Exploring the Functional Potential of the Xyrophytic Greek Carob (Ceratonia siliqua, L.) Cold Aqueous and Hydroethanolic Extracts
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Extraction
2.2. Evaluation of Antimicrobial Activity
2.3. Spectrophotometric Assays
2.3.1. Determination of Total Phenolic Content (TPC)
2.3.2. Ferric Reducing/Antioxidant Power Assay (FRAP)
2.3.3. Scavenging Activity on 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic Acid) Radical (ABTS•+)
2.4. Phenolic Profile by LC-QToF-MS Analysis
2.4.1. Reagents and Materials
2.4.2. Mass Spectra Analysis
2.5. In Vitro Antidiabetic Activity
2.6. Statistical Analysis of Results
3. Results
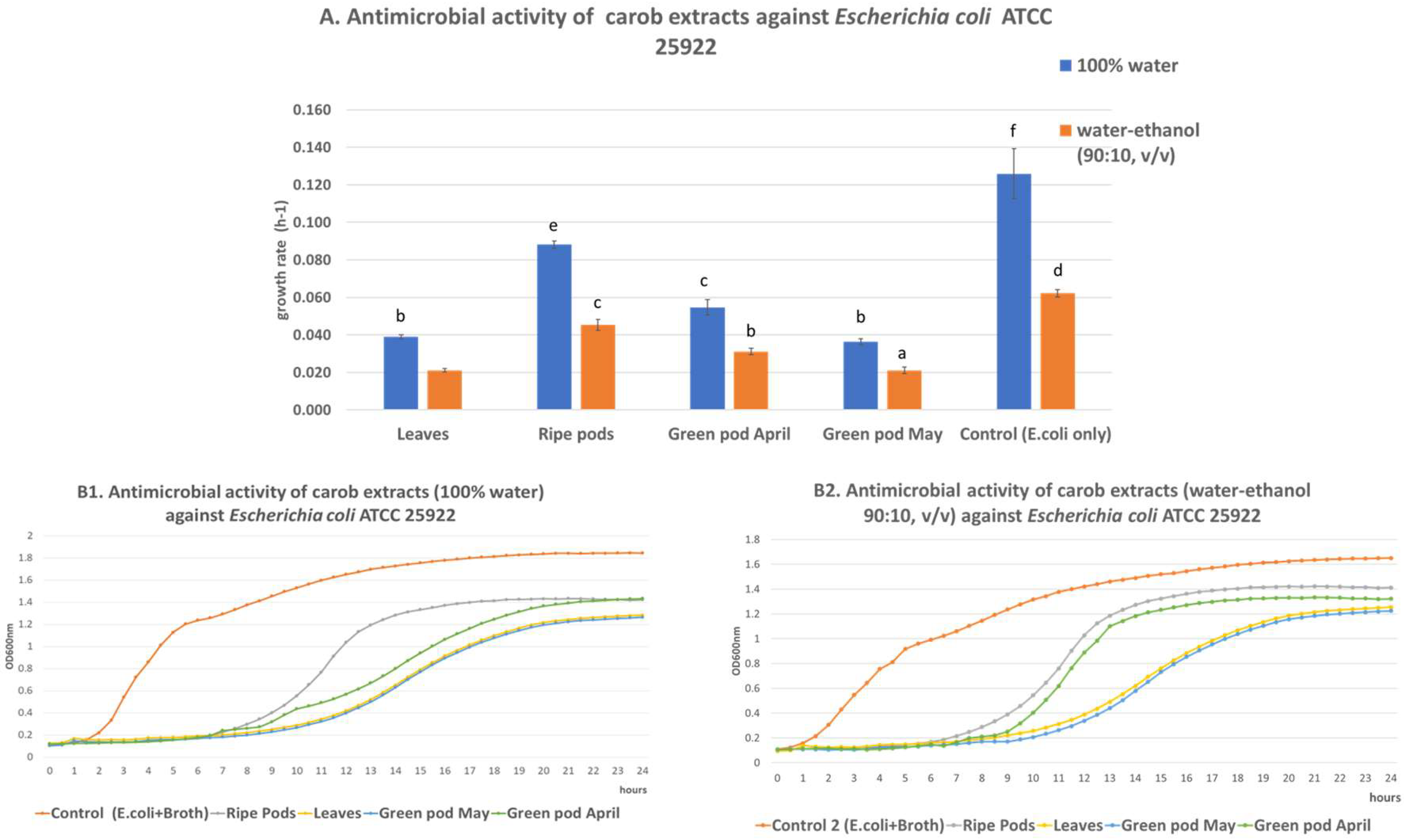
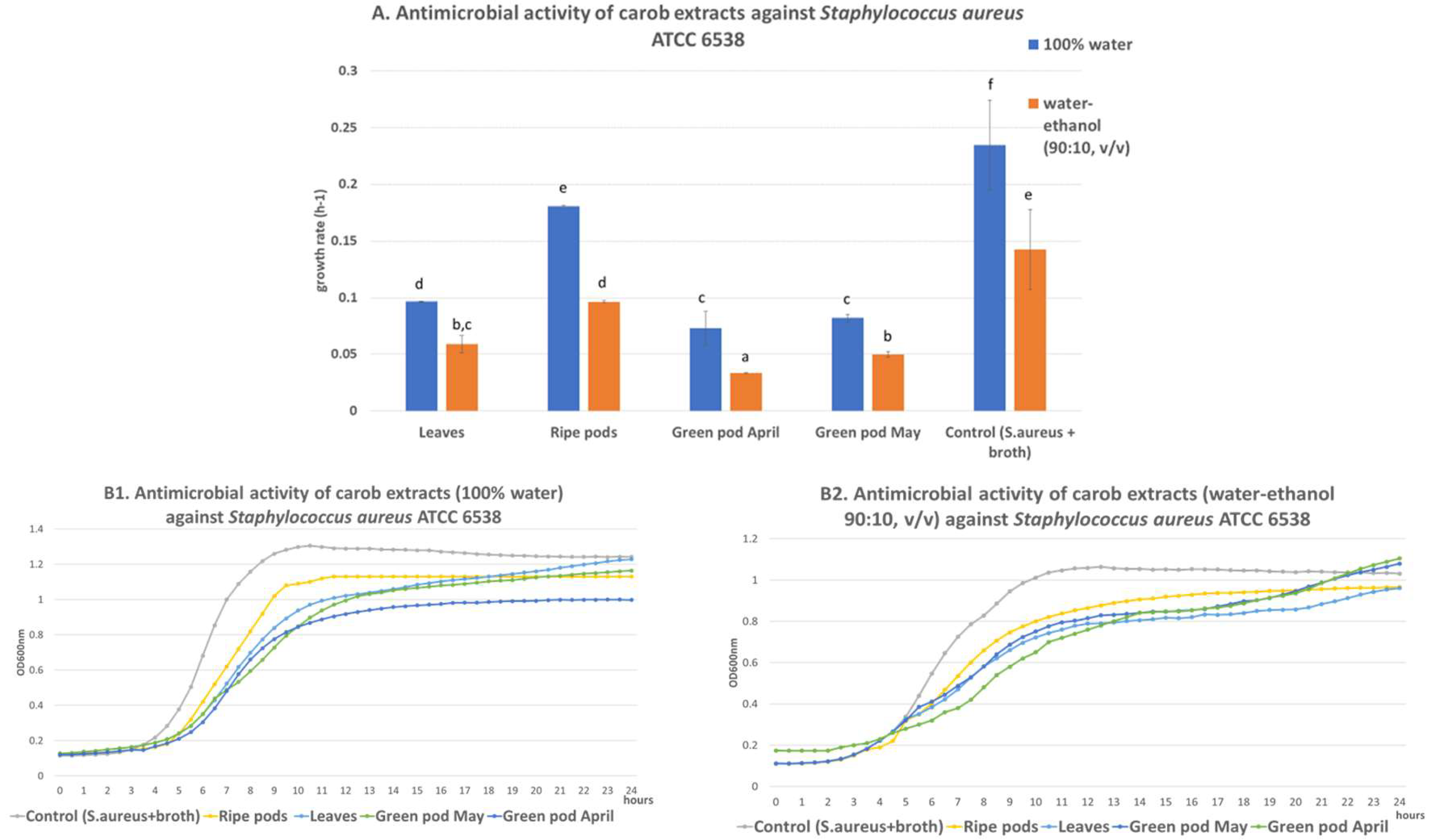
3.1. Antimicrobial Activity
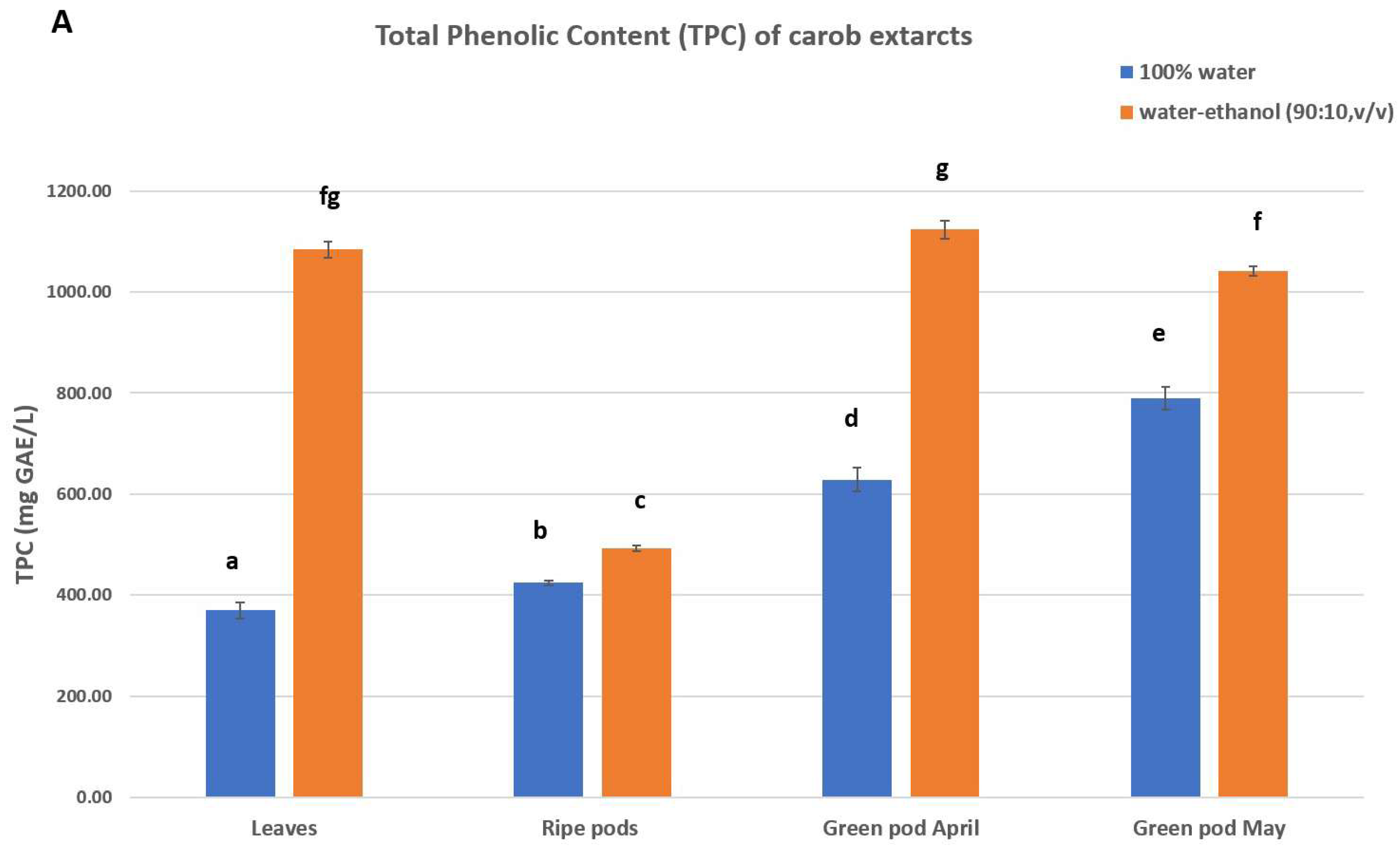
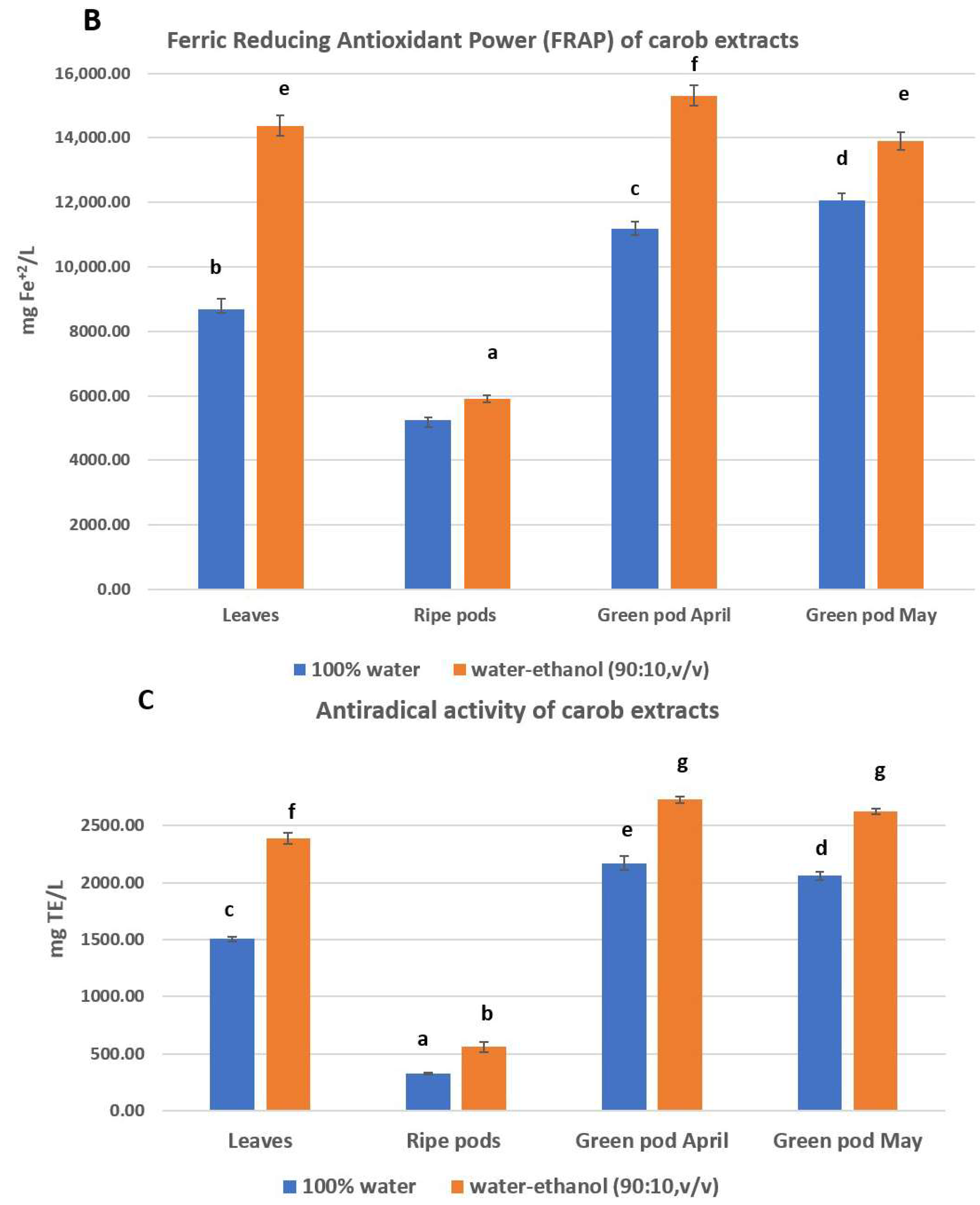
3.2. Total Phenolic Content (TPC), Antioxidant and Antiradical Activity
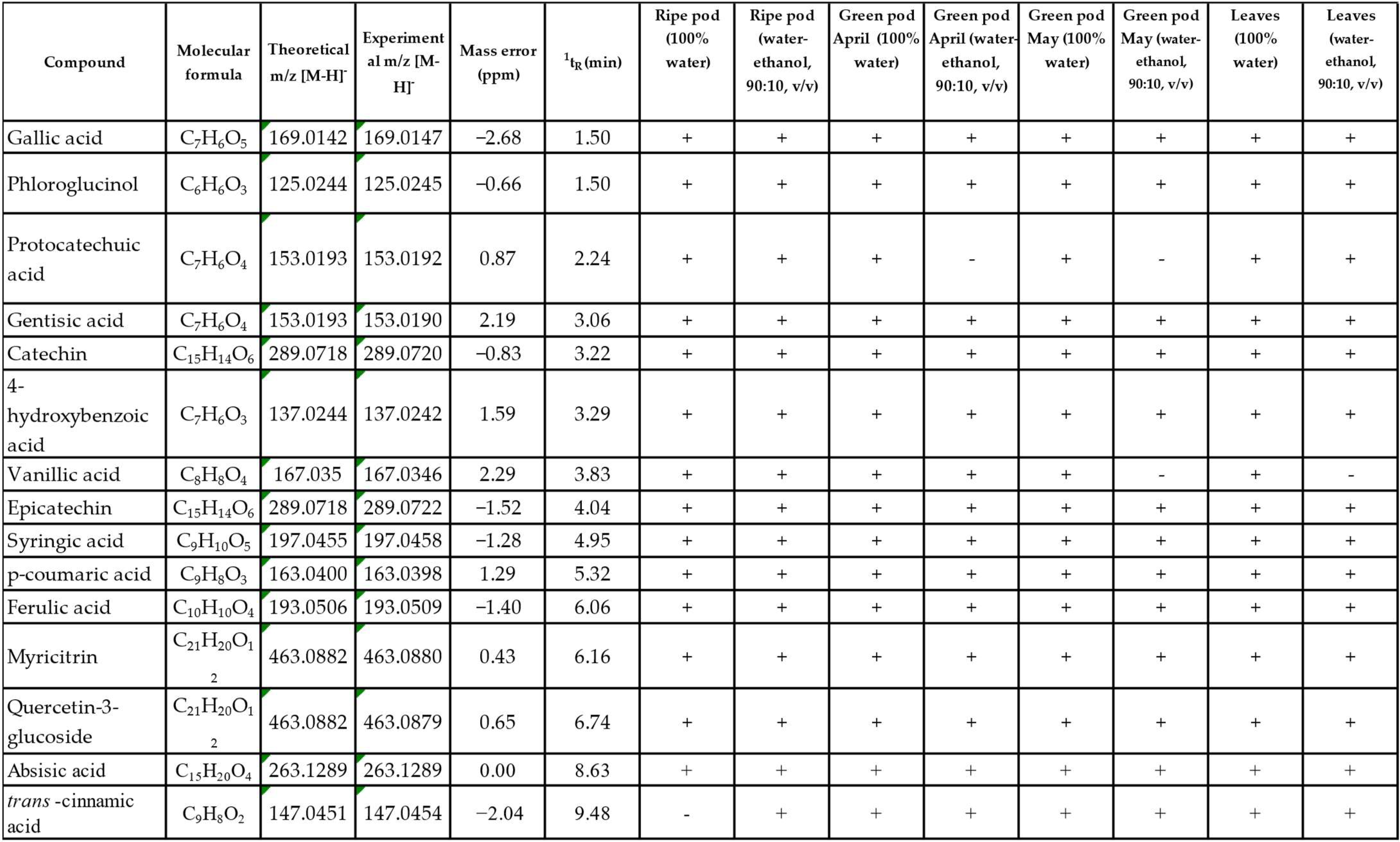
3.3. Phenolic Profile by LC-QToF-MS Analysis
3.4. In Vitro Inhibition of α-Amylase Activity
4. Discussion
4.1. Antimicrobial Activity
4.2. Polyphenolic Profile and Antioxidant Activity
4.3. Inhibition of α-Amylase/Antidiabetic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Andrikopoulos, N.K.; Kaliora, A.C. Carob: A Sustainable Opportunity for Metabolic Health. Foods 2022, 11, 2154. [Google Scholar] [CrossRef]
- Tzatzani, T.-T.; Ouzounidou, G. Carob as an Agrifood Chain Product of Cultural, Agricultural and Economic Importance in the Mediterranean Region. J. Innov. Econ. Manag. 2023, 42, 127–147. [Google Scholar] [CrossRef]
- Batlle, I.; Tous, J. Carob Tree; IPK and IPGRI: Rome, Italy, 1997. [Google Scholar]
