Eugenol@Natural Zeolite Nanohybrid vs. Clove Powder as Active and Reinforcement Agents in Novel Brewer’s Spent Grain/Gelatin/Glycerol Edible, High Oxygen Barrier Active Packaging Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BSG/Gel/Gl/xEG@NZ and BSG/Gel/Gl/xClP Films
2.3. Physicochemical Characterization of BSG/Gel/Gl, BSG/Gel/Gl/xEG@NZ, and BSG/Gel/Gl/xClP Films
2.4. Charactrization of Packaging Properties of BSG/Gel/Gl, BSG/Gel/Gl/xEG@NZ, and BSG/Gel/Gl/xClP Films
2.5. Antimicrobial Activity of BSG/Gel/Gl/10EG@NZ, BSG/Gel/Gl/10ClP Active Films
Disk Diffusion Susceptibility Test
2.6. Cytotoxic and Genotoxic Effects of Films in Human Lymphocytes
2.6.1. Ethics Statement and Approval
2.6.2. Whole Blood Collection and Cell Culture Preparation
2.6.3. CBMN Assay
2.7. Fresh Minced Pork Packaging Preservation Test with BSG/Gel/Gl/15EG@NZ, BSG/Gel/Gl/15ClP Active Films
2.8. Statistical Analysis
3. Results
3.1. XRD Analysis
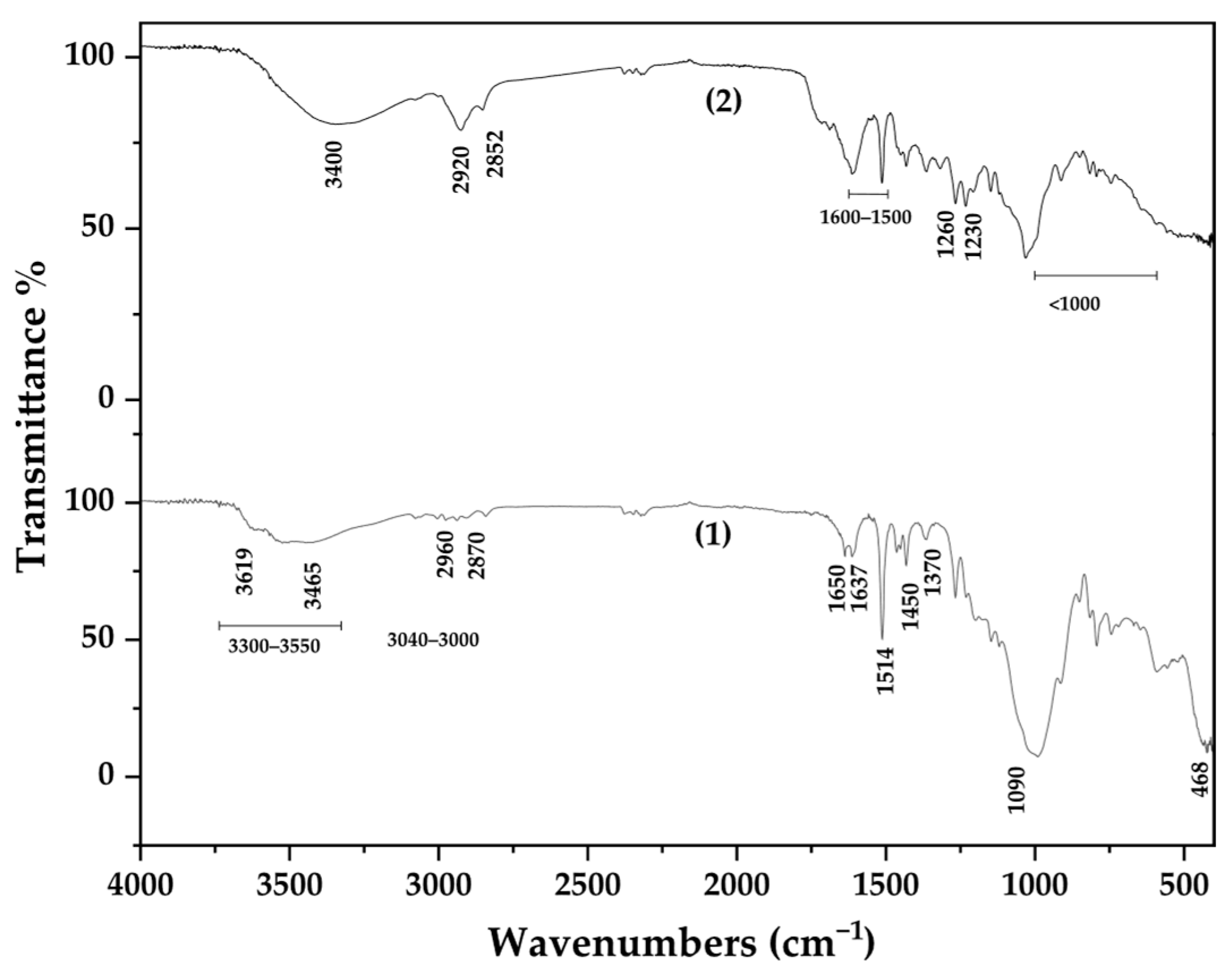
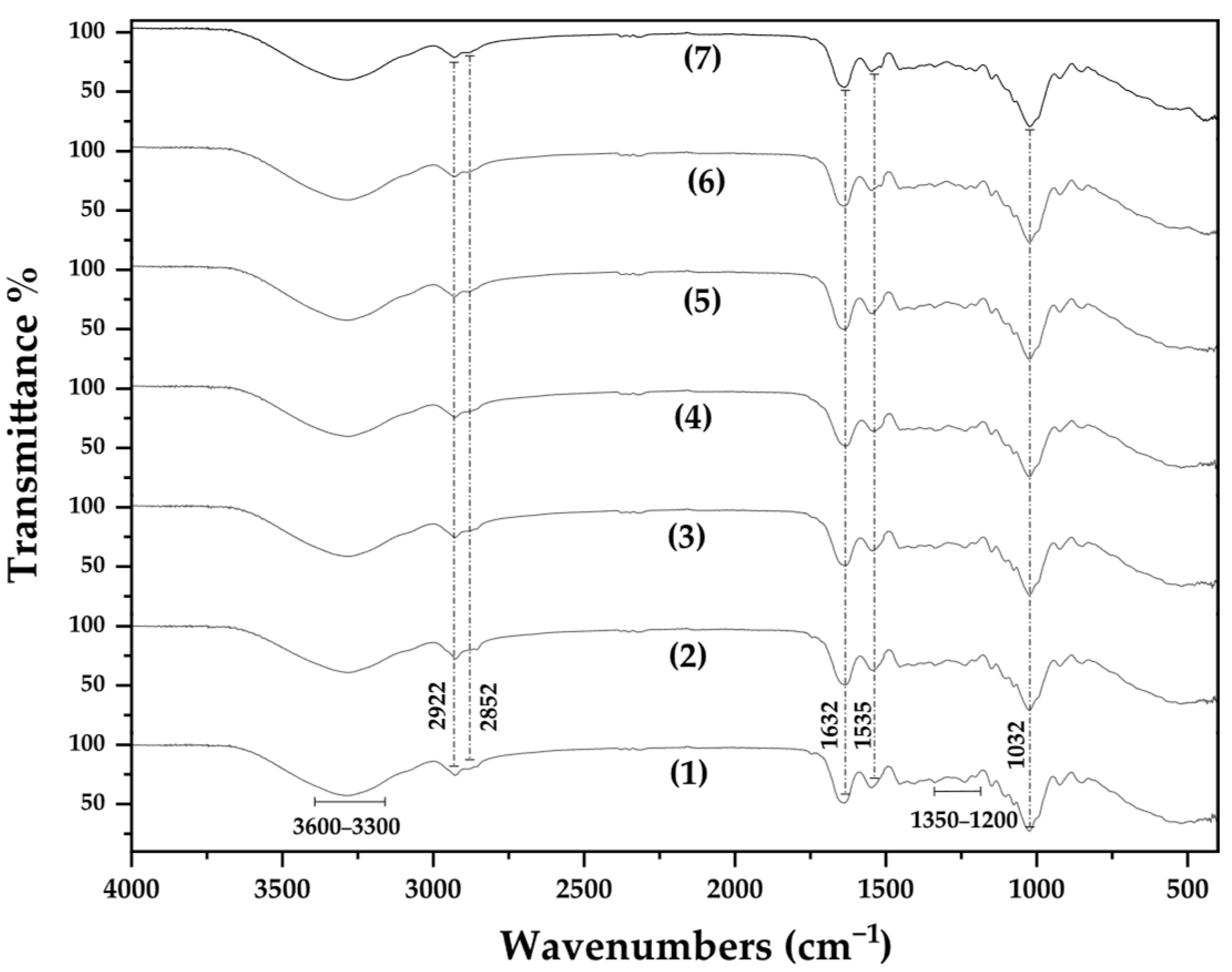
3.2. FTIR-ATR Spectroscopy
3.3. SEM Analysis
3.4. Tensile Properties of BSG/Gel/Gl/xEG@NZ and BSG/Gel/Gl/xClP Films
3.5. Oxygen Barrier Properties of BSG/Gel/Gl/xEG@NZ and BSG/Gel/Gl/xClP Films
3.6. Total Phenolic Content (TPC) of BSG/Gel/Gl/xEG@NZ and BSG/Gel/Gl/xClP Films
3.7. Antioxidant Activity of BSG/Gel/Gl/xEG@NZ and BSG/Gel/Gl/xClP Films
3.8. Antibacterial Activity of BSG/Gel/Gl/xEG@NZ and BSG/Gel/Gl/xClP Films
3.9. Cytotoxic and Genotoxic Effects of BSG/Gel/Gl/xEG@NZ and BSG/Gel/Gl/xClP Films in Human Lymphocytes
3.10. Packaging Preservation Test—Minced Pork Wrapped with BSG/Gel/Gl/10EG@NZ and BSG/Gel/Gl/10ClP Active Films
3.10.1. TVC
3.10.2. Lipid Oxidation
3.10.3. Heme Iron Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooney, R.; de Sousa, D.B.; Fernández-Ríos, A.; Mellett, S.; Rowan, N.; Morse, A.P.; Hayes, M.; Laso, J.; Regueiro, L.; Wan, A.H.L.; et al. A Circular Economy Framework for Seafood Waste Valorisation to Meet Challenges and Opportunities for Intensive Production and Sustainability. J. Clean. Prod. 2023, 392, 136283. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Fernández-Acero, F.J.; Amil-Ruiz, F.; Durán-Peña, M.J.; Carrasco, R.; Fajardo, C.; Guarnizo, P.; Fuentes-Almagro, C.; Vallejo, R.A. Valorisation of the Microalgae Nannochloropsis Gaditana Biomass by Proteomic Approach in the Context of Circular Economy. J. Proteom. 2019, 193, 239–242. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Branca, G.; Lipper, L.; McCarthy, N.; Jolejole, M.C. Food Security, Climate Change, and Sustainable Land Management. A Review. Agron. Sustain. Dev. 2013, 33, 635–650. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food Waste Generation and Industrial Uses: A Review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish Industry Waste: Treatments, Environmental Impacts, Current and Potential Uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Coppola, R. Recovery of Biomolecules of High Benefit from Food Waste. Curr. Opin. Food Sci. 2018, 22, 43–54. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Baldwin, C.J. Sustainability in the Food Industry; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-94926-8. [Google Scholar]
