrophylic nature, do not respond well to hydrophobic interactions.
Should better adsorbents be identified, however, adsorption represents a mere phase shift with several drawbacks, including the need for adsorbent regeneration/disposal, leading to costly, frequent adsorbent replacement, and potential for secondary waste generation, both of which will transfer issues downstream. Furthermore, PFASs can be released from adsorbent media during regeneration processes or if adsorbents are subject to particular conditions [54,55]. Regeneration can involve high-temperature heating (>1000 °C) to destroy or desorb PFASs, or solvent-based regeneration [54].
3.2. PFAS Filtration
Membrane processes are commonly adopted in multiple barrier treatment (MBT) schemes for wastewater and water treatment [56]. RO/NF filtration technology is effective for both SC- and LC-PFAS removal, often exceeding 90% efficiency; after filtration, PFASs are concentrated in the reject (retentate) stream. Earlier membranes were made of organic polymeric materials, prone to PFAS adsorption over time. Since membrane media is subject to progressive irreversible fouling, their substitution and disposal after their useful lifespan may require special procedures in the case of relevant accumulation of adsorbed contaminants [46].
NF and RO performance can be enhanced by engineered perm-selective materials with media surface modifications that combine the benefits of high PFAS rejection, reduced adsorption, and fouling with high water permeability. For example, increasing the density of carboxyl groups in a polyamide membrane can enhance the sorption of negatively charged PFAS compounds [57]. Membrane fabrication technology has recently evolved into metal oxide-based ceramic materials that exhibit excellent performance in mechanical stability and duration and increased fouling resistance and can be produced with geometric structures suitable to achieve extended ranges of PFAS mass transfer [58]. These were so far targeted to passive sampling applications but may find future scope in PFAS concentration strategies. Recent progress in nano-engineered composite membranes showed that these have superior capacity than conventional membranes in terms of permeability, reactivity, efficiency, and antifouling properties and better PFAS-removing efficiency through a combination of adsorption, degradation, and filtration mechanisms [59].
3.3. PFAS Destructive Methods
PFAS destruction is preferable to their “removal”, since it does not entail the formation of hazardous process residuals, such as, e.g., PFAS-saturated adsorbents, reject streams, or partial molecular decomposition into by-products of uncertain toxicity. For the purpose herein addressed, destruction is intended as the complete defluorination of the perfluoralkyl chain of the PFAS molecules, associated with the release of fluorine atoms in the form of fluoride (or HF) and of C as CO2 or acetate (CH3COO). This is also referred to as “mineralization”, regardless of the final carbon state.
3.3.1. Advanced Oxidation Processes
AOPs have been at the forefront of the technologies aimed at the destruction of recalcitrant chemicals. In this respect, however, PFASs present unique challenges that overcome the potential of conventional AOPs: their molecular decomposition in fact occurs through the combination of reductive and oxidative reactions, which include stepwise defluorination and the ultimate conversion of C-bonded F atoms to hydrogen fluoride (HF) or fluoride ions, subsequently stabilized as CaF2 or NaF. PFAS-reductive reactions are facilitated by aqueous (or hydrated) electrons (eaq−), essentially free electrons enveloped by water molecules, which react with electron-deficient sites (i.e., fluorinated C atoms) by tackling the C–F bond and forming intermediate radical ions (•CF) that ultimately release fluoride ions (F−) upon bond cleavage. It was shown that eaq− also target C=O bonds with reductive decomposition followed by oxidation to complete mineralization [60]. Processes generating both powerful reductive and oxidating species (advanced oxidation/reduction processes, AORPs) would therefore be ideal candidates for the destructive removal of these compounds [61].
A limit to the efficiency of PFAS degradation by AORP methods is that these contaminants’ concentrations in water/wastewater are usually in the order of ng to mg per liter. This makes technologies more expensive to implement (due to large volumes processed), demand higher specific energy to achieve the desired destruction (due to both the higher likely presence of scavenger compounds and the lower probability of radical/pollutant contact), and to technology-inherent limitations (due, e.g., to limited water column penetration of generated radicals, as in the case of irradiation processes). In terms of compound destruction, AORP technologies can be more cost effective for processing concentrated streams, but they are significantly less so (in terms of sheer observed compound removal) than, i.e., GAC, when treating high flows at very low concentrations.
It should be remembered that incomplete mineralization/sequential defluorination may lead to the formation of intermediate by-products, often unidentifiable and of currently unknown effects, but likely to prove recalcitrant to further processing, as a result of presence of residual C-F bonds. PFAS destructive reaction kinetics, furthermore, may be orders of magnitude lower than that of other organic matter in waste streams or of geochemical constituents in natural waters. These can thus act as potential scavengers of reactive radicals, lowering process efficiency and creating other by-products unrelated to PFASs [62].
3.3.2. Thermal Decomposition/Destruction
Other mechanisms tested for PFAS destruction are thermal oxidation (combustion) or pyrolysis [63]. Thermal destruction of PFOA and PFOS in water solutions has been attempted at the laboratory scale, for example, incineration at 300–350 °C [64] and 600–900 °C [65]; however, these temperatures were shown to be insufficient to fully defluorinate (mineralize) PFASs, which requires operating above water’s critical point, at >350 °C and ≈166 bar [66]. Few studies on full-scale PFAS incineration are available and often state that complete PFAS destruction requires temperatures >1000 °C [67]. Incomplete incineration may lead to by-products (e.g., perfluoroisobutylene, fluorocarbons, fluoroalkanes) with long atmospheric half-lives, or even to uncombusted PFAS release in flue gases [68]. The thermal destruction of SC-PFAAs requires higher temperatures than LC-PFAAs [69], leading to possible secondary recombination into LC-PFAA by-products.
Incineration tests of PFOA and PFOcDA (perfluorooctadecanoic acid) highlighted the correlation between their destruction level and process temperature: under combustion at 850 °C, at 2 s residence time in pure air atmosphere, a destruction efficiency of 99.999% was observed, not significantly different from results at 950 °C and 1000 °C. However, in tests at ≤700 °C, the destruction efficiency decreased significantly, with by-product generation increasing considerably, suggesting carbon chain reduction, recombination, and ether bonding as possible pathways. Both by-product species subject to regulation, and not, were detected at lower temperatures; therefore, incineration at ≥850 °C appears to be a minimum requirement for PFAS thermal treatment [70].
Even where complete mineralization is attained, however, it could result in highly corrosive HF formation, requiring careful flue gas management. Incineration is an established technology, but highly energy-intensive, especially where wastes with high water content are processed [71], and is generally carried out at large-scale facilities. Recently, a technical brief from the US-EPA on PFAS incineration concluded that “the effectiveness of incineration in their [i.e., PFAS] destruction and in terms of the potential mixed byproduct formation is still not clearly understood” [72]. This contributed to significant concern of incomplete PFAS destruction with the formation of partial combustion products, e.g., smaller PFAS-type molecules, which could also constitute a potential hazard; as a consequence, in 2022, the US Department of Defense (DoD) banned the incineration of wastes containing Aqueous Film Forming Foam (AFFF) (a PFAS-based fire suppressant), which, up to that moment, was deemed the only admissible management practice for these products’ disposal [73].
Pyrolysis, on the other hand, is a non-combustion thermal method used to process biosolids in an oxygen-free environment at elevated temperature (typically 500–800 °C). Pyrolysis is a scalable technology for biosolid processing; pyrolysis units are generally more compact than biosolid incinerators and require lower specific air flows, resulting in a lower footprint and capital expense for exhaust control equipment; the process produces biochar, py-oil and py-gas as final products that can be of reuse value [74]. Still, significant unknowns about PFASs and their by-products remain concerning those pyrolysis products and emissions. While biochar generally shows reduced levels of PFASs and other contaminants compared to the original feedstock, the potential for the PFAS air emission process is still uncertain [75].
In pyrolysis, C-C bond cleavage (BDE = 418 kJ/mol) occurs before C-F cleavage (BDE = 502 kJ/mol), resulting in complex mixtures of volatile compounds, including tetrafluoroethylene, difluorocarbene, trifluoromethyl radicals, 1H-perfluoroalkanes, and perfluorooleffins [76]. These may be released in exhausts, adsorbed to particulate matter, or remain embedded in biochar and condensates; SC-PFASs may volatilize due to rapid thermal desorption [77] and form long-chain precursors due to high-temperature reactions of sulfonate PFAS with excess ammonia and other amines [76].
A study investigating PFAS removal in a commercial biosolid processing pyrolysis facility targeted 41 PFAS compounds in feedstock and biochar, showing concentrations between 2 and 85 μg/kg of 21 target compounds in the feed, and no such PFAS detection in the produced biochar. Pyrolysis’ removal efficiencies of targeted PFASs were estimated between 81.3 and >99.9% (mean ≥ 97.4%) [77]; however, non-targeted PFAS compounds nor products of incomplete combustion were monitored in the study. Further investigation on these issues, especially concerning the incomplete mineralization products in biochar and flue gases, are needed to ascertain the effectiveness of pyrolysis technology towards PFAS final destruction.
4. Emerging Technologies for PFAS Destruction
As previously discussed, an ideal PFAS destructive removal method should avoid the creation of undesirable chemical by-products of uncertain toxicity and properties; furthermore, processing technologies should be able to deliver sufficient energy to overcome the high BDE of these compounds. Energy can be delivered to a solution, for example, by electromagnetic or sonic waves, and can be supplemented by the addition of appropriate reagents.
Table 2 shows the specific energy levels deliverable by various types of electromagnetic waves, from microwave to ionizing radiation [78]. Typically, high energy irradiation technology, such as electron beam, can deliver energy to individual electrons of up to a few thousand or million electron volts (eV; amount of kinetic energy gained by a single electron accelerating through an electric potential difference of 1 V, equal to ≈1.6 × 10−19 J). Non-thermal plasma can deliver specific energy up to 30 eV.

Table 2.
Specific energy levels of various electromagnetic waves (modified from [78]).
4.1. Emerging Advanced Oxidation and Oxidation/Reduction Processes
AOPs have been shown to be largely inefficient for the achievement of PFAS complete mineralization [79]. On the other hand, advanced reductive processes (ARPs) can achieve PFAS degradation due to the generation of eaq−, highly reactive radicals with redox potential of −2.9 eV. It was postulated that the small size of the eaq− radical could be the reason for its penetration and cleavage of the C—F bond, without being repelled by the fluorine atoms. Different AORP methods (electron beam, γ-rays, photolysis, UV radiation, plasma) [27] can split water molecules, generating both oxidizing and reducing species (e.g., hydroxyls, single hydrogen, reactive oxygen, hydroperoxyls, superoxides, solvated electrons) capable of inducing sequential defluorination of PFAS molecules.