- Papadopoulou, S.; Stefi, A.L.; Meletiou-Christou, M.S.; Christodoulakis, N.S.; Gkikas, D.; Rhizopoulou, S. Structural and Physiological Traits of Compound Leaves of Ceratonia siliqua Trees Grown in Urban and Suburban Ambient Conditions. Plants 2023, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Ioannou, G.D.; Savva, I.K.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Phenolic Profile, Antioxidant Activity, and Chemometric Classification of Carob Pulp and Products. Molecules 2023, 28, 2269. [Google Scholar] [CrossRef]
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20. [Google Scholar] [CrossRef]
- Ben Ayache, S.; Reis, F.S.; Inês Dias, M.; Pereira, C.; Glamočlija, J.; Soković, M.; Behija Saafi, E.; Ferreira, I.C.F.R.; Barros, L.; Achour, L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021, 351, 129263. [Google Scholar] [CrossRef]
- Toufeili, I.; Itani, M.; MonaZeidan, M.; Al Yamani, O.; Kharroubi, S. Nutritional and Functional Potential of Carob Syrup Versus Date and Maple Syrup. Food Technol. Biotechnol. 2022, 60, 266–278. [Google Scholar] [CrossRef]
- Aghajani, M.M.R.; Mahjoub, S.; Mojab, F.; Namdari, M.; Gorji, N.M.; Dashtaki, A.; Mirabi, P. Comparison of the Effect of Ceratonia siliqua L. (Carob) Syrup and Vitamin E on Sperm Parameters, Oxidative Stress Index, and Sex Hormones in Infertile Men: A Randomized Controlled Trial. Reprod. Sci. 2021, 28, 766–774. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob pulp: A nutritional and functional by-product worldwide spread in the formulation of different food products and beverages. A review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Moumou, M.; Mokhtari, I.; Milenkovic, D.; Amrani, S.; Harnafi, H. Carob (Ceratonia siliqua L.): A Comprehensive Review on Traditional Uses, Chemical Composition, Pharmacological Effects and Toxicology (2002–2022). J. Biol. Act. Prod. Nat. 2023, 13, 179–223. [Google Scholar] [CrossRef]