- Okino Delgado, C.H.; Fleuri, L.F. Orange and Mango By-Products: Agro-Industrial Waste as Source of Bioactive Compounds and Botanical versus Commercial Description—A Review. Food Rev. Int. 2016, 32, 1–14. [Google Scholar] [CrossRef]
- Umego, E.C.; Barry-Ryan, C. Review of the Valorization Initiatives of Brewing and Distilling By-Products. Crit. Rev. Food Sci. Nutr. 2024, 64, 8231–8247. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, P.-L.; Villain-Gambier, M.; Trébouet, D. By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy. Sustainability 2024, 16, 3472. [Google Scholar] [CrossRef]
- Nair, S.S.; Trafiałek, J.; Kolanowski, W. Edible Packaging: A Technological Update for the Sustainable Future of the Food Industry. Appl. Sci. 2023, 13, 8234. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Ludka, F.R.; Klosowski, A.B.; Camargo, G.A.; Justo, A.S.; Andrade, E.A.; Beltrame, F.L.; Olivato, J.B. Brewers’ Spent Grain Extract as Antioxidants in Starch-Based Active Biopolymers. Int. J. Food Sci. Technol. 2024, 59, 142–150. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s Spent Grain from Different Types of Malt: Evaluation of the Antioxidant Activity and Identification of the Major Phenolic Compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- Vieira, F.J.A.; Ludka, F.R.; Diniz, K.M.; Klosowski, A.B.; Olivato, J.B. Biodegradable Active Packaging Based on an Antioxidant Extract from Brewer’s Spent Grains: Development and Potential of Application. ACS Sustain. Resour. Manag. 2024, 1, 2413–2419. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Przybysz, M.; Ryl, J.; Saeb, M.R.; Piszczyk, Ł. Structural, Thermal and Physico-Mechanical Properties of Polyurethane/Brewers’ Spent Grain Composite Foams Modified with Ground Tire Rubber. Ind. Crops Prod. 2017, 108, 844–852. [Google Scholar] [CrossRef]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and Antimicrobial Films Based on Brewers Spent Grain Arabinoxylans, Nanocellulose and Feruloylated Compounds for Active Packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Proaño, J.L.; Salgado, P.R.; Cian, R.E.; Mauri, A.N.; Drago, S.R. Physical, Structural and Antioxidant Properties of Brewer’s Spent Grain Protein Films. J. Sci. Food Agric. 2020, 100, 5458–5465. [Google Scholar] [CrossRef]
- Lin, L.; Mirkin, S.; Park, H.E. Biodegradable Composite Film of Brewers’ Spent Grain and Poly(Vinyl Alcohol). Processes 2023, 11, 2400. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of Essential Oils as Preservatives in Food Systems: Challenges and Future Prospectives—A Review. Phytochem. Rev. 2022, 21, 1209–1246. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Varghese, S.A.; Siengchin, S.; Parameswaranpillai, J. Essential Oils as Antimicrobial Agents in Biopolymer-Based Food Packaging—A Comprehensive Review. Food Biosci. 2020, 38, 100785. [Google Scholar] [CrossRef]
- de Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite/Essential Oil Composites in a Multilayer Film Reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food Applications: A Review. Food Biosci. 2022, 46, 101577. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Development of Carvacrol@natural Zeolite Nanohybrid and Poly-Lactide Acid/Triethyl Citrate/Carvacrol@natural Zeolite Self-Healable Active Packaging Films for Minced Pork Shelf-Life Extension. Preprint 2024. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Leontiou, A.A.; Karydis-Messinis, A.; Moschovas, D.; Andritsos, N.D.