Abstract
Robotic-assisted rehabilitation has become an essential field in supporting the functional recovery of patients with neurological, musculoskeletal or post-traumatic conditions. This paper provides a systematic and applicative analysis of the control algorithms used in robotic rehabilitation systems, with a focus on the functional classification: position control, force, impedance, adaptive, artificial intelligence-based and hybrid schemes. The characteristics of each type of control, clinical applications, advantages and technical limitations are discussed in detail, illustrated by block diagrams and comparative graphs. The paper also includes a synthesis of existing commercial systems, a multi-criteria evaluation of the performance of the algorithms and an analysis of emerging trends in the recent literature (2020–2024). Current challenges regarding sensor integration, system standardization, real-time clinical feasibility and the applicability of brain–machine interfaces or adaptive myoelectric prostheses are discussed. The results obtained can support the development of efficient, safe and personalized solutions in the field of robotic rehabilitation.
1. Introduction
In recent decades, with the aging population and the increased incidence of stroke, traumatic brain injury, and neurodegenerative diseases, the demand for functional rehabilitation therapies has increased significantly [1,2]. Post-neurological injury motor rehabilitation involves repetitive, intensive, and personalized sessions, which require significant efforts on the part of therapists. To support these activities, robotic rehabilitation systems have been developed that allow active or passive training of patients while maintaining movement precision and the ability to objectively measure progress [3,4].
Robotic rehabilitation systems can operate at the upper-limb, lower-limb, or trunk level and can be end-effector, exoskeleton, soft wearable devices (soft robotics), or hybrid systems. They offer obvious advantages over conventional therapy: consistency, personalization, adaptability, and real-time feedback [5,6]. However, their effectiveness depends fundamentally on the implemented control algorithms, which must ensure both mechanical stability and a safe and intuitive interaction between the human and the robot [7,8].
The control of rehabilitation robots involves specific challenges, such as biological variability between patients, dynamic uncertainties of the human–robot system, adaptive assistance requirements, and physical safety [9,10]. In this context, multiple control strategies have been developed—from classical PID controllers to advanced methods such as impedance control, adaptive control, robust control, or algorithms based on artificial intelligence (especially neural networks and deep learning) [11,12].
Most studies in the literature classify these methods from a theoretical perspective, starting from control theory. However, for applied research in rehabilitation, a functional classification is more relevant, depending on the type of assisted movement, the nature of the human–robot interaction, and the clinical context (active, passive, and hybrid therapy) [13,14].
The objective of this paper is to provide an updated and applicative synthesis of the main categories of control algorithms used in robotic rehabilitation systems. This paper proposes an application-oriented classification and includes a critical analysis of the advantages and limitations of each method. Relevant examples from the specialized literature, comparative tables with commercial systems; illustrative figures of control architectures; and current challenges, such as computational feasibility, multimodal sensor integration, or algorithm standardization, are presented.
To perform the literature review and generate the statistics presented in this paper, a systematic search methodology inspired by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline was used. The searches were conducted in the main scientific databases—IEEE Xplore, ScienceDirect (Elsevier), MDPI, SpringerLink, and PubMed—covering the period from January 2020 to April 2024. Relevant keyword combinations were used, such as “robot-assisted rehabilitation”, “control algorithm”, “position control”, “force control”, “impedance control”, “adaptive control”, “AI-based control”, “prosthetic control”, “myoelectric interface”, and “brain–machine interface”.
After eliminating duplicates and papers that did not meet the eligibility criteria (e.g., reviews not focused on control, papers without practical application, and publications without peer-review), the articles were qualitatively analyzed and classified according to the type of control proposed, the clinical application, and the robotic platform used. The synthesized results were presented in the form of comparative graphs (Figures 8, 9 and 11). Although a systematic review in the full sense of the PRISMA methodology was not performed, our approach follows the PRISMA methodology in principle, encompassing the essential steps of selection, transparency, and rigorous classification of bibliographic sources.
The ultimate goal is to provide a comprehensive framework for researchers and practitioners, facilitating the understanding, comparison, and implementation of control strategies in modern robotic rehabilitation systems.
2. Classification of Rehabilitation Robots and Control Requirements
Robotic systems used in medical rehabilitation are designed to support voluntary or passive movement of patients, facilitating neuroplasticity and functional recovery. From a morphological and functional point of view, these systems can be classified into several categories, each with significant implications on the implemented control strategy.
A selection of the main commercial rehabilitation systems, along with their applications, level of interaction, and control schemes, is presented in Table 1.

Table 1.
Commercial rehabilitation systems and control schemes used.
2.1. Types of Robots Used in Rehabilitation
- (a)
- End-effector robots
These robots interact with the patient through a distal point (e.g., the palm or foot), without tracking joint alignment [21]. They are mechanically simple and easy to adapt to different patients. Well-known examples are the MIT-Manus for the upper limbs, and the Gait Trainer GT I for the lower limbs [22]. These robots generally require positional or force control, with limited possibilities for detailed biomechanical feedback.
- (b)
- Robotic exoskeletons
Exoskeletons are external structures that closely follow the patient’s anatomy and allow the training of each joint involved in movement [23]. Devices such as Lokomat, ArmeoPower, or HAL (Hybrid Assistive Limb) are used in gait or arm rehabilitation [24,25]. These systems offer a high degree of customization and joint control, but they require more complex algorithms for kinematic alignment and safety.
- (c)
- Soft wearable robots (soft robotic systems)
Soft systems, made of elastic materials or textiles integrated with pneumatic or cable actuators, allow for the creation of lightweight and comfortable devices, such as soft robotic gloves or walking exo-suits [26,27]. The interaction with the patient is more natural, but the control algorithms must compensate for mechanical compliance and manage dynamic nonlinearities induced by material deformability.
- (d)
- Body support platforms and walking robots
These include systems such as ZeroG, BalanceTrainer, or Rysen, which allow body-weight support during assisted walking or balance training [28,29]. The control algorithms for these systems focus on postural stability and active center-of-gravity monitoring, often integrating information from multiple sensors (IMU, force, and pressure).
2.2. Operating Modes
The drive mode directly influences the reaction speed, precision, and safety of the robotic system, as well as the choice of control algorithms.
Electric actuators (DC/BLDC motors) are the most widely used, being precise, compact, and relatively easy to control [30]. Most modern exoskeletons are based on this type of actuator.
Pneumatic actuators, especially those with compressed air or those that are of the McKibben type, offer compliance and safety in interaction, making them suitable for soft systems. However, they introduce significant delays and additional requirements for pressure control [31].
Hydraulic actuators are rare in clinical rehabilitation, but they provide large forces for industrial or research applications [32].
Bowden cable actuators are used in wearable systems, allowing the transfer of force from a static source to a mobile device, with minimal losses [33].
The type of actuator conditions the choice of control scheme; for example, pneumatic actuators often require predictive control or robust adaptive control to compensate for nonlinear behavior, while electric actuators allow for classic schemes with multiple control loops (position–velocity–current).
2.3. Functional and Algorithmic Requirements
Rehabilitation robots must satisfy multiple functional requirements, which impose direct constraints on control algorithms:
- -
- Safety of human–robot interaction: The system must react quickly and predictably in case of unexpected patient behavior. Therefore, many systems include force limitations, collision detection, and impedance control [34].
- -
- Adaptive assistance: The level of support should vary depending on the patient’s effort. Methods such as assist-as-needed, hybrid position–force control, or EMG-based schemes are often used [35].
- -
- Real-time and stability: The controller must operate at high frequencies (100–1000 Hz), maintaining numerical and physical stability [18]. AI algorithms can pose challenges in this regard.
- -
- Personalization and learning: The system must dynamically adapt to the patient’s ability, learning from previous movements. Recurrent neural networks, reinforcement-based controllers, and evolutionary optimization are explored [36].
- -
- Multimodal interfacing: The integration of sensors (force, position, EMG, and EEG) requires robust fusion and filtering algorithms, especially in the case of machine learning [37].
Therefore, the choice of control algorithm is not only dictated by theoretical performance, but also by the mechanical characteristics of the robot, the type of recovery targeted, and the practical conditions of clinical use. In the following sections, we will analyze these algorithms according to the application, illustrating with concrete examples.
3. Functional Classification of Control Algorithms
Figure 1 shows the general functional architecture of a robotic rehabilitation system. Control is based on multiple sensory inputs (EMG, IMU, EEG, force, and position), decision algorithms (PID, adaptive, AI, etc.), and multimodal feedback to the user. The integration of the therapist and the patient in the loop ensures the personalization of the intervention.
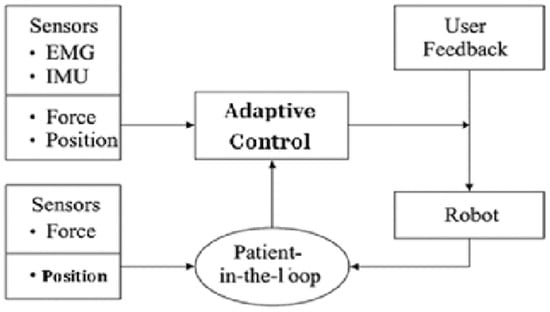
Figure 1.
