The Effects of Non-Viable Probiotic Lactobacillus paracasei on the Biotechnological Properties of Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of the Functional Efficacy of Paraprobiotics Using the Yeast Model
- Obtaining Paraprobiotics
- Obtaining the Yeast Cell Suspension
- Conditions for Yeast Multiplication
- Determination of Dry Matter Biomass Yield
- Parameters of Yeast Multiplication in the Presence of Paraprobiotics
- Evaluation of Yeast Cell Viability
- Evaluation of Yeast Stability During Storage
- Evaluation of the Fermentative Capacity of Yeast
2.2. Statistical Analysis
3. Results
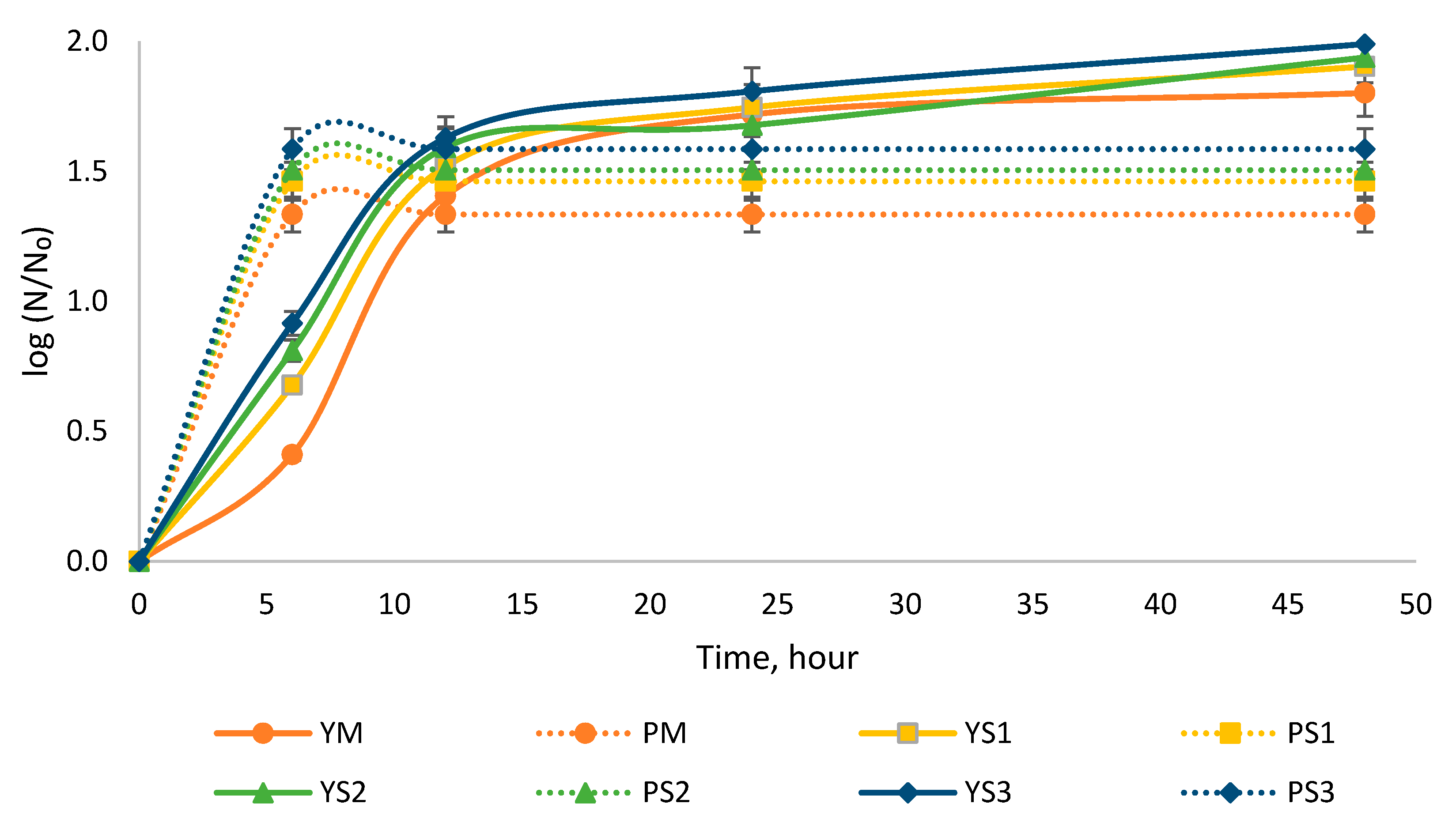
3.1. Evaluation of the Efficacy of Paraprobiotics on the Multiplication of S. cerevisiae
3.2. Evaluation of the Fermentative Activity of Yeast in the Presence of Paraprobiotics
3.3. The Effect of Paraprobiotics on Yeast Viability During Wet Biomass Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Saygili, D.; Yerlikaya, O.; Akpinar, A. The Effect of Using Different Yeast Species on the Composition of Carbohydrates and Volatile Aroma Compounds in Kefir Drinks. Food Biosci. 2023, 54, 102867. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tsao, M. Enhancement of Survival of Probiotic and Non-Probiotic Lactic Acid Bacteria by Yeasts in Fermented Milk under Non-Refrigerated Conditions. Int. J. Food Microbiol. 2009, 135, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Niu, Y.; Chen, Z.; Dong, S.; Li, H. Discovery of Novel Lactobacillus Plantarum Co-Existence-Associated Influencing Factor(s) on Saccharomyces Cerevisiae Fermentation Performance. LWT 2021, 135, 110268. [Google Scholar] [CrossRef]
- Nenciarini, S.; Reis-Costa, A.; Pallecchi, M.; Renzi, S.; D’Alessandro, A.; Gori, A.; Cerasuolo, B.; Meriggi, N.; Bartolucci, G.L.; Cavalieri, D. Investigating Yeast–Lactobacilli Interactions through Co-Culture Growth and Metabolite Analysis. Fermentation 2023, 9, 933. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, Y.; Xie, Y.; Zhang, H.; Jiang, K.; Pang, X.N.; Huang, M. Metabolic Characteristics of Lactic Acid Bacteria and Interaction with Yeast Isolated from Light-Flavor Baijiu Fermentation. Food Biosci. 2022, 50, 102102. [Google Scholar] [CrossRef]
- Tamang, J.P.; Lama, S. Probiotic Properties of Yeasts in Traditional Fermented Foods and Beverages. J. Appl. Microbiol. 2022, 132, 3533–3542. [Google Scholar] [CrossRef]
- Caetano, C.F.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A.; Rolo, J. The Role of Yeasts in Human Health: A Review. Life 2023, 13, 924. [Google Scholar] [CrossRef]
