1. Introduction
Over the first twenty years of the 21st century, the consumption of lithium compounds, calculated as lithium carbonate (Li
2CO
3), used as a standard market product, has tripled, while the price has increased from USD 2 to USD 8 per kilogram. More than 35% of lithium compounds are used in the production of batteries and chemical power sources, about 32% in the production of ceramics and glass. The main applications of lithium compounds, along with nuclear and military technologies, also include the production of catalysts, greases, ultra-light alloys, and aluminum electrolysis [
1,
2,
3,
4].
According to forecasts [
5], almost all demand for lithium will be driven by electric vehicle sales and will double by 2027 and triple by 2030 compared to 2023.
The discovery and development in the 1990s of the richest lithium brine deposit in Chile brought a fundamental transformation to the lithium market. Natural brines are becoming the dominant raw material for the production of Li
2CO
3 worldwide due to lower costs compared to extraction from solid ore [
6].
A number of methods are currently used in processing hydromineral lithium raw materials: natural evaporation and precipitation, and there are schemes using extraction, sorption, and electrolysis processes. For processing natural brines, a method of evaporation and crystallization has been described [
7]. For brines containing high concentrations of calcium and magnesium, ion exchange and extraction methods [
8], and others are used.
To extract lithium from lake brine and groundwater of hydromineral lithium raw materials, new technologies and methods are being developed that improve known processes: the use of selective reversible lithium sorbents [
9], sorption technologies for the complex extraction of useful components (including lithium) from oil waters [
10,
11].
Currently, there is a development for lithium extraction from geothermal brines using integrated membrane distillation and selective electrodialysis (MD–S-ED), which has demonstrated high Li
+/Mg
2+ selectivity and low energy consumption [
12].
In countries such as the USA, Italy, Israel, New Zealand, Australia, etc., research is underway to develop methods intended to extract lithium from specific geochemical types of natural waters [
13].
Geochemical types of natural waters may vary in mineralization. They are conventionally divided by chemical composition into three types: carbonate, sulfate, and chloride. Water mineralization is the total content of dissolved salts in water. In natural brines, mineralization varies widely: from 1 g/dm
3 (fresh water) to 400 g/dm
3 or more (concentrated brines). Their main physical properties directly depend on the mineralization and chemical composition of waters [
14].
For example, in the USA, lithium carbonate and phosphate are produced from Searles Lake brines (California, USA) with mineralization around 430 g/dm
3 and a maximum Li content of 81 mg/kg, along with by-products such as soda, boron, phosphoric acid, etc. Brines from Silver Peak Lake with 180 g/dm
3 mineralization are used to produce Li
2CO
3 and a range of compounds, while from brines of the Great Salt Lake (Salton Sea, California) with 310 g/dm
3 mineralization, lithium, magnesium, and sodium chlorides as well as potassium and sodium sulfates are produced. In Israel, lithium chloride is planned to be extracted from Dead Sea brines (mineralization 300–320 g/dm
3), with LiCl reserves amounting to 17.5 million tons [
15,
16].
In the USA, Chile, Bolivia, and Argentina, lithium salts are obtained from sodium chloride-type brines, where the total content of Mg
2+ and Ca
2+ to Li
+ concentration (
R) ranges from 3 to 24. The technology is based on solar concentration of brines in natural ponds, enabling high economic efficiency [
17,
18].
Brines with R > 24 cannot be processed by classical halurgical multi-stage evaporation technology, as the brine must first be purified from a relatively high amount of magnesium and alkaline earth metal ions (Ca
2+, Sr
2+, Ba
2+), whose carbonates are less soluble than lithium carbonate. Extraction of Li
+ from such brines may be based on various selective methods of sorption, ion exchange, and extraction [
19].
In Russia, lithium-containing sodium chloride-type brines enriched with Mg
2+ and Сa
2+ are found in the North Caucasus (R > 60) and Eastern Siberia (R > 200) [
15]. For extracting Li
+ from these types of brines, halurgical technology is not cost-effective due to the need for preliminary removal of significant amounts of Mg
2+ and Сa
2+.
Instead of natural concentration of sodium chloride-type (or sulfate-chloride-type) brines, widely used in industrial practice in the USA and Chile, sorption enrichment of solutions with lithium chloride is the only viable method for industrial processing of brines with a dominant CaCl
2 and MgCl
2 background and R-values of 120–140 or higher, found in Russia and China [
13].