AORPs can be energy-intensive and may demand long reaction times for PFAS mineralization; as AOPs, they may be susceptible to efficiency reduction due to the presence of geochemical constituents in the water matrix, inducing non-targeted reactions. Common by-products generated following ARPs’ incomplete PFAS mineralization may include short-chain PFAAs: Bentel et al. [80] explained that a lower BDE resulted in the faster defluorination achieved by ARPs along LC-PFAA perfluoroalkyl chains, compared to SC-PFAAs. These observations were confirmed by other studies on ARP rate kinetics that highlighted the longer mineralization times of shorter fluorinated alkyl chains [81].
Chemically based ARPs applied to PFAS-polluted water matrices use reagents, such as sulfite (SO32−), dithionite (S2O42−), sulfide (S2−), and ferrous iron (Fe2+), in conjunction with an activation energy source, to generate highly reactive reducing radicals. The addition of the chemicals, however, influences the final total dissolved solid (TDS) content of the treated solution, which increases following the production of inert salts. Typical reported doses of sulfite or iodide in chemical ARP treatment for the generation of eaq− range from few tenths to several mM, leading to TDS increases by hundreds or even thousands mg/L. Further TDS increases can be expected when acid and/or alkaline reagents are needed for pH adjustment; it follows that the direct application of these ARPs is not recommended for the treatment of drinking water or municipal wastewater targeted for reuse, as they typically have strict requirements on maximum salinity. They could be appropriate, however, for the treatment of RO’s PFAS-rich concentrates [82].
4.1.1. Electron Beam Technology
Electron beam (E-beam) is a high energy, reagent-less technique that utilizes ionizing radiation to breakdown recalcitrant chemicals. Ionizing radiation, such as γ-rays, is typically sourced from decaying radioactive isotopes (usually 60Co, or 137Ce) continuously produced by nuclear processes, which makes it expensive and inherently dangerous to operate, not to mention the transportation and storage issues of the isotopic materials. In contrast, E-beam consists of “on/off” electricity-generated beta radiation that uses a commercially available, mature technology used at industrial scales for many applications.
An E-beam device (electron beam accelerator) is schematized in Figure 2: a cathode in a sealed container under high vacuum releases electrons that are accelerated under high DC voltage or radiofrequency and are deflected to an exit window by electrostatic or magnetic fields. E-beam machines accelerate electrons to near the speed of light, achieving electron energies up to 100’s MeV. The electrons’ energy depends on the applied anode voltage and their quantity depends on the cathodic current intensity. Adjusting current and voltage allows for controlling beam penetration and the target-absorbed dose rate.
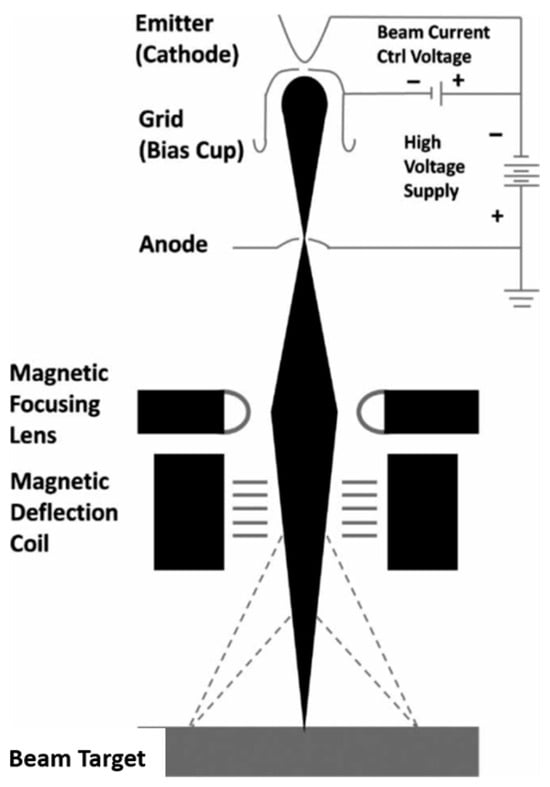
Figure 2.
Principle of E-beam devices.
Unlike other destructive techniques, E-beam generates both oxidizing and reducing radicals from H2O molecules splitting, due to the mere effect of irradiation: superoxides, O2•, •HO, hydrogen atoms, and solvated electrons carry out the degradation of pollutants in the solution. All these species are extremely reactive and very short-lived, with half-lives in the order of 10 μs at 10−4 M concentrations, leaving no residuals after reacting with solutes or recombining into water molecules [83]. E-beam AORP technology can be used to simultaneously destroy many other recalcitrant compounds in a solution, such as 1,4-dioxane, chloroform, bromoform, sulfamethoxazole, etc. [84].
A limited number of studies have applied E-beam technology to PFAS degradation [84,85]. A recent study individually irradiated PFCAs (PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA), PFSAs (PFBS, PFHxS, PFOS), and one fluorotelomer sulfonate (6:2 FTS): the results showed that E-beam is effective in treating PFASs with ≥90% removal of PFOA and PFOS from initial concentrations of 100 to 500 ppb, at doses of 250 kGy and 500 kGy (unit of absorbed radiation dose), respectively, under optimized conditions (pH = 2, D.O. = 2 mg/L). No SC-PFASs were detected after E-beam PFOS treatment; however, short-chain perfluoroalkyl carboxylates were detected after PFOA irradiation. E-beam’s degradation efficiency did not significantly change when treating PFASs with fluorinated carbon chain length 5–7, with removal ranging from 85 to 99% at 250 kGy. SC-PFASs (PFBA and PFBS) also showed 70–99% degradation; however, this required a higher irradiation dose of 1000 kGy [85].
The competition between different PFAS analytes for reactions involving eaq− and the variability of reaction kinetics with perfluorinated chain length, degree of fluorination, functional groups, and presence of highly reactive intermediates were highlighted by a study in which an equimolar solution of ten different PFASs (0.05 μM each) was treated at varying irradiation doses (125–1000 kGy). The results showed ≈ 30% degradation of total PFASs at 250 kGy, beyond which no further reduction was observed. Moreover, 6:2 FTS was degraded > 90% at 250 kGy; C8 compounds, PFOS and PFNA, showed degradation of ~50% and ~60% at 1000 kGy, respectively. PFOA (C7) and PFHxS (C6) featured ~30% degradation at 250 kGy, but no further degradation beyond that dose [86].
The calculated EEO for various PFAS degradation in different E-beam applications ranged from 48 to 1081 kWh/m3-order, with 6:2 FTS showing the lowest value and PFBS the highest [86]. These values are comparable and often much lower than those of other destructive techniques such as activated persulfate and ultrasound, as well as those of electrochemical oxidation, suggesting that E-beam could be an energy-sustainable technology for PFAS decomposition. E-beam efficiency in PFAS mineralization is affected by their concentration and matrix components, which can increase the scavenging of generated radicals.
E-beam could be used as the final step in a concentrate-and-destroy approach to PFAS waste management: the combination of a concentration step (e.g., RO, FF) followed by irradiation would enable large-scale applications of E-beam treatment of PFASs. As of today, most experimental work on PFAS destruction with E-beam was carried out with accelerators delivering a few kW of power; commercial accelerators, used for different industrial applications, can deliver 100’s kW of power, thus increasing achievable irradiation dose (quantity) and rate (time) manifolds and overcoming some of the limitations so far observed. Other irradiation methods (e.g., gamma rays) tested on PFASs and based on the same principles of E-beam are less efficient due to considerations of achievable dose rate delivery and required process times [87]. Gamma irradiation in fact delivers dose rates in the order of kGy/h, whereas E-beam delivers kGy/s, despite having limited penetration in water, which requires specific delivery modes [88].
4.1.2. Plasma Technology
Plasma is a partially or fully ionized state of matter formed of a mixture of electrons, free radicals, and ions, which constitutes an ideal reactive environment for pollutant molecule decomposition. Non-thermal plasma (NTP) technology operates at low temperatures using high voltage (5–20 kV) to generate electrical pulse discharges to electrodes exposed to an aqueous solution: the induced energy splits water molecules, generating oxidizing and reducing radicals [89]. Field trials of NTP systems for PFAS remediation in groundwater have shown destruction efficiencies of LC-PFASs ≥ 90% in single pass flow applications [90]. The limited penetration of plasma discharges in bulk water (a feature common to E-beam) requires its combination with gas bubbling or FF to transfer PFAS molecules to the surface where they are able to come in contact with the generated radicals.
NTP is a promising PFAA destruction technology at the laboratory level, with more significant results and shorter treatment times than other degradation technologies [91]. The presence of co-contaminants does not significantly impact its PFAS destruction effectiveness since the primary reactivity occurs at the plasma–liquid interface. Destruction of LC-PFAAs > 99% within 120 min and SC-PFAAs > 99% after 6 h of treatment were observed with EEOs for the mineralization of LC-PFAAs, SC-PFAAs, and their precursors, between 1560 and 2370 kWh/m3-order [92]. These values are higher than some of those reported for E-beam treatment [87], but still lower than other technologies.
Thermal plasma (TP) is one of the highest energy-intensive methods for toxic waste destruction, operating at very high temperatures (1700–2200 °C on average, reaching up to 11,000 °C at the reactor’s core). The plasma is generated by a gas (argon, steam, helium, or air) passing through an electric arc, where it absorbs energy to form a high-temperature plume; pollutants are mixed into the gas as aerosol mist or injected directly into the plume. PFAS molecules exposed to TP undergo thermal dissociation (mineralization) into thermodynamically stable end-products (CO, CO2, SOx, HF), which are then removed by scrubbing, converting HF into CaF2, SOx into CaSO4, and CO and CO2 to CaCO3 [32]. Thermal steam plasma (TSP) generates plasma using steam, forming an excess of hydrogen ions, allowing the formation of HF as the most favorable fluorine-carrying product. Neither of these techniques has proven to be commercially viable for widespread application for wastewater solids or solutions, so far.
4.1.3. Electrochemical Advanced Oxidation Processes (EAOPs)
Electrochemical oxidation methods remove organic pollutants by applying an electrical current between an anode and a cathode placed across a conductive solution: contaminants are either adsorbed and degraded at the electrodes’ surfaces or in the liquid medium [93]. Contrary to direct electrolysis, operated at low potentials, EAOP is operated at high anodic overpotential, generating strong oxidizing radicals to decompose persistent pollutants by indirect electrolysis. EAOPs proved effective for the treatment of LC-PFASs and precursors, with no need for the addition of reactants [94,95]. The needed potential (exceeding 3.5 V) for PFAS breakdown can however trigger undesired side reactions, with the formation of chlorate and perchlorate. Electrochemical reduction at the cathode by eaq− could limit these unwanted by-products but may promote excessive hydrogen evolution [96].
EAOP treatment of LC-PFASs showed removals > 99%; on the other hand, SC-PFASs proved generally more challenging to degrade, sometimes experiencing increases in total concentration after treatment due to precursor conversion [97].