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Novel Carvacrol@activated Carbon Nanohybrid for Innovative Poly(Lactide Acid)/Triethyl Citrate Based Sustainable Active Packaging Films. Polymers 2025, 17, 605. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Baikousi, M.; Karabagias, V.K.; Karageorgou, I.; Iordanidis, G.; Emmanouil-Konstantinos, C.; Leontiou, A.; Karydis-Messinis, A.; Zafeiropoulos, N.E.; Kehayias, G.; et al. Low-Density Polyethylene-Based Novel Active Packaging Film for Food Shelf-Life Extension via Thyme-Oil Control Release from SBA-15 Nanocarrier. Nanomaterials 2024, 14, 423. [Google Scholar] [CrossRef]
- Huang, X.; Ge, X.; Zhou, L.; Wang, Y. Eugenol Embedded Zein and Poly(Lactic Acid) Film as Active Food Packaging: Formation, Characterization, and Antimicrobial Effects. Food Chem. 2022, 384, 132482. [Google Scholar] [CrossRef]
- Navikaite-Snipaitiene, V.; Ivanauskas, L.; Jakstas, V.; Rüegg, N.; Rutkaite, R.; Wolfram, E.; Yildirim, S. Development of Antioxidant Food Packaging Materials Containing Eugenol for Extending Display Life of Fresh Beef. Meat Sci. 2018, 145, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, A.; Salmas, C.E.; Chalmpes, N.; Leontiou, A.A.; Karakassides, M.A.; Giannelis, E.P.; Giannakas, A.E. Laponite vs. Montmorillonite as Eugenol Nanocarriers for Low Density Polyethylene Active Packaging Films. Nanomaterials 2024, 14, 1938. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sahoo, J.; Chatli, K.M.; Biswas, A.K. Effect of Clove Powder and Modified Atmosphere Packaging on the Oxidative and Sensory Quality of Chicken Meat Caruncles During Ambient Storage (35 ± 2 °C) Conditions. J. Meat Sci. 2014, 10, 15–22. [Google Scholar]
- Mh, T. Effect of Clove Powder and Garlic Paste on Quality and Safety of Raw Chicken Meat at Refrigerated Storage. World J. Nutr. Food Sci. 2018, 1, 1002. [Google Scholar]
- Test No. 487: In Vitro Mammalian Cell Micronucleus Test. Available online: https://www.oecd.org/en/publications/test-no-487-in-vitro-mammalian-cell-micronucleus-test_9789264264861-en.html (accessed on 9 June 2025).
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN Project: Detailed Description of the Scoring Criteria for the Cytokinesis-Block Micronucleus Assay Using Isolated Human Lymphocyte Cultures. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Surrallés, J.; Xamena, N.; Creus, A.; Catalán, J.; Norppa, H.; Marcos, R. Induction of Micronuclei by Five Pyrethroid Insecticides in Whole-Blood and Isolated Human Lymphocyte Cultures. Mutat. Res. Toxicol. 1995, 341, 169–184. [Google Scholar] [CrossRef]
- Salmas, C.Ε.; Kollia, E.; Avdylaj, L.; Kopsacheili, A.; Zaharioudakis, K.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Kehayias, G.; Karakassides, A.; et al. Thymol@Natural Zeolite Nanohybrids for Chitosan/Poly-Vinyl-Alcohol Based Hydrogels Applied As Active Pads for Strawberries Preservation. Gels 2023, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, A.; Leontiou, A.A.; Oliinychenko, Y.K.; Stratakos, A.C.; Zaharioudakis, K.; Katerinopoulou, K.; Baikousi, M.; Andritsos, N.D.; Proestos, C.; Chalmpes, N.; et al. Eugenol@Natural-Zelolite vs Citral@Natural-Zeolite Nanohybrids for Gelatine-Based Edible-Active Packaging Films. Gels 2025, 11, 518. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Leontiou, A.A.; Moschovas, D.; Karydis-Messinis, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Shelf Life of Minced Pork in Vacuum-Adsorbed Carvacrol@Natural Zeolite Nanohybrids and Poly-Lactic Acid/Triethyl Citrate/Carvacrol@Natural Zeolite Self-Healable Active Packaging Films. Antioxidants 2024, 13, 776. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, G.; Auras, R.; Rubino, M.; Dolan, K.; Soto-Valdez, H. Determination of Eugenol Diffusion through LLDPE Using FTIR-ATR Flow Cell and HPLC Techniques. Polymer 2009, 50, 1470–1482. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Talande, S.; Bakandritsos, A.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Nanocarbon from Rocket Fuel Waste: The Case of Furfuryl Alcohol-Fuming Nitric Acid Hypergolic Pair. Nanomaterials 2021, 11, 1. [Google Scholar] [CrossRef]
- Parthipan, P.; AlSalhi, M.S.; Devanesan, S.; Rajasekar, A. Evaluation of Syzygium Aromaticum Aqueous Extract as an Eco-Friendly Inhibitor for Microbiologically Influenced Corrosion of Carbon Steel in Oil Reservoir Environment. Bioprocess Biosyst. Eng. 2021, 44, 1441–1452. [Google Scholar] [CrossRef]
- Dibazar, S.P.; Fateh, S.; Daneshmandi, S. Clove (Syzygium aromaticum) Ingredients Affect Lymphocyte Subtypes Expansion and Cytokine Profile Responses: An in Vitro Evaluation. J. Food Drug Anal. 2014, 22, 448–454. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Kremensas, A. Bio-Based Polyurethane Composite Foams with Improved Mechanical, Thermal, and Antibacterial Properties. Materials 2020, 13, 1108. [Google Scholar] [CrossRef] [PubMed]
- Shroti, G.K.; Saini, C.S. Development of Edible Films from Protein of Brewer’s Spent Grain: Effect of pH and Protein Concentration on Physical, Mechanical and Barrier Properties of Films. Appl. Food Res. 2022, 2, 100043. [Google Scholar] [CrossRef]
- Kechagias, A.; Leontiou, A.A.; Oliinychenko, Y.K.; Stratakos, A.C.; Zaharioudakis, K.; Proestos, C.; Giannelis, E.P.; Chalmpes, N.; Salmas, C.E.; Giannakas, A.E. Eugenol@Montmorillonite vs. Citral@Montmorillonite Nanohybrids for Gelatin-Based Extruded, Edible, High Oxygen Barrier, Active Packaging Films. Polymers 2025, 17, 1518. [Google Scholar] [CrossRef] [PubMed]
- Karydis-Messinis, A.; Moschovas, D.; Markou, M.; Gkantzou, E.; Vasileiadis, A.; Tsirka, K.; Gioti, C.; Vasilopoulos, K.C.; Bagli, E.; Murphy, C.; et al. Development, Physicochemical Characterization and in Vitro Evaluation of Chitosan-Fish Gelatin-Glycerol Hydrogel Membranes for Wound Treatment Applications. Carbohydr. Polym. Technol. Appl. 2023, 6, 100338. [Google Scholar] [CrossRef]
- Momtaz, M.; Momtaz, E.; Mehrgardi, M.A.; Momtaz, F.; Narimani, T.; Poursina, F. Preparation and Characterization of Gelatin/Chitosan Nanocomposite Reinforced by NiO Nanoparticles as an Active Food Packaging. Sci. Rep. 2024, 14, 519. [Google Scholar] [CrossRef]
- Pérez, C.D.; Flores, S.K.; Marangoni, A.G.; Gerschenson, L.N.; Rojas, A.M. Development of a High Methoxyl Pectin Edible Film for Retention of l -(+)-Ascorbic Acid. J. Agric. Food Chem. 2009, 57, 6844–6855. [Google Scholar] [CrossRef]