Lithium extraction from brines using selective reversible sorbents is the simplest and most technologically feasible method that can be easily scaled for industrial application [
20,
21].
There is a limited number of publications on lithium extraction from various hydro-mineral solutions using organic ion-exchange resins. These include paper [
21], which employed SK110 resin (sulfonated type) to remove divalent metal ions from concentrated solution.
In paper [
9], sorption was performed using industrial cation exchange resins TOKEM-160 (LLC “Trading House TOKEM”, Kemerovo, Russia) and AMBERLITE IR-120 (ROHM&HAGS, Dow Chemical, Midland, MI, USA). The sorbents were in H
+ form, spherical granules of 0.4–1.25 mm. Cation exchangers of gel-type structure were based on sulfonated polystyrene. Studies were conducted using geothermal water with high mineralization: Verkhne–Paratunskoe deposit—1.2 g/dm
3 and Pauzhetskoe deposit—2.3 g/dm
3 [
22,
23]. The heat carrier of Verkhne–Paratunskoe deposit (well 88) belongs to the sulfate–chloride, calcium–sodium, moderately siliceous thermal water group; the heat carrier of Pauzhetskoe deposit (well 103) belongs to the chloride–sodium group, high-silica solution.
Dependencies of cation concentrations in heat carriers on phase contact time under static conditions were obtained. The sorption capacities on TOKEM-160 and AMBERLITE IR-120 cation exchangers have similar values. The degree of lithium extraction from the solution of well 88 amounted to 85.7% for both cation exchange resins. The degree of lithium extraction from the solution of well 103 was 74.2% for TOKEM-160 and 73.4% for AMBERLITE IR-120; discrepancies are within the limits of analytical error [
9].
Currently, lithium raw material resources are estimated at 13 million tons, with global consumption of about 65 thousand tons. At the same time, approximately 22% of lithium is concentrated in ores and up to 78% in various types of hydro-mineral raw materials.
For Kazakhstan, due to limited lithium ore reserves, its extraction from brines enriched with calcium and magnesium chlorides and containing low metal concentrations is a pressing issue. In order to address this problem, it is necessary to determine the most economical method to process such brines, which requires a scientifically substantiated approach.
The aim of the work is to determine the capacitive properties of ionites and select the most effective ion exchange resins when performing lithium sorption from natural brines of the Aral Sea region, enriched with calcium and magnesium chlorides, with a Li concentration below the industrial one, under static conditions.
2. Materials and Methods
Materials: The following organic resins manufactured by LLC “Trading House TOKEM” (Kemerovo, Russia) were selected for this work: TOKEM-100, TOKEM-140, TOKEM 140-12, TOKEM 140-16, and TOKEM-160. The ion exchangers belong to the group of strong cation exchangers with gel structure, based on a styrene–divinylbenzene (DVB) matrix. pH range: 0–14.
Table 1 presents the characteristics of the investigated TOKEM cation exchangers.
Preparation of the ion exchangers was performed according to a standard procedure. The cationites used in the experiments are provided by the manufacturer in the ionic form H+ (hydrogen). The ion exchangers were purified from synthesis products and iron using sodium hydroxide and hydrochloric acid solutions. A certain volume of each ion exchanger pre-swollen in distilled water was loaded into glass columns. Then, 10 specific volumes of a 5% (mass) sodium hydroxide solution were passed through at a rate of 2 specific volumes/hour. To wash the ion exchangers from the alkaline solution, distilled water was passed through at a rate of 5 specific volumes/hour until a neutral reaction was achieved. Then, a 5% (volume) hydrochloric acid solution was passed through each column at a rate of 2 specific volumes/hour until a negative reaction for iron ions was achieved. The ion exchangers were washed from the acid with distilled water until pH = 6–7. Iron was monitored using ammonium thiocyanate, sodium hydroxide, and acid using phenolphthalein and methyl orange. Prior to testing, the ion exchangers underwent preliminary preparation, including backwashing with water (3–5 volumes of water per 1 volume of ion exchanger) until the filtrate became clear.
Preparation of natural brines for the experiments was conducted in several stages: preliminary settling, followed by decantation of the clarified part of the brine. The remaining solution with sediment and the clarified part were then filtered. Filtration was performed using a vacuum pump.