General functional architecture of a robotic rehabilitation system with adaptive control.
Control algorithms used in robotic rehabilitation systems are designed to safely, accurately, and adaptively manage human–robot interaction. In this section, we will analyze the main control categories, each illustrated with concrete applications in motor rehabilitation contexts. The classification is based on the functional control strategy, not on mathematical theory, thus aiming at clinical applicability.
3.1. Position Control
Positional control is the simplest and most widely used type of control in rehabilitation, being applied mainly in the passive phases of therapy. The aim is to guide the patient’s body segment along a predefined trajectory, with high precision and without requiring active muscular effort [38].
A classic example is the MIT-Manus robot, which uses PID control to follow two-dimensional trajectories in the horizontal plane, useful in post-stroke recovery [17]. Also, ArmeoSpring uses position control in gravity compensation mode for upper-limb rehabilitation [39].
This strategy is relatively easy to implement and requires a simplified mechanical model. However, it does not take into account the forces exerted by the patient, which can lead to a lack of active engagement and the phenomenon of “slacking” [15]. Strict control of position can limit the patient’s effort and reduce the effectiveness of active therapy [40].
Below, in the Figure 2, is a block diagram of a position control system. The desired command (setpoint) is compared with the actual position, and the error is processed by a PID controller. This generates the control signal applied to the robot actuator, while the position sensors provide feedback to close the control loop. This type of control is frequently used in systems with well-defined trajectories and demands for precision in movement.
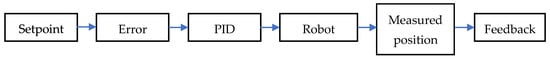
Figure 2.
Block diagram of a position control system.
The law governing position control is expressed by the classical PID relationship:
where u(t) is the command signal; e(t) = r(t) − y(t) represents the error between the reference trajectory r(t) and the current position y(t); and Kp, Ki, and Kd are the proportional, integral, and derivative gains.
u(t) = Kp × e(t) + Ki ∫ e(τ)dτ + Kd × de(t)/dt
Some of the most recent studies aimed at improving postural control are as follows:
Park et al. (2024) [41] addressed the problem of precise trajectory maintenance in post-stroke lower-limb rehabilitation, where simple PD control could not compensate for the disturbances resulting from human–robot interaction. The proposed solution combined a gravity-compensated PD control and a force feed-forward loop, implemented on a 3-DOF exoskeleton. Laboratory tests demonstrated stability and adaptability in passive and active exercises, but the authors emphasize the need for clinical validation.
Long and Peng (2021) [42] aimed to improve the position control of a lower-limb exoskeleton subject to dynamic uncertainties. They proposed a non-singular terminal sliding mode controller combined with an extended state observer and feed-forward compensation. Experimental results showed a significant reduction in tracking error and increased adaptability in the presence of disturbances, demonstrating superiority over classical methods.
Wang et al. (2025) [43] addressed the lack of torque sensors in a hip exoskeleton and the challenge of maintaining accuracy during slow walking. The solution integrated an extended linear state observer with a sliding mode controller, without requiring direct torque measurements. Results demonstrated stable trajectory tracking and robustness at various speeds, but maintaining performance at higher walking speeds remains a challenge.
Hasan and Dhingra (2022) [44] addressed the chattering problem in a sliding mode control of an 8-DOF exoskeleton. They proposed a super-twisting algorithm combined with a detailed friction model. This strategy reduced chattering, increased user comfort, and improved tracking accuracy, but real-time implementation requires further validation in a clinical context.
3.2. Force and Torque Control
Force control regulates the mechanical effort imposed by the robot so that the interaction is safe and comfortable. It is essential in applications such as postural support, resistance exercises, or assisted treadmill walking [45].
The Lokomat Pro system includes torque control loops at the hip and knee to adjust the degree of motor support, providing the patient with a variable level of challenge [46]. In the case of end-effector robots, force control is often combined with programmed trajectories to prevent tissue overload [47].
Implementing this control involves integrating force/torque sensors into the kinematic chain and actively compensating for system inertia. It also requires appropriate filtering to avoid instability caused by measurement noise [48].
Below, in the Figure 3, is a block diagram of a force control system. The desired force is compared with that measured by sensors (usually load cells), generating an error that is used by the controller. This adapts the robot’s actuation so that the human–robot mechanical interaction is finely controlled, especially in passive exercises or with haptic feedback. Force control is essential for patient safety in close physical interactions.

Figure 3.
Block diagram of a force control system.
Force control is based on adjusting the torque or force according to the measured error:
where Fcmd(t) is the force command applied to the actuator, Fref(t) is the reference force, and Fmeas(t) is the actual force measured at the human–robot interface.
Fcmd(t) = Kf × (Fref(t) − Fmeas(t))
Recent studies aimed at improving force and torque control are as follows:
Tong et al. (2024) [49] addressed the challenge of achieving a resistance-free interaction between the patient and the robot, in the case of an arm exoskeleton intended for post-stroke rehabilitation. The proposed solution was a “zero force” adaptive fuzzy–PID control, which allows the patient to initiate the movement. Compared to the classical PID control, the proposed algorithm reduced the errors (RMSE and MAE) by over 15% and increased the R2 coefficient by 4%, demonstrating a fluid interaction. However, the testing was performed only on healthy subjects, requiring additional clinical validations.
Pan et al. (2022) [50] proposed a force control based on an Assist-as-Needed (AAN) strategy with a force field for an upper-limb rehabilitation robot. The workspace is divided into patient-dominated and robot-dominated areas, and the applied forces are dynamically adjusted based on performance. Tests on healthy subjects showed that the robot automatically reduces the assist force when performance is good and increases it otherwise. This approach promotes active participation, but it has not been clinically tested on patients.
Chang et al. (2021) [51] addressed the problem of force control in a cable-actuated knee exoskeleton, where cable slack and force distribution are critical challenges. They implemented a hybrid control at two levels: a repetitive path-following control and robust voltage controllers based on admittance models. Experimental results showed a reduction in tracking errors and motor effort, with the elimination of mechanical play. However, the complexity of the implementation in regard to real hardware is a limitation.
Zhang et al. (2023) [52] developed a hybrid position–force control algorithm for a lower-limb rehabilitation robot. The solution combines a gravity-compensated PD control and a force feed-forward module capable of reproducing the therapist’s interaction. The system maintains trajectory accuracy in the passive phase and allows for active patient involvement in the later phases. The performances are promising, but fine sensor calibrations and clinical validations are needed for widespread applicability.
3.3. Impedance and Admittance Control
Impedance (or admittance) control defines a relationship between the robot’s position/force and the patient’s forces/movements. It is frequently used to reduce perceived stiffness and enable a “soft”, safe interaction [19].
The KUKA LWR robot, used in research for arm rehabilitation, offers advanced implementations of impedance control, mimicking the behavior of a virtual spring-damper [53]. Also, in soft systems, such as the SoftHand Pro robotic glove, admittance control allows for the transformation of EMG signals or motor intention into a dosed movement depending on the patient’s opposition [54].
This control is ideal for adaptive assistance, allowing the patient to influence the robot. However, tuning the impedance parameters is difficult and can lead to instability in physical contact [55]. Impedance control provides an elegant solution for safe physical interaction, but it is sensitive to delays in the control loop [56].
Below, in the Figure 4, is a block diagram of impedance control. The inputs are the desired trajectory, velocity, and acceleration, processed through a virtual mass-spring-brake model to generate a resultant force. This is then applied to the robotic system, allowing for a natural interaction that conforms to the patient’s biomechanical behavior. This type of control is common in walking exoskeletons and arm rehabilitation systems.

Figure 4.
Block diagram of impedance control.
The virtual mechanical model used to control the interaction is described by the classical impedance equation:
where M, B, and K are the virtual mass, damping, and stiffness parameters; and x(t) is the positional deviation of the system.
F(t) = M x″(t) + B x′(t) + K x(t)
Recently, improving impedance and admittance control has been addressed in several studies. Among the most relevant ones are the following:
Hu et al. (2023) [57] addressed the problem of fixed stiffness in impedance control, which does not take into account the patient’s progress during therapy. The proposed solution was a mass-spring-damper model (SDM) with real-time fuzzy-adaptive parameters, depending on the patient’s performance. Thus, the target trajectory changes according to the interaction force, providing a dynamic impedance-controlled environment. Experimental results showed increased active participation, but extensive clinical validation is needed.
Zhang et al. (2024) [58] proposed an adaptive impedance control for a 2-DOF arm exoskeleton, targeting uncertainties in human–robot interaction. The algorithm includes online estimation of patient parameters by recursive least squares with forgetting factor and selection of optimal stiffness by a reward function. Studies show superior performance compared to constant impedance control, but clinical validation on a larger sample of patients is needed.
Soltani-Zarrin et al. (2024) [59] developed a variable admittance assistive control for an upper-limb exoskeleton, designed to promote active participation without compensatory movements. The control uses two admittance ports: one dynamically adjusted at the wrist, and the other at the upper arm to control shoulder–elbow synergies. The strategy allows for minor deviations if the patient follows the trajectory correctly, but it provides corrective assistance otherwise. Experimental results demonstrate the effectiveness of the approach, with the need for reliable interaction sensors.