- Rico, D.; Alonso de Linaje, A.; Herrero, A.; Asensio-Vegas, C.; Miranda, J.; Martínez-Villaluenga, C.; de Luis, D.A.; Martin-Diana, A.B. Carob by-products and seaweeds for the development of functional bread. J. Food Process. Preserv. 2018, 42, e13700. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Antoniou, C.; Rouphael, Y.; Graziani, G.; Kyratzis, A. Mapping the primary and secondary metabolomes of carob (Ceratonia siliqua L.) fruit and its postharvest antioxidant potential at critical stages of ripening. Antioxidants 2021, 10, 57. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of Chemical Composition of Carob (Ceratonia siliqua): Protein, Fat, Carbohydrates, and Tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.S.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and pulsed ultrasound-assisted extraction of carob’s antioxidants: Processing parameters optimization and identification of polyphenolic composition. Ultrason. Sonochem. 2021, 76, 105630. [Google Scholar] [CrossRef]
- Benyaich, A.; Nouayti, A.; El Mekki, O.; Errafia, R.; Bahtat, F.; Aksissou, M. Phytochemical and pharmacological properties of Ceratonia siliqua L.: A comparative review of Moroccan and Mediterranean varieties. J. Appl. Pharm. Sci. 2025, in press. [Google Scholar] [CrossRef]
- Dahmani, W.; Akissi, Z.L.E.; Elaouni, N.; Bouanani, N.E.; Mekhfi, H.; Bnouham, M.; Legssyer, A.; Sahpaz, S.; Ziyyat, A. Carob leaves: Phytochemistry, antioxidant properties, vasorelaxant effect and mechanism of action. J. Ethnopharmacol. 2025, 340, 119226. [Google Scholar] [CrossRef]
- Dahmani, W.; Elaouni, N.; Abousalim, A.; Akissi, Z.L.E.; Legssyer, A.; Ziyyat, A.; Sahpaz, S. Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities. Plants 2023, 12, 3303. [Google Scholar] [CrossRef]