Experimental Procedure. To determine the static exchange capacity (SEC), 10 g of cation exchanger was placed in dry 1 dm
3 flasks, 0.5 dm
3 of solution was added, and the flasks were placed on a stirring device. Contact of the cation exchangers with the solution was maintained for 29 days. The phase ratio was selected so that the change in solution volume during sampling for analysis did not exceed 3%. The solutions were separated from the ion exchanger by vacuum filtration and systematically analyzed at certain intervals for lithium, magnesium, sodium, and calcium. SEC of the cation exchangers for metals was calculated based on the difference in concentrations of lithium, magnesium, sodium, and calcium in the initial solution and in the filtrate, taking into account the cation exchanger weight and solution volume, using the standard formula:
where C
0 and C—concentrations of lithium and other studied metals (Ca, Mg, and Na) in the original brine and after the sorption process, respectively, in mg/dm
3;
V—volume of brine, dm
3; and
W—weight of ion exchanger, g.
Extraction rate (E
Me) of metals from brine, %:
After contact with the solutions, the cation exchangers were dried at 50 °C to constant weight in a SNOL 60/300 drying oven.
Analytical Methods. Natural brines and post-sorption solutions were analyzed for lithium content using atomic emission spectroscopy on a dual-beam atomic absorption spectrometer Agilent 240FS/AA (Agilent Technologies, Santa Clara, CA, USA). The instrument is equipped with an automatic monochromator and flame slit width control (acetylene–air flame).
The quantitative content of the main elements in the solutions was determined using the Optima 8300DV (Perkin Elmer, Shelton, WA, USA) atomic absorption spectrometer with inductively coupled plasma (ICP) and the PFP 7 flame photometer (Jenway, UK).
Cation exchangers before and after sorption were studied using IR spectroscopy. Infrared absorption spectra were recorded on an Avatar 370 FT-IR (Thermo Nicolet Corporation, Madison, WI, USA) spectrometer in the 4000–250 cm−1 range using sample material and Vaseline oil on KRS-5 windows. Experimental attachment: “Transmission E.S.P.” accessory.
3. Results and Discussion
The object of this study was natural brines of the Pre-Aral region enriched with calcium and magnesium chlorides, composition in mg/dm3: Ca–560.6; Mg–644.5; and Na–2209.9. The lithium concentration in the brines was below industrial levels and amounted to 0.42 mg/dm3; the pH of the brine is 5.5–6.
Given the composition of the brines and the concentration of such macro impurities as magnesium and calcium, several methods were previously used to remove the present metals: precipitation with sulfuric acid and passing carbon dioxide (СO
2) through the brines. However, these approaches produced negative results. A method of purifying natural brines was selected, which included two stages: Initially, the solution was purified from magnesium. In the second stage, calcium carbonate was precipitated using soda ash. The effect of the amount of Ca(OH)
2 and Na
2СO
3 (100 ÷ 150%, step 10) according to stoichiometry at each stage of the impurity removal process was studied. The optimal purification conditions for brines were found to be 150% Ca(OH)
2, 110% Na
2СO
3 at room temperature. In the process of purifying brine samples from calcium and magnesium, their extraction amounted to 90% and 80%, respectively [
24]. During the two-stage purification of natural brines, there is a significant reduction in the lithium concentration to values well below 0.1 mg/dm
3.
It is known that in uranium production, preliminary purification of productive solutions is not performed before sorption on anion exchangers. After desorption, the enriched solutions are subjected to secondary concentration by removing such impurities as iron [
25]. A similar approach is used in the sorption of rhenium from sulfuric acid solutions without preliminary impurity removal [
26]. Therefore, further preliminary purification of the brines from magnesium and calcium impurities was not performed.
At the next stage, to obtain more detailed data, it was of interest to study the effect of natural brines on the sorption capacity of lithium, magnesium, sodium, and calcium on ion exchangers of the brand TOKEM TC LLC (Kemerovo, Russia), which are currently recommended for lithium extraction.