Cao et al. (2024) [60] aimed to increase user comfort in a knee exoskeleton using adaptive admittance control. The system includes a four-bar mechanism and a dual-loop controller: a position PID and an external loop that adapts the virtual mass and damping according to the contact force. Simulations and tests with volunteers showed more accurate joint tracking and reduced unwanted forces, significantly improving safety. Stability in real-world conditions remains a challenge due to sensor delays and noise.
3.4. Adaptive and Robust Control
Adaptive control adjusts algorithm parameters in real time, based on patient or environmental variations. It is useful in contexts where the system dynamics are unknown or vary significantly [61].
For example, the RUPERT IV exoskeleton integrates adaptive control to adjust the assistance effort based on muscle forces estimated through EMG [62]. Other systems use Model Reference Adaptive Control (MRAC) schemes to track trajectories without complete knowledge of the patient model [63].
Robust control (e.g., sliding mode control—SMC) is intended to maintain performance in the presence of uncertainties or disturbances. An example is the robotic ankle system developed by Zhang et al. that uses SMC to compensate for rapid load changes [64].
These methods provide consistent performance, but they may require large computational resources and may induce “chattering” effects (unwanted rapid vibrations) [20].
Below, in the Figure 5, is the block diagram for adaptive control. The system monitors the error between the desired and actual behavior and continuously adjusts the controller parameters through an adaptive module. This type of control is useful in personalized rehabilitation, where the patient’s condition changes over time, requiring continuous adjustments to maintain therapeutic effectiveness.

Figure 5.
Block diagram for adaptive control.
Adaptive control involves real-time adjustment of control parameters, depending on system dynamics or patient variations. A simplified example is as follows:
where θ(t) is the updated adaptive parameter, γ is the adaptation rate, e(t) is the error, and x(t) is an observable state.
θ′(t) = γ e(t) x(t)
Some recent studies that address these types of control are as follows:
Narayan et al. (2023) [65] addressed the issue of variation in patients’ biomechanical parameters during rehabilitation exercises. They proposed an adaptive backstepping controller with online estimation of limb mass and moment of inertia, implemented on a lower-limb exoskeleton. Tests on mannequins and human subjects demonstrated improved trajectory tracking accuracy and faster response to dynamic changes, though the precision of parameter estimation depended on sensor data quality.
Robust H∞H_\inftyH∞ control applied to an upper-limb rehabilitation robot can reject external perturbations and handle muscle and model uncertainties, ensuring a stable trajectory and efficient adaptation to unexpected forces. A potential drawback is the increased computational complexity and the need for careful tuning of weighting functions, which may limit real-time implementation [66].
Ahmadi et al. (2025) [67] developed a nonlinear observer-based adaptive control method for an ankle rehabilitation robot, using a sensor-less sliding mode estimator to handle model uncertainties and provide robust assistance. This scheme avoids the need for direct force sensors while maintaining trajectory accuracy under unmodeled dynamics.
Zhao et al. (2024) [68] proposed a fuzzy-adaptive impedance control layered on backstepping for an elbow exoskeleton. The method adapts stiffness/damping in real time based on patient effort to mitigate chattering, while enhancing user comfort and engagement. However, the scheme’s dual-layer complexity demands high computational power for real-world deployment.
3.5. Artificial Intelligence-Based Control
Machine learning (ML) and deep learning (DL) algorithms have been gradually integrated into rehabilitation control strategies, allowing for complex predictions and adaptations in real time.
A notable example is the robotic ankle prosthesis proposed by Fleming et al. (2023) that uses a neural controller to restore near-normative standing postural balance based on EMG and plantar pressure data [69]. Also, in BCI (Brain–Computer Interface) systems, ML classification algorithms interpret EEG signals to command robotic movement [70].
AI control allows for deep personalization and prediction of motor intent, but it involves challenges regarding the following:
- -
- Real-time feasibility,
- -
- Safety and interpretability of decisions,
- -
- The need for large labeled datasets [71].
Below, in the Figure 6, is a block diagram of an artificial intelligence-based control system. Data collected from sensors (EMG, EEG, IMU, etc.) is processed through a machine learning model (e.g., neural networks, LSTM, and CNN), which estimates movement intent and generates motor commands. This type of control offers advanced customization and the ability to adapt to complex patterns in user behavior.

Figure 6.
Block diagram of an artificial intelligence-based control system.
Control systems based on neural networks or reinforcement learning algorithms can be modeled as follows:
where Q(s,a) is the value of the decision function in state s with action a, r is the reward received, and γ is the attenuation factor.
Q(s,a) = r + γ × max Q(s′, a′)
Artificial intelligence-based control has been increasingly addressed recently. Relevant studies include the following:
Luo et al. (2023) [72] addressed the challenge of developing a robust assisted gait controller for lower-limb exoskeletons, capable of adapting to different motor impairments. The proposed solution consists of a deep reinforcement learning-based neural controller with three networks: one for movement policy, one for interaction force prediction, and one for muscle coordination. Training takes place in simulation, using “domain randomization” to generate generalized policies. The results demonstrated stability and symmetry in assisted gait, but sim-to-real transfer and clinical testing remain necessary steps.
Sambhus et al. (2023) [73] proposed a model-free force controller based on deep reinforcement learning for series elastic actuators (SEAs). The controller is trained in real time and adjusts the motor torque to generate the desired force at the joint, without requiring a precise dynamic model. Experimental results showed superior performance compared to a PID controller, especially under conditions of variability of mechanical characteristics. Limitations concern the safety of RL in clinical applications and the need for fine-tuning procedures in simulation.
Kumar et al. [74] developed a physics-informed neural network that maps EMG signals to muscle torque estimations, inspired by the Hill model. The system enables real-time, user-specific control and compensates for issues such as electrode misplacement or muscle fatigue. Validation on robotic prototypes confirmed its performance over classical control strategies.
Duong et al. (2023) [75] proposed an adaptive fuzzy sliding mode control strategy for a PAM-based actuator system, where fuzzy logic adjusts the sliding mode parameters based on human interaction force, helping to stabilize the impedance and reduce unwanted oscillations. The approach improved robustness and adaptability, though it increased implementation complexity given the need to design appropriate fuzzy functions and adaptive laws.
3.6. Control in Soft Systems (Soft Robotics)
Soft robots, being deformable structures, raise additional control problems. The flexibility of the material and the nonlinearities introduced by pneumatic actuators require robust and predictive methods [76].
Soft systems often integrate impedance control with sensing modalities (e.g., pressure or deformation sensors), enabling indirect position estimation in wearable devices such as ExoBoot and soft exo-gloves [77].
Control can be achieved through black-box models (neural networks) or predictive control based on prior identification of material behavior [16].
Although promising, these systems are still in their infancy in clinical applications and require significant optimization to ensure repeatability and safety.
Below, in the Figure 7, is the block diagram of a hybrid control system. It combines traditional schemes (e.g., PID) with those based on AI or adaptive control, either through logic switching or functional fusion. The goal is to exploit the robustness of classical control and the flexibility of intelligent control in the context of assisted rehabilitation.

Figure 7.
Block diagram of a hybrid control system.
A hybrid system combines two or more control strategies. For example, the combination of PID and admissibility control can be expressed as follows:
where α ∈ [0, 1] adjusts the weighting of the two control components, depending on the therapeutic context or patient burden.
u_total(t) = α × u_PID(t) + (1 − α) × u_imp(t)
There are studies that address this topic:
Zhao and Song (2020) [78] developed an active motion control strategy for a knee exoskeleton driven by antagonistic pneumatic muscle actuators. The proxy-based sliding mode control compensated for the system’s inherent nonlinearities and hysteresis, ensuring precise synchronization with the user’s movement and improved trajectory tracking, although implementation requires careful calibration of the controller parameters.
Polygerinos et al. (2022) [79] addressed force control in a soft device for post-stroke hand rehabilitation. The system uses integrated pneumatic chambers and a hybrid force–position control based on an empirical flow model. The challenge was to achieve a uniform pressure distribution and prevent finger hyperextension. Tests showed that the system allows guided flexion/extension with controlled force, but the accuracy is influenced by variations in the patient’s soft tissue structure.
Deng et al. (2023) [80] proposed a model predictive control (MPC) for a soft robot with elastic structures integrated in the ankle rehabilitation suite. MPC was used to predict and adjust the deformations of the soft material in real time. The solution led to a smoother trajectory and a prompter response compared to the classical PID control. The major disadvantage is the high computational cost, which limits the implementation on low-resource embedded systems.
Lee et al. (2024) [81] introduced an intent-driven upper-limb exoskeleton that employs soft pneumatic actuators combined with embedded soft bioelectronics. User intention is decoded in real time using a deep learning classifier driven by muscle activity (EMG), and pneumatic actuation provides movement assistance. Experimental tests show enhanced user comfort and active participation, though the system’s effectiveness depends on accurate, low-latency EMG sensing.
The main advantages and limitations of each control strategy used in robotic rehabilitation are synthesized in Table 2.

Table 2.
Comparison between the main control schemes used in robotically assisted rehabilitation.