4.1.4. UV-Based Processes
Photocatalysis is classified as an AOP: UV, or visible light (e.g., from Xenon or LED sources), activates suitable catalysts to drive chemical reactions by the molecular adsorption of photons, accelerating reaction rates [98,99]. Photocatalysis can be operated at ambient temperature generally with less energy input than thermal technologies; however, not all catalysts are ideally suited for PFAS treatment. For example, TiO2, generally considered an ideal catalyst due to high reactivity and chemical stability, is poorly effective for PFOS treatment. TiO2 is also challenging to recover from treated solutions, requiring extra processing steps [100]. Photocatalysis is selective with respect to target molecules: it was shown to be minimally effective on PFOS but more effective on PFOA [101]. In general, photocatalysis’ degradation efficiency is relatively low compared with other PFAS mineralization techniques, presenting a higher likelihood of toxic intermediate generation. PFAS photocatalytic degradation studies are still at the laboratory scale, mostly carried out with deionized (scavenger-free) water, thus lacking representative field applications [98,100,102].
Continued exposure of PFASs to radiation ≤ 320 nm can result in photodissociation through catalyst-free photodegradation: in direct PFAS photolysis, photons at certain wavelengths are directly absorbed by PFAS molecules. The degradation of PFOA was observed within 2 h of 185 nm vacuum ultraviolet (VUV) light irradiation from an initial concentration of 25 ppm, but incomplete degradation products, including perfluoroheptanoic acid, perfluorohexanoic acid, perfluoropentanoic acid, and perfluorobutanoic acid, were found in the treated solution, due to the successive losses of CF2 moieties at the selected irradiation wavelength [103].
The direct photolysis of 19 representative PFASs under far-UVC (222 nm) showed >81% decay of parent compounds and 31% defluorination after 4 h of exposure, an improvement compared to the results normally observed under conventional 254 nm UV irradiation [104]. It was shown that photolysis efficiency is boosted by higher temperature [105] and pH [106] and reduced by the presence of organic matter in the treated solution [107].
The sole use of UV radiation, however, is generally ineffective against PFASs, since these molecules lack chromophores capable of absorbing some of the wavelengths in the UV range. To photochemically degrade PFASs efficiently, a reduction mechanism through the generation of eaq− should be induced: these can be achieved by the addition of sulfite (SO32−) or iodide (I−) in solution: vacuum-UV irradiation then induces the photoionization of sulfite and water molecules, yielding eaq− [108]. Highly energy efficient, this technology can achieve partial to full decomposition (64–99%) of LC-PFAS molecules but may need additional steps for the decomposition of SC-PFASs. Furthermore, it requires highly alkaline conditions (pH ≈ 12), necessitating heavy pre- and post-treatment steps that increase chemical demand and process cost.
4.2. Other High Energy Destruction Technologies
Other high energy activation methods, such as sonolysis and supercritical water oxidation, can trigger PFAS destruction reactions.
4.2.1. Sonolysis
High-power ultrasounds, with frequencies between 20 kHz and a few hundred kHz, can induce very high energy input into solutions: a power density ≥ 0.67 W/cm2 is already sufficient to induce water cavitation; in pollutant destruction applications, densities > 10 W/cm2, with some reaching up to 1000 W/cm2, are used [109]. Ultrasonication offers great potential in processing liquids and slurries, improving mixing, increasing chemical reactions rates, and even causing the disintegration of biological cells. Ultrasound waves do not interact directly with the pollutants in solution, as their typical wavelengths (in the mm range) are too long compared to the molecules’ size; instead, the energy carried by the sonic wave causes the formation of small vacuum bubbles followed by violent collapse, generating extremes of temperature and pressure in their proximity. Such high intensity phenomena can induce chemical modifications, either by thermal or by direct mechanical action, and can inactivate harmful microorganisms.
Because PFASs are surface-active, they tend to accumulate at the gas–water interfaces of the cavitation bubbles; the latter’s subsequent collapse is violent, generating localized temperatures in excess of 5000 °C, physically tearing up or ionizing nearby molecules [109]. The exact mechanisms of sonochemical PFAS destruction have not been fully explained or agreed upon by researchers; however, the high temperatures achieved, well above those needed for PFAS incineration, suggest that thermal destruction phenomena could be a significant pathway, subjecting molecules onto or near the bubble surfaces to a pyrolysis-like process during implosion [110]. Sonochemical treatment can defluorinate PFAS molecules completely and without pre-treatment or the addition of chemicals, without specific temperature or pressure adjustments to the reactor. Correctly tuned sonolysis is effective for PFAS mineralization and is extremely easy to operate, but has high energy demand and reaction times. Applications so far have been described mostly at the bench scale: >99% mineralization was reported in a 2 L reactor after 2 h at 700–1040 kHz frequency, [111]; the largest reported application consisted of a 91 L reactor with multiple transducers at 500 kHz–1 MHz frequencies working simultaneously, showing significant PFOS degradation after 4 h treatment [112]. Neither of the cited studies estimated the energy input required for mineralization; however, long process times and generally high energy demand would make this type of treatment potentially best suited for the treatment of small waste streams, e.g., concentrates from separation processes.
4.2.2. Supercritical Water Oxidation (SCWO)
SCWO is an oxidative treatment applicable to a wide range of wet wastes, without the need for preliminary dewatering. The process occurs at high temperature and pressure, above the water critical point (374 °C and 218 atm). In its supercritical state, water is neither a liquid nor a gas; under these conditions, fluid molecules behave differently, organic solubility increases manifolds, oxidation is enhanced, and mass transfer is unrestricted, promoting chemical reactions that would not occur under standard conditions [113]. The technology was proven effective for the total destruction of toxic, persistent contaminants [114].
SCWO is a completely destructive treatment that degrades compounds in solution to their individual elements in the absence of reaction by-products, incomplete oxidization, or unreacted compounds. Compared to other technologies, SCWO is relatively fast, allowing smaller reactor sizes; however, the cost of heating the solution to the high process temperature and the technical challenge of operating at high pressure limit the practical scaling up possibilities of this technology. Furthermore, the process produces acidic species (H2SO4, HCl) that could cause reactor corrosion, requiring special high-grade materials for their construction [113]. In field tests, SCWO showed PFAS destruction > 99% at 590 °C and 234 atm, with considerable pH decrease (from 12.75 to 3.26) during the tests, despite the use of alkaline buffers [113]. Other laboratory studies showed the consistent destruction of influent total PFAS concentrations of up to 50 ppm to less than 70 ppt, at operating conditions of ≥600 °C and 238 atm, and at flowrates of up to 11.4 L/h [115].
4.3. Role of Catalysts in PFAS Degradation
An approximate range of specific energy demand for some of the mentioned treatments was given as 0.01–0.5 kWh/L [84]; however, the final requirements depend on the actual operating process conditions. Processing times associated with PFAS destruction in the literature range approximately from 30 min to 8 h, a broad interval depending on the specific destructive mechanism, influent concentration, chemical composition of the solution, and achieved degree of defluorination. The high energy intensity and large reactor volumes due to long treatment times confirm that optimal conditions for PFAS destruction involve high concentration/small volume applications.
Catalysts, both homogenous and heterogeneous, can improve reactive radical species generation in the treated solution by activating their chemical precursors (e.g., O3. H2O2, Na2CO3∙1.5H2O2, S2O82−, NH2Cl), thus reducing both process time and energy input requirements. Catalysts are applied in various destructive processes: in photocatalysis for example, electron–hole (e−/h+) pairs generated along incoming light absorption recombine very quickly, often before having the opportunity to induce any chemical reaction; catalysts provide electron sinks and/or stabilization of radical anions to foster further reactivity [116]. In addition to pure titanium oxide (TiO2), which is activated only by the UV spectrum, several TiO2-based nanomaterials showed potential for reaction enhancement: graphene oxide (GO) and transition and noble metallic nanoparticles have been used to modify TiO2, improving its photocatalytic activity [117]. Comparison studies of transition metal nanoparticle-modified TiO2 (Fe-TiO2 and Cu-TiO2) showed that under UV irradiation, Cu-TiO2 displayed better activity, resulting in higher photocatalytic rate constants, than pure TiO2 and Fe-TiO2 [118]. Other nanomaterials tested in PFAS photocatalysis include gallium oxide (Ga2O3), indium oxide (In2O3), nanoscale Zero Valent Iron (nZVI), bismuth oxychloride (BiOCl) or fluoride (BiOF) nanocomposites, and modified SiC nanophotocatalysts [119].
Catalysts can be implemented in electrochemical oxidation in the form of electrode modification (e.g., boron-doped diamond—BDD—electrodes) that have achieved the decomposition of both long- (C7–C18) and short-chain (C3–C6) PFASs [120]. Materials based on SnO2, PbO2, TiO2, and RuO2 showed high electron transfer ability and oxygen evolution in the mineralization of PFCAs and PFSAs [121]. Ce-doped porous nanocrystalline PbO2 film electrodes were also developed for the electrochemical degradation of PFOA, PFHpA, PFHxA, PFPeA, and PFBA in water, since CeO2 as a catalyst additive shows high electrical conductivity, diffusivity, and catalytic activity [121].
nZVI was exploited to remove and destroy PFASs by oxidative degradation in the presence of H2O2 thanks to its ability to behave as a heterogeneous Fenton catalyst, producing reactive oxygen species (ROS) by activating H2O2 without external energy input [122]. A study by Arvaniti et al. [123] employed magnesium-amino-clay-coated nZVI (Mg-amc@nZVI) for removing PFOA, PFNA, PFDA, and PFOS from solution, showing removals between 38 and 96% at pH = 3.
To date, there has been little attention paid to catalysts being potentially applicable to irradiation treatment (e.g., E-beam): unlike photocatalytic processes that activate catalysts with energy band gaps < 6.2 eV, E-beam could activate higher energy band gaps up to 1.24 MeV, with metal oxide catalysts, enhancing the process efficiency by lowering the activation energy of degradative reactions and increasing the generation rate of reactive species. The development of suitable catalysts for radiolysis could improve this technology’s efficiency and sustainability to achieve complete PFAS destruction [124].
4.4. Other Degradation Methods
Some bacteria (mostly of the Pseudomonas sp.) are able to bioaccumulate PFASs in aerobic and, to a lesser degree, anaerobic conditions. While there are no confirmed reports of the biological removal of fluorine atoms from PFASs, instances of bacterial defluorination of monofluorinated compounds were reported [125]. Bacteria isolated from PFAS-contaminated environments showed the ability to remove 32 and 28% of PFAS compounds, respectively, within 10 days of incubation under alkanotrophic conditions [126]. Mechanisms of PFAS biodegradation are still not well understood, and conflicting reports regarding the biodegradability of specific PFAS compounds exist. The biodegradation of PFASs appears to involve enzymes that directly remove fluorine atoms from these molecules, either by adding oxygen across the F-C bond or by adding electrons across it, allowing other assimilation enzymes to break down the rest of the compound [127].