- Serio, S.; Santoro, V.; Celano, R.; Fiore, D.; Proto, M.C.; Corbo, F.; Clodoveo, M.L.; Tardugno, R.; Piccinelli, A.L.; Rastrelli, L. Carob (Ceratonia siliqua) leaves: A comprehensive analysis of bioactive profile and health-promoting potential of an untapped resource. Food Chem. 2025, 468, 142392. [Google Scholar] [CrossRef]
- Ikram, A.; Khalid, W.; Wajeeha Zafar, K.; Ali, A.; Afzal, M.F.; Aziz, A.; Faiz ul Rasool, I.; Al-Farga, A.; Aqlan, F.; Koraqi, H. Nutritional, biochemical, and clinical applications of carob: A review. Food Sci. Nutr. 2023, 11, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Fayek, N.M.; Sabry, O.M.; El Zalabani, S.M.; Mohamed, A.F.; El-Askary, H.I. Carob Seeds as a Source of Bioactive Flavonoid Derivatives: Isolation, Network Pharmacology-Guided Anti-Cancer Activity, and HPLC Standardization. Chem. Biodivers. 2025, 22, e202401248. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Holgado, F.; Morales, F.J. Antioxidant and Antiglycation Properties of Carob Flour Extracts: Evaluating Their Potential as a Functional Ingredient in Health-Oriented Foods and Supplements. Appl. Sci. 2025, 15, 6556. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; AlJuhaimi, F.; Musa Özcan, M.; Uslu, N.; Karrar, E. The Effect of Decoction Times On Bioactive Properties and Phenolic Compounds of Carob (Ceratonia siliqua L.) Fruit Tea. Appl. Fruit Sci. 2025, 67, 95. [Google Scholar] [CrossRef]
- Davoudi, M.; Ahmadi Gavlighi, H.; Hashempour-Baltork, F.; Khosravi-Darani, K. In vitro antidiabetic and antioxidant activities of protein hydrolysates via alkaline autolysis of Fusarium venenatum mycoprotein. Sci. Rep. 2025, 15, 13287. [Google Scholar] [CrossRef]
- Trapali, M.; Papadopoulou, A. Genetic polymorphisms possibly implicated in Diabetes Mellitus. Rev. Clin. Pharmacol. Pharmacokinet. Int. Ed. 2023, 37, 43–48. [Google Scholar]