3.7. Trends in Research Illustrated Graphically
In recent years, various control algorithms have been applied in robotic rehabilitation, each serving different functional goals. Figure 8 provides a statistical overview of the distribution of control algorithm types across recent literature between 2020 and 2024.
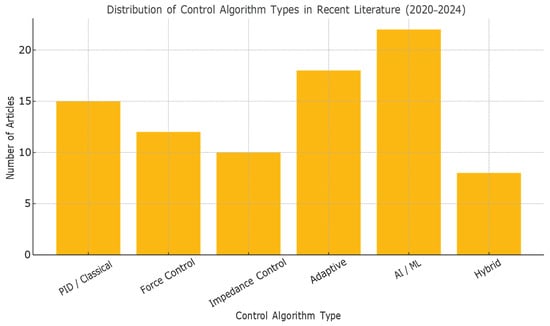
Figure 8.
Distribution of Control Algorithm Types in Recent Literature (2020–2024).
3.8. Distribution of Control Algorithms Across Clinical Applications Illustrated Graphic
Control algorithms in rehabilitation robotics are often tailored to specific clinical applications, such as post-stroke therapy, spinal cord injury, or musculoskeletal disorders. Figure 9 illustrates how various control strategies are distributed across these key application areas, based on recent literature.
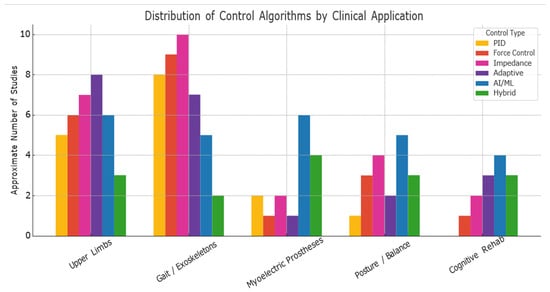
Figure 9.
Distribution of Control Algorithms by Clinical Application.
4. Control Algorithms for Emerging Rehabilitation Applications
In addition to the classical robotic systems used in therapy centers, a series of emerging technologies have recently emerged that extend the potential of rehabilitation by increasing interactivity, adaptability, and connectivity between the user and the system. These applications, such as smart myoelectric prostheses, brain–machine interfaces (BCIs), and multimodal sensor systems, require advanced control algorithms, often of the intelligent or adaptive type, capable of operating under dynamic and uncertain conditions.
4.1. Smart Myoelectric Prostheses (Ankle/Knee)
New-generation ankle and knee prostheses go beyond traditional passive mechanisms, using electrical actuators, EMG (electromyography) sensors, and adaptive or intelligent control algorithms to more closely replicate the biomechanics of natural gait [82,83].
A notable work is that of Fleming et al. (2023), who demonstrate that a neural controller trained on EMG and pressure signals can restore near-normative postural control in standing amputees without prior training [84]. The system in question integrates the following:
- -
- Deep learning algorithms (convolutional and temporal neural networks),
- -
- EMG sensors placed on the muscle stump,
- -
- Distributed plantar pressure for gait phase detection,
- -
- Adaptive feedback on the prosthetic motor.
These prostheses often use hybrid control schemes: torque control in the stance phase, and position control in the swing phase. Another example is the Empower Ankle, which adapts the propulsion impulse depending on the terrain and the user’s activity [85]. Myoelectrically controlled neural systems can learn complex balancing and adaptive responses without extensive training, if the architecture is properly calibrated [84].
However, these systems raise issues related to the following:
- -
- Robustness to EMG variations (sweating and electrode movement),
- -
- Real-time feasibility on portable hardware,
- -
- Safety of AI decisions (difficult to interpret).
4.2. Brain–Machine Interfaces (BCIs)
BCIs represent an approach in which robotic commands are generated directly from the user’s brain signals, usually recorded non-invasively (EEG) [86]. These signals reflect movement intention or cortical activation level and can be used to initiate assisted movements in robotic systems.
For example, a BCI system can detect motor imagery of hand movement and command an exoskeleton to reproduce that action, reinforcing cortical loops through visual and proprioceptive feedback [87].
The control algorithms involved include the following:
- -
- ML classifiers (SVM, LDA, and Random Forest) for EEG decoding,
- -
- Decision fusion algorithms with EMG or movement data,
- -
- Adaptive feedback loops for real-time recalibration [88].
An example is the MindWalker system, which uses EEG to control a lower-limb exoskeleton [89]. Other applications use BCIs in stroke recovery, automatically activating robots to amplify the patient’s intention [90].
Although promising, these systems suffer from high latency, limited accuracy (below 80% in real applications), and an increased dependence on daily calibrations [91].
4.3. Multimodal Sensor Integration and Data Fusion
Modern rehabilitation systems tend to integrate multiple sources of information to assess the patient’s condition and adjust the robot’s behavior in real time. Among the sensors commonly used are the following:
- -
- Force/torque sensors;
- -
- IMU (inertial units);
- -
- EMG, EEG, and EOG;
- -
- Plantar pressure sensors;
- -
- RGB-D or LiDAR cameras [92].
The fusion of these data allows for a more robust estimation of the patient’s state (intention, effort level, imbalance, etc.). This is achieved through algorithms such as the following:
- -
- Extended Kalman Filter or Particle Filter,
- -
- Hybrid neural networks (e.g., LSTM for EMG sequences + CNN for visual data),
- -
- Reinforcement Learning algorithms [93].
For example, in Ganesan et al. (2015), an upper-limb exoskeleton uses combined EMG and IMU feedback to adjust support levels dynamically during movement [94]. Also, in upper-limb therapies, the combination of EMG–IMU–visual feedback is used to adapt the level of resistance or challenge depending on the patient’s performance [95].
These approaches contribute to the following:
- -
- Personalization of the intervention,
- -
- Automatic detection of functional progress,
- -
- Prevention of accidents through anticipatory detection.
Challenges remain in the following areas:
- -
- Increased cost of equipment,
- -
- Temporary synchronization of sensors,
- -
- Complexity of real-time processing [96].
4.4. Functional Data from Clinical Studies
To highlight the concrete impact of these systems, below are some functional results reported in recent clinical studies.
The use of the Lokomat system resulted in significant increases in Fugl–Meyer score and walking speed after 4–6 weeks of assisted training [15]. Also, end-effector systems such as the InMotion ARM have shown improvements in the Box and Block Test score, indicating functional recovery of the upper limb [50].
In a randomized clinical trial, the use of the HAL (Hybrid Assistive Limb) exoskeleton was associated with increased Walking Index for Spinal Cord Injury II (WISCI II) scores and reduced walking fatigue [68].
Such results validate the integration of control algorithms into clinical protocols, emphasizing the need for personalization and adaptability according to the patient profile.
5. Current Challenges and Future Research Directions
Although robotic rehabilitation systems have experienced accelerated development over the past two decades, there are still many unresolved challenges that limit their large-scale clinical implementation. These concern not only the technical aspects of control, but also issues of standardization, clinical validation, adaptability, and economic feasibility.
5.1. Lack of Standardization and Benchmarking
A major obstacle to evaluating progress in robotic rehabilitation is the lack of a standardized framework for comparing algorithms, devices, and clinical outcomes [97]. Most studies are conducted on single prototypes, with closed datasets, and different evaluation methodologies. Without a common set of metrics, it is difficult to determine the superiority of one control method over another [98].
It takes the following:
- -
- Common testing protocols (e.g., functional gait assessment and Fugl–Meyer arm scores),
- -
- Objective metrics (accuracy, patient effort, and inter-session variability),
- -
- Open databases for algorithmic validation,
- -
- Competitions and community benchmarks.
Without such measures, the transition from research to the clinic remains slow and fragmented [99].
5.2. Computational Challenges and Real-Time Feasibility
Many advanced algorithms (especially those based on artificial intelligence) raise issues related to latency, complexity, and resource consumption. In clinical contexts, control must operate at high frequencies (above 500 Hz for force loops) and with minimal tolerances to delays [100].
For example, deep neural networks (CNN and LSTM) require dedicated processing units (e.g., GPU/TPU), which are difficult to integrate into portable systems and consume considerable power [101].
Potential solutions include the following:
- -
- Hybrid models (pre-trained offline and implemented online with optimizations),
- -
- Model compression (quantization and pruning),
- -
- Custom hardware accelerators (FPGAs for fast control) [102].
5.3. AI Safety, Interpretability, and Certification
The use of intelligent control algorithms raises ethical and technical questions regarding the safety of automated decisions. Unlike PID control, which is completely transparent, decisions made by neural networks are difficult to justify, thus complicating the process of clinical validation and medical approval [103]. The lack of interpretability and auditability of decisions based on neural networks is a major obstacle to their use in a medical context [104].
To mitigate these problems, the following are explored:
- -
- Use of interpretable networks (e.g., explainable AI),
- -
- Application of safety filters that can block dangerous exits,
- -
- Methods for formal verification of algorithm behavior [105].
Additionally, the lack of uniform regulations for AI-based medical software is delaying widespread adoption.
5.4. Patient-Centered Adaptation and the Challenge of Personalization
Each patient presents neurological, muscular, and cognitive peculiarities. An effective control algorithm for a young post-stroke patient may be ineffective or even dangerous for an elderly patient with severe neuropathy [106].