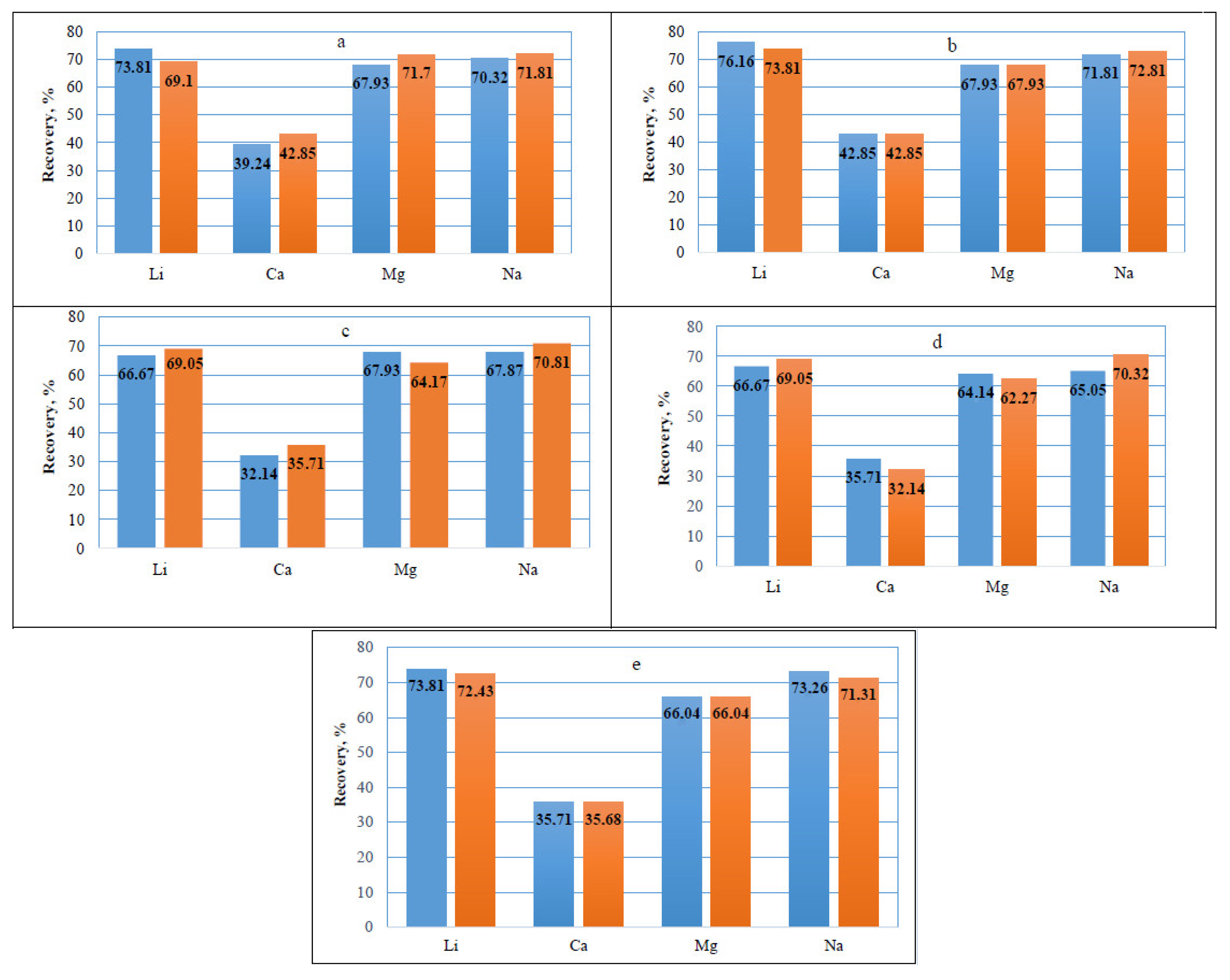
The histograms (
Figure 1) show the dependence of lithium, sodium, calcium, and magnesium extraction on the duration of the sorption process for the studied cation exchangers according to Formula (2).
As can be seen from
Figure 1a, sodium is also transferred into the ion exchange resin along with lithium. The extraction of Li and Na remains at nearly the same level for each of the applied cation exchangers. However, the degree of transfer of these metals from the solutions into TOKEM-140 (
Figure 1b) and TOKEM-160 (
Figure 1e), depending on the sorption duration, is higher than for any others and varies within the following limits, %: for TOKEM-140: Li from 76.2 to 73.8; Na from 71.8 to 72.8; for TOKEM-160: Li from 73.8 to 72.4; Na from 73.3 to 71.3, respectively.