- Tullio, V. Probiotic Yeasts: A Developing Reality? J. Fungi 2024, 10, 489. [Google Scholar] [CrossRef]
- Nambiar, R.B.; Perumal, A.B.; Shittu, T.; Sadiku, E.R.; Sellamuthu, P.S. Editorial: Probiotics, Prebiotics, Synbiotics, Postbiotics, & Paraprobiotics—New Perspective for Functional Foods and Nutraceuticals. Front. Nutr. 2023, 10, 1164676. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Schnorr, C.E.; de Pasquali, M.A.B. Paraprobiotics and Postbiotics—Current State of Scientific Research and Future Trends toward the Development of Functional Foods. Foods 2023, 12, 2394. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.L.; da Silva, T.F.; de Jesus, L.C.L.; Coelho-Rocha, N.D.; Barroso, F.A.L.; Tavares, L.M.; Azevedo, V.; Mancha-Agresti, P.; Drumond, M.M. Probiotics, Prebiotics, Synbiotics, and Paraprobiotics as a Therapeutic Alternative for Intestinal Mucositis. Front. Microbiol. 2020, 11, 544490. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Farhangfar, A.; Moradi, M.; Dalir-Naghadeh, B. Beyond Probiotics: Exploring the Potential of Postbiotics and Parabiotics in Veterinary Medicine. Res. Vet. Sci. 2024, 167, 105133. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liang, L.; Lv, P.; Liu, L.; Wang, S.; Wang, Z.; Chen, Y. Effects of Non-Viable Lactobacillus Reuteri Combining with 14-Day Standard Triple Therapy on Helicobacter Pylori Eradication: A Randomized Double-Blind Placebo-Controlled Trial. Helicobacter 2021, 26, e12856. [Google Scholar] [CrossRef]
- Sañudo, A.I.; Luque, R.; Díaz-Ropero, M.P.; Fonollá, J.; Bañuelos, Ó. In Vitro and in Vivo Anti-Microbial Activity Evaluation of Inactivated Cells of Lactobacillus Salivarius CECT 5713 against Streptococcus Mutans. Arch. Oral Biol. 2017, 84, 58–63. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, M.; Kang, T.; He, Y.; Zhang, J.; Zhao, Y.; Xiao, X. The Role of Inactivation Methods in Shaping Postbiotic Composition and Modulating Bioactivity: A Review. Foods 2025, 14, 2358. [Google Scholar] [CrossRef]
- Dangi, A.K.; Dubey, K.K.; Shukla, P. Strategies to Improve Saccharomyces Cerevisiae: Technological Advancements and Evolutionary Engineering. Indian J. Microbiol. 2017, 57, 378. [Google Scholar] [CrossRef]
- Pérez-Alvarado, O.; Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; Vinderola, G.; García-Cayuela, T. Role of Lactic Acid Bacteria and Yeasts in Sourdough Fermentation during Breadmaking: Evaluation of Postbiotic-like Components and Health Benefits. Front. Microbiol. 2022, 13, 969460. [Google Scholar] [CrossRef]