Currently, many systems only offer manually adjustable static parameters, which is not sufficient for personalized rehabilitation. It is necessary to develop algorithms that perform as follows:
- -
- Learn the patient’s progression over time,
- -
- Adjust difficulty automatically (e.g., through assist-as-needed),
- -
- Detect fatigue or frustration, and adapt the interaction [107].
An emerging concept is “Closed-Loop Patient-in-the-Loop Adaptive Control”, in which all components (sensors, algorithm, therapist, and patient) form a continuous adaptive loop, supported by data [108].
5.5. Costs, Complexity, and Barriers to Clinical Implementation
Even the most advanced rehabilitation systems may remain underutilized in practice due to the following:
- -
- High costs (equipment, personal training, and maintenance),
- -
- The complexity of the interfaces (which require specialized personnel),
- -
- Lack of interoperability with other medical systems [109].
The future depends on the following:
- -
- Simplification of robot–therapist interfaces,
- -
- Development of modular platforms,
- -
- Legislative support for reimbursement of robotic-assisted therapies.
5.6. Considerations Regarding International Standardization and Regulations
In the context of integrating robots into clinical therapy, compliance with international standards is essential. Robotic medical devices must comply with IEC 60601-1 requirements for electrical and electromagnetic safety [110], and personal robotic systems fall under the scope of the ISO 13482:2014 standard [111], which regulates the safety of personal assistance robots.
For neuromuscular rehabilitation devices, including exoskeletons and smart prostheses, IEC 80601-2-78 also applies, which covers the particular requirements for the functional safety of electromedical systems [112]. In addition, at the European level, the CE marking requires validation of compliance with the MDR 2017/745 directives (Medical Device Regulation), thus requiring rigorous assessments of performance, biocompatibility, clinical risk, and software–hardware interoperability [113].
Thus, control algorithms cannot be evaluated in isolation, but as part of a regulated ecosystem, in which reliability, traceability, and patient safety are priorities.
In conclusion, for control algorithms in robotic rehabilitation to have a real impact in the clinic, an interdisciplinary, patient-centered approach is needed, supported by rigorous scientific validation and responsible regulation.
5.7. Comparative Evaluation of Control Algorithms
To support the selection and development of control algorithms in rehabilitation applications, we conducted a comparative analysis based on four relevant criteria: accuracy, adaptability, safety, and implementation complexity. The Figure 10 presents a visual summary of these factors for the most commonly used control types.
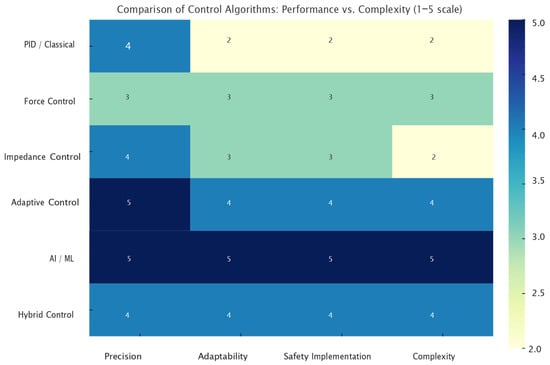
Figure 10.
Comparison of Control Algorithms: Performance vs. Complexity.
5.8. Emerging Trends in the Use of Control Algorithms
The analysis of the temporal distribution of control algorithms used in robotic-assisted rehabilitation indicates a clear transition from traditional methods (PID and force) to more sophisticated solutions, such as adaptive, hybrid control and artificial intelligence. The graph below, Figure 11, illustrates this trend, based on data synthesized from the literature published in the period of 2020–2024.
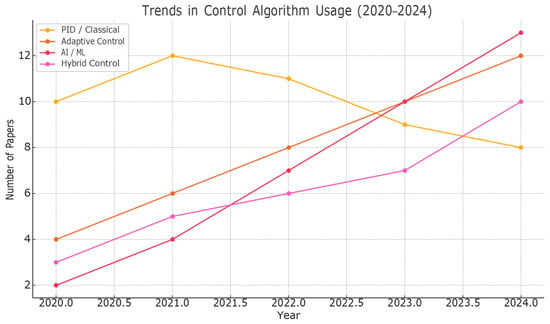
Figure 11.
Trends in Control Algorithm Usage (2020-2024).
6. Conclusions
This paper provided an applied and critical analysis of the main categories of control algorithms used in robotic medical rehabilitation systems, with a focus on how they influence the safety, efficiency, and personalization of therapy. The proposed approach was functionally oriented, classifying algorithms according to the mode of interaction and therapeutic purpose—a perspective more relevant for clinical applicability than purely theoretical approaches.
I highlighted the following facts:
- -
- Position control is effective in passive therapies, but limited in promoting active patient involvement;
- -
- Force and impedance/admittance control allow for a more natural and safe interaction, but require the integration of sensors and careful adjustment of parameters;
- -
- Adaptive and robust algorithms offer constant performance under uncertain conditions, but with increased computational costs;
- -
- AI-based control allows for prediction of patient intent and personalized learning, but raises issues of interpretability and validation.
Emerging applications such as smart myoelectric prostheses and brain–machine interfaces demonstrate the potential of advanced algorithms to restore near-normative functionality. Also, the integration of multimodal sensors and AI architectures contributes to the creation of intelligent systems capable of continuously adapting to the patient’s needs.
At the same time, we have identified major challenges that must be overcome to enable the widespread adoption of these technologies:
- -
- Lack of standardization and protocols for comparing algorithms,
- -
- Difficulties in implementing real-time control in portable contexts,
- -
- Uncertainties related to the safety of automated decisions in AI,
- -
- Barriers related to cost, complexity, and clinical integration.
For future research, we recommend the following:
- -
- Development of interoperable and open platforms, allowing comparative testing of algorithms,
- -
- Orientation towards hybrid adaptive control, combining classical schemes with elements of interpretable AI,
- -
- Implementation of standardized measures for clinical validation of algorithmic performance and functional impact,
- -
- Promoting therapist- and patient-centered interfaces to facilitate the real use of technology in the clinic.
Through this analysis, we hope to contribute to a better understanding of the complexity and opportunities in the field of robotic control applied in rehabilitation, providing a solid basis for engineering, clinical, and research decisions.
Author Contributions
Conceptualization, O.L.R. and C.B.; methodology, O.L.R. and C.B.; software, O.L.R. and C.B.; validation, O.L.R. and C.B.; formal analysis, O.L.R. and C.B.; investigation, O.L.R. and C.B.; resources, O.L.R. and C.B.; data curation, O.L.R. and C.B.; writing—original draft preparation, O.L.R. and C.B.; writing—review and editing, O.L.R. and C.B.; visualization, O.L.R. and C.B.; supervision, O.L.R. and C.B.; project administration, O.L.R. and C.B.; funding acquisition, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by IOSUD, UTCN.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C.J.L. The Global Burden of Neurological Disorders: Translating Evidence into Policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Maciejasz, P.; Eschweiler, J.; Gerlach, K.; Jansen, A.; Leonhardt, S. A Survey on Robotic Devices for Upper Limb Rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef]
- Vitiello, N.; Cipriani, L.; Carrozza, G.; Beccai, L.; Lotti, A. Wearable Robotic Devices for Rehabilitation and Assistance: Design Issues and Technological Challenges. Acta Polytech. Hung. 2021, 18, 45–66. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; van Wegen, E.E.H.; Meskers, C.G.M.; Kwakkel, G. Effects of Robot-Assisted Therapy for the Upper Limb After Stroke: A Systematic Review and Meta-Analysis. Neurorehabilit. Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef]
- Aprile, I.; Germanotta, M.; Cruciani, A.; Loreti, S.; Pecchioli, C.; Cecchi, F.; Montesano, A.; Galeri, S.; Diverio, M.; Falsini, C.; et al. Upper Limb Robotic Rehabilitation After Stroke: A Multicenter, Randomized Clinical Trial. J. Neurol. Phys. Ther. 2020, 44, 3–14. [Google Scholar] [CrossRef]