- Din, M.I.; Ghaffar, T.; Najeeb, J.; Hussain, Z.; Khalid, R.; Zahid, H. Potential Perspectives of Biodegradable Plastics for Food Packaging Application-Review of Properties and Recent Developments. Food Addit. Contam. Part A 2020, 37, 665–680. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of Mechanical Properties, Migration, and Potential Applications in Active Food Packaging Systems Containing Nanoclays and Nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and Aroma Barrier Properties of Edible Films: A Review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Cunha, M.; Berthet, M.-A.; Pereira, R.; Covas, J.A.; Vicente, A.A.; Hilliou, L. Development of Polyhydroxyalkanoate/Beer Spent Grain Fibers Composites for Film Blowing Applications. Polym. Compos. 2015, 36, 1859–1865. [Google Scholar] [CrossRef]
- Baddigam, K.R.; Chee, B.S.; Guilloud, E.; Venkatesh, C.; Koninckx, H.; Windey, K.; Fournet, M.B.; Hedenqvist, M.; Svagan, A.J. High Oxygen Barrier Packaging Materials from Protein-Rich Single-Celled Organisms. ChemRXiv 2025. [Google Scholar] [CrossRef]
- Chang, Y.; Joo, E.; Song, H.; Choi, I.; Yoon, C.S.; Choi, Y.J.; Han, J. Development of Protein-Based High-Oxygen Barrier Films Using an Industrial Manufacturing Facility. J. Food Sci. 2019, 84, 303–310. [Google Scholar] [CrossRef]
- Bull, M.K.; Steele, R.J.; Kelly, M.; Olivier, S.A.; Chapman, B. Packaging under Pressure: Effects of High Pressure, High Temperature Processing on the Barrier Properties of Commonly Available Packaging Materials. Innov. Food Sci. Emerg. Technol. 2010, 11, 533–537. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and Mechanical Properties of Compression Molded and Solution Casting Soybean Protein Concentrate Based Films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Moura-Alves, M.; Esteves, A.; Ciríaco, M.; Silva, J.A.; Saraiva, C. Antimicrobial and Antioxidant Edible Films and Coatings in the Shelf-Life Improvement of Chicken Meat. Foods 2023, 12, 2308. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Badeka, A.V. In Search of the EC60: The Case Study of Bee Pollen, Quercus Ilex Honey, and Saffron. Eur. Food Res. Technol. 2020, 246, 2451–2459. [Google Scholar] [CrossRef]
- Kowalewska, A.; Majewska-Smolarek, K. Eugenol-Based Polymeric Materials—Antibacterial Activity and Applications. Antibiotics 2023, 12, 1570. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of Cinnamaldehyde and Eugenol and Their Activity after Incorporation into Cellulose-Based Packaging Films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Narayanan, A.; Neera, M.; Ramana, K.V. Synergized Antimicrobial Activity of Eugenol Incorporated Polyhydroxybutyrate Films Against Food Spoilage Microorganisms in Conjunction with Pediocin. Appl. Biochem. Biotechnol. 2013, 170, 1379–1388. [Google Scholar] [CrossRef]
- Esenli, F.; Şans, B.E.; Erdoğan, B.; Sirkecioğlu, A. The Surface Characteristics of Natural Heulandites/Clinoptilolites with Different Extra-Framework Cations. Clay Miner. 2023, 58, 378–387. [Google Scholar] [CrossRef]
- Kalliampakou, K.I.; Athanasopoulou, E.; Spanou, A.; Flemetakis, E.; Tsironi, T. In Vitro Cytotoxicity Evaluation of a CMC-SA Edible Packaging Film for Migration and Safety Assessment. Sci. Rep. 2025, 15, 13304. [Google Scholar] [CrossRef]
- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Use of Formaldehyde as a Preservative during the Manufacture and Preparation of Food Additives|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/415 (accessed on 16 June 2025).