On the other hand, fluorinated precursors can be transformed into PFASs by a range of biological systems [128]; although the pathways for these biotransformations are still unclear, these phenomena have the potential of increasing the treated solution toxicity. While some PFASs can be partially degraded in solution by biological processes, complete mineralization is rare and challenging to obtain. It is currently understood and agreed upon that there are no readily accepted and practical biological mineralization pathways for all PFASs; even when such degradation occurred, it would happen very slowly, making it difficult to achieve significant removal in reasonable time frames. Bioremediation approaches for PFASs may ultimately not be successful due to process kinetics being very slow, and thus poorly suited to liquid wastes.
Bio-inspired systems using renewable plant-based, biomimetic nano-engineered materials are being studied to adsorb PFASs and subsequently biodegrade them into less harmful compounds [129]. These systems use low-cost feedstock (lignin and cellulose) that can efficiently adsorb PFASs, providing an ultimately self-degrading substrate for fungal and bacterial growth and facilitating their decomposition by redox processes, without the production of secondary contaminants.
4.5. PFAS Concentration for Improvement of Destruction Efficiency
In many degradation processes, the efficiency is generally proportional to the initial contaminant concentration. This is the case, for example, for common biological processes, up to the exceedance of inhibition limits [130]. At low concentrations, in fact, degradation is typically limited by the availability of either the contaminant (e.g., organic matter in biological processes) or the reactive species (e.g., reactive radicals in AOPs/AORPs). With increasing contaminant concentrations, degradation rates increase due to their greater availability (i.e., increased probability of contact) to the reactants’ molecules. At very high pollutant concentrations, however, processes can be limited by other factors, such as mass transfer rates or the ability to remove the degradation by-products.
Concentrated solutions therefore allow for applying more targeted and effective PFAS destructive technologies, as this increases short-lived reactive species’ action efficiency and reduces the need for their over-generation, which can be expensive and energy-intensive. Membrane treatment, for example, does not destroy PFASs, but generates a concentrated reject stream that can be more efficiently processed by advanced destructive methods [131]. PFAS rejection by NF or RO can range around 85–99% for LC-PFASs, and 20–70% for SC compounds, depending on operating pressure (transmembrane pressure, TMP) [132]. Studies of novel NF membranes revolve around the manipulation of the active separation layer to improve specific PFAS separation performance, e.g., by interfacial polymerization of membrane substrate. Composite membranes prepared by the polymerization of trimesoyl chloride and a piperazine and bipiperidine mixture showed retention of PFOA of ∼90% [133]; the inclusion of poly(piperazineamide) ultrathin film polymerized on top of polysulfone membrane substrate for PFBS and PFOS removal showed the differential rejection of PFBS (<69%) and PFOS (>88%) [134]. Poly(m-phenylene isophthalamide) (PMIA) hollow fiber NF membranes for PFOS removal at trace levels achieved overall rejection rates ranging from ≈92–99.40% under different operational parameters (TMP 0.4–1 MPa) and initial concentrations (50–500 μg/L) [135].
Although membranes have generally elevated PFAS removal efficiency, few are able to achieve PFAS concentrations below recommended health advisory limits (e.g., 70 ng/L, as per US EPA guidelines). Moreover, the complex composition of real wastewaters presents multiple pollutants that can affect membranes’ PFAS removal performance. Novel membrane technologies, e.g., adsorption-based membranes and forward osmosis (FO) filtration media, are currently under study [132].
PFAS fractionation is another pre-concentration technique used to separate these compounds from water, exploiting their surfactant properties: PFAS molecules tend to migrate and accumulate at air–water interfaces. Rising air bubbles in the water column can thus be used to concentrate and separate PFASs from the solution, generating lower volumes of PFAS-rich foam (the process is generally indicated as foam fractionation, FF) that can be treated more easily [136]. FF has shown effectiveness in removing most PFASs, although SC-PFASs may be less susceptible to this process: FF tests on AFFF solutions were optimized to remove 99.9% PFOS at a concentration 8400 times higher than the original solution; the process, however, was less effective for SC-PFASs (perfluorobutyl sulfonate, PFBS) due to their lower surface activity [137]. Higher ionic strength (≈5 mM NaCl) solutions slightly enhance FF efficiency [138]. An important advantage of FF is that it can be easily upscaled to meet different processing needs, making it a versatile solution to reduce contaminated volumes prior to further treatment. Recently, a study assessed the role of aeration-driven foaming in WWTP biological units on PFAS enrichment: the findings suggest that PFAS enrichment occurs in both phases (aqueous and solid) of the foamate, with the solid phase accounting for a significant fraction of these compounds (PFOA and PFOS in the foamate solid phase were as high as 60 and 95% of the total, respectively) [139]. FF with ozonated air showed higher PFAS fractionation than FF with air alone [137]. FF was also combined with photodegradation to destroy concentrated PFOS in the foamate [140]: this technique could be applied as a pre-treatment step in combination with most of the emerging technologies for PFAS destruction discussed in the previous sections.
In PFAS-related literature, the determination of F− ions associated with the reduction in solute compounds is commonly used as evidence of mineralization. Analytical techniques for PFAS determination continue to evolve as new PFASs are identified, matrix-specific methods validated, and analytical standards synthesized; still, the number and types of these compounds that can be individually determined is limited, as at the moment, there are no proven analytical methods that could detect all the potential fluoro-organic by-products generated by incomplete degradation [62]. Therefore, multiple considerations must be made when using “conventional” PFAS determination to understand the potential formation of by-products. The mass balance of F is the most reliable approach to check PFAS fate and possible by-product accumulation [141]. Advanced analytics, such as combustion ion chromatography measurement of extractable organofluorine, total oxidizable precursor (TOP) assay, or particle-induced γ-ray emission, can be used to verify the formation of precursors and the effectiveness of their destruction. The quantification of sequential short/ultrashort-chain PFAAs and fluoro-organic by-products (e.g., CF4, C2F6, C4F8), by GCMS, could be used to differentiate partial defluorination from the complete mineralization of LC-PFAAs [15].
Intentionally slow-operated NTP treatment of PFOA and PFOS was used to allow by-product accumulation in the treated solution and attempt their identification: several PFCAs (C4–C7), PFHxS, and PFBS were thus detected, together with significant concentrations of fluoride ions, inorganic carbon, smaller organic acids (trifluoroacetic, acetic, and formic acids), and trace amounts of 43 PFOA-related and 35 PFOS-related by-products [142].
The main issue is that practically all currently available technologies for PFAS destruction in complex aqueous solutions have the potential for generating SC-PFAAs, or other by-products, or to exert other secondary effects on solution chemistry. Non-detected (due to the absence of requirements) or unknown (due to the lack of analytical standards) partly degraded LC-PFASs can transform into detectable SC compounds, leading to observed increases in total PFAS concentrations in effluents ranging from 95% to 340% [143].
Thousands of precursor substances and perfluoroalkyl ethers will react differently to any of the discussed technologies both in concentrated and diluted waste streams. Current mainstream analytical techniques, including those developed for nondrinking matrices, are not capable of identifying all the individual potential treatment by-products. High-resolution MS may help identify a number of potential by-products within a few ppm; however, the issue is still far from being solved.
Artificial Intelligence (AI)-based platforms were created as PFAS screening tools, by combining rule-based classification systems with machine learning (ML) models. One such platform aligned with the latest OECD definitions was able to minimize unclassified PFASs, making the identification of their structure–function relationship easier [144]. Screen-and-search algorithms were developed for similar purposes; however, there is still limited information available on the toxicological effect of most of the by-products that could be identified [145]. Approaches based upon AI and ML can perform highly complex simulations of the molecules that are most likely to be generated as treatment by-products and their properties: an ML algorithm, trained on an existing set of PFAS molecules, generated over 260,000 novel possible molecules (PFAS-AI-Gen) to determine their possible effects using molecular descriptors with known relationships to toxicity [146].
The limited knowledge still available on PFAS partial degradation by-products indicates the need for a precautionary approach and for further specific research on these issues.
5. Discussion
PFAS treatment is technologically challenging, energy-intensive, and expensive, whether targeted at removal only or destruction.
An important issue to consider is that up to 75% of all (targeted and non-targeted) PFAS compounds, measurable though adsorbable organic fluorine (AOF) analysis, may not be detectable by common methodologies (e.g., EPA Method 533) in untreated matrices. Advancements in rapid targeted PFAS detection by optical or electrochemical sensing technologies could enable their discovery within minutes to hours at concentrations aligned within common ambient ones in water, soil, and sediments [147], and could potentially be integrated within in-stream waterborne pollutant detection systems [148]. Nevertheless, current treatment processes are optimized and monitored with focus on the few regulated (targeted) PFAS molecules; considering the variety of possible compounds potentially present in a waste stream and their different responses to both removal and destructive process technologies, the need for their precise tuning to the specific stream composition becomes ineluctable to minimize by-products and possibly achieve complete mineralization of all PFAS compounds and precursors.
For example, a study characterizing PFAS-contaminated raw water samples detected total targeted PFAS concentrations of 920 ng/L, while according to AOF measurements, the matrix content amounted to 2030 ng/L, a >120% difference [143]. The risk of undetected PFAS compounds undergoing recombination, cross-reactions, or simply breaking through the processes is always high. This can result in an increase in total target PFAS following treatment, due to the partial oxidation of non-detected LC-PFAS precursors. The literature reported an increase in PFBA, PFPeA, PFHxA, PFHpA, PFOA, and PFNA concentrations following TOP assays, which involve the oxidation of LC-PFAA precursors to PFCAs and PFSAs [149].
A major challenge in the effective management of PFAS contamination is thus related to the lack of standardized quantification methodologies. Recent analytical chemistry advancements, such as high-resolution mass spectrometry (HRMS), and non-target analysis (NTA) methods could allow the identification of unknown PFAS congeners in order to fine-tune removal and destructive remediation technologies and better assess exposure risk [150,151]. The precise quantification of total PFASs and precursors could be used to regulate the levels of energy input by the applied treatment: for example, higher UV doses in UV/H2O2 systems will normally lead to greater production of hydroxyl radicals, conductive to more precursor degradation, but also to the possible secondary formation of detectable compounds. By further raising the input energy, secondary compounds may also undergo degradation. In principle, both targeted and non-targeted PFASs could be completely destroyed by optimally tuned processes with very high, rapid energy input, such as E-beam or certain plasma technologies.
Despite intensive studies and a generally rapid improvement of treatment methods, only a few of them have reached a technological readiness level (TRL) mature enough for deployment at full-scale operation: for example, one of the most promising technologies for permanent PFAS destruction, due to its potential for rapid high energy delivery and fast reactivity, E-beam, is still confined to laboratory- or pilot-scale experiments. The main obstacles to its widespread diffusion include the need of larger pilot-scale demonstrations of its efficiency in real-world conditions and the current lack of suitable accelerator technology capable of operating at full-scale levels on water matrices with reliability and competitiveness comparable to other technologies [152].