- Marchal, L.; Reinkensmeyer, D.J. Review of Control Strategies for Robotic Movement Training after Neurologic Injury. J. Neuroeng. Rehabil. 2009, 6, 20. [Google Scholar] [CrossRef]
- Ji, G.; Zhou, Y.; Yu, H.; Wang, J.; Zhang, X. Human–Robot Interaction: A Review and Analysis on Variable Admittance Control and Safety in Physical HRI. Machines 2022, 10, 591. [Google Scholar] [CrossRef]
- Mahfouz, D.M.; Shehata, O.M.; Morgan, E.I.; Arrichiello, F. A Comprehensive Review of Control Challenges and Methods in End-Effector Upper-Limb Rehabilitation Robots. Robotics 2024, 13, 181. [Google Scholar] [CrossRef]
- Radder, E.L.; Meinhardt, U.; Van Meulen, F.; Dederer, H. Safety Assessment of Rehabilitation Robots: A Review Identifying Safety Skills and Gaps. Front. Robot. AI 2021, 8, 602878. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wang, Z.; Zhang, M. Research on Control Strategy Technology of Upper Limb Exoskeleton Robots: A Review. Machines 2023, 13, 207. [Google Scholar] [CrossRef]
- Alam, N.; Hasan, S.; Mashud, G.A.; Bhujel, S. Neural Network for Enhancing Robot-Assisted Rehabilitation: A Systematic Review. Actuators 2025, 14, 16. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wang, J. Research on adaptive impedance control technology of upper limb rehabilitation robots. Front. Bioeng. Biotechnol. 2023, 11, 1332689. [Google Scholar] [CrossRef]
- Huo, W.; Moon, H.; Alouane, M.A.; Bonnet, V.B.; Huang, J.; Amirat, Y.; Vaidyanathan, R.; Mohammed, S. Impedance Modulation Control of a Lower-Limb Exoskeleton to Assist Sit-to-Stand Movements. IEEE Trans. Robot. 2022, 38, 1230–1249. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Zhong, J.; Luo, S.; Lei, M.; Wang, H. Design and Implementation of an Impedance-Based Assist-As-Needed Controller for an End-Effector Upper-Limb Exoskeleton. Appl. Sci. 2021, 11, 340. [Google Scholar] [CrossRef]
- Liu, H.; Xie, S.; Zhao, Y. Model Predictive Control for Soft Robotic Manipulators: A Review. Sensors 2022, 22, 3765. [Google Scholar] [CrossRef]
- Krebs, H.I.; Hogan, N.; Aisen, M.L.; Volpe, B.T. Robot-Aided Neurorehabilitation: A Pilot Clinical Trial with MIT-MANUS. IEEE Trans. Rehabil. Eng. 1998, 6, 75–87. [Google Scholar] [CrossRef]
- Chen, J.; Hochstein, J.; Kim, C.; Tucker, L.; Hammel, L.E.; Damiano, D.L.; Bulea, T.C. A Pediatric Knee Exoskeleton with Real-Time Adaptive Control for Overground Walking in Ambulatory Individuals with Cerebral Palsy. Front. Robot. AI 2021, 8, 702137. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Xu, W.; Zhang, W.; Zhao, F.; Wang, B.; Du, H.; Zhang, C. Design and control of a low-cost non-backdrivable end-effector upper limb rehabilitation device. Front. Rehabil. Sci. 2024, 5, 1469491. [Google Scholar] [CrossRef]
- Rosales-Luengas, Y.; Salazar, S.; Rangel-Popoca, S.J.; Cortés-García, Y.; Flores, J.; Lozano, R. Active Gait Retraining with Lower Limb Exoskeleton Based on Robust Force Control. Appl. Sci. 2025, 15, 4032. [Google Scholar] [CrossRef]
- Brunet, M.; Pétriaux, M.; Di Meglio, F.; Petit, N. Fast replanning of a lower-limb exoskeleton trajectories for rehabilitation. arXiv 2022, arXiv:2209.04155. [Google Scholar] [CrossRef]
- Diego, P.; Herrero, S.; Macho, E.; Corral, J.; Diez, M.; Campa, F.J.; Pinto, C. Devices for Gait and Balance Rehabilitation: General Classification and a Narrative Review of End Effector-Based Manipulators. Appl. Sci. 2024, 14, 4147. [Google Scholar] [CrossRef]
- Cenciarini, M.; Dollar, A.M. Biomechanical Considerations in the Design of Lower-Limb Exoskeletons. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 318–323. [Google Scholar] [CrossRef]
- Atkinson, M.; Tully, A.; Maher, C.A.; Innes-Wong, C.; Russo, R.N.; Osborn, M.P. Safety, Feasibility and Efficacy of Lokomat® and Armeo®Spring Training in Deconditioned Paediatric, Adolescent and Young Adult Cancer Patients. Cancers 2023, 15, 1250. [Google Scholar] [CrossRef]
- Potašová, M.; Mačej, P.; Moraučíková, E.; Baňárová, P.S.; Kutiš, P. Lokomat vs. Conventional Therapy—Impact on Gait Symmetry in Hemiparetic Patients: Preliminary Clinical Study. Healthcare 2025, 13, 929. [Google Scholar] [CrossRef]
- Mohamad, H.; Ozgoli, S.; Kazemi, J. Optimization of Joint Space Trajectories for Assistive Lower Limb Exoskeleton Robots: Real-Time and Flexible Gait Patterns. J. Intell. Robot. Syst. 2024, 110, 122. [Google Scholar] [CrossRef]
- Morris, L.; Diteesawat, R.S.; Rahman, N.; Turton, A.; Cramp, M.; Rossiter, J. The state of the art of soft robotics to assist mobility: A review of physiotherapist and patient identified limitations of current lower-limb exoskeletons and the potential soft-robotic solutions. J. Neuroeng. Rehabil. 2023, 20, 18. [Google Scholar] [CrossRef]
- Sankar, A.; Dicianno, B.E. Overground Body Weight Support Systems for Gait and Balance Training: A Scoping Review. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100127. [Google Scholar] [CrossRef]
- Veneman, J.; Wessels, M.; Kloosterman, M. Development of the RYSEN: A 3D Body Weight Support System. Robotics 2020, 9, 90. [Google Scholar] [CrossRef]
- Bettella, F.; Goffredo, R.; Pellegrini, M.; Nanni, A.; Connor, A.G.; Cappello, L. A Scoping Review on Lower Limb Exoskeleton Actuations: Description and Characteristics. Robotica 2025, 43, 1572–1589. [Google Scholar] [CrossRef]
- Godage, I.S.; Chen, Y.; Walker, I.D. Dynamic Control of Pneumatic Muscle Actuators. arXiv 2018, arXiv:1811.04991. [Google Scholar] [CrossRef]
- Sun, M.; Ouyang, X.; Mattila, J.; Yang, H.; Hou, G. One Novel Hydraulic Actuating System for the Lower-Body Exoskeleton. Chin. J. Mech. Eng. 2021, 34, 31. [Google Scholar] [CrossRef]
- Shi, K.; Song, A.; Li, Y.; Chen, D.; Zhu, L. A Cable-Driven Three-DOF Wrist Rehabilitation Exoskeleton With Improved Performance. Front. Neurorobot. 2021, 15, 664062. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, S.; Fei, S. Collision Avoidance Based on Null-Space Impedance Control for Upper Limb Rehabilitation Robots. Med. Biol. Eng. Comput. 2023, 61, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Arantes, A.; Bressan, N.; Borges, L.R.; McGibbon, C.A. Evaluation of a Novel Real-Time Adaptive Assist-As-Needed Controller for Robot-Assisted Upper Extremity Rehabilitation Following Stroke. PLoS ONE 2023, 18, e0292627. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Huang, H.; Li, X.; Chen, J.; Si, J. Reinforcement learning-based motion control for a lower extremity rehabilitation exoskeleton performing collaborative squatting with unstable human-robot interaction. Front. Robot. AI 2021, 8, 702845. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Li, X.; Lei, Y.; Omalley, O. Advancing Biomedical Engineering through a Multi-Modal Sensor Fusion System for Enhanced Physical Training. AIMS Bioeng. 2023, 10, 364–383. [Google Scholar] [CrossRef]
- Qu, Z.; Tang, W.; Li, J.; Zhang, X. Fuzzy Adaptive Control for a 4-DOF Hand Rehabilitation Robot in Passive Mode. Actuators 2025, 14, 351. [Google Scholar] [CrossRef]
- Olczak, A.; Truszczyńska-Baszak, A.; Stępień, A. The Use of Armeo®Spring Device to Assess the Effect of Trunk Stabilization Exercises on the Functional Capabilities of the Upper Limb—An Observational Study of Patients after Stroke. Sensors 2022, 22, 4336. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Liu, X.; Yang, Y.; Huang, D. Adaptive Impedance Control of a Human–Robotic System Based on Motion Intention Estimation and Output Constraints. Appl. Sci. 2025, 15, 1271. [Google Scholar] [CrossRef]
- Park, C.; Kim, M.-S. Stratified predictions of upper limb motor outcomes after stroke. Front. Neurol. 2024, 14, 1323529. [Google Scholar] [CrossRef]
- Long, Y.; Peng, Y. Extended State Observer-Based Nonlinear Terminal Sliding Mode Control with Feed-forward Compensation for Lower Extremity Exoskeleton. IEEE Access 2021, 9, 3049879. [Google Scholar] [CrossRef]