- Bashkin, A.; Ghanim, M.; Abu-Farich, B.; Rayan, M.; Miari, R.; Srouji, S.; Rayan, A.; Falah, M. Forty-one plant extracts screened for dual antidiabetic and antioxidant functions: Evaluating the types of correlation between α-amylase inhibition and free radical scavenging. Molecules 2021, 26, 317. [Google Scholar] [CrossRef]
- Trapali, M.; Tebbi, S.O.; Karkalousos, P.; Debbache-Benaida, N.; Chaniotis, D.; Apostolopoulos, V. Antioxidant and antidiabetic potential of Acanthus mollis L. using choline chloride-based deep eutectic solvents. Rev. Clin. Pharmacol. Pharmacokinet. Int. Ed. 2024, 38, 19–25. [Google Scholar] [CrossRef]
- Tebbi, S.O.; Trapali, M.; Letsiou, S. Exploring the Anti-Diabetic, Antioxidant and Anti-Microbial Properties of Clematis flammula L. Leaves and Pistacia lentiscus L. Fruits Using Choline Chloride-Based Deep Eutectic Solvent. Waste Biomass Valor. 2024, 15, 2869–2879. [Google Scholar] [CrossRef]
- Letsiou, S.; Pyrovolou, K.; Konteles, S.J.; Trapali, M.; Krisilia, S.; Kokla, V.; Apostolaki, A.; Founda, V.; Houhoula, D.; Batrinou, A. Exploring the Antifungal Activity of Various Natural Extracts in a Sustainable Saccharomyces cerevisiae Model Using Cell Viability, Spot Assay, and Turbidometric Microbial Assays. Appl. Sci. 2024, 14, 1899. [Google Scholar] [CrossRef]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal distillates: A new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.; Proestos, C. Comparison of the Antioxidant and Antiradical Activity of Pomegranate (Punica granatum L.) Ultrasound-Assisted and Classical Extraction. Anal. Lett. 2016, 49, 969–978. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Tassou, C.C.; Drosinos, E.H.; Nychas, G.-J.E. Short Communication: Weak antimicrobial effect of carob (Ceratonia siliqua) extract against food-related bacteria in culture media and model food systems. World J. Microbiol. Biotechnol. 1997, 13, 479–481. [Google Scholar] [CrossRef]