Recently, however, a few commercial technologies have been proposed for PFAS treatment. These include classic adsorption-based processes but also destructive methods such as commercial-scale SCWO (“PFAS Annihilator” by Revive Environmental, Columbus, OH, USA), claiming to achieve 99.99% destruction of all PFAS types in varying liquid waste streams with no harmful by-product generation; micro-foam fractionation combined with electro-oxidation (“ForeverGone” by Gradiant, Boston, MA, USA a MIT startup); pyrolysis (“Sigma Pyrolysis” by Bioforcetech, San Francisco, CA, USA); and electroporation, consisting of the application of controlled electrical fields to disrupt PFAS chemical bonds (VVater, Austin, TX, USA) [153]. Electroporation, a new application in PFAS treatment, was previously used in the food and biomass processing industries at low energy intensity [154].
Despite the new options in the market, the water industry is still hesitant to adopt these technologies due to uncertainty about costs, effectiveness, and lack of specific regulatory mandates. Despite the commercial claims, it is uncertain which types of PFASs could be effectively destroyed by these technologies: non-targeted known or current compounds could soon face regulation, and destruction by these processes should be assessed. Not surprisingly, activated carbon adsorption, ion exchange resins, and separation by membrane technology are currently still the most used treatment methods for PFAS-contaminated water.
Treatment costs of PFAS-contaminated streams depend on discharge requirements and on treated wastewater volume. Specific treatment cost could be lower for large flows compared to small ones, since fixed costs may be largely independent of treated flows [155].
Generally, membrane removal can be more expensive (up to 0.16 USD/m3 permeate) than GAC adsorption (USD 0.025–0.11/m3) for PFAS removal, but this depends on applicable effluent standards and additional costs for retentate treatment or GAC recovery/disposal. Membrane/electrochemical oxidation costs have been assessed at 2.7–13.1 USD/m3 and photocatalysis at USD 295/m3 based on laboratory-scale tests [156]. Recent tests at the Fermi National Accelerator Laboratory in Chicago indicated a large-scale E-beam installation mass removal cost (≈500–800 USD/kg PFAS removed, depending on accelerator power rating) that is very competitive with those of GAC removal (27,530 USD/kg); GAC, however, showed lower volumetric costs (USD/L treated) in low concentration streams [157].
It is seldom clear, however, which of the various cost estimates reported in the literature include CAPEX and end-of-life residual disposal, as there are many cost factors to consider that vary based on the scale of the initial application on which the assessment is based. Data on the real costs of new commercial technologies have yet to be released and confirmed on real applications.
The refinement of emerging technologies is still needed. For example, a serious issue of AORPs—one of the apparently most efficient technologies at the moment for PFAS destruction—is that eaq− generated by these processes is highly reactive toward both target PFASs and several natural water matrix constituents, typically much more abundant in solution than PFASs. Consequently, the fraction of reactive species allocated for PFAS degradation is limited, leading to low treatment efficiency, either requiring higher energy for excess radical generation or preemptive minimization of the scavenging effects of the coexisting species. A reduction in ARPs’ heavy reliance on alkaline conditions can avoid costly pH adjustments; the treatment of pre-concentrated PFAS streams could improve ARP/AORP efficiency.
Combining ARPs with other physical/chemical or even biological processes could contribute to building more efficient multiple barrier treatment trains for PFASs [158]: reduction products generated from ARP treatment may be efficiently degraded by chemical oxidation—if sufficient reducing functional groups remain in solution—or by biological degradation, in the case that ARPs improved their biodegradability. Different technology combinations could be optimized by AI methods, as in the conventional wastewater treatment sector [159]: ML algorithms were used to simulate and predict the optimal conditions for the photocatalytic degradation of PFOA [160]. Various AI models had been previously applied to the detection, monitoring, and management of PFASs [161]; due to the nature of data-driven models, however, AI-based models do not always or necessarily exhibit superior performance to traditional approaches simply because they are advanced, as results depend on the characteristics and completeness of the data.
6. Conclusions and Future Directions
PFASs in their various forms are extremely stable chemicals; nevertheless, several established methods have been shown to remove them, but they are costly to operate and maintain. The most common removal method is adsorption onto GAC, followed by incineration of the spent medium. AORP methods (photocatalysis, EAOP, NTP, E-beam), pyrolysis, and SCWO have been proposed to achieve the final destruction of PFAS compounds. Ongoing research aims at reducing the cost of these technologies and improving their destruction efficiency; commercial PFAS destruction methods have been introduced recently, but their full-scale application is still unreported in the scientific literature. Concerns about the potential for some PFASs and precursors to break through current treatment methods, the partial degradation of targeted compounds to non-targeted ones, and potentially dangerous by-products have not been dismissed yet, due to the non-detectability of a large number of PFAS molecules beyond those targeted by regulations. Incomplete and uncertain degradation pathways following destructive treatment also raise issues about overall risk reduction.
A major challenge for the assessment of an effective reduction in PFAS contamination is related to the standardization of their quantification methodologies and to the development of analytical advancements to allow for the identification of PFAS congeners following specific treatments and fine-tune both removal and destructive remediation technologies.
Generally speaking, the most effective technologies for breaking down PFASs are those that can rapidly deliver high energy into a contaminated solution, in order to overcome the high C-F bond strength and avoid by-product accumulation during the destruction process. Since, in most instances, PFAS concentrations are very low, the pre-concentration of contaminated streams appears to be a suitable and efficient approach to reduce the volumes exposed to destructive treatment, with increased process yields. Some of the studied technologies, though capable of partial defluorination of target molecules, do not guarantee the complete mineralization of all organofluorine compounds; SCWO, EAOP, and pyrolysis seem to be the most reliable destructive methods available at the moment, as they can satisfy the requirements of high energy transfer to the contaminated matrices. Other highly effective technologies, NTP and E-beam, have demonstrated very promising potential. In particular, electron accelerators have the capability to deliver, very rapidly, large amounts of energy in high doses in the form of ionizing radiation, making complete targeted and non-targeted PFAS destruction possible, once issues with their practical implementation for liquid stream processing are resolved.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- EEA. PFAS Contamination in Europe. Available online: https://www.eea.europa.eu/en/european-zero-pollution-dashboards/indicators/treatment-of-drinking-water-to-remove-pfas-signal (accessed on 18 June 2025).
- Blake, M. They Poisoned the World; Random House: New York, NY, USA, 2025; p. 320. [Google Scholar]
- Lam, N.H.; Cho, C.R.; Kannan, K.; Cho, H.S. A nationwide survey of perfluorinated alkyl substances in waters, sediment and biota collected from aquatic environment in Vietnam: Distributions and bioconcentration profiles. J. Hazard. Mater. 2017, 323, 116–127. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Microplastics in the urban water cycle: A critical analysis of issues and of possible (needed?) solutions. Sci. Total. Environ. 2024, 954, 176580. [Google Scholar] [CrossRef] [PubMed]
- O’cOnnor, J.; Bolan, N.S.; Kumar, M.; Nitai, A.S.; Ahmed, M.B.; Bolan, S.S.; Vithanage, M.; Rinklebe, J.; Mukhopadhyay, R.; Srivastava, P.; et al. Distribution, transformation and remediation of poly- and per-fluoroalkyl substances (PFAS) in wastewater sources. Process. Saf. Environ. Prot. 2022, 164, 91–108. [Google Scholar] [CrossRef]
- Modiri, M.; Sasi, P.; Crone, B.; Potter, P.; Van Buren, J.; Doroshow, A.; Voit, J.; Mills, M.A. PFAS Precursors in Wastewater; University of Cincinnati Department of Chemical and Environmental Engineering Graduate Seminar: Cincinnati, OH, USA, 2024. [Google Scholar]
- Lenka, S.P.; Kah, M.; Padhye, L.P. A review of the occurrence, transformation, and removal of poly-and perfluoroalkyl substances (PFAS) in wastewater treatment plants. Water Res. 2021, 199, 117187. [Google Scholar] [CrossRef]
- Göckener, B.; Fliedner, A.; Rüdel, H.; Fettig, I.; Koschorreck, J. Exploring unknown per- and polyfluoroalkyl substances in the German environment–The total oxidizable precursor assay as helpful tool in research and regulation. Sci. Total. Environ. 2021, 782, 146825. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2019, 169, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Zhang, J.; Thiessen, P.A.; Chirsir, P.; Kondic, T.; Bolton, E.E. Per- and Polyfluoroalkyl Substances (PFAS) in PubChem: 7 Million and Growing. Environ. Sci. Technol. 2023, 57, 16918–16928. [Google Scholar] [CrossRef]
- NIH. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). National Institute of Health. Available online: https://www.niehs.nih.gov/health/topics/agents/pfc (accessed on 16 June 2025).
- US EPA. Per- and Polyfluoroalkyl Substances (PFAS) Final. PFAS National Primary Drinking Water Regulation. 40 CFR Parts 141 and 142; ANHE: Washington DC, USA, 2024. [Google Scholar]
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, E.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Yeung, L.W.; Stadey, C.; Mabury, S.A. Simultaneous analysis of perfluoroalkyl and polyfluoroalkyl substances including ultrashort-chain C2 and C3 compounds in rain and river water samples by ultra performance convergence chromatography. J. Chromatogr. A 2017, 1522, 78–85. [Google Scholar] [CrossRef]
- Vaalgamaa, S.; Vähätalo, A.V.; Perkola, N.; Huhtala, S. Photochemical reactivity of perfluorooctanoic acid (PFOA) in conditions representing surface water. Sci. Total. Environ. 2011, 409, 3043–3048. [Google Scholar] [CrossRef]
- Wang, N.; Liu, X.C.; Buck, R.C.; Korzeniowski, S.H.; Wolstenholme, B.W.; Folsom, P.W.; Sulecki, L.M. 6:2 Fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. Chemosphere 2011, 82, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Micro- and Nano-Plastics in DrinkingWater: Threat or Hype? Critical State-of-the-Art Analysis of Risks and Approaches. Xenobiotics 2025, 15, 85. [Google Scholar] [CrossRef]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J.; et al. Assessing the Ecological Risks of Per- and Polyfluoroalkyl Substances: Current State-of-the Science and a Proposed Path Forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Best Available Technologies and Small System Compliance Technologies for Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water. EPA Document Number: 815R24011. U.S. Environmental Protection Agency. Office of Water; Office of Groundwater and Drinking Water Standards and Risk Management Division: Washington, DC, USA, 2024.