A decrease in the lithium extraction rate and that of other macro impurities is also observed in monodisperse cation exchangers of the same gel structure class: from TOKEM-140, TOKEM-140-12 to TOKEM-140-16 (
Figure 1b–d). This may be related to the different amounts of crosslinking agents used to improve polymer properties. For example, lithium extraction into the TOKEM-140 resin after 2 weeks of sorption under static conditions is 76.2%, and under the same conditions, it decreases to 66.7% when using TOKEM-140-16 resin.
Calcium extraction into the cation exchangers (
Figure 1) varies on average within ~32 to 43%, and magnesium from 60 to 70%. By the degree of extraction into the resin, the metal ions can be arranged in the following sequence: Ca
2+ < Mg
2+ < Li
+, Na
+.
Analysis of the histograms (
Figure 1) showed that lithium and impurities such as magnesium, sodium, and calcium present in the brines are sorbed by the investigated ion exchanger brands differently.
Table 2 presents data on the static exchange capacity (SEC) of the sorption process for Li, Ca, Mg, and Na from the natural brine according to Formula (1).
As shown in
Table 2, the sorption of lithium is significantly influenced by ions of both alkali (Na
+) and alkaline earth metals (Ca
2+ and Mg
2+). The presence of sodium, magnesium, and calcium ions reduces the sorption of Li
+ ions.
The maximum static exchange capacity for sodium ions is explained by their higher concentration in the solution compared to other cations. The value of the static exchange capacity in relation to cations forms the following series: Li+ << Mg2+< Ca2+ << Na+ relative to the obtained concentrations of metal ions in natural brines.
It should be noted that the sorption capacity of gel-type ion exchangers differs, primarily due to their dispersity and the amount of crosslinking agent (e.g., for TOKEM-140). Unfortunately, manufacturers of cation exchangers do not provide their full specifications in certificates. These factors play an important role in ion exchange processes.