- Wang, R.; Lin, X.; Yin, C.; Liu, Z.; Zhang, Y.; Liu, W.; Du, F. Robust Continuous Sliding-Mode-Based Assistive Torque Control for Series Elastic Actuator-Driven Hip Exoskeleton. Actuators 2025, 14, 239. [Google Scholar] [CrossRef]
- Hasan, S.K.; Dhingra, A.K. Development of a Sliding Mode Controller with Chattering Suppressor for Human Lower Extremity Exoskeleton Robot. Results Control Optim. 2022, 7, 100123. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.; Jo, Y.; Moon, J.H.; Kim, J. A Robotic Treadmill System to Mimic Overground Walking Training with Body Weight Support. Front. Neurorobot. 2023, 17, 1089377. [Google Scholar] [CrossRef]
- Laszlo, C.; Munari, D.; Maggioni, S.; Knechtle, D.; Wolf, P.; De Bon, D. Feasibility of an Intelligent Algorithm Based on an Assist-as-Needed Controller for a Robot-Aided Gait Trainer (Lokomat) in Neurological Disorders: A Longitudinal Pilot Study. Brain Sci. 2023, 13, 612. [Google Scholar] [CrossRef]
- Hussain, A.; Khan, M.I.; Nazir, S.; Lee, S.; Kim, J.-H. Design and Force Control of an End-Effector-Based Upper-Limb Rehabilitation Robot. IEEE Access 2022, 10, 71593–71606. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Chen, F.; Zhang, Y.; Li, J.; Sun, H. Adaptive Force Control With Kalman Filtering for Robotic Upper-Limb Rehabilitation. Sensors 2023, 23, 2465. [Google Scholar] [CrossRef]
- Tong, L.; Cui, D.; Wang, C.; Peng, L. A novel zero-force control framework for post-stroke rehabilitation training based on fuzzy-PID method. Intell. Robot. 2024, 4, 125–145. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Sun, Q. Development of a Force-Field-Based Control Strategy for an Upper-Limb Rehabilitation Robot. Mech. Sci. 2022, 13, 949–959. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, X.; Wang, L.; Zhao, L.; Sun, H.; Zhang, Y. Hybrid Position-Force Control in Cable-Driven Knee Exoskeletons. Sensors 2023, 23, 3621. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J. Hybrid Control Strategy for Lower-Limb Rehabilitation Robot Combining Gravity Compensation and Feedforward Force. Appl. Sci. 2023, 13, 277. [Google Scholar] [CrossRef]
- Fang, Y.; Li, H.; Zhang, X.; Zhou, W.; Yang, M.; Sun, H. Human–Robot Collaborative Control Based on Impedance Modulation with KUKA LWR for Post-Stroke Rehabilitation. Sensors 2021, 21, 1576. [Google Scholar] [CrossRef]
- Sierotowicz, M.; Lotti, N.; Nell, L.; Missiroli, F.; Alicea, R.; Zhang, X.; Xiloyannis, M.; Rupp, R.; Papp, E.; Krzywinski, J.; et al. EMG-Driven Machine Learning Control of a Soft Assistive Glove for Grasping Assistance and Rehabilitation. IEEE Robot. Autom. Lett. 2022, 7, 3140–3147. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Wang, L.; Liu, M.; Zhou, Y.; Zhao, J. Fuzzy-Adaptive Impedance Control with Online Stiffness Adjustment in Rehabilitation Robots. Appl. Sci. 2023, 13, 2234. [Google Scholar] [CrossRef]
- Qin, T.; Wu, Z.; Lin, H.-Y.; Chen, Z.; Zhang, J. Impedance Control Stability Analysis Considering Networked Communication Delays. Sensors 2023, 23, 385. [Google Scholar] [CrossRef]
- Hu, J.; Zhuang, Y.; Meng, Q.; Yu, H. Active Training Control Method for Rehabilitation Robot Based on Fuzzy Adaptive Impedance Adjustment. Machines 2023, 11, 565. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Yang, W.; Liu, T.; Sun, H. Adaptive Impedance Control for Upper-Limb Exoskeletons Based on RLS and Stiffness Optimization. Sensors 2024, 24, 451. [Google Scholar] [CrossRef]
- Soltani-Zarrin, R.; Pourkand, A.; Kazemi, H.; Fattahi, N. A Dual-Port Admittance Control Scheme for Upper-Limb Rehabilitation Exoskeletons. Appl. Sci. 2024, 14, 78. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Wang, C.; Li, K.; Zhang, J.; Wang, G.; Ren, H. Variable Admittance Control of High Compatibility Exoskeleton Based on Human–Robotic Interaction Force. Chin. J. Mech. Eng. 2024, 37, 119. [Google Scholar] [CrossRef]
- Mashud, G.A.; Hasan, S.K.; Alam, N. Advances in Control Techniques for Rehabilitation Exoskeleton Robots: A Systematic Review. Actuators 2025, 14, 108. [Google Scholar] [CrossRef]
- Peternel, L.; Noda, T.; Petrič, T.; Ude, A.; Morimoto, J.; Babič, J. Adaptive Control of Exoskeleton Robots for Periodic Assistive Behaviours Based on EMG Feedback Minimisation. PLoS ONE 2016, 11, e0148942. [Google Scholar] [CrossRef]
- Amiri, M.S.; Ramli, R.; Barari, A. Optimally Initialized Model Reference Adaptive Controller of Wearable Lower Limb Rehabilitation Exoskeleton. Mathematics 2023, 11, 1564. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Yang, L.; Wu, S.; Chen, X.; Chen, S. Exoskeleton rehabilitation robot training for balance and lower limb function in sub-acute stroke patients: A pilot, randomized controlled trial. J. Neuroeng. Rehabil. 2024, 21, 98. [Google Scholar] [CrossRef]
- Narayan, J.; Abbas, M.; Dwivedy, S.K. Robust Adaptive Backstepping Control for a Lower-Limb Exoskeleton System with Model Uncertainties and External Disturbances. Automatika 2023, 64, 145–161. [Google Scholar] [CrossRef]
- Ghannadi, B.; Sharif Razavian, R.; McPhee, J. Configuration-Dependent Optimal Impedance Control of an Upper Extremity Stroke Rehabilitation Manipulandum. Front. Robot. AI 2018, 5, 124. [Google Scholar] [CrossRef]
- Ahmadi, A.; Aghababaeinejad, K.; Nopour, R.; Kamali Eigoli, A.; Naderi, E. Sensor-Less Sliding Mode Observer-Based Assistive Control of a Spherical Parallel Ankle Rehabilitation Robot Under Modeling Uncertainties. Iran. J. Sci. Technol. Trans. Mech. Eng. 2025, 49, 789–804. [Google Scholar] [CrossRef]
- Zhao, Z.; Hou, X.; Shan, D.; Liu, H.; Liu, H.; Hao, L. Application of Fuzzy Adaptive Impedance Control Based on Backstepping Method for PAM Elbow Exoskeleton in Rehabilitation. Polymers 2024, 16, 3533. [Google Scholar] [CrossRef]
- Fleming, A.J.; Huang, H.H.; Buxton, E.; Hodges, F.; Huang, S. Direct Continuous Electromyographic Control of a Powered Prosthetic Ankle for Improved Postural Control after Guided Physical Training: A Case Study. Wearable Technol. 2021, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, L.; Soriano-Segura, P.; Navarro, J.; Iáñez, M.; Azorín, J.M.; Contreras-Vidal, J.L. Brain–Machine Interface Based on Deep Learning to Control Asynchronously a Lower-Limb Robotic Exoskeleton: A Case-of-Study. J. NeuroEng. Rehabil. 2024, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Tjoa, E.; Guan, C. A Survey on Explainable Artificial Intelligence (XAI): Toward Medical XAI. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 4793–4813. [Google Scholar] [CrossRef]
- Luo, S.; Androwis, G.; Adamovich, S.; Nunez, E.; Su, H.; Zhou, X. Robust Walking Control of a Lower Limb Rehabilitation Exoskeleton Coupled with a Musculoskeletal Model via Deep Reinforcement Learning. J. NeuroEng. Rehabil. 2023, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Sambhus, R.; Gokce, A.; Welch, S.; Herron, C.W.; Leonessa, A. Real-Time Model-Free Deep Reinforcement Learning for Force Control of a Series Elastic Actuator. arXiv 2023, arXiv:2304.04911. [Google Scholar] [CrossRef]
- Kumar, R.; Muthukrishnan, S.P.; Kumar, L.; Roy, S. Predicting Multi-Joint Kinematics of the Upper Limb from EMG Signals Across Varied Loads with a Physics-Informed Neural Network. arXiv 2023, arXiv:2312.09418. [Google Scholar] [CrossRef]
- Duong, M.-D.; Pham, Q.-T.; Vu, T.-C.; Bui, N.-T.; Dao, Q.-T. Adaptive Fuzzy Sliding Mode Control of an Actuator Powered by Pneumatic Artificial Muscles. Sci. Rep. 2023, 13, 8242. [Google Scholar] [CrossRef] [PubMed]
- Della<monospace> </monospace>Santina, C.; Duriez, C.; Rus, D. Model-Based Control of Soft Robots: A Survey of the State of the Art and Open Challenges. IEEE Control Syst. 2023, 43, 30–65. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, W.; Lin, W.; Gao, Y. Soft Wearable Robots: Development Status and Technical Challenges. Sensors 2022, 22, 7584. [Google Scholar] [CrossRef]