- Crawford, S.; Thomas, R.; Taylor, F.; Dore, S.; Marley, M.; Crawford, L.; Occhialini, J.; McKnight, T.; Farmer, N. Early breakthrough of short-chain perfluoroalkyl substances in adsorptive media treatment. Remediation 2022, 32, 177–193. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Nayak, S.K.; Yamijala, S.S.R.K.C. Computing accurate bond dissociation energies of emerging per- and polyfluoroalkyl substances: Achieving chemical accuracy using connectivity-based hierarchy schemes. J. Hazard. Mater. 2024, 468, 133804. [Google Scholar] [CrossRef]
- da Silva, G.; Chen, C.C.; Bozzelli, J.W. Bond dissociation energy of the phenol OH bond from ab initio calculations. Chem. Phys. Lett. 2006, 424, 42–45. [Google Scholar] [CrossRef]
- O’HAgan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Capodaglio, A.G. Contaminants of emerging concern removal by high-energy oxidation-reduction processes: State of the art. Appl. Sci. 2019, 9, 4562. [Google Scholar] [CrossRef]
- Szreder, T.; Kisała, J.; Bojanowska-Czajka, A.; Kasperkowiak, M.; Pogocki, D.; Bobrowski, K.; Trojanowicz, M. High energy radiation – Induced cooperative reductive/oxidative mechanism of perfluorooctanoate anion (PFOA) decomposition in aqueous solution. Chemosphere 2022, 295, 133920. [Google Scholar] [CrossRef] [PubMed]
- Kosar, N.; Ayub, K.; Gilani, M.A.; Mahmood, T. Benchmark DFT studies on C–CN homolytic cleavage and screening the substitution effect on bond dissociation energy. J. Mol. Model. 2019, 25, 47. [Google Scholar] [CrossRef]
- Gou, Q.; Liu, J.; Su, H.; Guo, Y.; Chen, J.; Zhao, X.; Pu, X. Exploring an accurate machine learning model to quickly estimate stability of diverse energetic materials. iScience 2024, 27, 109452. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Meegoda, J.N.; de Souza, B.B.; Casarini, M.M.; Kewalramani, J.A. A Review of PFAS Destruction Technologies. Int. J. Environ. Res. Public. Health 2022, 19, 16397. [Google Scholar] [CrossRef]
- Chen, H.; Peng, H.; Yang, M.; Hu, J.; Zhang, Y. Detection, occurrence, and fate of fluorotelomer alcohols in municipal wastewater treatment plants. Environ. Sci. Technol. 2017, 51, 8953–8961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, H.; Li, F.; Zhou, Q. Occurrence and fate of perfluorinated acids in two wastewater treatment plants in Shanghai, China. Environ. Sci. Pollut. Res. 2015, 22, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Shigei, M.; Ahren, L.; Hazaymeh, A.; Dalahmeh, S.S. Per- and polyfluoroalkyl substances in water and soil in wastewater-irrigated farmland in Jordan. Sci. Total Environ. 2020, 716, 137057. [Google Scholar] [CrossRef]
- Gallen, C.; Eaglesham, G.; Drage, D.; Nguyen, T.H.; Mueller, J.F. A mass estimate of Perfluoroalkyl Substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 2018, 208, 975–983. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, J.; Zhang, M.; Chen, S.; He, B.; Zhao, H.; Li, Q.; Zhang, S.; Wang, T. Which type of pollutants need to be controlled with priority in wastewater treatment plants: Traditional or emerging pollutants? Environ. Int. 2019, 131, 104982. [Google Scholar] [CrossRef]
- Kibambe, M.G.; Momba, M.N.B.; Daso, A.P.; Coetzee, M.A.A. Evaluation of the efficiency of selected wastewater treatment processes in removing selected perfluoroalkyl substances (PFASs). J. Environ. Manag. 2020, 255, 109945. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Lewis, A.J.; Sales, C.M.; Suri, R.; McKenzie, E.R. Linking PFAS partitioning behavior in sewage solids to the solid characteristics, solution chemistry, and treatment processes. Chemosphere 2021, 271, 129530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, L.; Bergmann, D.; Bulatovic, T.; Surapaneni, A.; Gray, S. Review of influence of critical operation conditions on by-product/intermediate formation during thermal destruction of PFAS in solid/biosolids. Sci. Total. Environ. 2023, 854, 158796. [Google Scholar] [CrossRef] [PubMed]
- Moodie, D.; Coggan, T.; Berry, K.; Kolobaric, A.; Fernandes, M.; Lee, S.; Reichman, S.; Nugegoda, D.; Clarke, B.O. Legacy and emerging per- and polyfluoroalkyl substances (PFASs) in Australian biosolids. Chemosphere 2021, 270, 129143. [Google Scholar] [CrossRef]
- Cantoni, B.; Turolla, A.; Wellmitz, J.; Ruhl, A.S.; Antonelli, M. Perfluoroalkyl substances (PFAS) adsorption in drinking water by granular activated carbon: Influence of activated carbon and PFAS characteristics. Sci. Total. Environ. 2021, 795, 148821. [Google Scholar] [CrossRef]
- McCleaf, P.; Englund, S.; Östlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly-and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per-and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef]
- Vakili, M.; Cagnetta, G.; Deng, S.; Wang, W.; Gholami, Z.; Gholami, F.; Dastyar, W.; Mojiri, A.; Blaney, L. Regeneration of exhausted adsorbents after PFAS adsorption: A critical review. J. Hazard. Mater. 2024, 471, 134429. [Google Scholar] [CrossRef]
- Wu, C.; Klemes, M.J.; Trang, B.; Dichtel, W.R.; Helbling, D.E. Exploring the factors that influence the adsorption of anionic PFAS on conventional and emerging adsorbents in aquatic matrices. Water Res. 2020, 182, 115950. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, L.; Ren, X.; Sun, H. Synthesis of a perfluorooctanoic acid molecularly imprinted polymer for the selective removal of perfluorooctanoic acid in an aqueous environment. J. Appl. Polym. Sci. 2016, 133, 15. [Google Scholar] [CrossRef]
- Issaka, E.; Adams, M.; Baffoe, J.; Danso-Boateng, E.; Melville, L.; Fazal, A. Covalent organic frameworks: A review of synthesis methods, properties and applications for per- and poly-fluoroalkyl substances removal. Clean Technol. Environ. Policy 2025, 27, 833–860. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Andersen, H.R.; Thomaidis, N.S.; Stasinakis, A.S. Sorption of Perfluorinated Compounds onto different types of sewage sludge and assessment of its importance during wastewater treatment. Chemosphere 2014, 111, 405–411. [Google Scholar] [CrossRef]
- Ordonez, D.; Valencia, A.; Sadmani, A.H.M.A.; Chang, N.B. Green sorption media for the removal of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) from water. Sci. Total. Environ. 2022, 819, 152886. [Google Scholar] [CrossRef] [PubMed]
- Smaili, H.; Ng, C. Adsorption as a remediation technology for short-chain per- and polyfluoroalkyl substances (PFAS) from water—A critical review. Environ. Sci. Water Res. Technol. 2023, 9, 344–362. [Google Scholar] [CrossRef]
- Siriwardena, D.P.; James, R.; Dasu, K.; Thorn, J.; Darlington Iery, R.; Pala, F.; Schumitz, D.; Eastwood, S.; Burkitt, N. Regeneration of per- and polyfluoroalkyl substance-laden granular activated carbon using a solvent based technology. J. Environ. Manag. 2021, 289, 112439. [Google Scholar] [CrossRef]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1619–1666. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Lee, T. Cross-Flow Treatment of PFAS in Water: Materials Challenges and Potential Solutions. Accounts Mater. Res. 2021, 2, 129–133. [Google Scholar] [CrossRef]
- Qiang, Z.; Min, X.; Wang, Y.; Ma, X. Development of ceramic membranes with controllable PFAS mass transfer for passive sampling applications. Chem. Eng. J. Adv. 2023, 16, 100562. [Google Scholar] [CrossRef]
- Pervez, M.N.; Jiang, T.; Liang, Y. Structure and mechanism of nanoengineered membranes toward per- and polyfluoroalkyl substances (PFAS) removal from water: A critical review. J. Water Proc. Eng. 2024, 63, 105471. [Google Scholar] [CrossRef]
- Blotevogel, J.; Shukla, P. Breaking Down Barriers: Innovations in PFAS Destruction. Chem. Eng. 2025, 1005, 29. [Google Scholar]
- Capodaglio, A.G. High-energy oxidation process: An efficient alternative for wastewater organic contaminants removal. Clean Technol. Environ. Policy 2017, 19, 1995–2006. [Google Scholar] [CrossRef]
- Horst, J.; McDonough, J.; Ross, I.; Houtz, E. Understanding and managing the potential by-products of PFAS destruction. Groundw. Monit. Remediat. 2020, 40, 17–27. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 2009, 3, 129–151. [Google Scholar] [CrossRef]
- Krusic, P.J.; Roe, D.C. Gas-phase NMR technique for studying the thermolysis of materials: Thermal decomposition of ammonium perfluorooctanoate. Anal. Chem. 2004, 76, 3800–3803. [Google Scholar] [CrossRef]
- Yamada, T.; Taylor, P. Laboratory Scale Thermal Degradation of PFOS and Related Precoursors; Final Report—UDR-TR-03-00044; University of Dayton Research Institute (UDRI): Dayton, OH, USA, 2003. [Google Scholar]
- Wu, B.; Hao, S.; Choi, Y.; Higgins, C.P.; Deeb, R.; Strathmann, T.J. Rapid destruction and defluorination of perfluorooctanesulfonate by alkaline hydrothermal reaction. Environ. Sci. Technol. Lett. 2019, 6, 630–636. [Google Scholar] [CrossRef]
- Winchell, L.J.; Ross, J.J.; Wells, M.J.M.; Fonoll, X.; Norton, J.W.; Bell, K.Y. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water Environ. Res. 2021, 93, 826–843. [Google Scholar] [CrossRef]
- Watanabe, N.; Takata, M.; Takemine, S.; Yamamoto, K. Thermal mineralization behavior of PFOA, PFHxA, and PFOS during reactivation of granular activated carbon (GAC) in nitrogen atmosphere. Environ. Sci. Pollut. Res. Int. 2018, 25, 7200–7205. [Google Scholar] [CrossRef]
- Watanabe, N.; Takemine, S.; Yamamoto, K.; Haga, Y.; Takata, M. Residual organic fluorinated compounds from thermal treatment of PFOA, PFHxA and PFOS adsorbed onto granular activated carbon (GAC). J. Mater. Cycles Waste Manag. 2016, 18, 625–630. [Google Scholar] [CrossRef]
- Murakami, T.; Saito, N.; Matsukami, H.; Takaoka, M.; Fujimori, T. Destruction of perfluorooctanoic acid (PFOA) and perfluorooctadecanoic acid (PFOcDA) by incineration: Analysis of the by-products and their characteristics. Chemosphere 2025, 373, 144165. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef]
- US EPA. Per- and Polyfluoroalkyl Substances (PFAS): Incineration to Manage PFAS Waste Streams; US EPA Tech. Brief EPA-452-F/03-022; US EPA: Washington, DC, USA, 2020.