- Vanderwaeren, L.; Dok, R.; Voordeckers, K.; Nuyts, S.; Verstrepen, K.J. Saccharomyces Cerevisiae as a Model System for Eukaryotic Cell Biology, from Cell Cycle Control to DNA Damage Response. Int. J. Mol. Sci. 2022, 23, 11665. [Google Scholar] [CrossRef]
- Botstein, D.; Fink, G.R. Yeast: An Experimental Organism for 21st Century Biology. Genetics 2011, 189, 695–704. [Google Scholar] [CrossRef]
- Maehata, H.; Arai, S.; Iwabuchi, N.; Abe, F. Immuno-Modulation by Heat-Killed Lacticaseibacillus Paracasei MCC1849 and Its Application to Food Products. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008292. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1. [Google Scholar] [CrossRef]
- Cotârleț, M.; Pihurov, M.; Păcularu-Burada, B.; Vasile, A.M.; Bahrim, G.E.; Grigore-Gurgu, L. Selection of New Lactobacilli Strains with Potentially Probiotic Properties. Ann. Univ. Dunarea de Jos Galati. Fascicle VI—Food Technol. 2023, 47, 27–50. [Google Scholar] [CrossRef]
- Barros, C.P.; Pires, R.P.S.; Guimarães, J.T.; Abud, Y.K.D.; Almada, C.N.; Pimentel, T.C.; Sant’Anna, C.; De-Melo, L.D.B.; Duarte, M.C.K.H.; Silva, M.C.; et al. Ohmic Heating as a Method of Obtaining Paraprobiotics: Impacts on Cell Structure and Viability by Flow Cytometry. Food Res. Int. 2021, 140, 110061. [Google Scholar] [CrossRef] [PubMed]
- Stănciuc, N.; Borda, D.; Gurgu-Grigore, L.; Cotârleț, M.; Vasile, A.M.; Nistor, V.O.; Dumitrașcu, L.; Pihurov, M.; Păcularu-Burda, B.; Bahrim, G.E. Lactiplantibacillus Plantarum MIUG BL21 Paraprobiotics: Evidences on Inactivation Kinetics and Their Potential as Cytocompatible and Antitumor Alternatives. Food Chem. X 2024, 21, 101114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, F.; Ji, B.; Li, B.; Luo, Y.; Yang, L.; Li, T. Symbiosis between Microorganisms from Kombucha and Kefir: Potential Significance to the Enhancement of Kombucha Function. Appl. Biochem. Biotechnol. 2010, 160, 446–455. [Google Scholar] [CrossRef]
- Michelutti, L.; Bulfoni, M.; Nencioni, E. A Novel Pharmaceutical Approach for the Analytical Validation of Probiotic Bacterial Count by Flow Cytometry. J. Microbiol. Methods 2020, 170, 105834. [Google Scholar] [CrossRef]
- Kahr, H.; Helmberger, S.; Jäger, A.G. Yeast Adaptation on the Substrate Straw. In Proceedings of the Bioenergy Technology, World Reneable Energy Congress, Linkoping, Sweden, 8–13 May 2011. [Google Scholar]
- Molodoi, E.; Efremova, N.; Usatîi, A.; Fulga, L. The Optimization of Nutritive Medium Composition and Cultivation Conditions for Saccharomyces cerevisiae CNMN-Y-18 Yeast Strain-Mannans Producer. 2014. Available online: https://bioresearch.ro/2014-1/033-038-AUOFB.21.1.2014.MOLODOI.E.-The.optimization.of.pdf (accessed on 15 July 2025).