- Zhao, W.; Song, A. Active Motion Control of a Knee Exoskeleton Driven by Antagonistic Pneumatic Muscle Actuators. Actuators 2020, 9, 134. [Google Scholar] [CrossRef]
- Polygerinos, P.; Wang, Z.; Overvelde, J.T.B.; Galloway, K.C.; Wood, R.J.; Walsh, C.J. Soft Robotic Glove for Combined Assistance and At-Home Rehabilitation. Robot. Auton. Syst. 2014, 73, 135–143. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, T.; Li, M. Model Predictive Control for Soft Ankle Rehabilitation Robot. Mechatronics 2023, 92, 103015. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, K.; Soltis, I.; Matthews, J.; Lee, Y.J.; Kim, H.; Romero, L.; Zavanelli, N.; Kwon, Y.; Kwon, S.; et al. Intelligent Upper-Limb Exoskeleton Integrated with Soft Wearable Bioelectronics and Deep Learning for Human Intention-Driven Strength Augmentation. npj Flex. Electron. 2024, 8, 11. [Google Scholar] [CrossRef]
- Sup, F.C.; Bohara, A.; Goldfarb, M. Design and Control of a Powered Transfemoral Prosthesis. Int. J. Rob. Res. 2008, 27, 263–273. [Google Scholar] [CrossRef]
- Li, Z.; Fang, Y.; Luo, Y.; He, H.; Wang, X. A Survey on Myoelectric Control for Lower Limb Prostheses. Sensors 2022, 22, 2281. [Google Scholar] [CrossRef]
- Fleming, A.; Bryson, B.J.; Boone, A.D.; Kudrick, S.C.; Bjorlie, M.J.; Lum, P.S.; Dingwell, J.B.; Moran, C.E. Neural prosthesis control restores near-normative neuromechanics in postural control. Sci. Robot. 2023, 4, eaef5758. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.D.; Lee, S.C.K.; Swanson, B.T.; Esposito, E.R. Toward Developing a Powered Ankle-Foot Prosthesis with Electromyographic Control to Enhance Functional Performance: A Case Study in a U.S. Service Member. Mil. Med. 2022, 187, e534–e541. [Google Scholar] [CrossRef]
- Ding, Y.; Udompanyawit, C.; Zhang, Y.; He, B. EEG-based Brain–Computer Interface Enables Real-time Robotic Hand Control at Individual Finger Level. Nat. Commun. 2025, 16, 5401. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A Comprehensive Review of EEG-Based Brain–Computer Interface Paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef]
- Śliwowski, W.; Leroy, A.; Hueber, T.; Lainé, A.; Fantenberg, M.; Serre, A.; Roche, N.; Jutten, C.; Mattout, J.; Guan, C.; et al. Adaptive LDA Classifier Enhances Real-Time Control of an EEG-Based Imagined Speech Brain–Computer Interface. Brain Sci. 2024, 14, 196. [Google Scholar] [CrossRef]
- Cheron, G.; Coenen, V.; Grishin, A.; Leurs, K.; Lourenço, A.P.; Ivanenko, Y. MindWalker: BCI-Controlled Lower-Limb Exoskeleton for Gait Assistance. Brain Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Bundy, D.T.; Pandarinath, C.; Kirchner, J.; Franklin, R.J.; Hart, J.; Tucker, R.; Broderick, B.; Botvinick, M.M.; Contreras-Vidal, J.L. BCI–Robot Hybrid Systems for Stroke Rehabilitation: Current Trends and Challenges. Front. Hum. Neurosci. 2020, 14, 162. [Google Scholar] [CrossRef]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain–Computer Interfaces in Medicine. Mayo Clin. Proc. 2012, 87, 268–279. [Google Scholar] [CrossRef]
- Tramontano, M.; Li, X.; Miskovic, P.; Ijspeert, A.; Gassert, R. Multimodal Sensor Integration in Robotic Neurorehabilitation: A Review. Sensors 2021, 21, 4563. [Google Scholar] [CrossRef]
- An, Y.; Li, Y.; Wang, H.; Duffield, R.; Su, S.W. Human–Machine Co-Adaptation for Robot-Assisted Rehabilitation via Dual-Agent Multiple-Model Reinforcement Learning. arXiv 2024, arXiv:2407.21734. [Google Scholar] [CrossRef]
- Ganesan, Y.; Gobee, S.; Durairajah, V. Development of an Upper Limb Exoskeleton for Rehabilitation with Feedback from EMG and IMU Sensor. Procedia Comput. Sci. 2015, 76, 53–59. [Google Scholar] [CrossRef]
- Dissanayake, H.; Wijesundara, P.; Gunaratne, H.; Samaranayake, T.; Perera, C. Sensor Fusion Framework Using EMG, IMU, and Visual Feedback in Upper-Limb Rehabilitation Robots. Sensors 2022, 22, 5236. [Google Scholar] [CrossRef]
- Bahador, N.; Ferreira, D.; Tamminen, S.; Kortelainen, J. Deep Learning–Based Multimodal Data Fusion: Case Study in Food Intake Episodes Detection Using Wearable Sensors. JMIR Mhealth Uhealth 2021, 9, e21926. [Google Scholar] [CrossRef]
- Nicora, G.; Pe, S.; Santangelo, G.; Billeci, L.; Aprile, I.G.; Germanotta, M.; Bellazzi, R.; Parimbelli, E.; Quaglini, S. Systematic Review of AI/ML Applications in Multi-Domain Robotic Rehabilitation: Trends, Gaps, and Future Directions. J. NeuroEng. Rehabil. 2025, 22, 79. [Google Scholar] [CrossRef]
- Banyai, A.D.; Brișan, C. Robotics in Physical Rehabilitation: Systematic Review. Healthcare 2024, 12, 1720. [Google Scholar] [CrossRef]
- Esquenazi, A.; Talaty, M. From Bench to Bedside: Translating Robotic Rehabilitation into Clinical Practice. NeuroRehabilitation 2021, 48, 145–157. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Jaworski, W.; Gancarczyk, M.; Winiarski, S.; Rykała, Ł.; Przybyła, A.; Rojek, A.; Tabor, D.; Krawczyk, A.; Kostka, P.; et al. OpenExo: An Open-Source Modular Exoskeleton to Augment Human Walking. Sci. Robot. 2025, 10, eadt1591. [Google Scholar] [CrossRef]
- Coppola, M.; Rossi, M.; Bianchi, F.; Santini, P.; Rossi, L.; Ferrari, P. AI at the Edge: Challenges and Strategies for On-Device Deep Learning in Wearable Rehab Systems. Sensors 2022, 22, 3221. [Google Scholar] [CrossRef]
- Schütz, S.; Nejadfard, A.; Reichardt, M.; Berns, K. FPGA-Based Embedded System Designed for the Deployment in the Compliant Robotic Leg CARL. In Proceedings of the 16th International Conference on Informatics in Control, Automation and Robotics-Volume 2: ICINCO, Prague, Czech Republic, 29–31 July 2019; pp. 537–543. [Google Scholar] [CrossRef]
- Morley, J.; Floridi, L.; Kinros, G.; Lewis, D.; Vandemeulebroucke, T. Ethics of AI in Health Care: A Mapping Review. J. Am. Med. Inform. Assoc. 2021, 28, 394–405. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Huang, G.; Fu, C. Explainability for Artificial Intelligence in Healthcare: A Comprehensive Assessment. BMC Med. Inform. Decis. Mak. 2020, 20, 263. [Google Scholar] [CrossRef]
- Amodei, D.; Olah, C.; Steinhardt, J.; Christiano, P.; Schulman, J.; Mané, D. Concrete Problems in AI Safety. Commun. ACM 2020, 63, 54–64. [Google Scholar] [CrossRef]
- Chavarrías, A.; Rodriguez-Cianca, D.; Lanillos, P. Adaptive Torque Control of Exoskeletons under Spasticity Conditions via Reinforcement Learning. arXiv 2025, arXiv:2503.11433. [Google Scholar] [CrossRef]
- Khan, A.; Patel, R.; Singh, A.; Zhao, L.; Tan, W. Fatigue Detection and Adaptive Assistance in Upper-Limb Exoskeletons Using EMG and AI. Sensors 2022, 22, 8491. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, S.S.; Lei, M.; Lim, L.S.; Johan, H.; Zuo, B.; Ang, W.T. A Human-In-The-Loop Simulation Framework for Evaluating Control Strategies in Gait Assistive Robots. arXiv 2025, arXiv:2503.05825. [Google Scholar] [CrossRef]
- Nicora, G.; Parimbelli, E.; Mauro, M.C.; Falchini, F.; Germanotta, M.; Fasano, A.; Sgandurra, G.; Beani, E.; Gruppioni, E.; Bugané, F.; et al. Healthcare Practitioners and Robotic-Assisted Rehabilitation: Understanding Needs and Barriers. J. NeuroEng. Rehabil. 2025, 22, 112. [Google Scholar] [CrossRef]
- IEC 60601-1:2005; Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance. International Electrotechnical Commission: Geneva, Switzerland, 2005.
- ISO 13482:2014; Robots and Robotic Devices—Safety Requirements for Personal Care Robots. International Organization for Standardization: Geneva, Switzerland, 2014.
- IEC 80601-2-78:2019; Medical Electrical Equipment—Part 2-78: Particular Requirements for Basic Safety and Essential Performance of Robotic Medical Equipment. International Electrotechnical Commission: Geneva, Switzerland, 2019.
- Regulation (EU) 2017/745; Medical Device Regulation (MDR). European Parliament and Council: Brussels, Belgium, 2017.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).