- GHD. Understanding the US DoD’s Prohibition of AFFF Incineration and Your Disposal Responsibilities. Available online: https://www.ghd.com/en-au/insights/us-department-of-defenses-prohibition-of-afff-incineration (accessed on 17 August 2025).
- Callegari, A.; Hlavinek, P.; Capodaglio, A.G. Production of energy (biodiesel) and recovery of materials (biochar) from pyrolysis of urban waste sludge. Rev. Ambient. Água 2018, 13, 2. [Google Scholar] [CrossRef]
- Kundu, S.; Patel, S.; Halder, P.; Patel, T.; Marzbali, M.H.; Pramanik, B.K.; Paz-Ferreiro, J.; de Figueiredo, C.C.; Bergmann, D.; Surapaneni, A.; et al. Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water. Environ. Sci. Water Res. Technol. 2021, 7, 638–649. [Google Scholar] [CrossRef]
- McNamara, P.; Samuel, M.S.; Sathyamoorthy, S.; Moss, L.; Valtierra, D.; Lopez, H.C.; Nigro, N.; Somerville, S.; Liu, Z. Pyrolysis transports, and transforms, PFAS from biosolids to py-liquid. Environ. Sci. Water Res. Technol. 2023, 9, 386–395. [Google Scholar] [CrossRef]
- Thoma, E.D.; Wright, R.S.; George, I.; Krause, M.; Presezzi, D.; Villa, V.; Preston, W.; Deshmukh, P.; Kauppi, P.; Zemek, P.G. Pyrolysis processing of PFAS-impacted biosolids, a pilot study. J. Air Waste Manag. Assoc. 2022, 72, 309–318. [Google Scholar] [CrossRef]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Electromagnetic_radiation (accessed on 21 July 2025).
- Bruton, T.A.; Sedlak, D.L. Treatment of perfluoroalkyl acids by heat-activated persulfate under conditions representative of in situ chemical oxidation. Chemosphere 2018, 206, 457–464. [Google Scholar] [CrossRef]
- Bentel, M.J.; Yu, Y.; Xu, L.; Kwon, H.; Li, Z.; Wong, B.M.; Men, Y.; Liu, J. Defluorination of per- and polyfluoroalkyl substances (PFASs) with hydrated electrons: Structural dependence and implications to PFAS remediation and management. Environ. Sci. Technol. 2019, 53, 3718–3728. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Capodaglio, A.G. Can radiation chemistry supply a highly efficient AO(R)P process for organics removal from drinking and waste water? A review. Environ. Sci. Pollut. Res. 2017, 24, 20187–20208. [Google Scholar] [CrossRef]
- Londhe, K.; Lee, C.-S.; Zhang, Y.; Grdanovska, S.; Kroc, T.; Cooper, C.A.; Venkatesan, A.K. Energy Evaluation of Electron Beam Treatment of Perfluoroalkyl Substances in Water: A Critical Review. ACS ES&T Eng. 2021, 1, 827–841. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—a review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Cooper, C.; Paquette, D.; Mills, M.A.; Londhe, K.; Lee, C.S.; Grdanovska, S. Application of Electron Beam Technology to Decompose Persistent Emerging Drinking Water Contaminants: Poly- and Perfluoroalkyl Substances (PFAS) and 1,4-Dioxane; Final Report, Award #DE-SC0020277; State University of New York: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bartosiewicz, I.; Bojanowska-Czajka, A.; Kulisa, K.; Szreder, T.; Bobrowski, K.; Nichipor, H.; Garcia-Reyes, J.F.; Nałęcz-Jawecki, G.; Męczyńska-Wielgosz, S.; et al. Application of ionizing radiation in decomposition of perfluorooctanoate (PFOA) in waters. Chem. Eng. J. 2019, 357, 698–714. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Could EB irradiation be the simplest solution for removing emerging contaminants from water and wastewater? Water Pr. Technol. 2018, 13, 172–183. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Critical perspective on advanced treatment processes for water and wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Nau-Hix, C.; Multari, N.; Singh, R.K.; Richardson, S.; Kulkarni, P.; Anderson, R.H.; Holsen, T.M.; Thagard, S.M. Field Demonstration of a Pilot-Scale Plasma Reactor for the Rapid Removal of Poly- and Perfluoroalkyl Substances in Groundwater. ACS EST Water 2021, 1, 680–687. [Google Scholar] [CrossRef]
- Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Dickenson, E.R.V.; Mededovic Thagard, S. Plasma-based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol. 2017, 51, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Anderson, R.H.; Richardson, S.D.; Holsen, T.M.; Thagard, S.M. Rapid removal of poly- and perfluorinated compounds from investigation- derived waste (IDW) in a pilot-scale plasma reactor. Environ. Sci. Technol. 2019, 53, 11375–11382. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Blotevogel, J.; Thagard, S.M.; Mahendra, S. Scaling up water treatment technologies for PFAS destruction: Current status and potential for fit-for-purpose application. Curr. Opin. Chem. Eng. 2023, 41, 100944. [Google Scholar] [CrossRef]
- Fang, C.; Megharaj, M.; Naidu, R. Electrochemical advanced oxidation processes (EAOP) to degrade per- and polyfluoroalkyl substances (PFASs). J. Adv. Oxid. Technol. 2017, 20, 20170014–20170025. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Vo, P.H.N.; Shukla, P.; Ge, L.; Zhao, C.X. Electrochemical advanced oxidation of per- and polyfluoroalkyl substances (PFASs): Development, challenges and perspectives. Chem. Eng. J. 2024, 500, 157222. [Google Scholar] [CrossRef]
- Maldonado, V.Y.; Becker, M.F.; Nickelsen, M.G.; Witt, S.E. Laboratory and semi-pilot scale study on the electrochemical treatment of perfluoroalkyl acids from ion exchange still bottoms. Water 2021, 13, 2873. [Google Scholar] [CrossRef]
- Xia, C.; Lim, X.; Yang, H.; Goodson, B.M.; Liu, J. Degradation of per- and polyfluoroalkyl substances (PFAS) in wastewater effluents by photocatalysis for water reuse. J. Water Process. Eng. 2022, 46, 102556. [Google Scholar] [CrossRef]
- Cecconet, D.; Sturini, M.; Malavasi, L.; Capodaglio, A.G. Graphitic carbon nitride as a sustainable photocatalyst material for pollutants removal. State-of-the art, preliminary tests and application perspectives. Materials 2021, 14, 7368. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676. [Google Scholar] [CrossRef]
- Umar, M. Reductive and Oxidative UV Degradation of PFAS—Status, Needs and Future Perspectives. Water 2021, 13, 3185. [Google Scholar] [CrossRef]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, P.Y.; Jian, L. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci. 2007, 19, 387–390. [Google Scholar] [CrossRef]
- Xin, X.; Kim, J.; Ashley, D.C.; Huang, C.-H. Degradation and defluorination of perandpolyfluoroalkyl substances by direct photolysis at 222 nm. ACS EST Water 2023, 3, 2776–2785. [Google Scholar] [CrossRef]
- Lyu, X.J.; Li, W.W.; Lam, P.K.; Yu, H.Q. Boiling significantly promotes photodegradation of perfluorooctane sulfonate. Chemosphere 2015, 138, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, C.-J.; Chen, P.; Zhou, Q.; Zhang, W.-X. Effect of initial solution pH on photo-induced reductive decomposition of perfluorooctanoic acid. Chemosphere 2014, 107, 218–223. [Google Scholar] [CrossRef]
- Lyu, X.J.; Li, W.W.; Lam, P.K.; Yu, H.Q. Photodegradation of perfluorooctane sulfonate in environmental matrices. Sep. Purif. Technol. 2015, 151, 172–176. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.T.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total. Environ. 2022, 827, 153669. [Google Scholar] [CrossRef]
- Ciawi, E.; Rae, J.; Ashokkumar, M.; Grieser, F. Determination of temperatures within acoustically generated bubbles in aqueous solutions at different ultrasound frequencies. J. Phys. Chem. B 2006, 110, 13656–13660. [Google Scholar] [CrossRef]
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Singh Kalra, S.; Cranmer, B.; Dooley, G.; Hanson, A.J.; Maraviov, S.; Mohanty, S.K.; Blotevogel, J.; Mahendra, S. Sonolytic destruction of per- and polyfluoroalkyl substances in groundwater, aqueous film-forming foams, and investigation derived waste. Chem. Eng. J. 2021, 425, 131778. [Google Scholar] [CrossRef]
- Gole, V.L.; Fishgold, A.; Sierra-Alvarez, R.; Deymier, P.; Keswani, M. Treatment of perfluorooctane sulfonic acid (PFOS) using a large-scale sonochemical reactor. Sep. Purif. Technol. 2018, 194, 104–110. [Google Scholar] [CrossRef]
- Schlosky, K.M. Supercritical phase transitions at very high pressure. J. Chem. Educ. 1989, 66, 989. [Google Scholar] [CrossRef]
- Krause, M.J.; Thoma, E.; Sahle-Damesessie, E.; Crone, B.; Whitehill, A.; Shields, E.; Gullett, B. Supercritical water oxidation as an innovative technology for PFAS destruction. J. Environ. Eng. 2022, 148, 05021006. [Google Scholar] [CrossRef]
- Scheitlin, C.G.; Dasu, K.; Rosansky, S.; Espina Dejarme, L.; Siriwardena, D.; Thorn, J.; Mullins, L.; Haggerty, I.; Shqau, K.; Stowe, J. Application of Supercritical Water Oxidation to Effectively Destroy Per- and Polyfluoroalkyl Substances in Aqueous Matrices. ACS EST Water 2023, 3, 2053–2062. [Google Scholar] [CrossRef]
- Vadivel, D.; Dondi, D.; Capodaglio, A.G. Present achievements and future directions of advanced carbon dioxide reduction strategies. Curr. Opin. Chem. Eng. 2024, 45, 101029. [Google Scholar] [CrossRef]
- Yadav, M.; Osonga, F.J.; Sadik, O.A. Unveiling nano-empowered catalytic mechanisms for PFAS sensing, removal and destruction in water. Sci. Total. Environ. 2024, 912, 169279. [Google Scholar] [CrossRef]
- Chen, M.J.; Lo, S.L.; Lee, Y.C.; Huang, C.C. Photocatalytic decomposition of perfluorooctanoic acid by transition-metal modified titanium dioxide. J. Hazard. Mater. 2015, 288, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Barisci, S.; Suri, R. Electrooxidation of short and long chain perfluorocarboxylic acids using boron doped diamond electrodes. Chemosphere 2020, 243, 125349. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, J.; Zhao, G.; Fan, J.; Wang, Y.; Wang, Y. Fabrication of novel SnO2-Sb/carbon aerogel electrode for ultrasonic electrochemical oxidation of perfluorooctanoate with high catalytic efficiency. Appl. Catal. B Environ. 2013, 136–137, 278–286. [Google Scholar] [CrossRef]