- Râpeanu, G.; Bolocan, A.; Gazi, I.; Bahrim, G. Metabolic Activity Stimulation of the Wine Yeasts by Polyphenols Extracted from Red Grapes. Roum. Biotechnol. Lett. 2008, 13, 9–16. [Google Scholar]
- Gomes, C.S.; Strangfeld, M.; Meyer, M. Diauxie Studies in Biogas Production from Gelatin and Adaptation of the Modified Gompertz Model: Two-Phase Gompertz Model. Appl. Sci. 2021, 11, 1067. [Google Scholar] [CrossRef]
- Federal Agency for Technical Regulation and Metrology (Rosstandart). Baker’s Pressed Yeast—Technical Specifications; Standartinform: Moscow, Russia, 2013; p. 9. (In Russian) [Google Scholar]
- Yuraskina, T. Development of a Biotechnology for Producing Trace Element-Enriched Saccharomyces Cerevisiae Yeast; All-Russian Scientific Research Institute of Food Biotechnology: Moscow, Russia, 2024. (In Russian) [Google Scholar]
- Dabija, A.; Sion, I. The Increment of the Conservation Period for the Biotechnological Qualities of the Bakery Yeast. In Proceedings of the Integrated Systems for Agri-Food Production ISAP’03, Timișoara, Romania, 20–22 November 2003; pp. 52–56. [Google Scholar]
- Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A.I. Cellular Stress Impact on Yeast Activity in Biotechnological Processes—A Short Overview. Microorganisms 2023, 11, 2522. [Google Scholar] [CrossRef]
- Pejin, D.J.; Grujić, O.S.; Pejin, J.D.; Kocić-Tanackov, S.D. Effect of Yeast Storage Temperature and Flour Composition on Fermentative Activities of Baker’s Yeast. Zbornik Matice Srpske Za Prirodne Nauke 2009, 116, 305–313. [Google Scholar] [CrossRef]
- Melin, P.; Håkansson, S.; Eberhard, T.H.; Schnürer, J. Survival of the Biocontrol Yeast Pichia Anomala after Long-Term Storage in Liquid Formulations at Different Temperatures, Assessed by Flow Cytometry. J. Appl. Microbiol. 2006, 100, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, F. Non-Structured Kinetic Model for the Cell Growth of Saccharomyces Cerevisiae in a Batch Culture. Iran. J. Energy Environ. 2014, 5, 8–12. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Gioulatos, S.; Tsakalidou, E.; Kalantzopoulos, G. Interactions between Saccharomyces Cerevisiae and Lactic Acid Bacteria in Sourdough. Process Biochem. 2006, 41, 2429–2433. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of Probiotic Yeast and Lactic Acid Bacteria as Starter Culture to Produce Maize-Based Beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Zahidah, I.; Bölek, S.; Terzioğlu, Ö.T.; Adıgüzel, S. Determination of the Effects of Novel Paraprobiotic Supplement of Lactobacillus Plantarum on Soy Dairy-Free Beverage by Physicochemical, Antioxidant, Sensory Analyses, and Raman Spectroscopy Technique. J. Food Sci. 2024, 89, 7189–7202. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Song, H.J.; Park, S.Y.; Oh, D.N.; Kim, G.Y.; Been, N.Y.; Kim, D.Y.; Lee, E.J.; Nam, B.H.; Lee, J.M. Comparative Effects of Probiotics and Paraprobiotics Derived from Lactiplantibacillus Plantarum, Latilactobacillus Sakei, and Limosilactobacillus Reuteri in a DSS-Induced Ulcerative Colitis Mouse Model. J. Microbiol. Biotechnol. 2025, 35, e2411045. [Google Scholar] [CrossRef]
| Parameters | M | S1 | S2 | S3 |
|---|---|---|---|---|
| A | 1.334 ± 0.320 | 1.461 ± 0.273 | 1.505 ± 0.243 | 1.586 ± 0.235 |
| (µmax × e)/A | 195.800 ± 5.803 | 196.300 ± 4.922 | 196.500 ± 4.374 | 196.800 ± 4.247 |
| λ | −0.010 | |||
| R2 | 0.538 | 0.657 | 0.720 | 0.752 |
| MSD | 0.422 | |||
| Sample | Generation Numbers (n) | Multiplication Rate (v), 1/h | Generation Time (tg), h |
|---|---|---|---|
| M | 1.363 ± 0.101 b | 0.227 ± 0.060 b | 4.403 ± 0.474 a |
| S1 | 2.252 ± 0.320 ab | 0.375 ± 0.101 ab | 2.665 ± 0.653 b |
| S2 | 2.695 ± 0.379 a | 0.449 ± 0.014 a | 2.226 ± 0.133 b |
| S3 | 3.042 ± 0.536 a | 0.507 ± 0.094 a | 1.972 ± 0.081 b |
| Sample | M | S1 | S2 | S3 |
|---|---|---|---|---|
| Pick-up time, min | 14.450 ± 0.104 a* | 14.200 ± 0.153 a | 13.410 ± 0.108 b | 13.260 ± 0.168 b |
| Sample | Viability of Cells, % | ||
|---|---|---|---|
| 48 h | 7 day | 14 day | |
| M | 90.74 ± 0.63 a* | 89.44 ± 0.51 a | 88.24 ± 0.44 a |
| S1 | 93.11 ± 0.39 b | 92.68 ± 1.25 b | 91.57 ± 0.83 b |
| S2 | 94.48 ± 0.60 c | 94.18 ± 0.80 c | 94.15 ± 0.64 c |
| S3 | 95.57 ± 0.74 d | 95.39 ± 0.76 d | 95.45 ± 1.25 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pihurov, M.; Cotârleț, M.; Borda, D.; Bahrim, G.E. The Effects of Non-Viable Probiotic Lactobacillus paracasei on the Biotechnological Properties of Saccharomyces cerevisiae. Appl. Sci. 2025, 15, 9221. https://doi.org/10.3390/app15169221
Pihurov M, Cotârleț M, Borda D, Bahrim GE. The Effects of Non-Viable Probiotic Lactobacillus paracasei on the Biotechnological Properties of Saccharomyces cerevisiae. Applied Sciences. 2025; 15(16):9221. https://doi.org/10.3390/app15169221
Chicago/Turabian StylePihurov, Marina, Mihaela Cotârleț, Daniela Borda, and Gabriela Elena Bahrim. 2025. "The Effects of Non-Viable Probiotic Lactobacillus paracasei on the Biotechnological Properties of Saccharomyces cerevisiae" Applied Sciences 15, no. 16: 9221. https://doi.org/10.3390/app15169221
APA StylePihurov, M., Cotârleț, M., Borda, D., & Bahrim, G. E. (2025). The Effects of Non-Viable Probiotic Lactobacillus paracasei on the Biotechnological Properties of Saccharomyces cerevisiae. Applied Sciences, 15(16), 9221. https://doi.org/10.3390/app15169221