For this reason, it was not possible to obtain complete information on the influence of ion exchangers on the sorption of metals from natural brines.
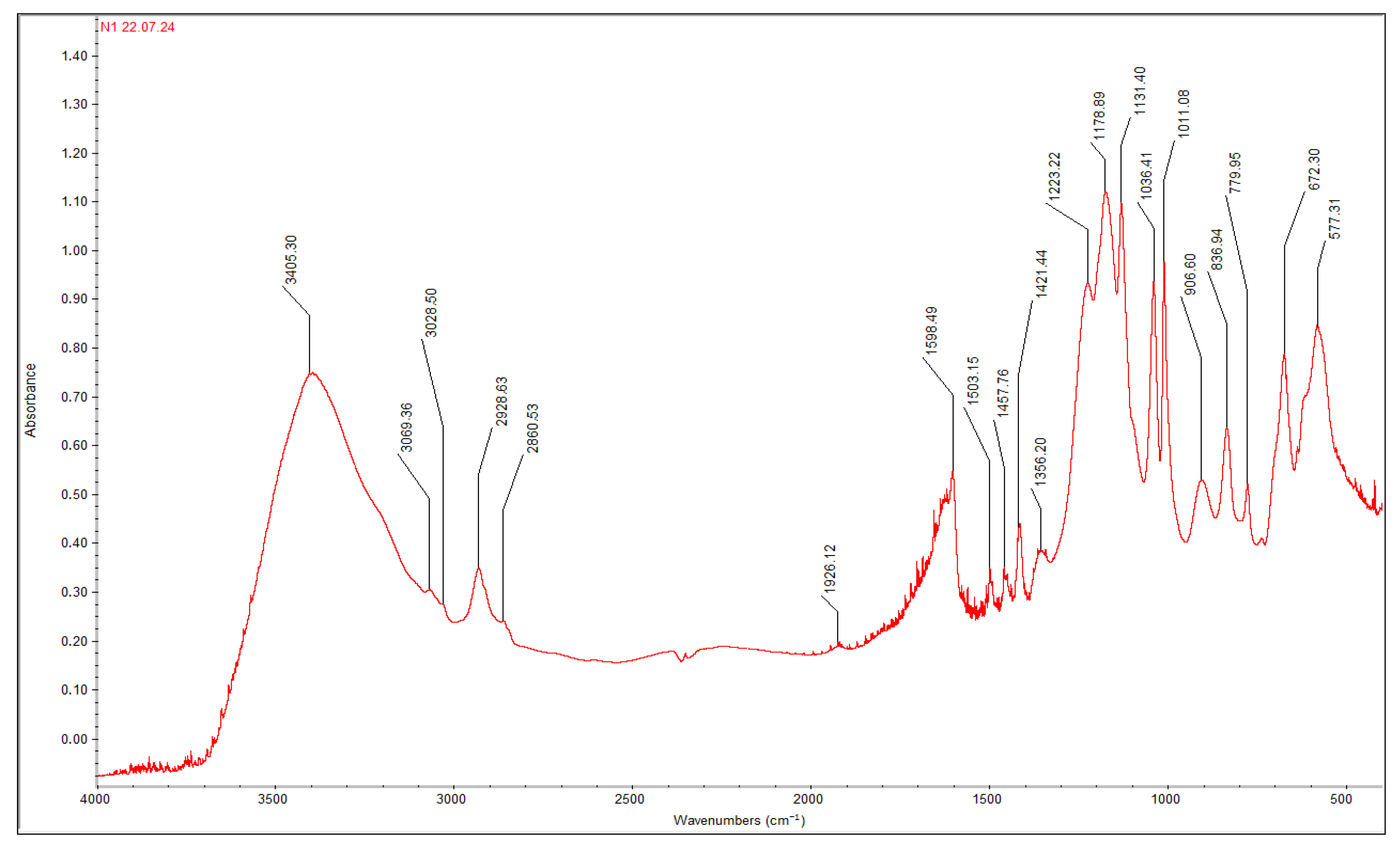
Figure 2 shows the IR spectrum of one representative cation exchanger—TOKEM-100. The spectrum contains bands at 1356.20, 1223.22, 1178.89, 1131.40, 1036.41, and 1011.08 cm
−1 [
27], which correspond to symmetric stretching S=O vibrations in the –SO
3H-group. There are two main types of vibrations: (1) stretching (ν), characterized by a change in bond lengths, and (2) deformation (δ), in which one or more bond angles change, while the bond lengths remain unchanged. The frequencies of stretching vibrations are usually higher than the frequencies of deformation vibrations. Both types of vibrations are divided into symmetrical (ν
s, δ
s), in which the symmetry of the atomic group does not change, that is, where both bonds are stretched or compressed simultaneously, and asymmetrical (ν
as, δ
as), in which the symmetry of the molecule or complex ion changes during the vibration [
27].
The band at 906.60 cm
−1 corresponds to S-OH single bond stretching vibrations. C-S vibrations in sulfonic cation exchangers based on polystyrene containing sulfo groups in the benzene ring (ortho-isomer) are characterized by a line at 577.31 cm
−1 [
27].
The spectrum contains stretching vibrations νOH of water molecules and νOH in SO
3H groups at 3405.30 cm
−1. Wavenumbers 3069.36 and 3028.50 cm
−1 correspond to C-H stretching in aromatic rings. СH
2 group stretching vibrations are characterized by wavenumbers 2928.63 and 2860.53 cm
−1. CH deformation vibrations in СH
2 groups are characterized by 1457.76 and 1421.44 cm
−1 [
27].
Out-of-plane CH deformation vibrations of para-substituted benzene rings in polystyrene-based ion exchangers are characterized by wavenumbers 836.94 and 779.95 cm−1. CH deformation vibrations in aromatic derivatives relate to wavenumber 672.30 cm−1.
Stretching vibrations of C=C in aromatic rings correspond to bands at 1598.49, 1503.15, and 1457.76 cm
−1 [
27].