- Niu, J.; Lin, H.; Xu, J.; Wu, H.; Li, Y. Electrochemical mineralization of perfluorocarboxylic acids (PFCAs) by Ce-doped modified porous nanocrystalline PbO2 film electrode. Environ. Sci. Technol. 2012, 46, 10191–10198. [Google Scholar] [CrossRef]
- Masud, A.; Guardian, M.G.E.; Travis, S.C.; Chavez Soria, N.G.; Jarin, M.; Aga, D.S.; Aich, N. Redox-active rGO-nZVI nanohybrid-catalyzed chain shortening of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). J. Hazard. Mater. Lett. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Hwang, Y.; Andersen, H.R.; Stasinakis, A.S.; Thomaidis, N.S.; Aloupi, M. Reductive degradation of perfluorinated compounds in water using Mg-aminoclay coated nanoscale zero valent iron. Chem. Eng. J. 2015, 262, 133–139. [Google Scholar] [CrossRef]
- Yu, S.; Kim, T.; Jo, S.H.; Son, J. Physico-Chemical Properties of Catalysts for the Mineralization of Sulfamethoxazole in a High Ionizing Radiation Treatment. In Proceedings of the 3rd International Conference on Applications of Radiation Science and Technology (ICARST-2025), Vienna, Austria, 7–11 April 2025. [Google Scholar]
- Huang, S.; Jaffé, P.R. Defluorination of Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the Ability of perfluorohexane sulfonate (PFHxS) bioaccumulation by two Pseudomonas sp. strains isolated from PFAS-contaminated environmental matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Aburto-Medina, A.; Ball, A.S. Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils. Front. Bioeng. Biotechnol. 2021, 8, 602040. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Tevlin, A.G.; Mabury, S.A.; Mabury, S.A. Fate of polyfluoroalkyl phosphate diesters and their metabolites in biosolids-applied soil: Biodegradation and plant uptake in greenhouse and field experiments. Environ. Sci. Technol. 2014, 48, 340–349. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Da, Y.; Yu, B.; Long, B.; Zhang, P.; Bakker, C.; McCarl, B.A.; Yuan, J.S.; Dai, S.Y. Sustainable environmental remediation via biomimetic multifunctional lignocellulosic nano-framework. Nat. Commun. 2022, 13, 4368. [Google Scholar] [CrossRef]
- Tinivella, R.; Bargiggia, R.; Zanoni, G.; Callegari, A.; Capodaglio, A.G. High-Strength, Chemical Industry Wastewater Treatment Feasibility Study for Energy Recovery. Sustainability 2023, 15, 16285. [Google Scholar] [CrossRef]
- Tow, E.W.; Ersan, M.S.; Kum, S.; Lee, T.; Speth, T.F.; Owen, C.; Bellona, C.; Nadagouda, M.N.; Mikelonis, A.M.; Westerhoff, P.; et al. Managing and treating per- and polyfluoroalkyl substances (PFAS) in membrane concentrates. AWWA Water Sci. 2021, 3, e1233. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Peydayesh, M.; Mezzenga, R. Membrane-based technologies for per- and poly-fluoroalkyl substances (PFASs) removal from water: Removal mechanisms, applications, challenges and perspectives. Environ. Int. 2021, 157, 106876. [Google Scholar] [CrossRef]
- Boo, C.; Wang, Y.; Zucker, I.; Choo, Y.; Osuji, C.O.; Elimelech, M. High performance nanofiltration membrane for effective removal of perfluoroalkyl substances at high water recovery. Environ. Sci. Technol. 2018, 52, 7279–7288. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Xu, C.; Zhi, R.; Miao, R.; Liang, T.; Yue, X.; Lv, Y.; Liu, T. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chem. Eng. J. 2018, 332, 787–797. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, C.; Li, P.; Li, Y.; Wang, J. Fabrication of novel poly (m-phenylene isophthalamide) hollow fiber nanofiltration membrane for effective removal of trace amount perfluorooctane sulfonate from water. J. Membr. Sci. 2015, 477, 74–85. [Google Scholar] [CrossRef]
- We, A.C.E.; Zamyadi, A.; Stickland, A.D.; Clarke, B.O.; Freguia, S. A review of foam fractionation for the removal of per- and polyfluoroalkyl substances (PFAS) from aqueous matrices. J. Hazard. Mater. 2024, 465, 133182. [Google Scholar] [CrossRef]
- Dai, X.; Xie, Z.; Dorian, B.; Gray, S.; Zhang, J. Comparative study of PFAS treatment by UV, UV/ozone, and fractionations with air and ozonated air. Environ. Sci. Water Res. Technol. 2019, 5, 1897–1907. [Google Scholar] [CrossRef]
- Meng, P.; Deng, S.; Maimaiti, A.; Wang, B.; Huang, J.; Wang, Y.; Cousins, I.T.; Yu, G. Efficient removal of perfluorooctane sulfonate from aqueous film-forming foam solution by aeration-foam collection. Chemosphere 2018, 203, 263–270. [Google Scholar] [CrossRef] [PubMed]
- We, A.C.E.; Zamyadi, A.; Stickland, A.D.; Clarke, B.O.; Freguia, S. The role of suspended biomass in PFAS enrichment in wastewater treatment foams. Water Res. 2024, 254, 121349. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.J.; Liu, Y.; Chen, C.; Sima, M.; Lyu, J.F.; Ma, Z.Y.; Huang, S. Enhanced use of foam fractionation in the photodegradation of perfluorooctane sulfonate (PFOS). Sep. Purif. Technol. 2020, 253, 117488. [Google Scholar] [CrossRef]
- Marsh, R.W.; Kewalramani, J.A.; Bezerra de Souza, B.; Meegoda, J.N. The use of a fluorine mass balance to demonstrate the mineralization of PFAS by high frequency and high power ultrasound. Chemosphere 2024, 352, 141270. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Mededovic Thagard, S.; Holsen, T.M. Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef]
- Ersan, M.S.; Wang, B.; Wong, M.S.; Westerhoff, P. Advanced oxidation processes may transform unknown PFAS in groundwater into known products. Chemosphere 2024, 349, 140865. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Cheng, Y.; Zhang, C.; Yang, Y.F.; She, Y.B.; Rajan, K. An artificial intelligence platform for automated PFAS subgroup classification: A discovery tool for PFAS screening. Sci. Total Environ. 2024, 921, 171229. [Google Scholar] [CrossRef]
- Baygi, S.F.; Crimmins, B.S.; Hopke, P.K.; Holsen, T.M. Comprehensive emerging chemical discovery: Novel polyfluorinated compounds in Lake Michigan trout. Environ. Sci. Technol. 2016, 50, 9460–9468. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.T.; Kuntz, D.; Wilson, A.K. Molecular Screening and Toxicity Estimation of 260,000 Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) through Machine Learning. J. Chem. Inf. Model. 2022, 62, 4569–4578. [Google Scholar] [CrossRef]
- McQueen, A.; Kimble, A.; Krupa, P.; Longwell, A.; Calomeni-Eck, A.; Moore, D. Review of Emerging and Nonconventional Analytical Techniques for Per- and Polyfluoroalkyl Substances (PFAS): Application for Risk Assessment. Water 2025, 17, 303. [Google Scholar] [CrossRef]
- Capodaglio, A.G. In-stream detection of waterborne priority pollutants, and applications in drinking water contaminant warning systems. Water Sci. Technol. Water Supply 2017, 17, 707–725. [Google Scholar] [CrossRef]
- Houtz, E.F.; Sutton, R.; Park, J.S.; Sedlak, M. Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res. 2016, 95, 142–149. [Google Scholar] [CrossRef]
- Shao, G.; Jiang, N.; Liu, T.; Xia, Y.; Liu, R.; Zhang, P.; Wang, Y. Target and Non-Target Screening of Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water: Focus on Alternatives and Health Risks. Environ. Res. 2025, 285, 122581. [Google Scholar] [CrossRef]
- Zahra, Z.; Song, M.; Habib, Z.; Ikram, S. Advances in per- and polyfluoroalkyl substances (PFAS) detection and removal techniques from drinking water, their limitations, and future outlooks. Emerg. Contam. 2025, 11, 100434. [Google Scholar] [CrossRef]
- Edgecock, R.; Chmielewski, A. Electron Accelerators for the Future. In Proceedings of the 3rd International Conference on Applications of Radiation Science and Technology (ICARST-2025), Vienna, Austria, 7–11 April 2025. [Google Scholar]
- Manufacturing Dive. What Are the Options for PFAS Destruction in Manufacturing? Available online: https://www.manufacturingdive.com/news/pfas-destruction-forever-chemicals-toxic-battelle/751656/ (accessed on 10 August 2025).
- Capodaglio, A.G. Pulse electric field technology for wastewater and biomass residues’ improved valorization. Processes 2021, 9, 736. [Google Scholar] [CrossRef]
- Belkouteb, N.; Franke, V.; McCleaf, P.; Köhler, S.; Ahrens, L. Removal of per- and polyfluoroalkyl substances (PFASs) in a full-scale drinking water treatment plant: Long-term performance of granular activated carbon (GAC) and influence of flow-rate. Water Res. 2020, 182, 115913. [Google Scholar] [CrossRef]
- Das, S.; Ronen, A. A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes. Membranes 2022, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Cooper, C. Degradation of Poly- and Perfluoroalkyl Substances (PFAS) in Water via High Power, Energy-Efficient Electron Beam Accelerator. Tech. Report DOE-3M—9132; USDOE Office of Energy Efficiency and Renewable Energy (EERE), Energy Efficiency Office: Washington, DC, USA; Industrial Efficiency & Decarbonization Office (IEDO): Washington, DC, USA, 2024. [CrossRef]
- Li, L.; Haak, L.; Guarin, T.C.; Teel, L.; Sundaram, V.; Pagilla, K.R. Per- and poly-fluoroalkyl substances removal in multi-barrier advanced water purification system for indirect potable reuse. Water Environ. Res. 2024, 96, e10990. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Callegari, A. Use, Potential, Needs, and Limits of AI in Wastewater Treatment Applications. Water 2025, 17, 170. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Hosseinzadeh, A.; Huang, Z.; Li, D.; Zhou, J.L. Application of machine learning algorithms in predicting the photocatalytic degradation of perfluorooctanoic acid. Catal. Rev. 2022, 66, 687–712. [Google Scholar] [CrossRef]
- Park, J.; Baik, J.H.; Adjei-Nimoh, S.; Lee, W.H. Advancements in artificial intelligence-based technologies for PFAS detection, monitoring, and management. Sci. Total Environ. 2025, 980, 179536. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).