- Kivcak, B.; Mert, T.; Öztürk, H.T. Antimicrobial and Cytotoxic Activities of Ceratonia siliqua L. Extracts. Turk. J. Biol. 2002, 26, 197–200. [Google Scholar]
- Aissani, N.; Coroneo, V.; Fattouch, S.; Caboni, P. Inhibitory effect of carob (Ceratonia siliqua) leaves methanolic extract on Listeria monocytogenes. J. Agric. Food Chem. 2012, 60, 9954–9958. [Google Scholar] [CrossRef]
- Meziani, S.; Oomah, B.D.; Zaidi, F.; Simon-Levert, A.; Bertrand, C.; Zaidi-Yahiaoui, R. Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum. Microb. Pathog. 2015, 78, 95–102. [Google Scholar] [CrossRef]
- Fidan, H.; Mihaylova, D.; Petkova, N.; Sapoundzhieva, T.; Slavov, A.; Krastev, L. Determination of chemical composition, antibacterial and antioxidant properties of products obtained from carob and honey locust. Turk. J. Biochem. 2019, 44, 316–322. [Google Scholar] [CrossRef]
- Hussein, A.M.S.; Shedeed, N.A.; Abdel-Kalek, H.H.; El-Din, M.H.A.S. Antioxidative, antibacterial and antifungal activities of tea infusions from berry leaves, carob and doum. Pol. J. Food Nutr. Sci. 2011, 61, 201–209. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Rivas-Gastélum, M.F.; Galindo-Castillo, P.A.; Esparza-Sánchez, J.; Jiménez-Pérez, M.I.; Perfecto-Avalos, Y.; Garcia-Amezquita, L.E.; Navarro-López, D.E.; López-Mena, E.R.; Sánchez-Arreola, E.; Tamayo-Martínez, J.P.; et al. Lyophilized and Oven-Dried Manilkara zapota Extracts: Characterization and In Vitro, In Vivo, and In Silico Analyses. Plants 2025, 14, 216. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid.-Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef]
- Jug, U.; Naumoska, K.; Vovk, I. (−)-epicatechin—An important contributor to the antioxidant activity of japanese knotweed rhizome bark extract as determined by antioxidant activity-guided fractionation. Antioxidants 2021, 10, 133. [Google Scholar] [CrossRef]