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay Evolution into a More Comprehensive Method to Measure Chromosomal Instability. Genes 2020, 11, 1203. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Rapid Detection of Total Viable Count (TVC) in Pork Meat by Hyperspectral Imaging. Food Res. Int. 2013, 54, 821–828. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Ding, W. Eugenol Nanocapsules Embedded with Gelatin-Chitosan for Chilled Pork Preservation. Int. J. Biol. Macromol. 2020, 158, 837–844. [Google Scholar] [CrossRef]
- Ding, Z.-G.; Shen, Y.; Hu, F.; Zhang, X.-X.; Thakur, K.; Khan, M.R.; Wei, Z.-J. Preparation and Characterization of Eugenol Incorporated Pullulan-Gelatin Based Edible Film of Pickering Emulsion and Its Application in Chilled Beef Preservation. Molecules 2023, 28, 6833. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sahoo, J.; Chatli, M.K.; Biswas, A.K. Shelf Life Evaluation of Raw Chicken Meat Emulsion Incorporated with Clove Powder, Ginger and Garlic Paste as Natural Preservatives at Refrigerated Storage (4 ± 1 °C). Int. Food Res. J. 2014, 21, 1327–1337. [Google Scholar]
- Kumudavally, K.V.; Tabassum, A.; Radhakrishna, K.; Bawa, A.S. Effect of Ethanolic Extract of Clove on the Keeping Quality of Fresh Mutton during Storage at Ambient Temperature (25 ± 2 °C). J. Food Sci. Technol. 2011, 48, 466–471. [Google Scholar] [CrossRef]
- Bilen, M.V.; Uzun, P.; Yıldız, H.; Fındık, B.T. Evaluation of the Effect of Active Essential Oil Components Added to Pickled-Based Marinade on Beef Stored under Vacuum Packaging: Insight into Physicochemical and Microbiological Quality. Int. J. Food Microbiol. 2024, 418, 110733. [Google Scholar] [CrossRef]
- Gengatharan, A.; Rahim, M.H.A. The Application of Clove Extracts as a Potential Functional Component in Active Food Packaging Materials and Model Food Systems: A Mini-Review. Appl. Food Res. 2023, 3, 100283. [Google Scholar] [CrossRef]
- Liang, M.; Yang, Z.; Xu, K.; Chen, X.; Yang, J.; Liu, W.; Zhao, S.; Xu, Y.; Zhang, J. Release Behavior and Kinetic Analysis of Eugenol from Clove Particles Using P&T–GC-MS Method. Ital. J. Food Sci. 2023, 35, 69–78. [Google Scholar] [CrossRef]
- Hendriks, F.C.; Valencia, D.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Zeolite Molecular Accessibility and Host–Guest Interactions Studied by Adsorption of Organic Probes of Tunable Size. Phys. Chem. Chem. Phys. 2017, 19, 1857–1867. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | BSG (g) | Gel (g) | Gl (g) | H2O (g) | EG@NZ (g) | ClP (g) |
|---|---|---|---|---|---|---|
| BSG/Gel/Gl | 3 | 1 | 1 | 1.6 | - | - |
| BSG/Gel/Gl/5EG@NZ | 3 | 1 | 1 | 1.6 | 0.347 | - |
| BSG/Gel/Gl/10EG@NZ | 3 | 1 | 1 | 1.6 | 0.733 | - |
| BSG/Gel/Gl/15EG@NZ | 3 | 1 | 1 | 1.6 | 1.160 | - |
| BSG/Gel/Gl/5ClP | 3 | 1 | 1 | 1.6 | - | 0.347 |
| BSG/Gel/Gl/10ClP | 3 | 1 | 1 | 1.6 | - | 0.733 |