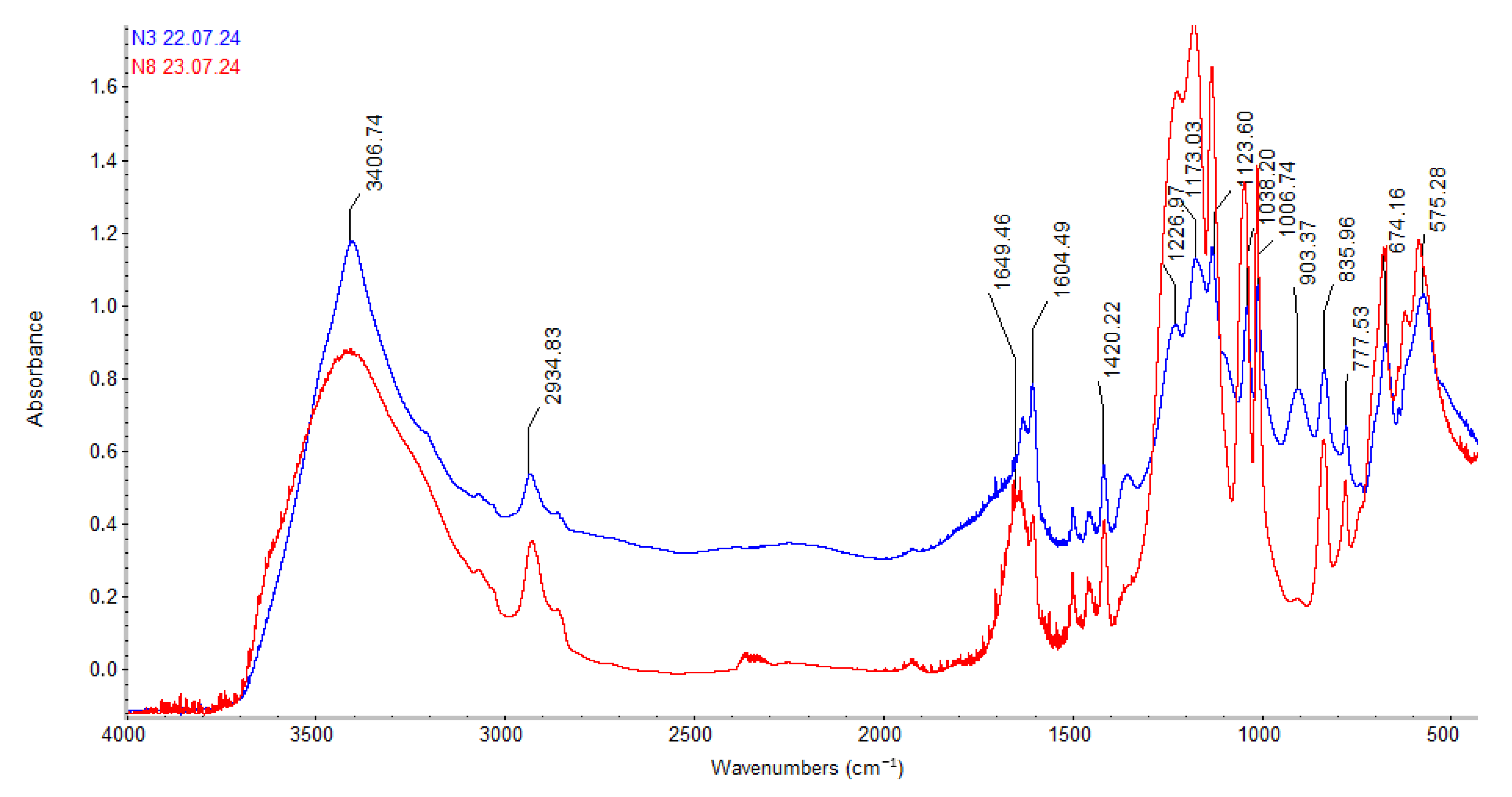
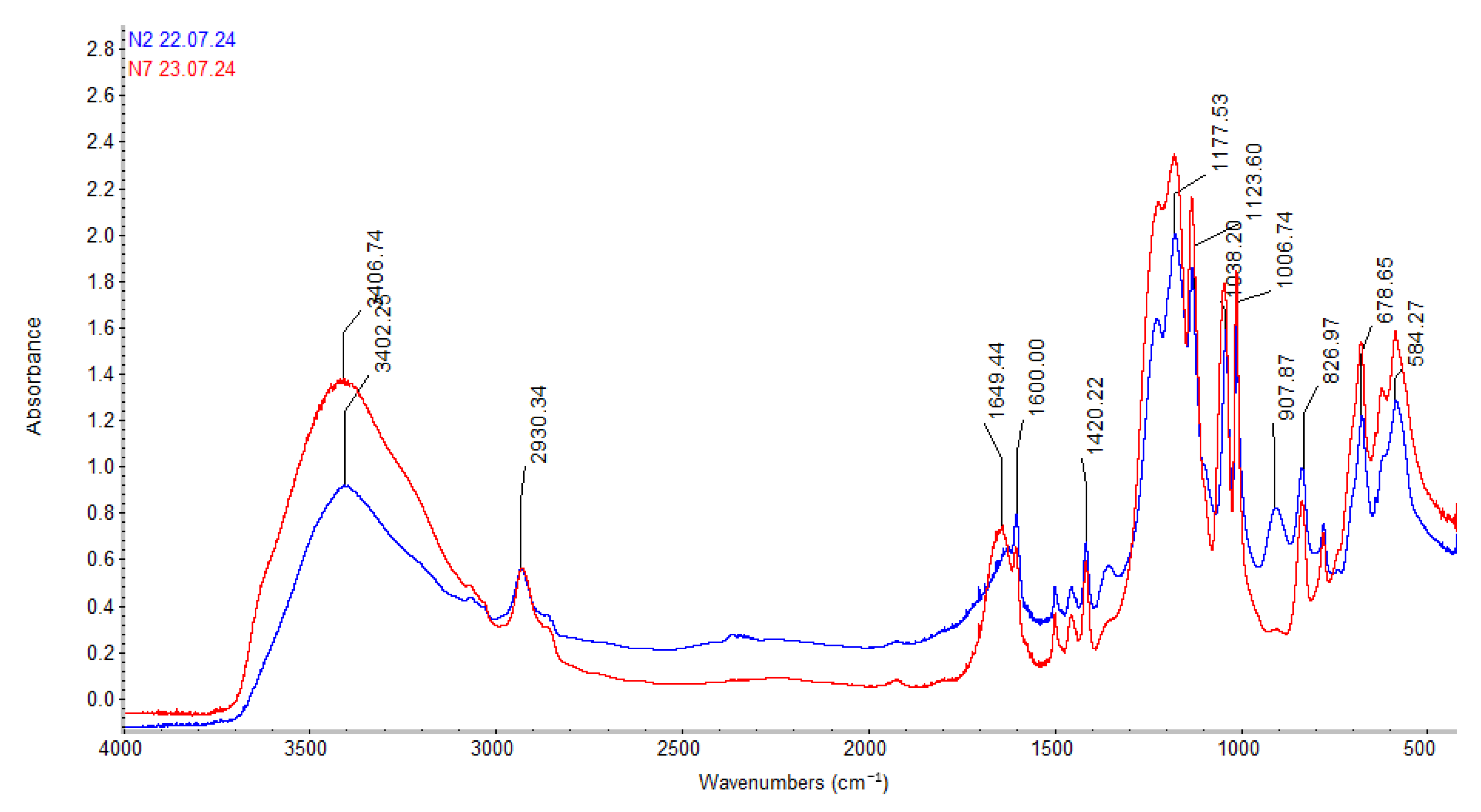
Figure 3 and
Figure 4 show IR spectra of TOKEM-140 and TOKEM-160 cation exchangers, respectively, in the original form (curve 1, blue) and after lithium sorption (curve 2, red) under static conditions.
As shown in
Figure 3, the IR spectrum profile of TOKEM-140 after sorption (curve 2, red) changes slightly. By comparing the wavenumbers (
Table 3), a slight shift of certain bands toward the high-frequency region can be observed, indicating the occurrence of the sorption process.
Table 3 presents the wavenumbers characterizing the IR spectra of TOKEM-140 cation exchanger before and after sorption.
A detailed study of the peaks in
Figure 3 shows (
Table 3) that some absorption bands at the corresponding wave numbers tend to either shift to the low- or high-wave region. This may indicate the occurrence of a sorption process from natural brines.
A similar pattern is observed in the IR spectrum of the TOKEM-160 cation exchanger (
Figure 4). The numerical values of the wavenumbers change within the same range (
Table 3).
Analysis of the obtained results and spectrograms (
Figure 3 and
Figure 4) showed that the sorption capacity of the studied ion exchangers toward lithium and macro impurities present in the natural brines of the Pre-Aral region varies. Lithium is sorbed more preferentially by the TOKEM-140 and TOKEM-160 cation exchangers.
 —2 weeks;
—2 weeks;  —4 weeks; TOKEM: (a)—100, (b)—140, (c)—140-12, (d)—140-16, and (e)—160.
—4 weeks; TOKEM: (a)—100, (b)—140, (c)—140-12, (d)—140-16, and (e)—160.
 —2 weeks;
—2 weeks;  —4 weeks; TOKEM: (a)—100, (b)—140, (c)—140-12, (d)—140-16, and (e)—160.
—4 weeks; TOKEM: (a)—100, (b)—140, (c)—140-12, (d)—140-16, and (e)—160.