- Magiera, A.; Kołodziejczyk-Czepas, J.; Olszewska, M.A. Antioxidant and Anti-Inflammatory Effects of Vanillic Acid in Human Plasma, Human Neutrophils, and Non-Cellular Models In Vitro. Molecules 2025, 30, 467. [Google Scholar] [CrossRef] [PubMed]
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant activity of syringic acid prevents oxidative stress in l-arginine-induced acute pancreatitis: An experimental study on rats. Int. Surg. 2015, 100, 891–896. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.R.; Jung, U.J. Myricitrin ameliorates hyperglycemia, glucose intolerance, hepatic steatosis, and inflammation in high-fat diet/streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2020, 21, 1870. [Google Scholar] [CrossRef]
- Bruzzone, S.; Magnone, M.; Mannino, E.; Sociali, G.; Sturla, L.; Fresia, C.; Booz, V.; Emionite, L.; De Flora, A.; Zocchi, E. Abscisic Acid Stimulates Glucagon-Like Peptide-1 Secretion from L-Cells and Its Oral Administration Increases Plasma Glucagon-Like Peptide-1 Levels in Rats. PLoS ONE 2015, 10, e0140588. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Darwish, W.S.; Khadr, A.E.S.; Kamel, M.A.E.N.; Abd Eldaim, M.A.; El Sayed, I.E.T.; Abdel-Bary, H.M.; Ullah, S.; Ghareeb, D.A. Phytochemical characterization and evaluation of biological activities of egyptian carob pods (Ceratonia siliqua L.) aqueous extract: In vitro study. Plants 2021, 10, 2626. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Tavares, C.S.; Roseiro, J.C.; Rauter, A.P. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crops Prod. 2013, 44, 119–126. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Saidani, K.; Sebai, H.; Amri, M.; Eto, B.; Marzouki, L. Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J. Sci. Food Agric. 2017, 97, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Qasem, M.A.; Noordin, M.I.; Arya, A.; Alsalahi, A.; Jayash, S.N. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ 2018, 6, e4788. [Google Scholar] [CrossRef]