| BSG/Gel/Gl/15ClP | 3 | 1 | 1 | 1.6 | - | 1.160 |
| Elastic Modulus E (MPa) Average ± Stdev | σuts (MPa) Average ± Stdev | Elongation at Break—%ε Average ± Stdev | |
|---|---|---|---|
| BSG/Gel/Gl | 17.57 ± 5.62 d | 1.83 ± 0.29 c | 27.25 ± 8.12 a |
| BSG/Gel/Gl/5EG@NZ | 43.97 ± 7.76 cd | 3.80 ± 0.96 bc | 17.71 ± 3.17 bc |
| BSG/Gel/Gl/10EG@NZ | 52.56 ± 7.75 bc | 5.07 ± 0.52 ab | 16.51 ± 1.32 bc |
| BSG/Gel/Gl/15EG@NZ | 77.01 ± 13.76 a | 5.82 ± 0.47 a | 14.28 ± 1.50 c |
| BSG/Gel/Gl/5ClP | 59.77 ± 7.45 abc | 5.74 ± 0.70 a | 23.08 ± 2.63 a |
| BSG/Gel/Gl/10ClP | 71.17 ± 12.60 a | 5.55 ± 0.72 a | 17.77 ± 1.59 b |
| BSG/Gel/Gl/15ClP | 70.29 ± 15.92 ab | 5.54 ± 0.59 a | 15.84 ± 2.57 bc |
| Sample Name | Average Thickness (mm) | O.T.R. (mL·m−2·day−1) | PeO2 (cm2·s−1) |
|---|---|---|---|
| BSG/Gel/Gl | 0.27 ± 0.01 a | 8301.2 ± 450.3 a | 2.59 × 10−7 ± 0.19 × 10−7 a |
| BSG/Gel/Gl/5EG@NZ | 0.25 ± 0.02 a | 0 b | 0 b |
| BSG/Gel/Gl/10EG@NZ | 0.27 ± 0.01 a | 0 b | 0 b |
| BSG/Gel/Gl/15EG@NZ | 0.25 ± 0.02 a | 0 b | 0 b |
| BSG/Gel/Gl/5ClP | 0.27 ± 0.02 a | 0 b | 0 b |
| BSG/Gel/Gl/10ClP | 0.25 ± 0.02 a | 22.8 ± 1.5 b | 5.87 × 10−10 ± 0.42 × 10−10 b |
| BSG/Gel/Gl/15ClP | 0.25 ± 0.01 a | 16.8 ± 1.1 b | 5.17 × 10−10 ± 0.32 × 10−10 b |
| Sample Name | EC60 mg/mL | TPC 1 (mg GAE/L) |
|---|---|---|
| BSG/Gel/Gl | 23.46 ± 3.13 a | 17.28 ± 0.86 d |
| BSG/Gel/Gl/5EG@NZ | 2.80 ± 0.55 bc | 95.28 ± 4.76 bcd |
| BSG/Gel/Gl/10EG@NZ | 1.83 ± 0.68 c | 256.15 ± 14.59 ab |
| BSG/Gel/Gl/15EG@NZ | 2.70 ± 0.40 bc | 291.77 ± 12.81 a |
| BSG/Gel/Gl/5ClP | 5.02 ± 0.25 ab | 67.28 ± 3.36 bcd |
| BSG/Gel/Gl/10ClP | 2.50 ± 0.26 c | 135.07 ± 6.75 abcd |
| BSG/Gel/Gl/15ClP | 3.00 ± 0.12 abc | 184.28 ± 9.21 abc |
| Samples | ZOI (mm) | |
|---|---|---|
| S. aureus | E. coli | |
| BSG/Gel/Gl | N.D. | N.D. |
| BSG/Gel/Gl/5EG@NZ | N.D. | N.D. |
| BSG/Gel/Gl/10EG@NZ | 25 | 20 |
| BSG/Gel/Gl/15EG@NZ | 45 | 30 |
| BSG/Gel/Gl/5ClP | N.D. | N.D. |
| BSG/Gel/Gl/10ClP | N.D. | N.D. |
| BSG/Gel/Gl/15ClP | 25 | N.D. |
| Concentrations (μg/mL) | BN | MN ± S.E. (‰) | CBPI ± S.E | Cytostasis (%) |
|---|---|---|---|---|
| Control * | 1000 | 2 ± 0 | 1.65 ± 0.03 | 0 |
| MMC (0.05) * | 1000 | 13 ± 2.8 | 1.48 ± 0.02 | 25.6 ± 2 |
| BSG/Gel/Gl | ||||
| 50 | 1000 | 1 ± 0 A,a | 1.64 ± 0.01 A,a | 2.3 ± 2 A,a |
| 100 | 1000 | 1 ± 0 A,a | 1.63 ± 0.00 AB,a | 3.1 ± 0 A,a |
| 500 | 1000 | 2 ± 0 A,ab | 1.54 ± 0.01 B,ab | 17.6 ± 1 A,ab |
| BSG/Gel/Gl/15ClP | ||||
| 50 | 1000 | 2 ± 0 AB,a | 1.57 ± 0.01 A,a | 12.4 ± 2 B,a |
| 100 | 1000 | 1 ± 0 B,a | 1.55 ± 0.01 AB,a | 15.3 ± 1 AB,a |
| 500 | 1000 | 3 ± 0 A,a | 1.51 ± 0.01 B,b | 22.1 ± 2 A,a |
| BSG/Gel/Gl/15EG@NZ | ||||
| 50 | 1000 | 1.5 ± 0.7 A,a | 1.65 ± 0.03 A,a | 0.00 ± 5 A,a |
| 100 | 1000 | 2 A,a | 1.63 ± 0.02 A,a | 3.5 ± 3 A,a |
| 500 | 1000 | 1.5 ± 0.7 A,b | 1.55 ± 0.01 A,a | 15.6 ± 2 A,b |
| Sample | 0th Day | 2nd Day | 4th Day | 6th Day |
|---|---|---|---|---|