- Micheletti, C.; Medori, M.C.; Bonetti, G.; Iaconelli, A.; Aquilanti, B.; Matera, G.; Bertelli, M. Effects of Carob Extract on the Intestinal Microbiome and Glucose Metabolism: A Systematic Review and Meta-Analysis. Clin. Ter. 2023, 174, 169–172. [Google Scholar] [PubMed]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrovolou, K.; Revelou, P.-K.; Trapali, M.; Strati, I.F.; Konteles, S.J.; Tarantilis, P.A.; Batrinou, A. Exploring the Functional Potential of the Xyrophytic Greek Carob (Ceratonia siliqua, L.) Cold Aqueous and Hydroethanolic Extracts. Appl. Sci. 2025, 15, 8909. https://doi.org/10.3390/app15168909
Pyrovolou K, Revelou P-K, Trapali M, Strati IF, Konteles SJ, Tarantilis PA, Batrinou A. Exploring the Functional Potential of the Xyrophytic Greek Carob (Ceratonia siliqua, L.) Cold Aqueous and Hydroethanolic Extracts. Applied Sciences. 2025; 15(16):8909. https://doi.org/10.3390/app15168909
Chicago/Turabian StylePyrovolou, Katerina, Panagiota-Kyriaki Revelou, Maria Trapali, Irini F. Strati, Spyros J. Konteles, Petros A. Tarantilis, and Anthimia Batrinou. 2025. "Exploring the Functional Potential of the Xyrophytic Greek Carob (Ceratonia siliqua, L.) Cold Aqueous and Hydroethanolic Extracts" Applied Sciences 15, no. 16: 8909. https://doi.org/10.3390/app15168909
APA StylePyrovolou, K., Revelou, P.-K., Trapali, M., Strati, I. F., Konteles, S. J., Tarantilis, P. A., & Batrinou, A. (2025). Exploring the Functional Potential of the Xyrophytic Greek Carob (Ceratonia siliqua, L.) Cold Aqueous and Hydroethanolic Extracts. Applied Sciences, 15(16), 8909. https://doi.org/10.3390/app15168909