| TVC (mg/kg) | ||||
| CONTROL | 4.30 ± 0.05 C,a | 5.7 ± 0.04 BC,a | 6.92 ± 0.06 AB,a | 8.18 ± 0.05 A,a |
| BSG/Gel/Gl/15EG@NZ | 3.55 ± 0.04 C,b | 4.70 ± 0.04 BC,b | 5.71 ± 0.05 AB,b | 6.67 ± 0.10 A,b |
| BSG/Gel/Gl/15ClP | 3.74 ± 0.04 C,ab | 4.959 ± 0.04 BC,ab | 6.01 ± 0.05 AB,ab | 7.18 ± 0.05 A,ab |
| Sample | 0th Day | 2nd Day | 4th Day | 6th Day |
| TBARS (mg/kg) | ||||
| CONTROL | 0.46 ± 0.01 C,a | 0.59 ± 0.02 BC,a | 0.75 ± 0.02 AB,a | 0.81 ± 0.02 A,a |
| BSG/Gel/Gl/15EG@NZ | 0.46 ± 0.01 B,a | 0.47 ± 0.02 B,b | 0.60 ± 0.02 AB,b | 0.65 ± 0.02 A,b |
| BSG/Gel/Gl/15ClP | 0.46 ± 0.01 C,a | 0.53 ± 0.02 BC,ab | 0.68 ± 0.02 AB,ab | 0.73 ± 0.02 A,ab |
| Sample | 0th Day | 2nd Day | 4th Day | 6th Day |
| Heme iron (μg/g) | ||||
| CONTROL | 7.67 ± 0.16 A,a | 6.26 ± 0.21 AB,b | 5.55 ± 0.15 BC,b | 4.20 ± 0.25 C,b |
| BSG/Gel/Gl/15EG@NZ | 7.67 ± 0.16 A,a | 7.51 ± 0.25 AB,a | 6.67 ± 0.18 ABC,a | 5.04 ± 0.30 C,a |
| BSG/Gel/Gl/15ClP | 7.67 ± 0.16 A,a | 6.89 ± 0.23 AB,ab | 6.11 ± 0.17 BC,ab | 4.62 ± 0.28 C,ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntari, Z.; Kechagias, A.; Leontiou, A.A.; Vardakas, A.; Dormousoglou, M.; Angelari, T.; Zaharioudakis, K.; Stathopoulou, P.; Karahaliou, P.; Beligiannis, G.; et al. Eugenol@Natural Zeolite Nanohybrid vs. Clove Powder as Active and Reinforcement Agents in Novel Brewer’s Spent Grain/Gelatin/Glycerol Edible, High Oxygen Barrier Active Packaging Films. Appl. Sci. 2025, 15, 9282. https://doi.org/10.3390/app15179282
Ntari Z, Kechagias A, Leontiou AA, Vardakas A, Dormousoglou M, Angelari T, Zaharioudakis K, Stathopoulou P, Karahaliou P, Beligiannis G, et al. Eugenol@Natural Zeolite Nanohybrid vs. Clove Powder as Active and Reinforcement Agents in Novel Brewer’s Spent Grain/Gelatin/Glycerol Edible, High Oxygen Barrier Active Packaging Films. Applied Sciences. 2025; 15(17):9282. https://doi.org/10.3390/app15179282
Chicago/Turabian StyleNtari, Zoe, Achilleas Kechagias, Areti A. Leontiou, Alexios Vardakas, Margarita Dormousoglou, Tarsizia Angelari, Konstantinos Zaharioudakis, Panagiota Stathopoulou, Panagiota Karahaliou, Grigorios Beligiannis, and et al. 2025. "Eugenol@Natural Zeolite Nanohybrid vs. Clove Powder as Active and Reinforcement Agents in Novel Brewer’s Spent Grain/Gelatin/Glycerol Edible, High Oxygen Barrier Active Packaging Films" Applied Sciences 15, no. 17: 9282. https://doi.org/10.3390/app15179282
APA StyleNtari, Z., Kechagias, A., Leontiou, A. A., Vardakas, A., Dormousoglou, M., Angelari, T., Zaharioudakis, K., Stathopoulou, P., Karahaliou, P., Beligiannis, G., Proestos, C., Salmas, C. E., & Giannakas, A. E. (2025). Eugenol@Natural Zeolite Nanohybrid vs. Clove Powder as Active and Reinforcement Agents in Novel Brewer’s Spent Grain/Gelatin/Glycerol Edible, High Oxygen Barrier Active Packaging Films. Applied Sciences, 15(17), 9282. https://doi.org/10.3390/app15179282














